Abstract
Scirrhous gastric cancer is associated with abundant stroma and frequently develops into peritoneal carcinomatosis with malignant ascites. Although malignant ascites is among the most deadly diseases worldwide, its molecular pathogenesis is poorly understood. We investigated the role of hepatocyte growth factor (HGF) in the production of peritoneal carcinomatosis with malignant ascites. We examined three scirrhous and three non‐scirrhous human gastric cancer cell lines for the production of peritoneal carcinomatosis in vivo and responses to HGF in vitro. Furthermore, clinical scirrhous gastric cancer specimens were examined for HGF production. Among the six cell lines examined, only two scirrhous cell lines (NUGC4 and GCIY) produced peritoneal carcinomatosis with massive ascites after intraperitoneal injection in nude mice. Their proliferation was stimulated by exogenous HGF in vitro. On the other hand, a non‐scirrhous cell line, MKN45, with MET amplification generated peritoneal tumors but not ascites. MET tyrosine kinase inhibitors, crizotinib and TAS‐115, inhibited HGF‐stimulated proliferation of NUGC4 and GCIY as well as constitutive proliferation of MKN45. Furthermore, crizotinib and TAS‐115 prolonged the survival of mice bearing established tumors by NUGC4 or MKN45. In clinical specimens, HGF was markedly produced by stromal fibroblasts. Malignant ascitic fluids from patients with peritoneal carcinomatosis contained high levels of HGF. Our results strongly suggest that paracrine HGF‐induced activation of MET‐mediated signaling pathways plays an important role in the pathogenesis of peritoneal carcinomatosis in scirrhous gastric cancer. Thus, MET signaling pathway may be a potential therapeutic target for peritoneal carcinomatosis of gastric cancer, even without MET amplification.
We demonstrated the role of the paracrine HGF in peritoneal carcinomatosis with massive ascites produced in scirrhous gastric cancer, and the therapeutic potential of MET‐TKIs by using preclinical models. Clinical trials of MET‐TKIs for peritoneal carcinomatosis are therefore warranted to improve the prognosis of scirrhous gastric cancer.
Gastric cancer is the second leading cause of cancer‐related death worldwide. Scirrhous cancer, which accounts for 10% of all gastric cancers, is characterized by extensive stromal fibrosis with diffuse infiltration of cancer cells and often poorly differentiated adenocarcinoma cells.1 Scirrhous gastric cancer also frequently develops into peritoneal carcinomatosis, consisting of peritoneal dissemination of cancer cells and massive ascitic fluid, which lead to extremely poor prognoses.2, 3 However, the molecular pathogenesis by which scirrhous gastric cancer undergoes peritoneal carcinomatosis remains mostly unknown.
MET, a tyrosine kinase‐type receptor of hepatocyte growth factor (HGF), is widely expressed and activated in various types of solid tumors, including gastric cancer.4, 5 The activation of MET signaling pathways promotes migration, invasiveness, proliferation, and anti‐apoptotic activities,6 and its activation is associated with aggressive phenotypes.7, 8 MET is activated through several mechanisms, including the binding of its ligand, HGF, amplification of its gene, and transactivation by other receptors, such as epidermal growth factor receptor (EGFR).6, 9 MET amplification is a frequent molecular abnormality in gastric cancer.10 MET signaling is essential for the survival of MET amplification‐positive gastric cancer cells.11 Recent studies have indicated, however, that MET amplification is rare (2%) in advanced gastric cancers.12, 13 On the other hand, HGF is reportedly overexpressed in a large proportion of gastric cancers—elevated serum HGF is associated with poor prognoses14, 15—suggesting the involvement of HGF in the progression of gastric cancer. However, the role of HGF in peritoneal carcinomatosis in gastric cancer, especially scirrhous cancer, is not well defined. This study investigated the possible role of the HGF‐MET axis in the pathogenesis of peritoneal carcinomatosis caused by human gastric cancer.
Materials and Methods
Cell lines and reagents
Human gastric cancer cell lines NUGC4, OCUM‐1, MKN45, and MKN‐7 were obtained from the Health Science Research Resources Bank (Osaka, Japan). The GCIY line was purchased from the RIKEN Cell Bank (Tsukuba, Japan), and GCR1 was kindly provided by Dr Yoshio Endo (Cancer Research Institute, Kanazawa University, Kanazawa, Japan). The characteristics of these cell lines are shown in Table 1. All cell lines were maintained in RPMI‐1640 media supplemented with 10% fetal bovine serum, 100 units/mL penicillin G, and 100 units/mL streptomycin. Cells were regularly screened for Mycoplasma using a MycoAlert Mycoplasma Detection kit (Lonza). Recombinant human HGF was prepared as described previously.16 Goat anti‐human HGF neutralizing antibody, control antibody, and recombinant mouse HGF were purchased from R&D Systems (Minneapolis, MN, USA). Crizotinib was purchased from Selleck Chemicals (Houston, TX, USA). TAS‐115 was obtained from Taiho Pharmaceutical (Tsukuba, Japan).
Table 1.
Evaluation of tumorigenicity and the development or peritoneal carcinomatosis in nude mice
| Cell line | Histological typinga | Primary tumor typing | MET amplification | Tumorb formation | Peritonealc dissemination | Ascites |
|---|---|---|---|---|---|---|
| Route of injecton | s.c. | i.p. | ||||
| MKN45 | por | Not scirrhous | + | 5/5 | 5/5 | 0/5 |
| NUGC4 | por/sig | Scirrhous cancer | − | 5/5 | 5/5 | 5/5 (+) |
| GCIY | por/sig | Scirrhous cancer | − | 5/5 | 5/5 | 5/5 (+) |
| GCR1 | tub2 | Not scirrhous | − | 0/5 | 0/5 | 0/5 |
| MKN7 | tub1 | Not scirrhous | − | 0/5 | 0/5 | 0/5 |
| OCUM‐1 | por | Scirrhous cancer | − | 0/5 | 0/5 | 0/5 |
Histological typing: por, poorly differentiated adenocarcinoma; sig, signet ring cell carcinoma; tub1, well differentiated adenocarcinoma; tub2, moderately differentiated adenocarcinoma.
Animals were injected subcutaneously with 1 × 106 cells in 200 μL phosphate buffered saline (PBS). Four weeks later the animals were evaluated tumor formation at the flunk of nude mice. Number of mice with tumors/number of mice given injection.
Animals were injected intraperitoneally with 1 × 106 cells in 200 μL PBS. Four weeks later, the animals were sacrificed and evaluated peritoneal dissemination in the abdominal cavities of nude mice.
Tissue samples
Primary tumor tissue samples were obtained from the Division of Surgical Oncology, Cancer Research Institute, Kanazawa University, during gastrectomies of previously untreated gastric cancer patients. The histological diagnosis of each patient was confirmed with the specimens. In addition, primary cultured fibroblasts were isolated from surgical specimens of human stomachs and were identified through immunostaining with monoclonal antibodies against cytokeratin and vimentin. This study was approved by the ethical committee of Kanazawa University, and all patients provided written informed consent for the research use of their samples.
Proliferation assay
Cell proliferation was measured using the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) dye reduction method.17 Tumor cells were seeded at 5 × 103 per well in 96‐well plates and incubated at 37°C. After 6 h culture, crizotinib, TAS‐115, HGF, mouse‐HGF, anti‐HGF neutralizing antibody, or control immunoglobulin G was added to each well at indicated concentrations, and the culture was continued for an additional 72 h. Next, 50 μL of MTT solution (2 mg/mL; Sigma, St. Louis, MO, USA) was added to each well and incubated for 2 h at 37°C. The media were removed, and the dark blue crystals in each well were dissolved in 100 μL of membrane dimethyl sulfoxide. Absorbance was measured on a microplate reader at test and reference wavelengths of 550 and 630 nm, respectively. The percentage of growth is shown relative to untreated controls. Each experiment was performed at least three times independently.
Western blot analysis
Cells were cultured in RPMI 1640 supplemented with 0.1% fetal bovine serum in the presence or absence of HGF and/or TAS‐115 or crizotinib. After the indicated treatments, cell lysates were prepared with sample buffer (20 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L ethylene glycol tetraacetic acid, 1% Triton X‐100, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β‐glycerophosphate, 1 mmol/L Na3VO4, 1 μg/mL leupeptin, and 1 mmol/L phenylmethylsulfonyl fluoride) and flash‐frozen on dry ice. For western blotting assays, cell lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a membrane (Bio‐Rad, Hercules, CA, USA). Membranes were blocked with Blocking One (Nacalai Tesque, Kyoto, Japan) for 1 h at room temperature and then incubated at 4°C overnight with anti‐MET (25H2), anti‐phospho‐MET (Y1234/Y1235;3D7), anti‐Akt, and anti‐phospho‐Akt (Ser473) in 1:1000 dilution (Cell Signaling Technology, Danvers, MA, USA); anti‐human/mouse/rat extracellular signal‐related kinase (ERK)1/2 (0.2 μg/mL) and anti‐phospho‐ERK1/2 (T202/Y204; 0.1 μg/mL) (R&D Systems); or anti‐GAPDH antibody (Trevigen, Gaithersburg, MD, USA). After 3 washes, membranes were incubated for 1 h at room temperature with horseradish peroxidase‐conjugated species‐specific antibodies. Immunoreactive bands were visualized with SuperSignal West Dura Extended Duration Substrate enhanced chemiluminescence substrate (Pierce Biotechnology, Rockford, IL, USA).
Animal study
Pathogen‐free, 6‐week‐old Balb/c (nu/nu) mice were obtained from Japan Clea (Tokyo, Japan) and quarantined for 1 week under pathogen‐free conditions at the Advanced Science Research Center of Kanazawa University. All animal experiments complied with the guidelines for the care and use of laboratory animals of Kanazawa University. Tumorigenicity and experimental peritoneal carcinomatosis were established via subcutaneous (s.c.) and intraperitoneal (i.p) inoculations, respectively, of 1 × 106 human gastric cancer cell line cells in 200 μL phosphate‐buffered saline (PBS) into nude mice. Tumor growth was measured weekly after s.c. injection, and peritoneal tumors and/or formation of ascitic fluid were determined 4 weeks later. The mice were killed and peritoneal tumors were evaluated from the number of tumor nodules in the greater omentum and the mesentery.
To determine the inhibitory effects of the MET tyrosine kinase inhibitors (TKIs) crizotinib and TAS‐115, mice were injected i.p. with 1 × 106 cells of NUGC4 or MKN45 in 200 μL PBS. Mice were treated with crizotinib (25 mg/kg per day), TAS‐115 (200 mg/kg per day), or vehicle alone via oral gavage 3–4 weeks after recognizable macroscopic peritoneal tumors and/or formation of ascitic fluid in the peritoneal cavity. To monitor the extent of peritoneal carcinomatosis, we measured body weight routinely.
Enzyme‐linked immunosorbent assay
The concentrations of human HGF and mouse HGF were measured using Quantikine ELISA kits (R&D Systems) according to the manufacturer protocol. Optical density at 450 nm was measured on an ELISA plate reader (Bio‐Rad).
Immunohistochemistry
Surgical specimens of primary gastric tumors were routinely fixed in formalin and embedded in paraffin. Tissue sections (3 μm) were deparaffinized in xylene, rehydrated in an ethanol series, and treated for 30 min with 0.3% hydrogen peroxide to block endogenous peroxidase activity. Sections were subsequently washed with PBS and unmasked in citrate antigen unmasking solution (Target Retrieval Solution High pH; DakoCytomation, Carpinteria, CA, USA) in an autoclave for 20 min at 120°C or for 20 min at 95°C. Sections were immunohistochemically stained with goat anti‐human HGF polyclonal antibody AF‐294‐NA (R&D Systems) or polyclonal rabbit anti‐MET antibody (Invitrogen, Carlsbad, CA, USA) using a standard indirect avidin‐biotin horseradish peroxidase method. Sections were considered positive when the intensity of staining was ≥10%.
Statistical analysis
Statistical analysis was done using GraphPad Prism Ver. 5.01 (GraphPad Software, San Diego, CA, USA); Comparisons were made using two‐tailed Student's t‐test and significant difference was defined as P < 0.05. Data are shown as mean ± SE.
Results
Production of peritoneal carcinomatosis with malignant ascites by human scirrhous gastric cancer cell lines in nude mice
We examined the generation of peritoneal carcinomatosis in six human gastric cancer cell lines. As summarized in Table 1, only NUGC4 and GCIY, derived from scirrhous gastric cancer patients, developed peritoneal tumors with massive ascites after i.p. inoculation into nude mice, strikingly mimicking the clinical phenotype of scirrhous gastric cancer. On the other hand, MKN45, a non‐scirrhous gastric cancer cell line with MET amplification, produced only peritoneal tumors.
Exogenous HGF‐stimulated proliferation of scirrhous gastric cancer cell lines that produced peritoneal tumors with massive ascites
We first examined autocrine HGF production in the gastric cancer cell lines. We performed western blotting of the cell lysates and ELISA of the conditioned media. The HGF protein was barely detectable in any of the six human gastric cancer cell lines tested, whereas normal fibroblasts produced a large amount of HGF (Fig. S1).
We next examined the effect of exogenous HGF on proliferation of the gastric cancer cell lines (Fig. 1a–c). Hepatocyte growth factor enhanced proliferation of only NUGC4 and GCIY, which generated peritoneal carcinomatosis with massive ascites in nude mice (Fig. 1b,c). On the other hand, HGF had no effect on MKN45 with MET amplification or on the other gastric cancer cell lines (GCR1, MKN7, OCUM‐1) (Fig. 1a,c). Moreover, we evaluated the effect of HGF on anoikis/anchorage‐independent growth as well as cell migration for NUGC4, GCIY, and MKN45 to demonstrate the role of HGF and MET in other phenotypes associated with the formation of peritoneal metastasis and ascites. Hepatocyte growth factor also enhanced proliferation of NUGC4 and GCIY cells, even in an anoikis/anchorage‐independent growth assay. Hepatocyte growth factor also had no effect on MKN45 with MET amplification (Fig. S2). Moreover, on cell migration assay, all gastric cancer cell lines examined except OCUM‐1 showed significant migratory responses to HGF (Fig. S3).
Figure 1.
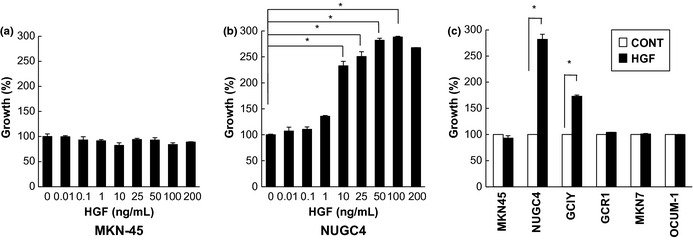
Hepatocyte growth factor (HGF) enhances the proliferation of human gastric cancer cell lines. A non‐scirrhous cell line with MET amplification, MKN45 (a), and a scirrhous cancer cell line without MET amplification, NUGC4 (b), were treated with increasing concentrations of HGF, and cell growth was determined after 72 h using an 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay. (c) Six human gastric cancer cell lines (MKN45, NUGC4, GCIY, GCR1, MKN7, and OCUM‐1) were treated with HGF (50 ng/mL), and cell growth was determined after 72 h using MTT assay. Representative results from three independent experiments are shown. Bars are SDs. *P < 0.01.
We then examined the effect of the MET‐TKIs crizotinib and TAS‐115 on HGF‐enhanced proliferation of NUGC4 and GCIY, as well as on the high baseline proliferation of MKN45 (Fig. 2a). Both crizotinib and TAS‐115 potently inhibited the HGF‐enhanced proliferation of NUGC4 and GCIY as well as the high baseline proliferation of MKN45. We also confirmed that anti‐HGF antibody blocked HGF‐enhanced but not baseline proliferations of NUGC4 and GCIY or the high baseline proliferation of MKN45 (Fig. 2b).
Figure 2.
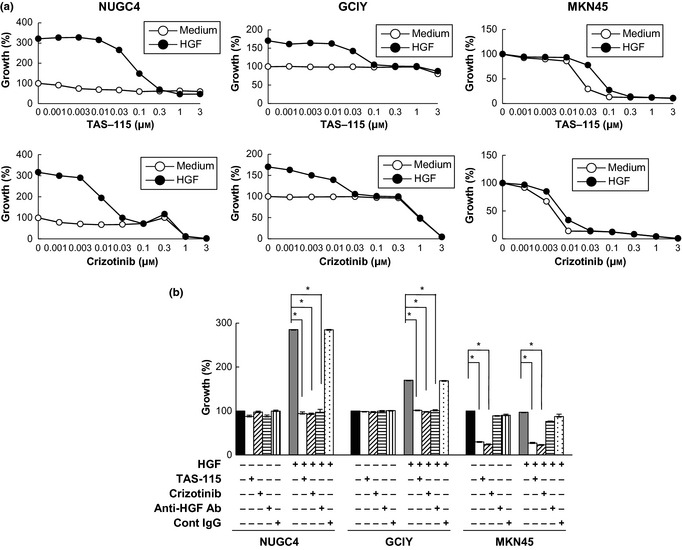
In vitro effect of MET‐tyrosine kinase inhibitors (MET‐TKIs) on cell proliferation of gastric cancer cell lines. The MET‐TKIs crizotinib and TAS‐115 inhibited hepatocyte growth factor (HGF)‐stimulated proliferation of NUGC4 and GCIY, the scirrhous gastric cancer cell lines whose proliferation was stimulated by exogenous HGF, as well as the high baseline proliferation of MKN45, a non‐scirrhous gastric cancer cell line harboring MET amplification. (a) Dose‐dependent growth inhibition of HGF‐stimulated proliferation of NUGC4 and GCIY. High baseline proliferation of MKN45 was also inhibited. (b) Effects of the MET‐TKIs crizotinib (0.1 μmol/L) and TAS‐115 (1 μmol/L) and a neutralizing anti‐HGF antibody (Ab; 5 μg/mL) on HGF‐stimulated proliferation of NUGC4 and GCIY and their baseline proliferation and the high baseline proliferation of MKN45. Representative results from three independent experiments are shown. Bars are SDs. *P < 0.01.
Effect of exogenous HGF on MET phosphorylation and MET‐mediated intracellular signaling
We next examined the effect of exogenous HGF on MET‐mediated intracellular signaling in the six gastric cancer cell lines using western blot analysis (Fig. 3a). MKN45 with MET amplification expressed a large amount of MET protein, which was also constitutively highly phosphorylated and unaffected by exogenous HGF. By contrast, HGF stimulated MET phosphorylation in the other five cells lines, although the degree of phosphorylation varied among them. Furthermore, HGF stimulated the phosphorylation of Akt and ERK in these five cell lines. Both crizotinib and TAS‐115 suppressed HGF‐induced MET phosphorylation in NUGC4 and GCIY cells, as well as constitutive MET phosphorylation in MKN45 with MET amplification (Fig. 3b). Furthermore, crizotinib and TAS‐115 inhibited ERK and Akt phosphorylation in HGF‐stimulated NUGC4 and GCIY cells, as well as the constitutive phosphorylation of ERK and Akt in MKN45 (Fig. 3b).
Figure 3.
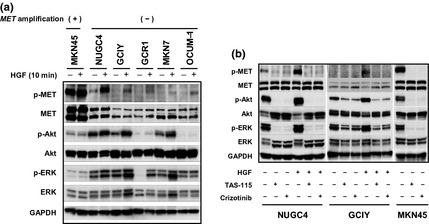
Immunoblotting analysis of MET, Akt, and extracellular signal‐related kinase (ERK) phosphorylations. (a) Six human gastric cancer cell lines were seeded in 10‐cm dishes and stimulated with hepatocyte growth factor (HGF) (50 ng/mL) for 10 min. Whole cell lysates were electrophoresed, blotted onto filter membranes, and probed with primary antibodies against phospho‐MET, MET, phospho‐Akt, Akt, phospho‐ERK, ERK, and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH). (b) NUGC4, GCIY, and MKN45 were treated with or without crizotinib (0.1 μmol/L) or TAS‐115 (1 μmol/L) and/or HGF (50 ng/mL) for 1 h. The cell lysates were harvested, and phosphorylation of indicated proteins was determined with immunoblotting analysis. Representative results from three independent experiments with similar results are shown.
Crizotinib and TAS‐115 prolonged the survival of nude mice inoculated with NUGC4 or MKN45
The therapeutic effects of the MET inhibitors were evaluated in nude mice inoculated i.p. with NUGC4 or MKN45. Daily oral treatments were initiated after the development of peritoneal tumors and/or malignant ascites, mimicking clinical situations of treatment for inoperable scirrhous gastric cancer. In each model, crizotinib and TAS‐115 were well tolerated at 25 mg/kg per day and 200 mg/kg per day, respectively, for as long as 2 months with no evidence of overt toxicity.
In the NUGC4 model, control mice developed discernible ascites as early as day 21 and all died within 60 days after tumor inoculation. Under these conditions, both crizotinib and TAS‐115 administration, which started after the development of peritoneal carcinomatosis with discernible ascites, decreased ascites gradually within 14 days and significantly prolonged survival compared to that in control mice (Fig. 4a). In the MKN45 model, although both crizotinib and TAS‐115 were associated with no significant difference in survival compared to outcomes in control mice, they prolonged survival even when treatments were initiated after the development of peritoneal tumors (Fig. 4a).
Figure 4.
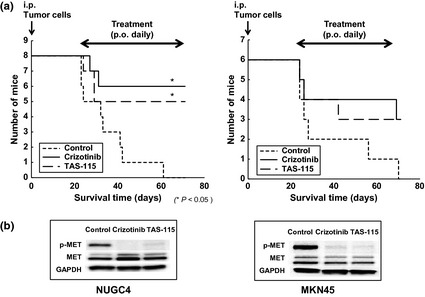
The MET‐tyrosine kinase inhibitors (MET‐TKIs) crizotinib and TAS‐115 prolonged survival of nude mice inoculated with NUGC4 or MKN45. NUGC4 (1.0 × 106 cells) or MKN45 (1.0 × 106 cells) were inoculated intraperitoneally (i.p.) into nude mice. After confirmation of peritoneal tumors with ascitic fluid formation for NUGC4 or peritoneal tumors for MKN45 3 weeks after i.p. inoculation, mice were treated orally (p.o.) with crizotinib (25 mg/kg per day) or TAS‐115 (200 mg/kg per day). (a) Survival of nude mice inoculated with NUGC4 or MKN45. Real survival curves according to the Kaplan–Meier method and log‐rank test analysis. (b) Effects of crizotinib and TAS‐115 on MET phosphorylation in NUGC4 and MKN45 tumors. Tumors were harvested on day 4 of MET‐TKIs administration after the development of peritoneal tumors and/or malignant ascites, and phosphorylated MET and MET protein contents were detected with immunoblotting using anti‐phosphotyrosine antibody and anti‐MET antibody, respectively.
To confirm the effects of crizotinib and TAS‐115, we evaluated NUGC4 and MKN45 tumors for MET phosphorylation 4 days after initiating the treatments of MET‐TKIs crizotinib and TAS‐115. Using western blotting analysis, we demonstrated that crizotinib (25 mg/kg per day) and TAS‐115 (200 mg/kg per day) remarkably inhibited MET phosphorylation in NUGC4 and MKN45 tumors (Fig. 4b).
We further determined the levels of human HGF and mouse HGF in ascites that developed in nude mice inoculated with NUGC4. Interestingly, high levels of mouse HGF, but not human HGF, were detected in the ascites (Fig. S4A), indicating that mouse HGF was produced by host stromal cells. We therefore examined whether mouse HGF could stimulate proliferation of NUGC4 in vitro. Indeed, mouse HGF stimulated proliferation of NUGC4 and GCIY cells in a dose‐dependent manner (Fig. S4B and S4D). We also confirmed that crizotinib and TAS‐115 inhibited proliferation of NUGC4 and GCIY when stimulated by mouse HGF (Fig. S4C and S4E). Collectively, our results indicate that the proliferation and formation of ascites by NUGC4 in nude mice is likely to be stimulated by mouse HGF produced by host stromal cells in a paracrine manner.
Production of HGF by stromal fibroblasts and presence of HGF in malignant ascites in human gastric cancers
To determine the clinical relevance of the HGF‐MET axis in peritoneal carcinomatosis of gastric cancer, we performed immunohistochemical staining of MET and HGF in 23 stage IV scirrhous‐type gastric cancers with peritoneal carcinomatosis. Representative results are shown in Figure 5. Of the 23 scirrhous‐type primary gastric tumors, 17 (73.5%) scored positively for MET. By contrast, none (0%) of the 23 primary tumors scored positively for HGF. On the other hand, HGF scored positively in stromal cells in 20 (87.0%) primary tumors. We also examined the HGF protein content in malignant ascitic fluids. We found higher concentrations of HGF in malignant ascites from patients with gastric cancer (mean, 1.45 ng/mL; range 0.47–2.63, n = 20) compared to those in nonmalignant peritoneal exudate fluids (mean, 0.80 ng/mL; range, 0.55–0.96, n = 10). These results support the notion that HGF expressed by stromal fibroblasts promotes the development of peritoneal carcinomatosis in gastric cancer in a paracrine manner.
Figure 5.
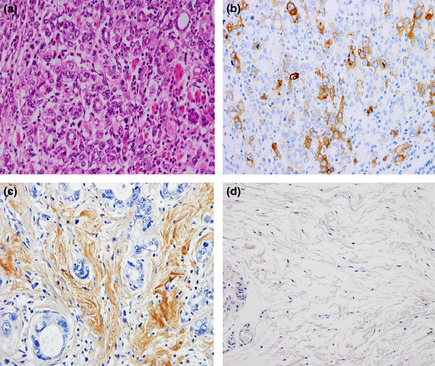
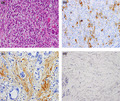
Immunohistochemical staining of MET and hepatocyte growth factor (HGF) in human primary gastric tumors and of HGF in normal human gastric tissues. Anti‐MET, anti‐HGF, and H&E staining of a representative MET‐ and HGF‐positive primary gastric tumors with peritoneal dissemination (a–c), and representative HGF‐negative normal human gastric tissues (d). MET was strongly immunolocalized in the membrane and was weakly immunolocalized in the cytoplasm of primary gastric cancer cells (b). HGF was strongly immunolocalized in the cytoplasm of stromal fibroblasts but scarcely so in gastric cancer cells (c). HGF was barely detectable in human stromal fibroblasts in normal human gastric tissues (d). Magnification, 200×.
Discussion
We previously showed the involvement of the CXCL12‐CXCR4 chemokine axis and EGFR activation by EGFR ligands in the development of peritoneal carcinomatosis with ascites in scirrhous gastric cancer.18, 19 In this study, we have further demonstrated the critical role of MET activation by HGF in this process. Interestingly, only the scirrhous gastric cancer cell lines (NUGC4 and GCIY) developed peritoneal tumors with massive ascites (Table 1 and Fig. 1). Neither NUGC4 nor GCIY produced HGF (Fig. S1). Exogenous HGF enhanced proliferation of NUGC4 and GCIY and induced phosphorylation of MET, Akt, and ERK (Fig. 3). The MET‐TKIs crizotinib and TAS‐115 inhibited HGF‐stimulated proliferation in vitro and prolonged the survival of nude mice bearing peritoneal tumors and ascites (Fig. 4). A high level of mouse HGF, but not human HGF, was detected in malignant ascites that developed in nude mice (Fig. S4A). Although HGF is believed to induce species‐specific biological activities,20 mouse HGF efficiently, but slightly less effectively than human HGF, stimulated proliferation of NUGC4 and GCIY in vitro (Fig. S4B and D). These findings indicate that paracrine HGF produced by host stromal cells activated ERK and Akt via MET phosphorylation, and thereby stimulated proliferation of NUGC4 and GCIY, which facilitated the development of peritoneal tumors with massive ascites (Fig. S5). On the other hand, another scirrhous gastric cancer cell line, OCUM‐1, did not produce peritoneal tumors or ascites (Table 1). Although HGF discernibly stimulated MET phosphorylation, it did not enhance ERK and Akt phosphorylation (Fig. 3a). Therefore, HGF‐induced activation of ERK and Akt may be necessary for scirrhous gastric cancer cells to produce peritoneal tumors and ascites.
MKN45, a non‐scirrhous gastric cancer cell line, harbors MET amplification and is known to produce peritoneal tumors after intraperitoneal inoculation.21 Amplified MET was constitutively highly phosphorylated, and MET‐TKIs dramatically reduced the survival of this cell line (Figs. 2 and 3). Therefore, MKN45 is addicted to signals from the amplified MET. Interestingly, whereas the amplified MET was highly phosphorylated, the levels of phosphorylated ERK and Akt were relatively low compared with those in other gastric cancer cell lines (Fig. 3). Moreover, phosphorylations of MET, ERK, and Akt in MKN45 were not further stimulated by exogenous HGF. Thus, MET is already fully activated in MKN45, and HGF is unable to further stimulate its phosphorylation and downstream signal transduction (Fig. S5). It is also unclear at present why MKN45 cells failed to produce ascites even though they efficiently developed peritoneal tumors in nude mice. One possibility is that amplified MET and paracrine HGF‐activated MET might differ with respect to their downstream signals. There may also be other important differences between scirrhous and non‐scirrhous gastric cancer cell lines. Understanding such differences will provide valuable information for the development of new strategies to control malignant ascites caused by gastric cancer.
MET can be activated by various mechanisms, including binding by its ligand, HGF, amplification of the MET gene itself, and transactivation by other receptors, such as EGFR.6, 9 We observed that although human scirrhous gastric cancer NUGC4 cells did not show MET amplification or produce detectable levels of HGF, MET was phosphorylated at an appreciable level (Fig. 3a). We also observed that the MET‐TKIs crizotinib and TAS‐115 inhibited baseline phosphorylations of Akt and ERK in NUGC4 and GCIY cells in the absence of HGF (Fig. 3b). We found that proliferation activity at basal levels with no stimulation was scarcely abrogated by pretreatment with anti‐HGF neutralizing antibody in NUGC4 and GCIY cells (Fig. 2b), suggesting spontaneous transactivation of MET in these scirrhous gastric cancer cells. As we reported previously,18 NUGC4 cells express EGFR and its ligand amphiregulin in an autocrine manner and the mean concentration of amphiregulin protein was markedly high in malignant ascites from gastric cancer patients (3.06 ng/mL). GCIY cells also expressed EGFR and its ligand amphiregulin (data not shown). Human scirrhous gastric cancer NUGC4 and GCIY cells produced peritoneal tumors and massive ascites in nude mice upon i.p. inoculation. These results suggested that MET activation by transactivation with the EGFR ligand amphiregulin may be the other activation pathway of MET and may be necessary for survival of scirrhous gastric cancer cells in the development of peritoneal carcinomatosis.
Crizotinib has been approved in several countries, including the United States and Japan, for treatment of lung cancer with anaplastic lymphoma kinase rearrangements. It also shows activity against ROS1, and a recent report has shown remarkable responses of crizotinib in lung cancers with ROS1 rearrangements.22 Moreover, crizotinib has high inhibitory activity to MET because it was initially developed as a MET‐TKI.23 Recent studies have demonstrated remarkable clinical responses of crizotinib in various tumor types (esophagus, lung, and brain) with MET amplification.11, 24, 25 Herein, we report that crizotinib, as well as another orally available MET‐TKI, TAS‐115, prolonged survival both in mice bearing peritoneal tumors with MET‐amplified MKN45 and in mice bearing peritonitis with massive ascites caused by scirrhous gastric cancer cell lines (Fig. 4). Importantly, these compounds showed remarkable survival prolongation even when treatment was initiated after the development of peritoneal tumors with or without massive ascites. We also confirmed that NUGC4‐inoculated mice receiving MET‐TKIs before the development of discernible ascites lived significantly longer without ascites than mice treated with MET‐TKIs after ascites occurrence (Fig. S6). These observations suggest that the compounds have therapeutic potential for inoperable scirrhous gastric cancer with peritoneal carcinomatosis. Patient selection with MET‐related biomarkers, such as elevated HGF concentrations in ascites, MET amplification in cancer cells, and HGF‐induced MET phosphorylation in cancer cells, may be useful in the successful clinical applications of these compounds. We are now working to establish simple assays to evaluate susceptibility to MET‐TKIs or HGF‐stimulated proliferation of cancer cells under ex vivo conditions.
In conclusion, we demonstrated the role of the paracrine HGF in peritoneal carcinomatosis with massive ascites produced in scirrhous gastric cancer, and the therapeutic potential of MET‐TKIs by using preclinical models. Clinical trials of MET‐TKIs for peritoneal carcinomatosis are therefore warranted to improve the prognosis of scirrhous gastric cancer.
Disclosure Statement
Seiji Yano received a research grant from the Taiho Pharmaceutical Co., Ltd.
Supporting information
Fig. S1. Expression of hepatocyte growth factor (HGF) protein in human gastric cancer cell lines and normal stromal fibroblasts.
Fig. S2. The effects of hepatocyte growth factor (HGF) on anoikis/anchorage‐independent growth in the NUGC4, GCIY and MKN45 cell lines.
Fig. S3. Hepatocyte growth factor (HGF) induces cell migratory responses in human gastric cancer cells.
Fig. S4. Mouse hepatocyte growth factor (HGF) efficiently stimulates proliferation of NUGC4 and GCIY in vitro.
Fig. S5. Model of the distinct mechanisms of MET phosphorylation in human gastric cancer cells with or without MET amplification.
Fig. S6. Survival data for NUGC4‐inoculated mice treated with MET inhibitors before the development of discernable ascites.
Acknowledgements
This study was supported by Grants‐in‐Aid of Cancer Research (K. Yasumoto, 23591921) and Scientific Research on Innovative Areas “Integrative Research on Cancer Microenvironment Network” (S. Yano, 22112010) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
(Cancer Sci 2013; 104: 1640–1646)
References
- 1. Duarte I, Llanos O. Patterns of metastases in intestinal and diffuse types of carcinoma of the stomach. Hum Pathol 1981; 12: 237–42. [DOI] [PubMed] [Google Scholar]
- 2. Maehara Y, Moriguchi S, Kakeji Y et al Pertinent risk factors and gastric carcinoma with synchronous peritoneal dissemination or liver metastasis. Surgery 1991; 110: 820–3. [PubMed] [Google Scholar]
- 3. Takahashi I, Matsusaka T, Onohara T et al Clinicopathological features of long–term survivors of scirrhous gastric cancer. Hepatogastroenterology 2000; 47: 1485–8. [PubMed] [Google Scholar]
- 4. Huang TJ, Wang JY, Lin SR, Lian ST, Hsieh JS. Overexpression of the c‐met protooncogene in human gastric carcinoma – Correlation to clinical features. Acta Oncol 2001; 40: 638–43. [DOI] [PubMed] [Google Scholar]
- 5. Inoue T, Kataoka H, Goto K et al Activation of c‐Met (hepatocyte growth factor receptor) in human gastric cancer tissues. Cancer Sci 2004; 95: 803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trusolino L, Berrotti A, Comoglio PM. MET signaling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 2010; 11: 834–48. [DOI] [PubMed] [Google Scholar]
- 7. Tsugawa K, Yonemura Y, Hirono Y et al Amplification of the c‐met, c‐erbB‐2 and epidermal growth factor receptor gene in human gastric cancers: correlation to clinical features. Oncology 1998; 55: 475–81. [DOI] [PubMed] [Google Scholar]
- 8. Graziano F, Galluccio N, Lorenzini P et al Genetic activation of the MET pathway and prognosis of patients with high‐risk, radically resected gastric cancer. J Clin Oncol 2011; 29: 4789–95. [DOI] [PubMed] [Google Scholar]
- 9. Fischer OM, Giordano S, Comoglio PM, Ullrich A. Reactive oxygen species mediates Met receptor transactivation by G protein‐coupled receptors and the epidermal growth factor receptor in human carcinoma cells. J Biol Chem 2004; 279: 28970–8. [DOI] [PubMed] [Google Scholar]
- 10. Kuniyasu H, Yasui W, Kitadai Y, Yokozaki H, Ito H, Tahara E. Frequent amplification of the c‐met gene in scirrhous type stomach cancer. Biochem Biophys Res Commun 1992; 189: 227–32. [DOI] [PubMed] [Google Scholar]
- 11. Okamoto W, Okamoto I, Arao T et al Antitumor action of the MET tyrosine kinase inhibitor crizotinib (PF‐02341066) in gastric cancer positive for MET amplification. Mol Cancer Ther 2012; 11: 1557–64. [DOI] [PubMed] [Google Scholar]
- 12. Lennerz JK, Kwak EL, Ackerman A et al MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol 2011; 29: 4803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janjigian YY, Tang LH, Coit DG et al MET expression and amplification in patients with localized gastric cancer. Cancer Epidermiol Biomarkers Prev 2011; 20: 1021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amemiya H, Kono K, Itakura J et al c‐Met expression in gastric cancer with liver metastasis. Oncology 2002; 63: 286–96. [DOI] [PubMed] [Google Scholar]
- 15. Park WS, Oh RR, Kim YS et al Absence of mutations in the kinase domain of the Met gene and frequent expression of Met and HGF/SF protein in primary gastric carcinomas. APMIS 2000; 108: 195–200. [DOI] [PubMed] [Google Scholar]
- 16. Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast‐derived epithelial morphogen as hepatocyte growth factor. Cell 1991; 67: 901–8. [DOI] [PubMed] [Google Scholar]
- 17. Green LM, Reade JL, Ware CF. Rapid colorimetric assay for cell viability: application to the quantitation of cytotoxic and growth inhibitory lymphokines. J Immunol Methods 1984; 70: 257–68. [DOI] [PubMed] [Google Scholar]
- 18. Yasumoto K, Koizumi K, Kawashima A et al Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res 2006; 66: 2181–7. [DOI] [PubMed] [Google Scholar]
- 19. Yasumoto K, Yamada T, Kawashima A et al The EGFR ligands amphiregulin and heparin‐binding EGF‐like growth factor promote peritoneal carcinomatosis in CXCR4‐expressing gastric cancer. Clin Cancer Res 2011; 17: 3619–30. [DOI] [PubMed] [Google Scholar]
- 20. Bhargava M, Joseph A, Knesel J et al Scatter factor and hepatocyte growth factor: activities, properties, and mechanism. Cell Growth Differ 1992; 3: 11–20. [PubMed] [Google Scholar]
- 21. Nakagawa T, Tohyama O, Yamaguchi A et al E7050: a dual c‐Met and VEGFR‐2 tyrosine kinase inhibitor promotes tumor regression and prolongs survival in mouse xenograft models. Cancer Sci 2010; 101: 210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bergethon K, Shaw AT, Ou SH et al Ros1 rearrangements define a unique molecular class of lung cancer. J Clin Oncol 2012; 30: 863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zou HY, Li Q, Lee JH et al An orally available small‐molecule inhibitor of c‐Met, PF‐2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 2007; 67: 4408–17. [DOI] [PubMed] [Google Scholar]
- 24. Chi AS, Batchelor TT, Kwak EL, Clark JW, Wang DL. Rapid radiographic and clinical improvement after treatment of a MET‐amplified recurrent glioblastoma with a mesenchymal‐epithelial transition inhibitor. J Clin Oncol 2012; 30: 30–3. [DOI] [PubMed] [Google Scholar]
- 25. Ou SH, Kwak EL, Siwak‐Tapp C et al Activity of crizotinib (PF02341066), a dual mesenchymal‐epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non‐small cell lung cancer patient with de novo MET amplification. J Thorac Oncol 2011; 6: 942–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Expression of hepatocyte growth factor (HGF) protein in human gastric cancer cell lines and normal stromal fibroblasts.
Fig. S2. The effects of hepatocyte growth factor (HGF) on anoikis/anchorage‐independent growth in the NUGC4, GCIY and MKN45 cell lines.
Fig. S3. Hepatocyte growth factor (HGF) induces cell migratory responses in human gastric cancer cells.
Fig. S4. Mouse hepatocyte growth factor (HGF) efficiently stimulates proliferation of NUGC4 and GCIY in vitro.
Fig. S5. Model of the distinct mechanisms of MET phosphorylation in human gastric cancer cells with or without MET amplification.
Fig. S6. Survival data for NUGC4‐inoculated mice treated with MET inhibitors before the development of discernable ascites.


