Abstract
Retrospective studies have suggested that UDP‐glucuronosyltransferase (UGT)1A1,UGT1A7, and UGT1A9 predict severe toxicity and efficacy of irinotecan‐containing regimens. We prospectively evaluated the impact of UGT1A genotypes and haplotypes on severe toxicity and efficacy in patients treated with fluorouracil, leucovorin, and irinotecan combination chemotherapy (FOLFIRI) for metastatic colorectal cancer (mCRC) from the two prospective multicenter phase II studies in Japan. The FLIGHT1 study was a first‐line FOLFIRI trial, and FLIGHT2 was a FOLFOX‐refractory, second‐line FOLFIRI trial. A total of 73 patients agreed to additional analysis, and were genotyped for UGT1A polymorphisms, UGT1A1*28 (TA6>TA7), UGT1A1*6 (211G>A), UGT1A1*27 (686C>A), UGT1A1*60 (−3279T>G), UGT1A1*93 (−3156G>A), UGT1A7 (−57T>G), UGT1A7*3 (387T>G, 622T>C), and UGT1A9*22 (T9>T10). Of 73 patients, 34 developed G3/4 severe hematological toxicities. The toxicities were significantly more frequent in patients with UGT1A1*6 (211A), UGT1A7 (387G), and UGT1A9*22 reference alleles (T9). Haplotype I, which consists of all favorable alleles, was associated with a significant reduction in hematologic toxicity (P = 0.031). In contrast, haplotype II, which contains four high‐risk alleles, showed significantly higher hematologic toxicity than the other haplotypes (P = 0.010). Six out of seven patients who were homozygous for UGT1A1*28 or *6 experienced severe hematological toxicity despite the fact that their response rate was not impaired (42.9%). We concluded that UGT1A polymorphisms, especially UGT1A1*6, are important for the prediction of severe toxicity of FOLFIRI in northeast Asian populations. In this regard, haplotype analyses should substantially impact the prediction of severe hematological toxicities of FOLFIRI. (Clinical Trial Registration: UMIN000002388 and UMIN000002476).
The toxicities were significantly more frequent in patients with UGT1A1*6, UGT1A7 (387G), and UGT1A9*22 reference alleles (T9). Six out of 7 patients who were homozygous for UGT1A1*28 or *6 experienced severe hematological toxicity despite the fact that their response rate was not impaired (42.9%).

Irinotecan with continuous fluorouracil plus leucovorin (FOLFIRI) has been approved as a first‐line therapy for metastatic colorectal cancer (mCRC).1, 2, 3 Although this regimen can result in prolonged survival, 20–35% of FOLFIRI‐treated patients develop severe neutropenia. Irinotecan is activated by hydrolysis to SN‐38, a potent topoisomerase I inhibitor that is primarily inactivated through biotransformation into SN‐38 glucuronide (SN‐38G) by UDP‐glucuronosyltransferase (UGT)1A1.4, 5 The toxicity of irinotecan is correlated to polymorphisms in the number of TA repeats in the promoter region of UGT1A1*28 that affect transcriptional efficiency.6 Other UGT1A polymorphisms are related to the efficiency of the detoxification of SN‐38. The UGT1A9 isoform contributes to SN‐38 glucuronidation.7 The *22 variant (a T insert at position_118) of hepatic UGT1A9 is associated with increased gene expression8 and reduced hematologic toxicity.9 The UGT1A7 isoform is mainly expressed in the gastrointestinal tract, and a gene variant with impaired enzyme function is UGT1A7*3. The effect of the UGT1A7 polymorphisms on irinotecan pharmacology has been studied.10, 11, 12, 13 Recently, the toxicity and tumor response of FOLFIRI has been correlated with the UGT1A1, UGT1A7, and UGT1A9 genetic variants and their haplotypes in Caucasians patients.9, 14 There are ethnic differences in UGT1A genes and their linkage disequilibrium (LD) between Caucasian and Asian populations. UGT1A1*6 and UGT1A1*27 were strongly associated with severe neutropenia, especially among Asian patients.11, 12, 15, 16 The relative contribution of polymorphisms, especially UGT1A1*6, to the prediction of the outcome of FOLFIRI therapy in Asian patients must be determined. Moreover, the location of these variants in the same UGT1A gene cluster and their LD indicate that haplotype‐based studies should be carried out to determine the potential interaction among UGT1A variants. Hence, the aim of this study is to evaluate whether UGT1A alleles might be involved in the risk of toxicity and response in Japanese mCRC patients treated with FOLFIRI.
Patients and Methods
Study design and patient eligibility
The objective of this study was to assess the relationship between UGT1A polymorphisms and toxicity as well as tumor response. The study was carried out as an ancillary investigation of two prospective studies that involved 20 treatment centers in Japan. The study participants have been described in detail elsewhere.17, 18 This study was carried out according to the Declaration of Helsinki. The study protocol was approved by the Ethics and Scientific Committee and the institutional review board of each participating institution, and all patients gave written informed consent before entering the study. Eligibility criteria included: histologically proven mCRC; no prior chemotherapy for metastatic disease in the case of FLIGHT117 (UMIN000002388), or resistance to FOLFOX as the first‐line chemotherapy in the case of FLIGHT218 (UMIN000002476); aged 20–80 years; Eastern Cooperative Oncology Group performance status of 0–1; at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.019; and adequate organ function.
Thirty‐nine of 53 patients from the FLIGHT1 study and 36 of 50 patients from the FLIGHT2 study agreed to the additional investigation. Two patients who were homozygous for UGT1A1*28 and received a reduced dosage of irinotecan were excluded from the whole analysis of toxicities and efficacies and analyzed individually.
Treatment
All patients were treated with the modified FOLFIRI regimen as described by Tournigand et al.3 Briefly, the regimen consisted of irinotecan 150 mg/m2 (instead of 180 mg/m2 commonly used in North America and Europe) given i.v. for 90 min on day 1 + 400 mg/m2 fluorouracil bolus followed by 2400 mg/m2 fluorouracil continuous infusion during 46 h + 200 mg/m2 leucovorin on day 1 every 2 weeks. Blood tests and clinical evaluation were carried out every 2 weeks before treatment. Chemotherapy could be given if patients had a leukocyte count >3000 mm3, a neutrophil count >1500 mm3, a platelet count >75 000 mm3, and clinical toxicity that was resolved or grade 1. In patients who were homozygous for UGT1A1*28, the starting dose of irinotecan was reduced to 100 mg/m2, based on the recommendation of an advisory meeting by the subcommittee of the Food and Drug Administration Center for Drug Evaluation and Research held November 200420 and our previous phase I study.21 Two patients were homozygous for *28 and therefore excluded from the whole analysis of toxicities and efficacies and analyzed individually.
Efficacy and toxicity assessment
Objective clinical evaluation, blood counts, and hepatic and renal function tests were carried out within 48 h before each cycle. Computed tomography scans of measurable lesions were assessed at baseline and then repeated at least every four cycles. Objective tumor response and duration of response were assessed by RECIST version 1.0. Toxicity was evaluated according to Common Toxicity Criteria for Adverse Events version 3.0.
Uridine diphosphate–glucuronosyltransferase genetic analyses
Genomic DNA was extracted from peripheral blood anticoagulated with EDTA‐2Na using a conventional NaI method.22 UGT1A1*28 (TA6>TA7), UGT1A7*3 (387T>G, 622T>C)/UGT1A9*22 (*1b [T9>T10]), and UGT1A1*93 (−3156G>A)/UGT1A1*6 (211G>A)/UGT1A1*27 (686C>A)/UGT1A1*60 (−3279T>G)/UGT1A7 (−57T>G) were genotyped as described previously by fragment size analysis, direct sequencing, and TaqMan assay, respectively.21, 23 UGT1A7*1, UGT1A7*2, UGT1A7*3, and UGT1A7*4 can be tagged by sequencing of 387T>G and 622T>C.24 For fragment size analysis, PCR reactions were carried out in a total volume of 10 μL containing template DNA (80 ng/μL), according to the manufacturer's instructions (Ex Taq; Takara, Tokyo, Japan). The amplification was carried out with a GeneAmp PCR System PC808 (ASTEC, Tokyo, Japan) with an initial denaturation at 95°C for 2 min followed by 27 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 20 s, and extension at 72°C for 30 s. The PCR products of TA6 and TA7, whose sizes were 94 and 96 bp, respectively, were mixed with Hi‐Di formamide, including the internal size standard (GeneScan 500; Applied Biosystems, Foster City, CA, USA) at a 1:10 ratio. Then, samples were run in the ABI Prism 3100 Genetic Analyzer (Applied Biosystems). Fragment sizes were determined by comparison with the internal size standard (GeneScan LIZ‐500) by using the local Southern algorithm, and the data were analyzed by GeneMapper software version 3.5 (Applied Biosystems). For direct sequencing, PCR amplifications were carried out by using the Gene Amp PCR System PC808 (ASTEC) with Ex Taq polymerase. Amplification conditions were 30 cycles of 95°C for 30 s, each annealing temperature for 20 s, and 72°C for 30 s. The PCR products were purified using ExoSAP‐IT (Amersham Bioscience, Tokyo, Japan) for 20 min at 37°C and then for 20 min at 80°C. Sequencing reactions were carried out by using a BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Tokyo, Japan). After purification with ethanol, the reaction products were analyzed by an ABI 3100‐Avant Genetic Analyzer (Applied Biosystems). TaqMan assays of PCR products were carried out according to the manufacturer's protocol. Specific forward/reverse PCR primers and TaqMan probes for UGT1A1*93 were custom synthesized by Applied Biosystems. Primers and probes for UGT1A1*6, UGT1A1*27, UGT1A1*60, and UGT1A7 (−57) were purchased from Applied Biosystems (TaqMan SNP Genotyping Assays). Reaction mixtures were loaded into 384‐well plates and placed in the ABI Prism 7900HT Sequence Detection System (Applied Biosystems). The PCR amplifications were carried out as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles of PCR with a denaturation at 95°C for 15 s, and one‐step annealing/extension for 1 min at 60°C.
Statistical analysis
These analyses are an additional investigation of the FLIGHT117 and FLIGHT218 studies, which were prospectively designed to evaluate the associations between UGT1A genotypes/haplotypes and outcomes of toxicity (severe toxicity at first cycle and in all cycles) and efficacy (response rate [complete response, partial response] and progression‐free survival [PFS]). In the present study, we evaluated associations between genotypes/haplotypes as well as clinical variables (sex, age, and performance status), toxicity, and response rate by Fisher's exact test and the Cochran–Armitage trend test (C.A.‐test). Logistic regression models were investigated for toxicity. Genetic variants were included in a stepwise logistic regression analysis in the multivariate model. Calculations were carried out by using R version 2.13.0 software.25 Each nucleotide polymorphism was evaluated for Hardy–Weinberg equilibrium and LD, and case–control haplotype analyses were carried out with Haploview 4.2 software.26 Lewontin's coefficient D' and correlation coefficient r 2 were calculated as measures of LD. P < 0.05 was considered statistically significant.
Results
Clinical features, genotype/haplotype frequency, and LD
In the 75 Japanese colorectal cancer patients, genotypes of UGT1A1*28 and UGT1A1*93 as well as UGT1A7 (622T>C) and UGT1A7 (−57T>G) matched perfectly, so UGT1A1*93 and UGT1A7 (−57T>G) were excluded from further examination (Table 1, Fig. 1). Minor allele frequencies of seven remaining UGT1A polymorphisms are shown in Table S1. The P‐values of all seven UGT1A polymorphisms were >0.05, under the Hardy–Weinberg equilibrium. High LD was also observed between UGT1A1*6 and UGT1A7 (622T>C), UGT1A1*6 and UGT1A7 (−57T>G), and UGT1A7 (387T>G) and UGT1A9*22(*1b) (Fig. 1).
Table 1.
Clinical features and genotype frequencies in Japanese patients with metastatic colorectal cancer
| Clinical feature or genotype | Detail | Frequency (%) |
|---|---|---|
| Sex | Male | 50 (66.7) |
| Female | 25 (33.3) | |
| Age, years | ≤60 | 28 (37.3) |
| >60 | 47 (62.7) | |
| Performance statusa | 0 | 58 (77.3) |
| 1 | 17 (22.7) | |
| Hematologic toxicity (entire course) | No | 40 (53.3) |
| Yes | 35 (46.7) | |
| Hematologic toxicity(first cycle) | No | 63 (84.0) |
| Yes | 12 (16.0) | |
| Objective response | CR + PR | 24 (33.3) |
| SD + PD | 48 (66.7) | |
| UGT1A1*6 | −/− | 50 (66.7) |
| −/*6 | 23 (30.7) | |
| *6/*6 | 2 (2.7) | |
| UGT1A1*27 | −/− | 73 (97.3) |
| −/*27 | 2 (2.7) | |
| UGT1A1*28 | −/− | 59 (78.7) |
| −/*28 | 14 (18.7) | |
| *28/*28 | 2 (2.7) | |
| UGT1A1*60 | −/− | 40 (53.3) |
| −/*60 | 29 (38.7) | |
| *60/*60 | 6 (8.0) | |
| UGT1A1*93 | −/− | 59 (78.7) |
| −/*93 | 14 (18.7) | |
| *93/*93 | 2 (2.7) | |
| UGT1A7 (−57T>G) | −57T/T | 40 (53.3) |
| −57T/G | 29 (38.7) | |
| −57G/G | 6 (8.0) | |
| UGT1A7*3 (387T>G) | 387T/T | 25 (33.3) |
| 387T/G | 37 (49.3) | |
| 387G/G | 13 (17.3) | |
| UGT1A7*3 (622T>C) | 622T/T | 40 (53.3) |
| 622T/C | 29 (38.7) | |
| 622C/C | 6 (8.0) | |
| UGT1A9*22 (UGT1A9*1b) | *22/*22 | 26 (34.7) |
| −/*22 | 36 (48.0) | |
| −/− | 13 (17.3) |
Assessed using Eastern Cooperative Oncology Group criteria. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 1.
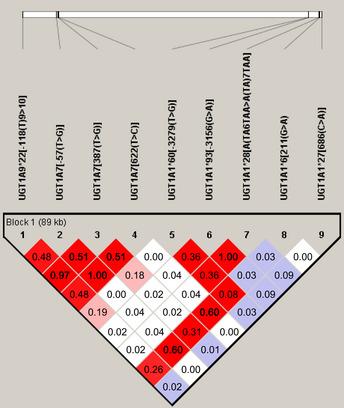
Pairwise linkage disequilibrium relationships between UGT1A polymorphisms in Japanese patients with metastatic colorectal cancer. Lewontin's coefficient D' is represented by the color scheme: log of the odds (LOD) ≥ 2 shown in pink/red; LOD < 2 and D' = 1 shown in blue; and LOD < 2 and D' < 1 shown in white. The correlation coefficient r 2 is shown in each box.
The frequency of UGT1A1*28 was lower (Table 1) in the Japanese patients than Caucasians as reported previously.9, 27 UGT1A1*6 frequency was discriminatively high, but it was rare among Caucasians.28 The frequency of UGT1A1*60 and UGT1A7*3 was low in Japanese patients, UGT1A9*22 had the same frequency in Japanese and Caucasian patients, and UGT1A1*27 had very low frequency and no homozygosity.
Haplotype frequencies are listed in Table 2. The major haplotypes in the Japanese population were I, II, and III, and our analyses were performed on these.
Table 2.
UGT1A haplotype frequencies in Japanese patients with metastatic colorectal cancer
| Hp | UGT1A1 | UGT1A7 | UGT1A9 | |||||
|---|---|---|---|---|---|---|---|---|
| *60 | *28 | *6 | *27 | 387T>G | 622T>C | *22 | Frequency | |
| I | T | TA6 | G | C | T | T | T10 | 0.520 |
| II | T | TA6 | Aa | C | Ga | Ca | T9 a | 0.173 |
| III | Ga | TA6 | G | C | Ga | T | T9 a | 0.127 |
| IV | Ga | TA7 a | G | C | Ga | Ca | T9 a | 0.053 |
| V | Ga | TA7 a | G | C | T | T | T10 | 0.053 |
| VI | T | TA6 | G | C | Ga | Ca | T9 a | 0.020 |
| VII | Ga | TA7 a | G | Aa | Ga | T | T9 a | 0.013 |
| VIII | Ga | TA6 | G | C | Ga | Ca | T9 a | 0.013 |
| IX | Ga | TA6 | G | C | T | T | T10 | 0.013 |
Association allele was estimated by Haploview. Hp, haplotype.
UGT1A genotypes/haplotypes and severe toxicity
Correlations between UGT1A genotypes/haplotypes and severe (grade 3–4) toxicity were analyzed. Of 73 patients, 34 had G3/4 hematological toxicity. Severe hematological toxicity during the entire course of therapy was more frequent in patients with UGT1A1*6 (211A, P = 0.018 by C.A.‐test) and UGT1A7 (387G, P = 0.039 by C.A.‐test) alleles than in patients without these variant alleles. In the UGT1A9*22 polymorphism, the variant T10 allele was a marker for reduced toxicity (P = 0.028 by C.A.‐test), as previously reported.8, 9 Severe hematologic toxicity was trend toward higher among the UGT1A7 (622C) allele (P = 0.058 by Fisher's exact test). For severe hematological toxicity during the entire course of therapy, multivariate analysis indicated that UGT1A1*6 was the only significant predictor (P = 0.022, odds ratio 3.00, 95% confidence interval 1.17–7.69; Table S5). Older age (60 years or more) was also a risk factor for severe toxicity (Table 3).
Table 3.
UGT1A genotypes/clinical features and severe hematologic toxicity in Japanese patients with metastatic colorectal cancer treated with FOLFIRI
| Genotype or clinical feature | Detail | Severe hematologic toxicity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| After first cycle | During entire course of therapy | ||||||||
| Toxicity | P‐value | Toxicity | P‐value | ||||||
| Yes | No | Fisher's exact test | C.A.‐trend test | Yes | No | Fisher's exact test | C.A.‐trend test | ||
| UGT1A1*6 | −/− | 6 | 42 | 0.175 | 0.132 | 18 | 30 | 0.049 | 0.018 |
| −/*6 | 5 | 18 | 14 | 9 | |||||
| *6/*6 | 1 | 1 | 2 | 0 | |||||
| UGT1A1*27 | −/− | 11 | 60 | 0.304 | ND | 33 | 38 | 1.000 | ND |
| −/*27 | 1 | 1 | 1 | 1 | |||||
| UGT1A1*28 | −/− | 8 | 51 | 0.227 | ND | 27 | 32 | 1.000 | ND |
| −/*28 | 4 | 10 | 7 | 7 | |||||
| UGT1A1*60 | −/− | 4 | 36 | 0.114 | 0.313 | 16 | 24 | 0.402 | 0.279 |
| −/*60 | 8 | 21 | 16 | 13 | |||||
| *60/*60 | 0 | 4 | 2 | 2 | |||||
| UGT1A7 (387T>G) | 387T/T | 2 | 23 | 0.360 | 0.328 | 7 | 18 | 0.079 | 0.039 |
| 387T/G | 8 | 28 | 20 | 16 | |||||
| 387G/G | 2 | 10 | 7 | 5 | |||||
| UGT1A7 (622T>C) | 622T/T | 4 | 36 | 0.191 | 0.162 | 14 | 26 | 0.058 | 0.105 |
| 622T/C | 7 | 21 | 18 | 10 | |||||
| 622C/C | 1 | 4 | 2 | 3 | |||||
| UGT1A9*22 (UGT1A9*1b) | *22/*22 | 2 | 24 | 0.356 | 0.296 | 7 | 19 | 0.044 | 0.028 |
| −/*22 | 8 | 27 | 20 | 15 | |||||
| −/− | 2 | 10 | 7 | 5 | |||||
| Haplotype I | 0 | 3 | 12 | 0.041 | 0.080 | 9 | 6 | 0.072 | 0.031 |
| 1 | 9 | 29 | 20 | 18 | |||||
| 2 | 0 | 20 | 5 | 15 | |||||
| Haplotype II | 0 | 6 | 43 | 0.170 | 0.106 | 18 | 31 | 0.025 | 0.010 |
| 1 | 5 | 17 | 14 | 8 | |||||
| 2 | 1 | 1 | 2 | 0 | |||||
| Haplotype III | 0 | 10 | 45 | 0.765 | 0.449 | 24 | 31 | 0.488 | 0.282 |
| 1 | 2 | 15 | 9 | 8 | |||||
| 2 | 0 | 1 | 1 | 0 | |||||
| Sex | Male | 8 | 40 | 1.000 | ND | 22 | 26 | 1.000 | ND |
| Female | 4 | 21 | 12 | 13 | |||||
| Age, years | ≤60 | 2 | 26 | 0.114 | ND | 8 | 20 | 0.018 | ND |
| >60 | 10 | 35 | 26 | 19 | |||||
| PS | 0 | 9 | 48 | 0.718 | ND | 27 | 30 | 1.000 | ND |
| 1 | 3 | 13 | 7 | 9 | |||||
–, reference allele. C.A., Cochran–Armitage; ND, not done; PS, Eastern Cooperative Oncology Group performance status.
Haplotype I, which consisted of all favorable alleles, was associated with low hematologic toxicity both after the first cycle (P = 0.080 by C.A.‐test) and during the entire course of therapy (P = 0.031 by C.A.‐test). In contrast, haplotype II, which contains four high‐risk alleles, was significantly associated with high hematologic toxicity during the entire course of therapy (P = 0.010 by C.A.‐test) (Table 3).
In the trial of first‐line therapy (FLIGHT1, n = 38, Table 4), UGT1A1*28 (P = 0.063 by Fisher's exact test) genotypes were risk factors for severe hematological toxicity after the first cycle, and the allele number of UGT1A7 (387G, P = 0.026 by C.A.‐test) and UGT1A9*22 (T10, P = 0.016 by C.A.‐test) were risk factors for severe hematological toxicity during the entire course of therapy.
Table 4.
Associations between UGT1A genotypes and irinotecan toxicity and objective responses in Japanese patients with metastatic colorectal cancer treated with FOLFIRI as first‐line therapy (FLIGHT1)
| Genotype or clinical feature | Detail | Severe hematologic toxicity | Objective responses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After first cycle | During entire course of therapy | During entire course of therapy | |||||||||||
| Toxicity | P‐value | Toxicity | P‐value | Responses | P‐value | ||||||||
| Yes | No | Fisher's exact test | C.A.‐ trend test | Yes | No | Fisher's exact test | C.A.‐ trend test | CR + PR | SD + PD | Fisher's exact test | C.A.‐ trend test | ||
| UGT1A1*6 | –/– | 4 | 21 | 0.243 | 0.266 | 10 | 15 | 0.384 | 0.150 | 11 | 12 | 0.725 | 1.000 |
| –/*6 | 2 | 10 | 7 | 5 | 7 | 5 | |||||||
| *6/*6 | 1 | 0 | 1 | 0 | 0 | 1 | |||||||
| UGT1A1*27 | –/– | 6 | 31 | 0.184 | ND | 17 | 20 | 0.474 | ND | 17 | 18 | 1.000 | ND |
| –/*27 | 1 | 0 | 1 | 0 | 1 | 0 | |||||||
| UGT1A1*28 | –/– | 4 | 28 | 0.063 | ND | 14 | 18 | 0.395 | ND | 16 | 15 | 1.000 | ND |
| –/*28 | 3 | 3 | 4 | 2 | 2 | 3 | |||||||
| UGT1A1*60 | –/– | 1 | 18 | 0.079 | 0.133 | 7 | 12 | 0.206 | 0.095 | 8 | 11 | 0.109 | 0.781 |
| –/*60 | 6 | 11 | 9 | 8 | 10 | 5 | |||||||
| *60/*60 | 0 | 2 | 2 | 0 | 0 | 2 | |||||||
| UGT1A7 (387T>G) | 387T/T | 1 | 12 | 0.490 | 0.213 | 4 | 9 | 0.065 | 0.026 | 7 | 5 | 0.678 | 0.815 |
| 387T/G | 4 | 14 | 8 | 10 | 7 | 10 | |||||||
| 387G/G | 2 | 5 | 6 | 1 | 4 | 3 | |||||||
| UGT1A7 (622T>C) | 622T/T | 2 | 19 | 0.113 | 0.079 | 7 | 14 | 0.132 | 0.102 | 10 | 10 | 1.000 | 1.000 |
| 622T/C | 4 | 11 | 10 | 5 | 7 | 7 | |||||||
| 622C/C | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| UGT1A9*22 (UGT1A9*1b) | *22/*22 | 1 | 13 | 0.413 | 0.183 | 4 | 10 | 0.057 | 0.016 | 8 | 5 | 0.474 | 0.646 |
| –/*22 | 4 | 13 | 8 | 9 | 6 | 10 | |||||||
| –/– | 2 | 5 | 6 | 1 | 4 | 3 | |||||||
| Haplotype I | 0 | 3 | 5 | 0.100 | 0.040 | 7 | 1 | 0.028 | 0.019 | 4 | 4 | 1.000 | 1.000 |
| 1 | 4 | 16 | 8 | 12 | 9 | 9 | |||||||
| 2 | 0 | 10 | 3 | 7 | 5 | 5 | |||||||
| Haplotype II | 0 | 4 | 22 | 0.249 | 0.202 | 10 | 16 | 0.212 | 0.080 | 12 | 12 | 1.000 | 0.755 |
| 1 | 2 | 9 | 7 | 4 | 6 | 5 | |||||||
| 2 | 1 | 0 | 1 | 0 | 0 | 1 | |||||||
| Haplotype III | 0 | 6 | 22 | 0.720 | 0.398 | 12 | 16 | 0.568 | 0.253 | 13 | 13 | 1.000 | 0.747 |
| 1 | 1 | 8 | 5 | 4 | 5 | 4 | |||||||
| 2 | 0 | 1 | 1 | 0 | 0 | 1 | |||||||
| Sex | Male | 5 | 19 | 1.000 | ND | 12 | 12 | 0.745 | ND | 13 | 10 | 0.489 | ND |
| Female | 2 | 12 | 6 | 8 | 5 | 8 | |||||||
| Age, years | ≤60 | 2 | 12 | 1.000 | ND | 5 | 9 | 0.328 | ND | 6 | 7 | 1.000 | ND |
| >60 | 5 | 19 | 13 | 11 | 12 | 11 | |||||||
| PS | 0 | 5 | 28 | 0.223 | ND | 15 | 18 | 0.653 | ND | 17 | 15 | 0.603 | ND |
| 1 | 2 | 3 | 3 | 2 | 1 | 3 | |||||||
–, reference allele. C.A., Cochran–Armitage; CR, complete response; ND, not done; PD, progressive disease; PR, partial response; PS, Eastern Cooperative Oncology Group performance status; SD, stable disease.
In the trial of second‐line therapy (FLIGHT2, n = 35, Table 5), older age (60 years and older, P = 0.069 by Fisher's exact test) trends to a risk factor for severe hematological toxicity after the first cycle. The severe hematological toxicity during the entire course of therapy was significantly different among the genotypes of UGT1A7 (387G, P = 0.047 by Fisher's exact test), UGT1A9*22 (T9, P = 0.047 by Fisher's exact test), and patient age (P = 0.036 by Fisher's exact test).
Table 5.
Associations between UGT1A genotypes and irinotecan toxicity and objective responses in Japanese patients with metastatic colorectal cancer treated with FOLFIRI as second‐line therapy (FLIGHT2)
| Genotype or clinical feature | Detail | Severe hematologic toxicity | Objective responses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After first cycle | During entire course of therapy | During entire course of therapy | |||||||||||
| Toxicity | P‐value | Toxicity | P‐value | Responses | P‐value | ||||||||
| Yes | No | Fisher's exact test | C.A.‐trend test | Yes | No | Fisher's exact test | C.A.‐trend test | CR + PR | SD + PD | Fisher's exact test | C.A.‐trend test | ||
| UGT1A1*6 | −/− | 2 | 21 | 0.400 | 0.306 | 8 | 15 | 0.105 | 0.054 | 2 | 21 | 0.058 | 0.021 |
| −/*6 | 3 | 8 | 7 | 4 | 3 | 8 | |||||||
| *6/*6 | 0 | 1 | 1 | 0 | 1 | 0 | |||||||
| UGT1A1*27 | −/− | 5 | 29 | 1.000 | ND | 16 | 18 | 1.000 | ND | 6 | 28 | 1.000 | ND |
| −/*27 | 0 | 1 | 0 | 1 | 0 | 1 | |||||||
| UGT1A1*28 | −/− | 4 | 23 | 1.000 | ND | 13 | 14 | 0.700 | ND | 6 | 21 | 0.299 | ND |
| −/*28 | 1 | 7 | 3 | 5 | 0 | 8 | |||||||
| UGT1A1*60 | −/− | 3 | 18 | 1.000 | 0.819 | 9 | 12 | 0.414 | 0.859 | 6 | 15 | 0.106 | 0.041 |
| −/*60 | 2 | 10 | 7 | 5 | 0 | 12 | |||||||
| *60/*60 | 0 | 2 | 0 | 2 | 0 | 2 | |||||||
| UGT1A7 (387T>G) | 387T/T | 1 | 11 | 0.528 | 1.000 | 3 | 9 | 0.047 | 0.542 | 2 | 10 | 1.000 | 0.893 |
| 387T/G | 4 | 14 | 12 | 6 | 3 | 15 | |||||||
| 387G/G | 0 | 5 | 1 | 4 | 1 | 4 | |||||||
| UGT1A7 (622T>C) | 622T/T | 2 | 17 | 0.612 | 0.831 | 7 | 12 | 0.403 | 0.491 | 2 | 17 | 0.317 | 0.228 |
| 622T/C | 3 | 10 | 8 | 5 | 3 | 10 | |||||||
| 622C/C | 0 | 3 | 1 | 2 | 1 | 2 | |||||||
| UGT1A9*22 (UGT1A9*1b) | *22/*22 | 1 | 11 | 0.528 | 1.000 | 3 | 9 | 0.047 | 0.542 | 2 | 10 | 1.000 | 0.893 |
| −/*22 | 4 | 14 | 12 | 6 | 3 | 15 | |||||||
| −/− | 0 | 5 | 1 | 4 | 1 | 4 | |||||||
| Haplotype I | 0 | 0 | 7 | 0.095 | 0.765 | 2 | 5 | 0.049 | 0.501 | 1 | 6 | 1.000 | 0.753 |
| 1 | 5 | 13 | 12 | 6 | 3 | 15 | |||||||
| 2 | 0 | 10 | 2 | 8 | 2 | 8 | |||||||
| Haplotype II | 0 | 2 | 21 | 0.400 | 0.306 | 8 | 15 | 0.105 | 0.054 | 2 | 21 | 0.058 | 0.021 |
| 1 | 3 | 8 | 7 | 4 | 3 | 8 | |||||||
| 2 | 0 | 1 | 1 | 0 | 1 | 0 | |||||||
| Haplotype III | 0 | 4 | 23 | 1.000 | ND | 12 | 15 | 1.000 | ND | 6 | 21 | 0.299 | ND |
| 1 | 1 | 7 | 4 | 4 | 0 | 8 | |||||||
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Sex | Male | 3 | 21 | 0.640 | ND | 10 | 14 | 0.716 | ND | 4 | 20 | 1.000 | ND |
| Female | 2 | 9 | 6 | 5 | 2 | 9 | |||||||
| Age, years | ≤60 | 0 | 14 | 0.069 | ND | 3 | 11 | 0.036 | ND | 2 | 12 | 1.000 | ND |
| >60 | 5 | 16 | 13 | 8 | 4 | 17 | |||||||
| PS | 0 | 4 | 20 | 1.000 | ND | 12 | 12 | 0.493 | ND | 5 | 19 | 0.640 | ND |
| 1 | 1 | 10 | 4 | 7 | 1 | 10 | |||||||
–, reference allele. C.A., Cochran–Armitage; CR, complete response; ND, not done; PD, progressive disease; PR, partial response; PS, Eastern Cooperative Oncology Group performance status; SD, stable disease.
Only two patients, one in FLIGHT1 and one in FLIGHT2, developed grade 3 diarrhea. Both of them had UGT1A diplotype I/II.
UGT1A genotypes/haplotypes and response/PFS
Objective tumor responses were analyzed in 71 patients. Of 36 patients, 18 had complete or partial tumor responses in FLIGHT 1, and 6 out of 35 patients had objective responses in FLIGHT 2. No genotype/haplotype was associated with tumor response in FLIGHT 1. In FLIGHT2, the numbers of UGT1A1*6 and haplotype II were positive factors (P = 0.021 by C.A.‐test) and UGT1A1*60 was a negative factor (P = 0.041 by C.A.‐test) for objective responses (Table 5).
Effects of UGT1A polymorphisms on PFS in FLIGHT1 and FLIGHT2 were analyzed. There was no association between UGT1A polymorphisms and PFS. Only female sex was the negative factor for PFS (P = 0.016) (Table S6).
UGT1A1*28 and UGT1A1*6 homozygous
Homozygosity of UGT1A1*28 or *6 and compound heterozygosity of *28 and *6 were reported as high‐risk factors for hematologic toxicity, especially in Asia,29 therefore, we examined these genotypes (Table S7). Although the initial dose of irinotecan was reduced to 100 mg/m2 for patients with homozygous UGT1A1*28, one of two patients suffered from severe toxicity. In all cases, six of seven patients suffered from severe hematological toxicity. Although in these patients the toxicity was very severe, the response rate was not impaired (3/7, 42.9%).
Discussion
The present study evaluated whether UGT1A polymorphisms influence the toxicity and efficacy of the FOLFIRI regimen in Japanese mCRC patients. This study was carried out as an integrated investigation of two prospective studies that involved 20 treatment centers in Japan.17, 18 To the best of our knowledge, this is the first prospective study to assess the role of UGT1A polymorphisms on the efficacy and toxicity of FOLFIRI.
The UGT1A1, UGT1A7, and UGT1A9 genetic variants and their haplotypes play a predictive role in the outcome of FOLFIRI treatments in Caucasian mCRC patients.9 There are ethnic differences in UGT1A genotypes. Among the Japanese population, UGT1A1*28 has a low frequency and UGT1A1*6 has a discriminatively high frequency, unlike the frequencies among Caucasians.11, 12, 16, 17 Therefore, the relationship between UGT1A genetics and the outcome of FOLFIRI should be evaluated in each ethnic group.
In our analysis of Japanese colorectal cancer patients treated with FOLFIRI, severe hematological toxicity during the entire course of therapy was more frequent in patients with UGT1A1*6 (211A) and UGT1A7*3 (387G) genotypes. In contrast, the variant T10 allele of the UGT1A9*22 (*1b) polymorphism was a marker for reduced toxicity (Table 3).8, 9 In addition to certain genotypes, older age was also a risk factor for severe hematological toxicity. Unlike findings from Caucasian patients, the UGT1A1*28 allele was not necessarily related to hematological toxicity in Japanese patients. On the contrary, UGT1A1*6 was strongly correlated with severe hematological toxicity, which is consistent with other clinical studies of Asian populations.11, 12, 16 We can assume that both UGT1A9*22 (reference T9 genotype) and UGT1A7*3 (387G) are risk alleles that have strong LD (correlation coefficient r 2 = 0.97) for each other (Fig. 1), as previously reported.9 Interestingly, there was no significant relationship between hematological toxicity and female sex, unlike the previous findings of Cecchin et al.9 for Caucasians.
The close LD between UGT1A1*6 and UGT1A7*3 (387T>G, 0.32; 622T>C, 0.64), as well as between UGT1A9*22 and UGT1A7*3 (387T>G, 0.97; 622T>C, 0.46) was thought to be associated with toxicity profiles of each genotype. Therefore, the influence of not only genotypes but also haplotypes must be examined.9, 14, 30 For haplotype I, which consists of all favorable alleles, there was a significant reduction in hematologic toxicity during the entire course of therapy (P = 0.031) as well as after the first cycle (P = 0.080). This protective haplotype was the most frequent haplotype (0.520) in Japanese patients.9 In contrast, haplotype II, which contains four high‐risk alleles (UGT1A1*6, UGT1A7*3 [387G and 622C] and UGT1A9*1 [T9]), showed significantly higher hematologic toxicity during the entire course of therapy (haplotype frequency, 0.173; P = 0.010). The odds ratio for that toxicity was extremely high, and this haplotype was the second most common haplotype in Japanese patients, which differs considerably from that of Caucasians. This haplotype may be the leading cause of ethnic differences in irinotecan toxicity. Consequently, haplotype analysis seemed to have a stronger impact than each genotype analysis. Although toxicity was very severe in these patients, the response rate was not impaired.
There were some differences in profiles between first‐line and second‐line FOLFIRI treatments. In the first‐line group, UGT1A1*28 was a risk factor for severe hematological toxicity after the first cycle of FOLFIRI, and UGT1A7 (387G) and UGT1A9*22 (T9) genotypes were risk factors during the entire course of therapy. In the second‐line group, older age was a risk factor after the first cycle, and UGT1A7 (387G) and UGT1A9 (T9) alleles and older age were risk factors during the entire course of therapy for severe hematological toxicity. In the second‐line therapy group, older patients could not tolerate the sequential treatments. Objective tumor responses were predicted by UGT1A1*6 and UGT1A1*60, but these results might be controversial.
Homozygosity of UGT1A1*28 or *6 and compound heterozygosity of *28 and *6 have been reported as high‐risk factors for hematologic toxicity, especially in Asia.29 In our study, all *28/*6 and *6/*6 patients developed severe hematological toxicity. Moreover, one of two *28 homozygous patients suffered from severe hematological toxicity despite the reduced dosage of irinotecan (100 mg/m2) used for these patients (Table S7). Although toxicity was very severe in these patients, the response rate was not impaired (3/7: 42.9%). For these patients, although the FOLFIRI regimen should be given in a careful manner, it is effective, and its use for advanced colorectal cancer patients should not be precluded from the treatment options.
In conclusion, assessment of UGT1A1*28 and *6 and also UGT1A7*3 and UGT1A9*22 is very important to predict the toxicity of irinotecan in Japanese (or Asian) patients. Although each UGT1A1*6 (211A), UGT1A7*3 (387G), and UGT1A9*22 (T9) allele might predict hematological toxicity, haplotype II (containing four risk alleles, UGT1A1*6, UGT1A7*3 [387G and 622C], UGT1A9*1 [T9]) and homozygosity of UGT1A1*28 and *6 were better predictors of toxicity. For these patients, the FOLFIRI regimen should be given carefully. We will attempt further haplotype analysis by carrying out a new prospective study and using bioinformatics.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Table S1. Minor allele frequency in Japanese patients with metastatic colorectal cancer.
Table S2. UGT1A polymorphisms and severe toxicity by univariate analysis in Japanese patients with metastatic colorectal cancer.
Table S3. UGT1A polymorphisms and severe toxicity and objective responses by univariate analysis (FLIGHT1) in Japanese patients with metastatic colorectal cancer.
Table S4. UGT1A polymorphisms and severe toxicity and objective responses by univariate analysis (FLIGHT2) in Japanese patients with metastatic colorectal cancer.
Table S5. Multivariable logistic regression analysis in Japanese patients with metastatic colorectal cancer.
Table S6. UGT1A genotypes and progression‐free survival in Japanese patients with metastatic colorectal cancer.
Table S7. UGT1A1*28 and *6 polymorphisms and toxicity/objective responses in Japanese patients with metastatic colorectal cancer.
Acknowledgments
This study was supported in part by a non‐profit organization Epidemiological and Clinical Research Information Network (ECRIN), as well as a Grant‐in‐Aid for Scientific Research of the Ministry of Education, Science, Sports and Culture of Japan (project No. 21591725 and 19591545). We thank Ms. Mai Hatta for her excellent clinical research coordination.
(Cancer Sci 2013; 104: 1662–1669)
References
- 1. Douillard JY, Cunningham D, Roth AD et al Irinotecan combined with fluorouracil compared with fluorouracil alone as first‐line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000; 355: 1041–7. [DOI] [PubMed] [Google Scholar]
- 2. Saltz LB, Cox JV, Blanke C et al Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000; 343: 905–14. [DOI] [PubMed] [Google Scholar]
- 3. Tournigand C, Andre T, Achille E et al FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004; 22: 229–37. [DOI] [PubMed] [Google Scholar]
- 4. Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN‐38, a metabolite of the camptothecin derivative CPT‐11, in the antitumor effect of CPT‐11. Cancer Res 1991; 51: 4187–91. [PubMed] [Google Scholar]
- 5. Iyer L, King CD, Whitington PF et al Genetic predisposition to the metabolism of irinotecan (CPT‐11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN‐38) in human liver microsomes. J Clin Invest 1998; 101: 847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beutler E, Gelbart T, Demina A. Racial variability in the UDP‐glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc Natl Acad Sci U S A 1998; 95: 8170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gagne JF, Montminy V, Belanger P, Journault K, Gaucher G, Guillemette C. Common human UGT1A polymorphisms and the altered metabolism of irinotecan active metabolite 7‐ethyl‐10‐hydroxycamptothecin (SN‐38). Mol Pharmacol 2002; 62: 608–17. [DOI] [PubMed] [Google Scholar]
- 8. Yamanaka H, Nakajima M, Katoh M et al A novel polymorphism in the promoter region of human UGT1A9 gene (UGT1A9*22) and its effects on the transcriptional activity. Pharmacogenetics 2004; 14: 329–32. [DOI] [PubMed] [Google Scholar]
- 9. Cecchin E, Innocenti F, D'Andrea M et al Predictive role of the UGT1A1, UGT1A7, and UGT1A9 genetic variants and their haplotypes on the outcome of metastatic colorectal cancer patients treated with fluorouracil, leucovorin, and irinotecan. J Clin Oncol 2009; 27: 2457–65. [DOI] [PubMed] [Google Scholar]
- 10. Ando M, Ando Y, Sekido Y, Ando M, Shimokata K, Hasegawa Y. Genetic polymorphisms of the UDP‐glucuronosyltransferase 1A7 gene and irinotecan toxicity in Japanese cancer patients. Jpn J Cancer Res 2002; 93: 591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujita K, Ando Y, Nagashima F et al Genetic linkage of UGT1A7 and UGT1A9 polymorphisms to UGT1A1*6 is associated with reduced activity for SN‐38 in Japanese patients with cancer. Cancer Chemother Pharmacol 2007; 60: 515–22. [DOI] [PubMed] [Google Scholar]
- 12. Han JY, Lim HS, Shin ES et al Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non‐small‐cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol 2006; 24: 2237–44. [DOI] [PubMed] [Google Scholar]
- 13. Lankisch TO, Schulz C, Zwingers T et al Gilbert's Syndrome and irinotecan toxicity: combination with UDP‐glucuronosyltransferase 1A7 variants increases risk. Cancer Epidemiol Biomarkers Prev 2008; 17: 695–701. [DOI] [PubMed] [Google Scholar]
- 14. Levesque E, Belanger AS, Harvey M et al Refining the UGT1A haplotype associated with irinotecan‐induced hematological toxicity in metastatic colorectal cancer patients treated with 5‐fluorouracil/irinotecan‐based regimens. J Pharmacol Exp Ther 2013; 345: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ando Y, Saka H, Ando M et al Polymorphisms of UDP‐glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 2000; 60: 6921–6. [PubMed] [Google Scholar]
- 16. Innocenti F, Vokes EE, Ratain MJ. Irinogenetics: what is the right star? J Clin Oncol 2006; 24: 2221–4. [DOI] [PubMed] [Google Scholar]
- 17. Okuyama Y, Hazama S, Nozawa H et al Prospective phase II study of FOLFIRI for mCRC in Japan, including the analysis of UGT1A1 28/6 polymorphisms. Jpn J Clin Oncol 2011; 41: 477–82. [DOI] [PubMed] [Google Scholar]
- 18. Kato T, Okuyama Y, Nagata N et al Multicenter phase II studies of FOLFIRI with reduced starting dose of irinotecan in metastatic CRC patients with homozygous for UGT1A1*28/*6. Presented at the ASCO Gastrointestinal Cancers Symposium; 14–17 Jan 2009, San Francisco, CA. [Google Scholar]
- 19. Therasse P, Arbuck SG, Eisenhauer EA et al New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 20. The Food and Drug Administration Center for Drug Evaluation and Research held in November 2004. [Cited 21 Jul 2005.] Available from URL: http://www.fda.gov/2004.
- 21. Hazama S, Nagashima A, Kondo H et al Phase I study of irinotecan and doxifluridine for metastatic colorectal cancer focusing on the UGT1A1*28 polymorphism. Cancer Sci 2010; 101: 722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang L, Hirayasu K, Ishizawa M, Kobayashi Y. Purification of genomic DNA from human whole blood by isopropanol‐fractionation with concentrated Nal and SDS. Nucleic Acids Res 1994; 22: 1774–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kobayashi M, Hazama S, Takahashi K et al Is there diversity among UGT1A1 polymorphism in Japan? World J Gastrointest Oncol 2011; 4: 170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guillemette C, Ritter JK, Auyeung DJ, Kessler FK, Housman DE. Structural heterogeneity at the UDP‐glucuronosyltransferase 1 locus: functional consequences of three novel missense mutations in the human UGT1A7 gene. Pharmacogenetics 2000; 10: 629–44. [DOI] [PubMed] [Google Scholar]
- 25. The R Project for Statistical Computing. [Cited 20 May 2013.] Available from URL: http://www.r-project.org/.
- 26. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–5. [DOI] [PubMed] [Google Scholar]
- 27. Akiyama Y, Fujita K, Nagashima F et al Genetic testing for UGT1A1*28 and *6 in Japanese patients who receive irinotecan chemotherapy. Ann Oncol 2008; 19: 2089–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Innocenti F, Undevia SD, Iyer L et al Genetic variants in the UDP‐glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 2004; 22: 1382–8. [DOI] [PubMed] [Google Scholar]
- 29. Satoh T, Ura T, Yamada Y et al Genotype‐directed, dose‐finding study of irinotecan in cancer patients with UGT1A1*28 and/or UGT1A1*6 polymorphisms. Cancer Sci 2011; 102: 1868–73. [DOI] [PubMed] [Google Scholar]
- 30. Martinez‐Balibrea E, Abad A, Martinez‐Cardus A et al UGT1A and TYMS genetic variants predict toxicity and response of colorectal cancer patients treated with first‐line irinotecan and fluorouracil combination therapy. Br J Cancer 2010; 103: 581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Minor allele frequency in Japanese patients with metastatic colorectal cancer.
Table S2. UGT1A polymorphisms and severe toxicity by univariate analysis in Japanese patients with metastatic colorectal cancer.
Table S3. UGT1A polymorphisms and severe toxicity and objective responses by univariate analysis (FLIGHT1) in Japanese patients with metastatic colorectal cancer.
Table S4. UGT1A polymorphisms and severe toxicity and objective responses by univariate analysis (FLIGHT2) in Japanese patients with metastatic colorectal cancer.
Table S5. Multivariable logistic regression analysis in Japanese patients with metastatic colorectal cancer.
Table S6. UGT1A genotypes and progression‐free survival in Japanese patients with metastatic colorectal cancer.
Table S7. UGT1A1*28 and *6 polymorphisms and toxicity/objective responses in Japanese patients with metastatic colorectal cancer.


