This randomized clinical trial assesses the impact of a brief home-visiting approach, Family Spirit Nurture, on sugar-sweetened beverage consumption, responsive parenting and infant feeding practices, and optimal infant growth through 12 months post partum.
Key Points
Question
What is the impact of a nutrition-focused home-visiting intervention on early childhood obesity and associated risk factors?
Findings
In this randomized clinical trial of 134 Navajo mothers and their infants enrolled 3 to 12 months post partum, mothers who received the Family Spirit Nurture infant nutrition and responsive feeding home-visiting intervention vs those who did not reported feeding children substantially fewer sugar-sweetened beverages and having better responsive feeding practices. In turn, their infants had lower body mass index z scores.
Meaning
Results of this trial suggest that a home-visiting intervention created in partnership with and for Native American individuals is an effective strategy for promoting healthy infant feeding and growth in the first year of life.
Abstract
Importance
Early childhood obesity disproportionately affects Native American communities. Home visiting is a promising strategy for promoting optimal infant growth in this population.
Objective
To assess the impact of a brief home-visiting approach, Family Spirit Nurture (FSN), on sugar-sweetened beverage (SSB) consumption, responsive parenting and infant feeding practices, and optimal growth through 12 months post partum.
Design, Setting, and Participants
This study was a 1:1 randomized clinical trial comparing FSN with an injury prevention education control condition in a reservation-based community. Participants were Navajo mothers 13 years or older with infants younger than 14 weeks recruited between March 22, 2017, and May 18, 2018, and followed up through 12 months post partum. Intent-to-treat analyses were conducted.
Interventions
The 6-lesson FSN curriculum, delivered 3 to 6 months post partum by Navajo paraprofessionals, targeted optimal responsive and complementary feeding practices and avoidance of SSBs. The control group received 3 injury prevention lessons.
Main Outcomes and Measures
Primary outcomes established a priori were infant SSB consumption and responsive parenting and complementary feeding practices (responsive feeding scale, age at complementary food introduction, and percentage of mothers who introduced complementary food to infants at 6 months of age or older). The secondary outcome was the effect of the intervention on infant body mass index z scores (zBMIs).
Results
A total of 134 Navajo mothers of infants younger than 14 weeks were enrolled in the randomized clinical trial, including 68 (mean [SD] maternal age at enrollment, 27.4 [6.4] years) in the intervention group and 66 (mean [SD] maternal age at enrollment, 27.5 [6.1] years) in the control group. Intervention participants reported statistically significantly lower infant SSB consumption through 12 months post partum (mean [SE], 0.56 [0.12] cups per week in the intervention group and 1.78 [0.18] cups per week in the control group; incidence rate ratio, 0.31; 95% CI, 0.19-0.50). Improvements in responsive feeding practices were observed through 9 months post partum (mean [SE], 3.48 [0.07] in the intervention group and 3.22 [0.08] in the control group) (difference, 0.26; 95% CI, 0.06-0.47); statistical significance was lost at 12 months post partum. Age at which the infant was given first food was younger in the intervention group (mean [SE] age, 4.61 [0.21] months in the intervention group and 5.28 [0.23] months in the control group) (difference, −0.67; 95% CI, −0.04 to −1.29). Infants in the intervention group had lower zBMI at 6 and 9 months compared with those in the control group (mean [SE] at 9 months, 0.27 [0.14] in the intervention group and 0.81 [0.14] in the control group; difference, −0.54; 95% CI, −0.94 to −0.14). The 12-month between-group difference was meaningful but not statistically significant (mean [SE], 0.61 [0.16] in the intervention group and 1.07 [0.20] in the control group; difference, −0.46; 95% CI, −0.92 to 0.01).
Conclusions and Relevance
Infants of Native American mothers who participated in a home-visiting intervention had substantially lower SSB consumption and improvements in responsive feeding practices and infant zBMI scores, suggesting the intervention is effective for promoting healthy infant feeding and growth.
Trial Registration
ClinicalTrials.gov Identifier: NCT03101943
Introduction
Between 1980 and 2015, obesity rates doubled, or more than doubled, in more than 70 countries around the world, including the United States.1,2 The United States is one of the most obese nations in the world.2 The World Health Organization reported that 36.2% of US adults and 21.4% of US children (age range, 5-19 years) in 2016 were obese.2 If trends continue, more than one-half of US children who were 2 years old in 2016 will be obese by the time they are 35 years old.3 Risk for obesity begins in utero, requiring intergenerational solutions.4 A critical point of intervention to prevent obesity is in the first 1000 days (from pregnancy through the first 24 months of life) when there is greater developmental plasticity.5,6,7
Native American (NA) children have the highest rates of obesity in the United States, leading to increased risk for obesity and obesity-related morbidity and mortality across the life course.8 Resulting underlying conditions (eg, diabetes and cardiovascular disease) also exacerbate disparities in poor infectious disease outcomes (eg, COVID-19).9 The latest data indicate that 20.7% of NA children 2 to 5 years old are obese8 compared with 13.7% of US children of all other races.10 These disparities are rooted in a history of colonization and loss of lands that disrupted NA traditional family networks, lifestyles favoring rigorous physical activity, and healthy food systems.11,12 These effects manifest in food and water insecurity, unhealthy commodity foods, chronic stress,13 a paucity of grocery stores on reservation lands, and ubiquitous fast-food chains and convenience stores marketing sugar-sweetened beverages (SSBs) and processed foods.14,15,16,17
Children in the United States are exceeding guidelines for daily intake of added sugar, mostly through consumption of SSBs.18 Despite guidance by the American Academy of Pediatrics to avoid SSBs before age 2 years—they have even gone so far as to encourage excise taxes on SSBs to reduce consumption19—it is well known that very young children are being introduced to SSBs.20 Unpublished data from a prior study conducted in southwestern US reservation communities showed that 87% of participating Navajo mothers fed SSBs to infants by age 12 months (A. Barlow, PhD, MPH, MA, written communication, July 11, 2016). Published research related to early childhood obesity prevention is lacking; existing studies21,22,23,24 among NA populations have mostly focused on older children. More empirical knowledge is needed on the effects of early childhood nutrition intervention among this population, one of the most at-risk and understudied groups in the country.25,26
The brief Family Spirit Nurture (FSN) early childhood home-visiting intervention, which was designed in partnership with tribal communities, addressed specific parent feeding practices in infancy associated with increased risk for early childhood obesity, including the following: (1) infant SSB consumption,27 (2) introduction of complementary foods before 6 months post partum,28 and (3) nonresponsive feeding styles.29,30 Prior studies31,32 have shown that home visiting is an effective and acceptable method of intervention in NA communities. However, no previous evidence-based home-visiting interventions in NA communities have targeted early childhood obesity prevention. The present randomized clinical trial (RCT) addresses this gap in prevention science by evaluating the effect of the brief FSN intervention on mothers’ responsive feeding, initiation of complementary foods, infant SSB intake, and infant weight status 3 to 12 months post partum.
Methods
Study Design and Objectives
This trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. Parent or guardian written informed consent and participant assent were obtained for participants younger than 18 years, and written informed consent was obtained for those 18 years or older. The study was approved by the Navajo Nation Human Research Review Board and the Johns Hopkins Bloomberg School of Public Health Institutional Review Board. The trial protocol is available in Supplement 1.
The FSN intervention was evaluated among a reservation-based community in Shiprock, New Mexico, on the Navajo Nation, through a 1:1 RCT comparing FSN with an injury prevention education (IPE) control condition. Family Spirit Nurture built on the previously published, evidence-based Family Spirit home-visiting early childhood intervention.31,32,33 Family Spirit is the only national evidence-based home-visiting model recognized by the Maternal, Infant, and Early Childhood Home Visiting (MIECHV) Program34 designed by and for tribal communities. The program is a bipartisan, congressionally legislated strategy to promote maternal and early childhood health and well-being.35,36 Using the same format and delivery system as Family Spirit, FSN content (6 lessons) focused on early childhood feeding and avoidance of SSBs, which were not included in the original Family Spirit curriculum. The control group received IPE content (3 lessons) from the previous Family Spirit intervention. All home-visiting lessons were delivered by Navajo paraprofessionals (L.C., L.N., K.S-Y., and S.Y.) from 3 to 6 months post partum. To examine how water insecurity impacts infant feeding practices, all families received potable water delivery 6 to 9 months post partum. In this article, we intentionally do not present study time points impacted by water delivery (7 and 8 months post partum). Those findings are beyond the scope of this study and will be reported in a separate article.
Evaluation assessments conducted at baseline (<14 weeks post partum) and 4, 6, 7, 8, 9, and 12 months post partum included the following: sociodemographic data; maternal and infant beverage intake; mothers’ SSB knowledge, infant feeding practices, responsive feeding practices, perceptions of infant weight and eating, and infant temperament; and maternal stress and depression. Pregnancy and delivery data were abstracted from medical records, as were maternal and infant physiologic and anthropometric data.
Primary objectives were to assess the effect of a brief home-visiting approach, FSN, on SSB consumption, responsive parenting and infant feeding practices, and optimal infant growth through 12 months post partum. The ultimate goal was to improve healthy growth in the first year of life.
Eligibility, Randomization, and Blinding
Eligibility criteria included maternal age 13 years or older, self-reported NA race/ethnicity, mother to an infant younger than 14 weeks, and residence within 50 miles of the Northern Navajo Medical Center, located in Shiprock, New Mexico. Mothers were excluded if they were unable to fully participate or were unwilling to undergo randomization. Between March 22, 2017, and May 18, 2018, mothers were recruited from the local pediatric clinic and the local Women, Infants, and Children program as well as through word of mouth. Participants were followed up through 12 months post partum.
Participants who completed a baseline assessment were randomized 1:1 to the FSN intervention or the IPE control. Randomization was stratified by water security status using random block size to ensure equal numbers of water-insecure households in each arm.37 Randomization status was delivered by text message or phone call to staff (L.C., L.N., K.S-Y., and S.Y.) who enrolled the participant. Participants and study staff (L.C., L.N., K.S-Y., and S.Y.) were not blind to randomization status.
Intervention and Control
Family Spirit Nurture content was developed through an iterative process between the Johns Hopkins Center for American Indian Health and a community advisory board. The community advisory board provided cultural and contextual guidance and ensured continuous engagement of Navajo stakeholders.
The FSN curriculum included 6 lessons delivered 3 to 6 months post partum by Navajo paraprofessionals covering the following: optimal infant feeding practices, responsive feeding, avoiding SSBs, optimal complementary feeding practices, and whole family healthy eating practices (Table 1). Curriculum content followed American Academy of Pediatrics guidelines for feeding infants.38 Trained Navajo paraprofessional family health coaches (L.C., L.N., K.S-Y., and S.Y.) taught lessons to mothers and other invited caregivers using table-top flip charts in participants’ homes or other private locations. Lessons are highly visual, are interactive, and incorporate cultural teachings related to infant feeding and nutrition that support study aims. Each lesson included a hands-on activity (eg, examination of the actual amount of sugar in specific SSBs) and exercises focused on goal setting and self-esteem. Each 45-minute lesson included a warm-up, lesson content and activities, a question and answer period, referrals as needed, and summary handouts. Visits occurred every 2 weeks 3 to 6 months post partum.
Table 1. Overview of the 6-Lesson Family Spirit Nurture Curriculum.
| Lesson No. | Lesson title | Description | Timing of lesson |
|---|---|---|---|
| 1 | What You and Baby Eat = Your Baby’s Future | Importance of healthy eating for the whole family, with additional information on moderating sugar intake | 3 mo Post partum |
| 2 | Infant Feeding for Mind, Body, and Spirit | Infants’ and parents’ roles in feeding during early infancy; identifying infant’s hunger and fullness cues; delaying introduction of solid foods until 6 mo post partum; includes supplemental information for breastfeeding moms | 3.5 mo Post partum |
| 3 | I Am Me! | Identifying infant’s personality traits to aid in feeding and create a stronger bond between mom and infant | 4 mo Post partum |
| 4 | Rethink That Drink | Avoiding sugary drinks in infant’s diet and in whole family’s diet as healthy eating role models; considering the traditional Native American diet | 4.5 mo Post partum |
| 5 | Infant Feeding Part 2 | Preparing to introduce solid foods at 6 mo post partum | 5 mo Post partum |
| 6 | Be a Healthy Eating Role Model | Healthy eating starts with parents | 6 mo Post partum |
Control group mothers received 3 educational lessons, at 3, 4, and 5 months post partum, on injury prevention drawn from the original Family Spirit intervention. This subject was a high-priority topic for the community that avoided confounding FSN content and outcomes. Content included childproofing, safe travel and outings with an infant, and strategies to avoid abuse and neglect. Control lessons used the same format as FSN lessons.
Data Collection, Adverse Event Reporting, and Primary Outcomes
Assessments were administered in participants’ homes or other private locations on tablets using research electronic data capture (REDCap). REDCap is a secure, Health Insurance Portability and Accountability Act–adherent, web-based mobile application designed to support data capture for research studies.39,40 Data collection included interviews, maternal self-reports, study staff observations, and medical record review. Over the course of the study, participants received $20 in gift cards and 2 gift packages valued at $25 for completion of assessments at baseline and 4 and 12 months post partum.
At each visit (lesson or assessment), participants were asked whether they or their infant had visited the emergency department or had been hospitalized since the last visit. If the participant disclosed an adverse event, the incident was reported to relevant ethical review boards. None of the adverse events reported to review boards were considered a result of participation in the study.
The following primary outcomes were established a priori: infant SSB consumption41,42 and responsive parenting and complementary feeding practices (responsive feeding scale,41 age at complementary food introduction, and percentage introduced complementary food at 6 months of age or older). Infant SSB consumption was measured using the adapted Pre-School Beverage Intake Questionnaire. Mothers reported how many times per week their infant drank specific beverages in the past month and the approximate amount they drank each time. Visual aids (physical cups of different sizes and pictures with examples of SSBs) were used to help specify the quantity of fluid and categorize beverages as SSBs or not. For each beverage, the number of times consumed per week was multiplied by the quantity consumed to obtain cups per week. The number of cups per week of beverages categorized as SSBs were added to obtain total SSB cups per week. The SSB categories were sugar-sweetened juice beverages, Kool-Aid, soda (nondiet), sports or energy drinks, flavored milk, sweet tea, punch, and other drinks (specified). The secondary outcome was the preliminary effect of the intervention on infant body mass index z scores (zBMIs). Infant zBMI was calculated at 1 to 2 months, 3, 6, 9 and 12 months of age (± 1 month) using length and weight measurements abstracted from medical charts. If multiple measurements were available at a given time point, the average of those measurements was used. Measures were selected for their wide standard use, strong psychometric properties, and appropriate cross-cultural validity. Table 2 lists maternal and infant baseline characteristics (based on scales and calculated variables) and outcome measures.37,41,42,43,44,45,46,47,48,49,50
Table 2. Data Collection Time Points and Measures.
| Variable | Brief description and purpose | Instrument or mode of administration and source | α Level | Baseline | Post partum, mo | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 7 | 8 | 9 | 12 | |||||
| Baseline characteristics | ||||||||||
| Household crowding | No. of people living in the home per bedroom >2 | Demographics assessment: interview: US Department of Housing and Urban Development43 | NA | X | X | |||||
| Multigenerational household | Mother lives in a household with ≥3 generations | Demographics assessment: interview: developed by study team | NA | X | X | |||||
| Financial hardship index | 5-Item index (range, 0-5) to assess financial hardship in the past 6-12 moa | Demographics assessment: interview: adapted indicators of financial stress index44 | .5732 | X | X | |||||
| Food security | 6-Item index (range, 0-6) to assess home food availability in the past 30 db | Demographic assessment: interview: National Health and Nutrition Examination Survey45 | .8009 | X | X | X | X | X | ||
| Water security | Dichotomous variable categorizing family water security statusc | Water availability assessment: self-report: developed by study team but based on water assessments developed by the World Bank46 | NA | X | X | X | X | X | X | X |
| Prepregnancy maternal BMI | Calculated using prepregnancy maternal height and weight measurements from medical recordsd | Maternal medical record review: Centers for Disease Control and Prevention47 | NA | X | ||||||
| Infant birth weight status | Sex-specific categorization of birth weighte | Infant medical record review: INTERGROWTH-21st network standard cutoffs were used48,49 | NA | X | ||||||
| Early parent feeding practices | ||||||||||
| Responsive feeding scale | Mean of a 6-item scale (range, 0-4), with higher scores indicating more responsive feeding stylef | Responsive feeding scale assessment: self-report: Maryland Infant Feeding Study41 | .6343 | X | X | X | X | X | ||
| Age at complementary food introduction | Maternal report of the age infant was given first food | Infant feeding history interview: developed by study team | NA | X | X | X | X | X | X | X |
| % Introduced complementary food at 6 mo post partum or later | Age at complementary food introduction was dichotomized into ≥6 mo and <6 mo post partum | Infant feeding history interview: developed by study team | NA | X | X | X | X | X | X | X |
| SSB consumption | ||||||||||
| Maternal SSB knowledge index | A 14-item knowledge quiz with 32 possible points for correct answers | SSB knowledge quiz: self-report: developed by study team based on lesson content | .8526 | X | X | X | X | X | ||
| % Ever introduced SSBs (6, 9, and 12 mo post partum) | % Of mothers who had ever given their infant SSBs (excluding 100% fruit juice) at 6, 9, and 12 mo post partum | Infant feeding history interview: adapted BEVQ-PS42 | NA | X | X | X | X | X | X | X |
| Infant SSB consumption, cups per week | Mean weekly SSB consumption was calculated in cups per week | Infant feeding history interview: adapted BEVQ-PS42 | NA | X | X | X | X | X | X | X |
| Infant growth | ||||||||||
| zBMI | Infant length and weight measurements were abstracted from infant medical records | Infant medical record review: World Health Organization Child Growth Standards50 | NA | X | ||||||
Abbreviations: BEVQ-PS, Beverage Intake Questionnaire–Pre-School; BMI, body mass index; NA, not applicable; SSB, sugar-sweetened beverage; X, the time point at which relevant data were collected; zBMI, body mass index z score.
Items included (1) family did not always have enough money to make ends meet at the end of the month (in the past 12 months); (2) household pawned or sold items (in the past 6 months); (3) was unable to always pay utility or phone bill; (4) was unable to always heat home when needed; and (5) sought financial assistance from friends or family. Index was broken into the following categories: no financial hardship, 1 financial hardship, and 2 to 5 financial hardships reported.
Items included (1) cut meal size, (2) ate less than thought you should, (3) hungry but did not eat, (4) skipped meal, (5) did not eat for a whole day, and (6) cut size or skipped infant’s meal because of insufficient money or food. Index was broken into the following categories: high or marginal food security (0-1 item), low food security (2-3 items), and very low food security (4-6 items).
Coded as water insecure if participants answered affirmatively to any of the following 3 questions: (1) home was without drinking water 1 or more days in the past month, (2) not always comfortable drinking available water, and (3) not always comfortable using available water to feed infant or make formula.
Measurements closest to but before estimated conception were used. Measurements more than 2 years before estimated date of conception were excluded.
Small for gestational age (birth weight <10th percentile), average weight for gestational age (birth weight 10th-90th percentile), or large for gestational age (birth weight >90th percentile).
Responsive feeding is 1 of 5 domains of the adapted 24-item responsive feeding scale.
Sample Size and Statistical Analysis
The primary outcome for determining sample size was the proportion of infants ever introduced to SSBs at 12 months post partum. With a sample size of 136 mother-infant dyads (68 per study arm), the study was powered to detect a 24% between-group absolute difference (assuming 80% introduction among infants in the control group), accounting for 10% attrition at 12 months post partum (based on previous Family Spirit trials31,32) with 80% power and 5% significance.51
Sample size was calculated using Stata, version 14 (StataCorp).37 In addition, the study was powered to detect a 0.48 between-group difference in the mean zBMI with 80% power and 5% significance. This calculation assumes a mean (SD) zBMI of 1.09 (1.24) in the control group. The calculation was conducted via a power simulation (run 10 000 times) using a random draw from real site population data and random treatment assignment. Infant zBMI was not listed as a primary outcome given the large minimum detectable difference, but it was included in the original analysis plan.
Sociodemographic and relevant maternal and infant medical record data were examined for meaningful baseline between-group imbalances. Mixed-effects models were used to examine between-group differences in maternal and infant outcomes at 6, 9, and 12 months post partum and between-group differences in trajectory when appropriate and power was sufficient. Mixed-effects models incorporate all available data rather than relying on complete case analyses and provide more efficient estimates of time-specific comparisons in longitudinal RCTs than cross-sectional comparisons.52,53 For continuous outcomes (responsive feeding scale and infant zBMI), linear mixed-effects models were used, with a random effect at the individual level. For the proportion of infants ever given SSBs, a time-discrete survival analysis was used. Kaplan-Meier estimates and Cox proportional hazards are presented. For count data (infant SSB consumption in cups per week), mixed-effects Poisson regression, with a random effect at the individual level, was used. The zBMI was calculated from infant length and weight measurements obtained from medical records and included an additional time point before study enrollment (length and weight measurement 1-2 months post partum) for a more complete depiction of infant growth. Multiple imputation was used to address missing zBMI values.
Study retention was high through 12 months (>90%); therefore, multiple imputation was not required for primary outcomes. All mixed-effects models included study group, time point, and an interaction term between study group and time point. For time-invariant outcomes, linear or logistic regression was used to compare differences between study groups. All models controlled for water security status (stratification variable used for randomization), and birth weight was included as a control variable for infant zBMI. The threshold for interpretation of statistical significance was set at 2-sided P = .0125 using a Bonferroni correction to adjust for multiple comparisons.
Results
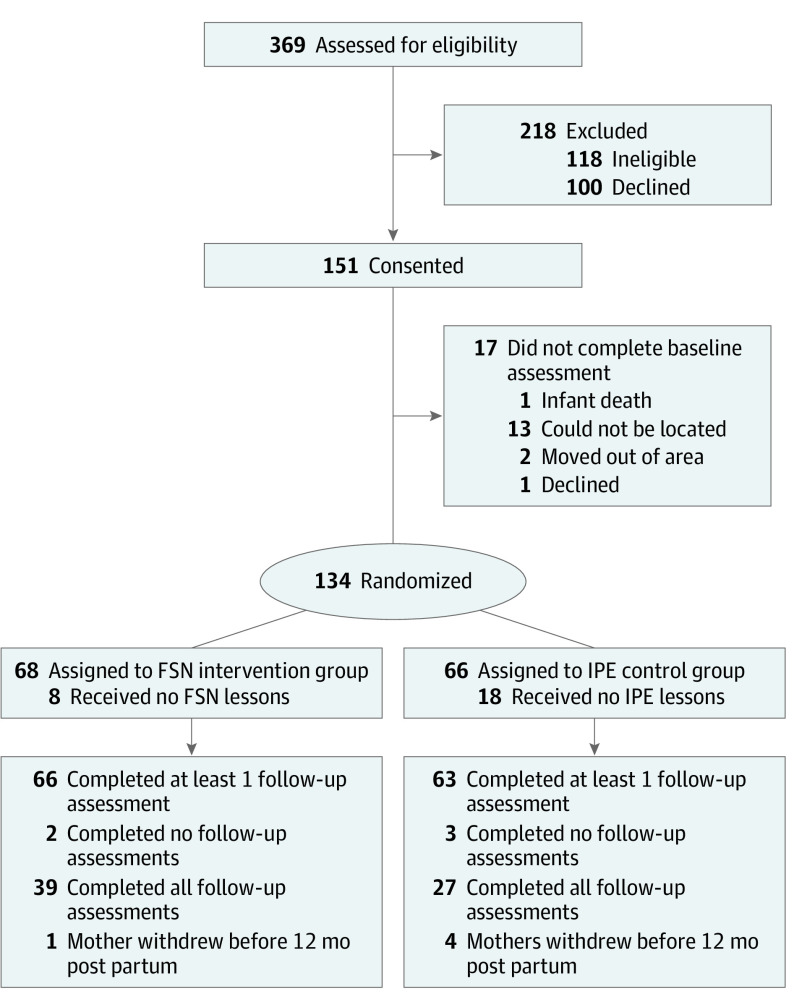
Of the 151 eligible participants who consented, 134 Navajo mothers of infants younger than 14 weeks enrolled in the RCT, completed baseline assessments, and were randomly assigned to the FSN intervention (n = 68) (mean [SD] age at enrollment, 27.4 [6.4] years) or IPE control group (n = 66) (mean [SD] age at enrollment, 27.5 [6.1] years). Between March 2017 and August 2019, follow-up assessments were completed by 77% (103 of 134) of participants at 6 months post partum, 78% (105 of 134) of participants at 9 months post partum, and 92% (123 of 134) of participants at 12 months post partum (Figure). There were no differences in attrition between study groups.
Figure. CONSORT Flow Diagram.
FSN indicates Family Spirit Nurture; IPE, injury prevention education.
Baseline Characteristics
The mean maternal age at enrollment was 27.4 years (range, 13-40 years), and the mean infant age was approximately 2 months. One-half of enrolled infants were girls (52% [70 of 134]). At baseline, most mothers (84% [112 of 134]) had completed education through at least high school or were still attending high school, and almost one-half (49% [66 of 134]) had completed education beyond high school. More than 80% (107 of 132) reported being unmarried. Household instability was high at baseline, with 44% (59 of 134) reporting they had lived in more than 1 home in the past year. Almost one-half (49% [65 of 133]) reported living in a multigenerational household (≥3 generations), and 55% (73 of 134) categorized their homes as crowded (>2 people sleeping per room).43 More than one-half (55% [74 of 134]) of enrolled mothers reported at least 1 indicator of financial hardship at baseline,44 almost one-quarter (23% [30 of 133]) reported low or very low food security,45 and more than one-third (35% [47 of 134]) reported being water insecure in the past month at baseline.46
Medical record review revealed that, of the women for whom prepregnancy BMI data were available (72% [97 of 134]), 27% (n = 26) were overweight, and 56% (n = 54) were obese. In addition, 21% (25 of 121) of enrolled mothers had diabetes during pregnancy (compared with 6.9% reported nationally in 2016),47 and 1 in 5 infants (21% [26 of 123]) were born large for gestational age.48,49 There were no meaningful differences between the intervention and control groups in baseline characteristics, and baseline equivalence was maintained at 12 months post partum (Table 3).
Table 3. Maternal and Infant Baseline Characteristics.
| Variable | No. | Total (N = 134) | No. | Intervention (n = 68) | No. | Control (n = 66) |
|---|---|---|---|---|---|---|
| Maternal baseline characteristics | ||||||
| Mother’s age at enrollment, mean (SD), y | 134 | 27.4 (6.2) | 68 | 27.4 (6.4) | 66 | 27.5 (6.1) |
| Parity, mean (SD) | 134 | 2.4 (1.5) | 68 | 2.4 (1.6) | 66 | 2.4 (1.4) |
| Educational level, No. (%) | 134 | 68 | 66 | |||
| Less than high school | 22 (16) | 8 (12) | 14 (21) | |||
| Still attending or completed high school or GED | 46 (34) | 27 (40) | 19 (29) | |||
| Completed more than high school | 66 (49) | 33 (49) | 33 (50) | |||
| Married, No. (%) | 132 | 25 (19) | 67 | 10 (15) | 65 | 15 (23) |
| Employment status, No. (%) | 134 | 68 | 66 | |||
| Not employed | 113 (84) | 54 (79) | 59 (89) | |||
| Full-time | 12 (9) | 8 (12) | 4 (6) | |||
| Part-time | 9 (7) | 6 (9) | 3 (5) | |||
| No. of people in home, mean (SD) | 134 | 6.3 (2.4) | 68 | 6.3 (2.5) | 66 | 6.3 (2.4) |
| Households crowded, No. (%) | 134 | 73 (55) | 68 | 38 (56) | 66 | 35 (53) |
| Multigenerational household (≥3 generations), No. (%) | 133 | 65 (49) | 68 | 30 (44) | 65 | 35 (54) |
| Lived in >1 home in the past year, No. (%) | 134 | 59 (44) | 68 | 27 (40) | 66 | 32 (49) |
| Received financial help with groceries, No. (%) | 134 | 92 (69) | 68 | 47 (69) | 66 | 45 (68) |
| Enrolled in WIC, No. (%) | 134 | 60 (45) | 68 | 31 (46) | 66 | 29 (44) |
| Enrolled in SNAP or EBT, No. (%) | 134 | 74 (55) | 68 | 37 (54) | 66 | 37 (56) |
| Enrolled in TANF, No. (%) | 134 | 3 (2) | 68 | 1 (2) | 66 | 2 (3) |
| Financial hardship reported, No. (%) | 134 | 68 | 66 | |||
| No financial hardship | 60 (45) | 33 (49) | 27 (41) | |||
| 1 Financial hardship | 32 (24) | 13 (19) | 19 (29) | |||
| 2-5 Financial hardships | 42 (31) | 22 (32) | 20 (30) | |||
| Water insecure in the past 30 d, No. (%) | 134 | 47 (35) | 68 | 24 (35) | 66 | 23 (35) |
| Food security in the past 30 d, No. (%) | 133 | 67 | 66 | |||
| High or marginal food security (0-1 items) | 103 (77) | 57 (85) | 46 (70) | |||
| Low food security (2-3 items) | 21 (16) | 6 (9) | 15 (23) | |||
| Very low food security (4-6 items) | 9 (7) | 4 (6) | 5 (8) | |||
| Prepregnancy maternal BMI, mean (SD) | 97 | 31.2 (7.0) | 51 | 31.5 (7.7) | 46 | 30.9 (6.1) |
| Diabetes during pregnancy, No. (%) | 121 | 25 (21) | 63 | 11 (18) | 58 | 14 (24) |
| Gestational diabetes, No. (%) | 120 | 16 (13) | 62 | 9 (15) | 58 | 7 (12) |
| Type 2 diabetes, No. (%)a | 120 | 9 (8) | 62 | 2 (3) | 58 | 7 (12) |
| Maternal SSB knowledge, median (IQR) | 134 | 29 (26-30) | 68 | 29 (27-30) | 66 | 29 (25-30) |
| Infant baseline characteristics | ||||||
| Girls, No. (%) | 134 | 70 (52) | 68 | 35 (52) | 66 | 35 (53) |
| Age at enrollment, mean (SD), mo | 132 | 2.0 (1.1) | 68 | 1.9 (1.1) | 64 | 2.0 (1.0) |
| Birth weight, mean (SD), g | 123 | 3326.7 (488.0) | 64 | 3315.3 (448.0) | 59 | 3339.1 (531.6) |
| Infant birth weight status, No. (%) | 123 | 64 | 59 | |||
| Small for gestational age | 3 (2) | 2 (3) | 1 (2) | |||
| Average weight for gestational age | 94 (76) | 50 (78) | 44 (75) | |||
| Large for gestational age | 26 (21) | 12 (19) | 14 (24) | |||
| Gestational age, mean (SD), wk | 125 | 38.6 (1.6) | 64 | 38.5 (1.7) | 61 | 38.7 (1.5) |
| Premature (gestational age <37 wk), No. (%) | 125 | 13 (10) | 64 | 8 (13) | 61 | 5 (8) |
| Initiated breastfeeding, No. (%) | 133 | 116 (87) | 68 | 61 (90) | 65 | 55 (85) |
Abbreviations: BMI, body mass index; EBT, electronic benefit transfer; GED, general equivalency diploma; IQR, interquartile range; SNAP, Supplemental Nutrition Assistance Program; SSB, sugar-sweetened beverage; TANF, Temporary Assistance for Needy Families; WIC, Women, Infants, and Children program.
Data were not available for type 1 diabetes status in the study population.
Maternal and Infant Primary Outcomes
Early Parent Feeding Practices
Responsive feeding was higher (higher indicates more responsive) in the intervention group vs the control group at 6 months (mean [SE], 3.54 [0.07] in the intervention group and 3.29 [0.08] in the control group) (adjusted mean difference, 0.25; 95% CI, 0.04-0.47) and at 9 months post partum (mean [SE], 3.48 [0.07] in the intervention group and 3.22 [0.08] in the control group) (adjusted mean difference, 0.26; 95% CI, 0.06-0.47). By age 12 months, the between-group difference was no longer statistically significant. These results are summarized in Table 4. On average, infants in the intervention group were introduced to complementary foods 20 days earlier than infants in the control group, and a higher proportion of infants in the intervention group were introduced before age 6 months. Age at which an infant was given first food was mean (SE) 4.61 (0.21) months in the intervention group and 5.28 (0.23) months in the control group (difference, −0.67; 95% CI, −0.04 to −1.29).
Table 4. Maternal and Infant Outcomesa.
| Variable | No. | Intervention | No. | Control | Adjusted mean difference (95% CI) | P value |
|---|---|---|---|---|---|---|
| Early parent feeding practices | ||||||
| Responsive feeding, mean (SE)b | ||||||
| Baseline | 67 | 3.41 (0.07) | 61 | 3.34 (0.07) | 0.07 (−0.12 to 0.26) | .45 |
| 6 mo Post partum | 55 | 3.54 (0.07) | 41 | 3.29 (0.08) | 0.25 (0.04 to 0.47) | .02 |
| 9 mo Post partum | 58 | 3.48 (0.07) | 47 | 3.22 (0.08) | 0.26 (0.06 to 0.47) | .01 |
| 12 mo Post partum | 61 | 3.40 (0.07) | 57 | 3.21 (0.07) | 0.19 (0.00 to 0.38) | .05 |
| Age infant given first food, mean (SE), mo | 65 | 4.61 (0.21) | 61 | 5.28 (0.23) | −0.67 (−0.04 to −1.29) | .04 |
| Infant given first food ≥6 mo post partum, No. (%) | 65 | 17 (26) | 61 | 27 (44) | OR, 0.45 (0.21 to 0.96) | .04 |
| SSB consumption | ||||||
| Ever introduced SSBs, No. (%)c | ||||||
| 6 mo Post partum | 66 | 5 (7) | 62 | 5 (8) | HR, 0.95 (0.28 to 3.28) | .94 |
| 9 mo Post partum | 66 | 25 (38) | 62 | 21 (34) | HR, 1.14 (0.63 to 2.03) | .67 |
| 12 mo Post partum | 66 | 40 (63) | 62 | 33 (53) | HR, 1.25 (0.79 to 1.98) | .34 |
| Infant SSB consumption, mean (SE), cups per weekb | ||||||
| 6 mo Post partum | 55 | 0.01 (0.02) | 42 | 0.16 (0.08) | IRR, 0.09 (0.01 to 0.89) | .04 |
| 9 mo Post partum | 58 | 0.09 (0.04) | 47 | 0.43 (0.10) | IRR, 0.21 (0.08 to 0.57) | .002 |
| 12 mo Post partum | 62 | 0.56 (0.12) | 60 | 1.78 (0.18) | IRR, 0.31 (0.19 to 0.50) | <.001 |
| Infant growth | ||||||
| Infant zBMI, mean (SE)b,d | ||||||
| <2 mo Post partum | 62 | −0.44 (0.13) | 58 | −0.40 (0.13) | −0.04 (−0.41 to 0.33) | .83 |
| 3 mo Post partum | 62 | −0.03 (0.17) | 58 | 0.32 (0.14) | −0.35 (−0.79 to 0.08) | .11 |
| 6 mo Post partum | 62 | 0.16 (0.14) | 58 | 0.63 (0.14) | −0.47 (−0.87 to −0.06) | .02 |
| 9 mo Post partum | 62 | 0.27 (0.14) | 58 | 0.81 (0.14) | −0.54 (−0.94 to −0.14) | .009 |
| 12 mo Post partum | 62 | 0.61 (0.16) | 58 | 1.07 (0.20) | −0.46 (−0.92 to 0.01) | .05 |
Abbreviations: HR, hazard ratio; IRR, incidence rate ratio; OR, odds ratio; SSB, sugar-sweetened beverage; zBMI, body mass index z score.
All models control for water security status at baseline.
All mixed-effects models included study group, time point, and an interaction term between study group and time point and included a random effect at the individual level.
Percentages represent Kaplan-Meier estimates controlling for baseline water insecurity.
Model was adjusted for birth weight, and multiple imputation was used to address missing values.
SSB Consumption
Maternal SSB knowledge, assessed via the maternal SSB knowledge index (a 14-item curriculum-based assessment created by the study team), was high (>90% of questions answered correctly) in both study groups throughout the study. There was almost no SSB consumption among infants in either study group through 6 months post partum. By the final assessment (12 months post partum), more than one-half of infants in both groups had been given SSBs, with an average age at initiation of 9 months (8.83 months in the intervention group and 9.19 months in the control group, P = .59). Mothers in the intervention group reported statistically significantly lower infant SSB consumption at 9 months of age (mean [SE], 0.09 [0.04] cups per week in the intervention group vs 0.43 [0.10] cups per week in the control group; incidence rate ratio, 0.21; 95% CI, 0.08-0.57) and at 12 months of age (mean [SE], 0.56 [0.12] cups per week in the intervention group vs 1.78 [0.18] cups per week in the control group; incidence rate ratio, 0.31; 95% CI, 0.19-0.50). These results are summarized in Table 4.
Infant Growth
The overall target of the study was to improve infant growth and reduce early childhood obesity. Infant growth measures were not indicated as primary outcomes because this study was only powered to detect large between-study group differences, which could lead to missing meaningful small to moderate differences. We present the results herein to inform future directions for study. Infant birth weight was comparable between the study groups (mean [SD], 3315.3 [448.0] g in the intervention group vs 3339.1 [531.6] g in the control group) (Table 3).
The zBMI50 was lower in the intervention group vs the control group at 6 months (mean [SE], 0.16 [0.14] in the intervention group and 0.63 [0.14] in the control group; difference, −0.47; 95% CI, −0.87 to −0.06) and at 9 months post partum (mean [SE], 0.27 [0.14] in the intervention group and 0.81 [0.14] in the control group; difference, −0.54; 95% CI, −0.94 to −0.14). Although the magnitude of the between-group difference at 12 months remained meaningful, statistical significance was not achieved (mean [SE], 0.61 [0.16] in the intervention group [73rd percentile] at 12 months and 1.07 [0.20] in the control group [86th percentile]; difference, −0.46; 95% CI, −0.92 to 0.01). These results are summarized in Table 4. In addition, growth trajectories for infants in the control group were greater when tracking zBMI from 2 months to 9 months post partum (adjusted mean difference, −0.49; 95% CI, −0.94 to −0.06; P = .03).
Discussion
Results from this study provide preliminary evidence of effectiveness of this brief, low-cost home-visiting intervention to prevent early childhood obesity. Improvements were achieved in overall infant feeding practices that are known to protect against early childhood obesity, including quantity of infant SSB consumption and mothers’ responsive feeding practices. In a future article, we will explore whether water delivery to both groups 6 to 9 months post partum impacted timing of SSB introduction and quantity consumed.
Extensive evidence over many decades and multiple trials supports that home visiting provides a wide range of benefits to mothers and their children.35 We believe that this study adds important findings to a limited body of literature regarding the benefits of home-visiting interventions to promote healthy infant and toddler growth and reduce early childhood obesity risk.24,54,55,56,57,58 It also further supports the effectiveness of culturally matched, community-based paraprofessionals in fostering positive change in their communities.31 Although brief, the intervention focused on modifiable obesogenic behaviors. In communities where improvements in diet are difficult to achieve because of lack of access to high-quality foods, it is critical to note that home-visiting interventions targeting better feeding practices and decreased SSB intake may be sufficient to improve children’s weight status. A zBMI of 1.04 corresponds to the 85th percentile, the threshold for being categorized as overweight.59 At 12 months post partum, the mean zBMI for control infants (1.07 [86th percentile]) exceeded this threshold, whereas the mean zBMI for intervention infants did not (0.61 [73rd percentile]).
Limitations
This trial has some limitations. This research was conducted at a single site, which affects the generalizability of study results. Participants were only followed up from 3 to 12 months post partum. To fully understand the impact of improvements in feeding practices and SSB consumption on early childhood obesity and the durability of positive effects, a longer multisite study is needed. In addition, the infant responsive feeding scale may have been limited in its application to infants at 12 months because decreases in responsive feeding were observed in both study groups. Transitioning to the toddler responsive feeding scale may be needed to continue to capture the intervention’s effect on responsive feeding as infants become more independent eaters. Furthermore, there was a missed opportunity to intervene during pregnancy and the first 3 months of life, which could enhance outcomes by impacting metabolic reprogramming and promotion of optimal feeding practices from birth.60 We are addressing all of these limitations through a longer, multisite RCT61 across 3 reservation sites with 2 tribal nation partners.62
Conclusions
This trial assesses the first home-visiting intervention to date created in partnership with NA communities that has documented positive effects on healthy infant feeding and growth during the first year of life. Native American populations have the largest early childhood obesity disparities in the US,8 which are associated with increased risk for obesity and obesity-related morbidity and mortality across the life course. We believe that this trial provides evidence for an innovative, promising, and frugal avenue for prevention. More than 130 tribal communities and 4 non-NA communities in 21 US states63 have been trained to implement the original Family Spirit program in their communities, which provides critical opportunities for scaling the brief FSN intervention.
Trial protocol
Data sharing statement
References
- 1.Afshin A, Forouzanfar MH, Reitsma MB, et al. ; GBD 2015 Obesity Collaborators . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13-27. doi: 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Global Health Observatory (GHO) data: overweight and obesity. Updated 2019. Accessed February 28, 2020. https://www.who.int/gho/ncd/risk_factors/overweight/en/
- 3.Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med. 2017;377(22):2145-2153. doi: 10.1056/NEJMoa1703860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moussa HN, Alrais MA, Leon MG, Abbas EL, Sibai BM. Obesity epidemic: impact from preconception to postpartum. Future Sci OA. 2016;2(3):FSO137. doi: 10.4155/fsoa-2016-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med. 2016;50(6):761-779. doi: 10.1016/j.amepre.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 6.Redsell SA, Edmonds B, Swift JA, et al. Systematic review of randomised controlled trials of interventions that aim to reduce the risk, either directly or indirectly, of overweight and obesity in infancy and early childhood. Matern Child Nutr. 2016;12(1):24-38. doi: 10.1111/mcn.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blake-Lamb TL, Locks LM, Perkins ME, Woo Baidal JA, Cheng ER, Taveras EM. Interventions for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med. 2016;50(6):780-789. doi: 10.1016/j.amepre.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullock A, Sheff K, Moore K, Manson S. Obesity and overweight in American Indian and Alaska Native children, 2006-2015. Am J Public Health. 2017;107(9):1502-1507. doi: 10.2105/AJPH.2017.303904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999-2016. Pediatrics. 2018;141(3):e20173459. doi: 10.1542/peds.2017-3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bombay A, Matheson K, Anisman H. The intergenerational effects of Indian Residential Schools: implications for the concept of historical trauma. Transcult Psychiatry. 2014;51(3):320-338. doi: 10.1177/1363461513503380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemke S, Delormier T. Indigenous Peoples’ food systems, nutrition, and gender: conceptual and methodological considerations. Matern Child Nutr. 2017;13(S3)(suppl 3):e12499. doi: 10.1111/mcn.12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gone JP, Trimble JE. American Indian and Alaska Native mental health: diverse perspectives on enduring disparities. Annu Rev Clin Psychol. 2012;8:131-160. doi: 10.1146/annurev-clinpsy-032511-143127 [DOI] [PubMed] [Google Scholar]
- 14.Fretts AM, Huber C, Best LG, et al. Availability and cost of healthy foods in a large American Indian community in the north-central United States. Prev Chronic Dis. 2018;15:E03. doi: 10.5888/pcd15.170302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berryhill K, Hale J, Chase B, Clark L, He J, Daley CM. Food security and diet among American Indians in the Midwest. J Community Health. 2018;43(5):901-907. doi: 10.1007/s10900-018-0501-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown MC, Shrestha U, Huber C, et al. Characterizing the local food environment and grocery-store decision making among a large American Indian community in the north-central USA: qualitative results from the Healthy Foods Healthy Families Feasibility Study. Public Health Nutr. 2019;22(14):2653-2661. doi: 10.1017/S1368980019001095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connell M, Buchwald DS, Duncan GE. Food access and cost in American Indian communities in Washington State. J Am Diet Assoc. 2011;111(9):1375-1379. doi: 10.1016/j.jada.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey RL, Fulgoni VL, Cowan AE, Gaine PC. Sources of added sugars in young children, adolescents, and adults with low and high intakes of added sugars. Nutrients. 2018;10(1):102. doi: 10.3390/nu10010102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muth ND, Dietz WH, Magge SN, Johnson RK; American Academy of Pediatrics; Section on Obesity; Committee on Nutrition; American Heart Association . Public policies to reduce sugary drink consumption in children and adolescents. Pediatrics. 2019;143(4):e20190282. doi: 10.1542/peds.2019-0282 [DOI] [PubMed] [Google Scholar]
- 20.Kay MC, Welker EB, Jacquier EF, Story MT. Beverage consumption patterns among infants and young children (0-47.9 months): data from the Feeding Infants and Toddlers Study, 2016. Nutrients. 2018;10(7):825. doi: 10.3390/nu10070825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaRowe TL, Adams AK, Jobe JB, Cronin KA, Vannatter SM, Prince RJ. Dietary intakes and physical activity among preschool-aged children living in rural American Indian communities before a family-based healthy lifestyle intervention. J Am Diet Assoc. 2010;110(7):1049-1057. doi: 10.1016/j.jada.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lytle LA, Dixon LB, Cunningham-Sabo L, et al. Dietary intakes of Native American children: findings from the Pathways Feasibility Study. J Am Diet Assoc. 2002;102(4):555-558. doi: 10.1016/S0002-8223(02)90129-X [DOI] [PubMed] [Google Scholar]
- 23.Stroehla BC, Malcoe LH, Velie EM. Dietary sources of nutrients among rural Native American and White children. J Am Diet Assoc. 2005;105(12):1908-1916. doi: 10.1016/j.jada.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 24.Karanja N, Lutz T, Ritenbaugh C, et al. The TOTS community intervention to prevent overweight in American Indian toddlers beginning at birth: a feasibility and efficacy study. J Community Health. 2010;35(6):667-675. doi: 10.1007/s10900-010-9270-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Healthy Eating Research A national research agenda to reduce consumption of sugar-sweetened beverages and increase safe water access and consumption among zero- to five-year-olds. Published August 2018. Accessed February 12, 2020. https://healthyeatingresearch.org/research/national-research-agenda-zero-to-five-beverage-consumption/ [DOI] [PMC free article] [PubMed]
- 26.Duffy EW, Lott MM, Johnson EJ, Story MT. Developing a national research agenda to reduce consumption of sugar-sweetened beverages and increase safe water access and consumption among 0- to 5-year-olds: a mixed methods approach. Public Health Nutr. 2020;23(1):22-33. doi: 10.1017/S1368980019002106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan L, Li R, Park S, Galuska DA, Sherry B, Freedman DS. A longitudinal analysis of sugar-sweetened beverage intake in infancy and obesity at 6 years. Pediatrics. 2014;134(suppl 1):S29-S35. doi: 10.1542/peds.2014-0646F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97(12):1019-1026. doi: 10.1136/archdischild-2012-302263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spill MK, Callahan EH, Shapiro MJ, et al. Caregiver feeding practices and child weight outcomes: a systematic review. Am J Clin Nutr. 2019;109(suppl 7):990S-1002S. doi: 10.1093/ajcn/nqy276 [DOI] [PubMed] [Google Scholar]
- 30.Black MM, Aboud FE. Responsive feeding is embedded in a theoretical framework of responsive parenting. J Nutr. 2011;141(3):490-494. doi: 10.3945/jn.110.129973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barlow A, Mullany B, Neault N, et al. Paraprofessional-delivered home-visiting intervention for American Indian teen mothers and children: 3-year outcomes from a randomized controlled trial. Am J Psychiatry. 2015;172(2):154-162. doi: 10.1176/appi.ajp.2014.14030332 [DOI] [PubMed] [Google Scholar]
- 32.Barlow A, Mullany B, Neault N, et al. Effect of a paraprofessional home-visiting intervention on American Indian teen mothers’ and infants’ behavioral risks: a randomized controlled trial. Am J Psychiatry. 2013;170(1):83-93. doi: 10.1176/appi.ajp.2012.12010121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullany B, Barlow A, Neault N, et al. The Family Spirit trial for American Indian teen mothers and their children: CBPR rationale, design, methods and baseline characteristics. Prev Sci. 2012;13(5):504-518. doi: 10.1007/s11121-012-0277-2 [DOI] [PubMed] [Google Scholar]
- 34.Health Resources & Services Administration Home visiting. Accessed October 5, 2020. https://mchb.hrsa.gov/maternal-child-health-initiatives/home-visiting-overview
- 35.Sama-Miller E, Akers L, Mraz-Esposito A, Coughlin R, Zukiewicz M Home Visiting Evidence of Effectiveness Review: Executive Summary. US Dept of Health and Human Services, Administration for Children and Families, Office of Planning, Research, and Evaluation; 2019. OPRE Report 2019-93.
- 36.Michalopoulos C, Shea Crowne S, Portilla XA, et al. A summary of results from the MIHOPE and MIHOPE–Strong Start studies of evidence-based home visiting. Published January 18, 2019. Accessed February 10, 2020. https://www.acf.hhs.gov/opre/resource/a-summary-of-results-from-the-mihope-and-mihope-strong-start-studies-of-evidence-based-home-visiting
- 37.StataCorp Stata statistical software: release 14. StataCorp; 2015. [Google Scholar]
- 38.American Academy of Pediatrics Infant food and feeding. Accessed February 10, 2020. https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/HALF-Implementation-Guide/Age-Specific-Content/Pages/Infant-Food-and-Feeding.aspx
- 39.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap Consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurley KM, Black MM, Papas MA, Caulfield LE. Maternal symptoms of stress, depression, and anxiety are related to nonresponsive feeding styles in a statewide sample of WIC participants. J Nutr. 2008;138(4):799-805. doi: 10.1093/jn/138.4.799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lora KR, Davy B, Hedrick V, Ferris AM, Anderson MP, Wakefield D. Assessing initial validity and reliability of a beverage intake questionnaire in Hispanic preschool-aged children. J Acad Nutr Diet. 2016;116(12):1951-1960. doi: 10.1016/j.jand.2016.06.376 [DOI] [PubMed] [Google Scholar]
- 43.Blake KS, Kellerson RL, Simic A; Econometrica, Inc; ICF International Measuring overcrowding in housing. Prepared for US Dept of Housing and Urban Development, Office of Policy Development and Research. Published September 2007. Accessed February 12, 2020. https://www.huduser.gov/publications/pdf/measuring_overcrowding_in_hsg.pdf
- 44.Bray JR Number 4: Hardship in Australia: an analysis of financial stress indicators in the 1998-99 Australian Bureau of Statistics Household Expenditure Survey. Accessed February 12, 2020. https://www.dss.gov.au/about-the-department/publications-articles/research-publications/occasional-paper-series/number-4-hardship-in-australia-an-analysis-of-financial-stress-indicators-in-the-1998-99-australian-bureau-of-statistics-household-expenditure-survey
- 45.Centers for Disease Control and Prevention National Health and Nutrition Examination Survey (NHANES): food security-FSQ. Updated 2009. Accessed October 9, 2020. https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/questionnaires/ai_fsq_f.pdf
- 46.World Bank Living Standards Measurement Study. Updated 2019. Accessed March 11, 2020. http://surveys.worldbank.org/
- 47.Centers for Disease Control and Prevention About adult BMI. Updated 2017. Accessed February 12, 2020. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html
- 48.Villar J, Cheikh Ismail L, Victora CG, et al. ; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) . International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857-868. doi: 10.1016/S0140-6736(14)60932-6 [DOI] [PubMed] [Google Scholar]
- 49.The Global Health Network International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) standards and tools. Updated 2020. Accessed February 10, 2020. https://intergrowth21.tghn.org/standards-tools/
- 50.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. World Health Organization; 2006. [Google Scholar]
- 51.Bleich SN, Vercammen KA. The negative impact of sugar-sweetened beverages on children’s health: an update of the literature. BMC Obes. 2018;5:6. doi: 10.1186/s40608-017-0178-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashbeck EL, Bell ML. Single time point comparisons in longitudinal randomized controlled trials: power and bias in the presence of missing data. BMC Med Res Methodol. 2016;16:43. doi: 10.1186/s12874-016-0144-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Twisk J, de Vente W. Attrition in longitudinal studies: how to deal with missing data. J Clin Epidemiol. 2002;55(4):329-337. doi: 10.1016/S0895-4356(01)00476-0 [DOI] [PubMed] [Google Scholar]
- 54.Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Effectiveness of home based early intervention on children’s BMI at age 2: randomised controlled trial. BMJ. 2012;344:e3732. doi: 10.1136/bmj.e3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paul IM, Savage JS, Anzman SL, et al. Preventing obesity during infancy: a pilot study. Obesity (Silver Spring). 2011;19(2):353-361. doi: 10.1038/oby.2010.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wen LM, Baur LA, Simpson JM, et al. Sustainability of effects of an early childhood obesity prevention trial over time: a further 3-year follow-up of the Healthy Beginnings Trial. JAMA Pediatr. 2015;169(6):543-551. doi: 10.1001/jamapediatrics.2015.0258 [DOI] [PubMed] [Google Scholar]
- 57.Karanja N, Aickin M, Lutz T, et al. A community-based intervention to prevent obesity beginning at birth among American Indian children: study design and rationale for the PTOTS study. J Prim Prev. 2012;33(4):161-174. doi: 10.1007/s10935-012-0278-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ordway MR, Sadler LS, Holland ML, Slade A, Close N, Mayes LC. A home visiting parenting program and child obesity: a randomized trial. Pediatrics. 2018;141(2):e20171076. doi: 10.1542/peds.2017-1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention Defining childhood obesity. Updated 2018. Accessed June, 2020. https://www.cdc.gov/obesity/childhood/defining.html
- 60.Lane RH Fetal programming, epigenetics, and adult onset disease. Clin Perinatol. 2014;41(4):815-831. doi: 10.1016/j.clp.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 61.Piloting a precision approach to home visiting. ClinicalTrials.gov. identifier: NCT03975530. Updated August 10, 2020. Accessed September 27, 2020. https://clinicaltrials.gov/ct2/show/NCT03975530
- 62.Ingalls A, Rosenstock S, Foy Cuddy R, et al. Family Spirit Nurture (FSN): a randomized controlled trial to prevent early childhood obesity in American Indian populations: trial rationale and study protocol. BMC Obes. 2019;6:18. doi: 10.1186/s40608-019-0233-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johns Hopkins Bloomberg School of Public Health Our affiliates. Accessed October 5, 2020. https://www.jhsph.edu/research/affiliated-programs/family-spirit/training/our-affiliates/index.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Data sharing statement