This cohort study assesses the antibiotic susceptibility patterns of Escherichia coli among infants admitted to US neonatal intensive care units from 2009 to 2017.
Key Points
Question
To what antibiotics are Escherichia coli isolates susceptible among infants admitted to neonatal intensive care units, and how has this susceptibility changed over time?
Findings
In this cohort study of 721 infants with E coli infection admitted to 69 neonatal intensive care units across the US from 2009 to 2017, a mean of 66.8% of isolates from blood, cerebrospinal fluid, and urine specimens were not susceptible to ampicillin, 16.8% were not susceptible to aminoglycosides, and 5.0% had an extended-spectrum β-lactamase phenotype.
Meaning
These findings may inform empirical antibiotic prescribing for newborns across the US.
Abstract
Importance
Escherichia coli is a leading cause of serious infection among term and preterm newborn infants. Surveillance of antibiotic susceptibility patterns of E coli among infants admitted to neonatal intensive care units should inform empirical antibiotic administration.
Objective
To assess the epidemiologic characteristics and antibiotic susceptibility patterns of E coli in infants admitted to neonatal intensive care units in the US over time.
Design, Setting, and Participants
This retrospective cohort study used the Premier Health Database, a comprehensive administrative database of inpatient encounters from academic and community hospitals across the US. Participants included newborn infants admitted to centers contributing microbiology data from January 1, 2009, to December 31, 2017, with E coli isolated from blood, cerebrospinal fluid, or urine cultures. Data were collected and analyzed from December 1, 2018, to November 30, 2019.
Main Outcomes and Measures
Changes in annual antibiotic susceptibility of E coli during the study period. The proportion of infants with nonsusceptible organisms (resistant or intermediate susceptibility) in antibiotic categories by year, birth weight, infection source, and timing of infection and patient and center characteristics associated with neonatal E coli infection and antibiotic susceptibility were assessed.
Results
A total of 721 infants (434 male [60.2%]; median age at E coli infection, 14 days [interquartile range, 1-33 days]) from 69 centers had at least 1 episode of E coli infection and available susceptibility results. No significant changes were observed over time in the overall annual proportions of antibiotic nonsusceptibility to ampicillin (mean [SD], 66.8% [1.5%]; range, 63.3% to 68.6%; estimated yearly change, −0.28% [95% CI, −1.75% to 1.18%]), nonsusceptibility to aminoglycosides (mean [SD], 16.8% [4.5%]; range, 10.7% to 23.2%; estimated yearly change, −0.85% [95% CI, −1.93% to 0.23%]), or extended-spectrum β-lactamase phenotype (mean [SD], 5.0% [3.7%]; range, 0% to 11.1%; estimated yearly change, 0.46% [95% CI, −0.18% to 1.11%]). No isolates with nonsusceptibility to carbapenems were identified. Among 218 infants with early-onset infection, 22 (10.1%) had isolates with nonsusceptibility to both ampicillin and gentamicin, the antibiotics most commonly administered to newborns as empirical therapy.
Conclusions and Relevance
In this cohort study, nonsusceptibility to commonly administered antibiotics was found in substantial proportions of neonatal E coli isolates, with no significant change from 2009 to 2017. These findings may inform empirical antibiotic choices for newborn infants.
Introduction
Escherichia coli causes invasive systemic infections in newborn infants, defined as early-onset sepsis (EOS) when occurring in the first 3 days after birth or late-onset sepsis (LOS) when occurring after 3 days.1 Among infants born preterm, E coli is the most common cause of EOS and a significant cause of LOS and urinary tract infection.2,3 E coli is the second most common cause of EOS in term infants after group B Streptococcus (GBS).3,4 Guidelines from the American Academy of Pediatrics for the management of EOS recommend that standard empirical antibiotic regimens include ampicillin and gentamicin dual therapy to provide coverage for common neonatal pathogens, including E coli and GBS.2,4 In contrast, empirical therapy for suspected LOS typically varies by center because it is based on epidemiological surveillance of infecting organisms as well as national and local antibiotic susceptibility patterns.5
Newborn infants admitted to neonatal intensive care units (NICUs) are at increased risk of colonization and infection with antibiotic-resistant organisms. This risk may be increased with maternal perinatal antibiotic administration, which promotes selection of antibiotic-resistant organisms within the maternal flora and subsequently affects the initial colonization of the newborn microbiome.6,7 Recommended obstetric practices for prevention of neonatal GBS disease, management of suspected intra-amniotic infection, and surgical prophylaxis for cesarean delivery are associated with receipt of antenatal antibiotics among approximately 50% of pregnant women.8,9,10,11 Antibiotics directly administered to the newborn for risk of infection may further select for resistant species.
Neonatal clinicians must balance concerns of inadequate empirical coverage for suspected infection with the risks of indiscriminate antibiotic use. Although, to our knowledge, widespread resistance to ampicillin has not been described for GBS, evolving reports of gram-negative bacteria resistant to both ampicillin and gentamicin have been published.12,13,14,15,16,17 Reports of cephalosporin resistance, extended-spectrum β-lactamase (ESBL)–producing Enterobacteriaceae, and carbapenem-resistant Enterobacteriaceae are emerging in all settings, including NICUs.15,18,19,20 Evolving evidence suggests an increasing prevalence of major multidrug-resistant gram-negative bacteria during the past 2 decades, including clonal lineages, such as E coli sequence type 131 and Klebsiella pneumoniae sequence type 258.21,22,23
There is a paucity of contemporary, large-scale, neonatal-specific antibiotic susceptibility data for E coli in the US. Individual risks associated with broad-spectrum antibiotic exposure and population concerns for the emergence of species resistant to all available antimicrobial agents underscore the need for data to inform empirical antibiotic recommendations.24,25,26 In this study, we assessed patterns of antibiotic susceptibility of E coli among infants admitted to NICUs across the US from January 1, 2009, to December 31, 2017.
Methods
Data Source and Study Population
We conducted a retrospective cohort study using data from the Premier Health Database (Premier Inc), a large administrative database of inpatient encounters from participating academic and community hospitals across the US. Patient-level data include diagnosis and procedure codes, demographics, billed services, insurance provider, disposition, daily pharmacy charge data, and, from a subset of centers, detailed microbiology data. This study was deemed exempt from review, with a waiver of informed consent, by the Children’s Hospital of Philadelphia institutional review board, owing to use of deidentified data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. Data collection and analysis took place from December 1, 2018, to November 30, 2019.
We identified inborn infants from January 1, 2009, to December 31, 2017, admitted to NICUs contributing microbiology data during the study period calendar years. We defined the day of birth as the day of infant hospital admission and analyzed admitted infants from birth to 1 year of age.
Study Definitions
Episode of E coli Infection
Isolation of E coli in 1 or more cultures of blood, cerebrospinal fluid, or urine specimens was considered an episode. We defined an episode of E coli infection as the first culture with E coli growth; if multiple culture types with E coli growth were collected on the same day, we counted the isolate in the blood culture as the first. We defined new episodes of E coli infection in the same patient as subsequent cultures positive for E coli more than 30 days after the previous episode regardless of specimen source.
Antibiotic Susceptibility of E coli
An E coli isolate was defined as susceptible to an antibiotic when the final interpretation report indicated S (susceptible) and nonsusceptible when the report indicated R (resistant) or I (intermediate). Specific susceptibility results (eg, minimum inhibitory concentrations) were not consistently reported and were therefore excluded from this analysis. For polymicrobial results, only susceptibilities of E coli were analyzed.
Antibiotic Resistance Categories for E coli
We analyzed all available antibiotic susceptibility data and focused on 4 priority antibiotic susceptibility categories for E coli: (1) ampicillin nonsusceptibility, defined as any isolate with nonsusceptibility to ampicillin; (2) aminoglycoside nonsusceptibility, defined as any isolate with at least 1 nonsusceptibility result to gentamicin, amikacin, or tobramycin; (3) ESBL phenotype, defined as any isolate with at least 1 nonsusceptibility result to cefotaxime, ceftriaxone, ceftazidime, or cefepime; and (4) carbapenem-resistant Enterobacteriaceae, defined as any isolate with at least 1 nonsusceptibility result to imipenem, meropenem, doripenem, or ertapenem sodium. Definitions of ESBL and carbapenem-resistant Enterobacteriaceae were based on updated Centers for Disease Control and Prevention (CDC) definitions and accounted for cascade reporting.27 The ESBL definition assumed isolates to be susceptible to third- and fourth-generation cephalosporins if no susceptibility results to these agents were reported but the isolate was susceptible to more than 1 of the following: ampicillin, piperacillin, aztreonam, or cefazolin.27 The carbapenem-resistant Enterobacteriaceae definition assumed isolates to be carbapenem susceptible if no carbapenem susceptibility result was reported but the isolate was susceptible to more than 1 of the following: ampicillin, combined ampicillin and sulbactam, combined amoxicillin and clavulanic acid, combined piperacillin and tazobactam, cefazolin, cefoxitin, or cefotetan.27 A fifth category was added for EOS that included nonsusceptibility to both ampicillin and gentamicin because this is the standard empirical regimen for infants at risk of EOS.2,4
Statistical Analysis
We determined the proportion of infants with nonsusceptible isolates within each category. The denominator included unique infants with a first episode of E coli infection and susceptibility testing for at least 1 drug in that category. The numerator included infants with a nonsusceptible isolate. To estimate the mean absolute change per year in the proportion of nonsusceptibility in each category and its 95% CI, generalized linear models were used to model nonsusceptibility (yes or no) as a function of year. For the EOS analysis, we used the χ2 test and analysis of variance to compare demographics and clinical characteristics between groups and logistic regression to compare in-hospital mortality. Two-sided P < .05 was considered statistically significant. Statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc).
Results
Characteristics of the Study Participants and Centers
We identified 117 484 infants admitted to 170 NICUs that contributed microbiology data from 2009 to 2017. Of these, 733 infants (0.6%) had at least 1 episode of E coli infection; 721 (98.4%) from 69 centers had available susceptibility results and were included in the analysis. The median number of infants with E coli infection included per center was 5 (interquartile range, 2-12). Most infants (694 [96.3%]) with E coli infection had only 1 episode. Infected infants had predominantly very low birth weight (<1500 g) and were predominantly male (434 [60.2%] vs 287 [39.8%] female) (Table 1). Most of the infections (501 [69.5%]) occurred as LOS. Among 721 first episodes of E coli infection, the specimen source in 393 (54.5%) was classified as blood; in 322 (44.7%), as urine; and in 6 (0.8%), as cerebrospinal fluid. Most infants had only a positive blood culture (358 [49.7%]) or urine culture (322 [44.7%]) result, although not all infants had all 3 culture types performed with each episode (eTable 1 in the Supplement). The first episode of E coli infection occurred at a median age of 14 (interquartile range, 1-33) days and ranged from hospital days 1 to 240.
Table 1. Patient- and Center-Level Characteristics of Infants With Escherichia coli Infection.
| Characteristic | Patient data (n = 721)a |
|---|---|
| Birth weight category, g | |
| <1500 | 437 (60.6) |
| ≥1500 | 284 (39.4) |
| Sex | |
| Male | 434 (60.2) |
| Female | 287 (39.8) |
| Race/ethnicity | |
| Black | 206 (28.6) |
| White | 301 (41.7) |
| Hispanic | 7 (1.0) |
| Other | 190 (26.4) |
| Unknown | 17 (2.4) |
| Timing of first episodeb | |
| Early onset | 220 (30.5) |
| Late onset | 501 (69.5) |
| Specimen source for first episode | |
| Blood | 393 (54.5) |
| Cerebrospinal fluid | 6 (0.8) |
| Urine | 322 (44.7) |
| No. of episodes during admission | |
| 1 | 694 (96.3) |
| 2 | 24 (3.3) |
| 3 | 3 (0.4) |
| Length of stay, median (IQR), d | 55 (26-94) |
| Disposition | |
| Home | 583 (80.9) |
| Died | 61 (8.5) |
| Other | 77 (10.7) |
| Geographic region | |
| Midwest | 124 (17.2) |
| Northeast | 77 (10.7) |
| South | 389 (54.0) |
| West | 131 (18.2) |
| Geographic classification | |
| Urban setting | 693 (96.1) |
| Rural setting | 28 (3.9) |
| Hospital academic classification | |
| Teaching hospital | 486 (67.4) |
| Nonteaching hospital | 235 (32.6) |
| Hospital size | |
| ≥200 beds | 681 (94.5) |
| <200 beds | 40 (5.5) |
Abbreviation: IQR, interquartile range.
Unless otherwise indicated, data are expressed as number (percentage) of patients. Percentages were rounded and may not total 100.
Early onset was considered as 3 days or less after birth and late onsent as more than 3 days after birth.
Antibiotic Susceptibility Trends of E coli
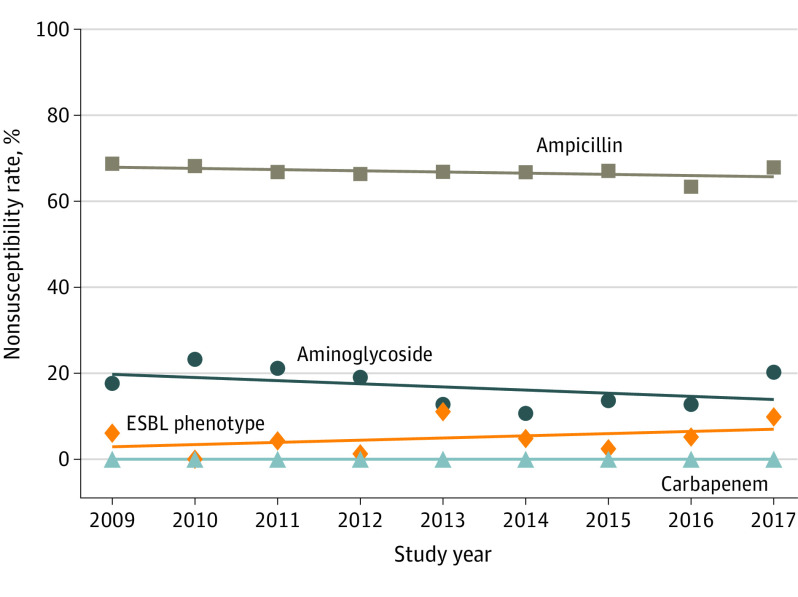
Nearly all isolates were tested against ampicillin (720 [99.9%]) and gentamicin (718 [99.6%]); 691 (95.8%) were tested against cefazolin, 660 (91.5%) against ceftriaxone, and 679 (94.2%) against combined trimethoprim and sulfamethoxazole therapy (Table 2). Nonsusceptibility counts and rates by year for each subcategory (ie, birth weight status, specimen source, and timing of infection) and for each antibiotic category of interest are reported in eTable 2 in the Supplement. No significant changes were observed over time in the overall annual proportions of antibiotic nonsusceptibility to ampicillin (mean [SD], 66.8% [1.5%]; range, 63.3% to 68.6%; estimated yearly change, −0.28% [95% CI, −1.75% to 1.18%]), nonsusceptibility to aminoglycosides (mean [SD], 16.8% [4.5%]; range, 10.7% to 23.2%; estimated yearly change, −0.85% [95% CI, −1.93% to 0.23%]), or ESBL phenotype (mean [SD], 5.0% [3.7%]; range, 0% to 11.1%; estimated yearly change, 0.46% [95% CI, −0.18% to 1.11%]) (Figure and eTable 3 in the Supplement). No isolates were nonsusceptible to any tested carbapenem antibiotic. We performed similar temporal trend analyses by birth weight, specimen source, and timing of infection (eTable 3 and eFigure in the Supplement). There was a small but statistically significant decrease in aminoglycoside nonsusceptibility over time for E coli causing LOS (estimated yearly change, −1.53% [95% CI, −2.88% to −0.18%]); no other significant trends were identified. The rates of cefazolin resistance (118 of 691 [17.1%]), fluoroquinoline resistance (57 of 558 [10.2%] for ciprofloxacin, 56 of 509 [11.0%] for levofloxacin, and 17 of 107 [15.9%] for moxifloxacin), and combined trimethoprim-sulfamethoxazole resistance (209 of 679 [30.8%]) were substantial (Table 2).
Table 2. Rates of Antibiotic Testing and Nonsusceptibility Rates of Escherichia coli Isolates.
| Antibiotic category | Isolate, No. (%)a | |
|---|---|---|
| Tested | Nonsusceptibility result | |
| Priority | ||
| Ampicillin | 720 (99.9) | 480 (66.7) |
| Aminoglycosidesb | 719 (99.7) | 119 (16.6) |
| ESBL phenotypec | 695 (96.4) | 34 (4.9) |
| Carbapenemsd | 662 (91.8) | 0 |
| Individuale | ||
| Penicillin | ||
| Ampicillin-sulbactam | 507 (70.3) | 311 (61.3) |
| Piperacillin-tazobactam | 574 (79.6) | 26 (4.5) |
| Amoxicillin-clavulanate | 312 (43.3) | 38 (12.2) |
| Piperacillin | 133 (18.4) | 81 (60.9) |
| Ticarcillin | 94 (13.0) | 56 (59.6) |
| Aminoglycosides | ||
| Gentamicin | 718 (99.6) | 109 (15.2) |
| Tobramycin | 572 (79.3) | 83 (14.5) |
| Amikacin | 376 (52.1) | 3 (0.8) |
| Cephalosporins | ||
| Cefazolin | 691 (95.8) | 118 (17.1) |
| Ceftriaxone | 660 (91.5) | 33 (5.0) |
| Cefepime | 465 (64.5) | 15 (3.2) |
| Ceftazidime | 318 (44.1) | 14 (4.4) |
| Cefuroxime | 312 (43.3) | 17 (5.4) |
| Cefoxitin | 240 (33.3) | 13 (5.4) |
| Cefotaxime | 212 (29.4) | 7 (3.3) |
| Cefotetan | 135 (18.7) | 0 |
| Cephalothin | 128 (17.8) | 77 (60.2) |
| Cefpodoxime | 112 (15.5) | 9 (8.0) |
| Ceftizoxime | 91 (12.6) | 4 (4.4) |
| Carbapenems | ||
| Meropenem | 369 (51.2) | 0 |
| Imipenem | 315 (43.7) | 0 |
| Ertapenem | 291 (40.4) | 0 |
| Doripenem | 104 (14.4) | 0 |
| Other | ||
| Trimethoprim-sulfamethoxazole | 679 (94.2) | 209 (30.8) |
| Nitrofurantoin | 353 (49.0) | 15 (4.2) |
| Aztreonam | 344 (47.7) | 14 (4.1) |
| Tetracycline | 334 (46.3) | 90 (26.9) |
| Tigecycline | 147 (20.4) | 0 |
| Fluoroquinolones | ||
| Ciprofloxacin | 558 (77.4) | 57 (10.2) |
| Levofloxacin | 509 (70.6) | 56 (11.0) |
| Moxifloxacin | 107 (14.8) | 17 (15.9) |
Abbreviation: ESBL, extended-spectrum β-lactamase.
Includes 721 cases of E coli isolates. Nonsusceptibility is expressed as number (percentage) of the category tested.
Includes testing for gentamicin, amikacin, and/or tobramycin.
Includes testing for cefotaxime, ceftriaxone, ceftazidime, and/or cefepime.
Includes testing for imipenem, meropenem, doripenem, and/or ertapenem.
For individual antibiotics, only those with at least 10% of specimens tested are reported.
Figure. Nonsusceptibility Trends of Neonatal Escherichia coli From 2009 to 2017.
The overall nonsusceptibility trends for the categories of interest (blood, cerebrospinal fluid, and urine cultures combined) are shown stratified by antibiotic category. Nonsusceptibility counts and rates for each antibiotic category by year are shown in eTable 2 in the Supplement. ESBL indicates extended-spectrum β-lactamase.
Twenty-seven infants (3.7%) had more than 1 episode of E coli infection; 24 infants had 2 episodes, and 3 infants had 3 episodes. In 6 instances, the initial infecting isolate was susceptible to an antibiotic but the subsequent infecting isolate was not (3 to ampicillin and 3 to aminoglycosides).
Susceptibility of E coli Isolates Causing EOS
Because the empirical combination of ampicillin and gentamicin is commonly used for newborns at risk for EOS, we examined cases of EOS due to E coli separately (Table 3). Of the 220 EOS episodes, 2 (0.9%) were diagnosed based solely on positive urine culture results and were excluded. Of 218 analyzed isolates, 69 (31.7%) were susceptible to both ampicillin and gentamicin, 127 (58.3%) were nonsusceptible to ampicillin and susceptible to gentamicin, 22 (10.1%) were nonsusceptible to both ampicillin and gentamicin, and 0 were susceptible to ampicillin and nonsusceptible to gentamicin. Of 197 infants with available drug charges, 180 (91.4%) received empirical ampicillin and gentamicin. Of the 22 infants with EOS caused by E coli with nonsusceptibility to combined ampicillin-gentamicin, 18 were receiving both drugs at the time the culture was performed, 2 were receiving ampicillin alone, 1 was receiving gentamicin alone, and 1 did not have available pharmacy data. Greater proportions of infants infected with combined ampicillin-gentamicin–nonsusceptible E coli isolates had very low birth weight, were Black, and were born in the southern US, but only birth in a teaching hospital was significantly different among groups (ampicillin and gentamicin nonsusceptible: 20 of 22 [90.9%]; ampicillin nonsusceptible and gentamicin susceptible: 80 of 127 [63.0%]; ampicillin and gentamicin susceptible: 38 of 69 [55.1%]; P = .01) in unadjusted analyses among the 3 groups (Table 3). Among all 218 infants with EOS due to E coli, 22 (10.1%) died; of these infants, 20 (90.9%) died in the first week after birth and 2 (9.1%) died 8 to 30 days after birth. A larger proportion of infants with EOS caused by isolates with nonsusceptibility to combined ampicillin-gentamicin died (4 of 22 [18.2%]; ampicillin nonsusceptible and gentamicin susceptible: 12 of 127 [9.4%]; ampicillin and gentamicin susceptible: 6 of 69 [8.7%]) (Table 3), but this difference was not significant in unadjusted analyses among the 3 groups (P = .41) or when the dual nonsusceptibility group was compared with the other 2 groups combined (P = .18) (Table 3). After adjusting for birth weight, sex, and teaching hospital status, the risk of mortality was not associated with susceptibility patterns (eTable 4 in the Supplement).
Table 3. Characteristics of Infants with Early-onset Sepsis Due to Escherichia coli by Ampicillin and Gentamicin Susceptibility Profilea.
| Characteristic | Susceptibility profile groupa | P valueb | P valuec | ||
|---|---|---|---|---|---|
| Ampicillin and gentamicin susceptible (n = 69) | Ampicillin nonsusceptible and gentamicin susceptible (n = 127) | Ampicillin and gentamicin nonsusceptible (n = 22) | |||
| Birth weight category, g | |||||
| <1500 | 27 (39.1) | 50 (39.4) | 12 (54.5) | .39 | .17 |
| ≥1500 | 42 (60.9) | 77 (60.6) | 10 (45.5) | ||
| Sex | |||||
| Male | 33 (47.8) | 78 (61.4) | 11 (50.0) | .16 | .55 |
| Female | 36 (52.2) | 49 (38.6) | 11 (50.0) | ||
| Race/ethnicity | |||||
| Black | 13 (18.8) | 31 (24.4) | 10 (45.5) | .44 | .18 |
| White | 25 (36.2) | 46 (36.2) | 6 (27.3) | ||
| Hispanic | 1 (1.4) | 3 (2.4) | 0 | ||
| Other | 28 (40.6) | 42 (33.1) | 6 (27.3) | ||
| Unknown | 2 (2.9) | 5 (3.9) | 0 | ||
| Geographic region | |||||
| Midwest | 16 (23.2) | 29 (22.8) | 3 (13.6) | .76 | .50 |
| Northeast | 6 (8.7) | 16 (12.6) | 1 (4.5) | ||
| South | 32 (46.4) | 52 (40.9) | 12 (54.5) | ||
| West | 15 (21.7) | 30 (23.6) | 6 (27.3) | ||
| Urban setting | 67 (97.1) | 125 (98.4) | 21 (95.5) | .64 | .46 |
| Teaching hospital | 38 (55.1) | 80 (63.0) | 20 (90.9) | .01 | .005 |
| Hospital size ≥200 beds | 66 (95.7) | 125 (98.4) | 21 (95.5) | .45 | .59 |
| Length of stay, median (IQR), d | 21 (10-42) | 24 (13-52) | 42 (12-83) | .36 | .19 |
| Received empirical ampicillin and gentamicind | 54 (87.1) | 108 (94.7) | 18 (85.7) | .13 | .33 |
| Died within 7 de | 6 (8.7) | 10 (7.9) | 4 (18.2) | .43 | .28 |
| Died | 6 (8.7) | 12 (9.4) | 4 (18.2) | .41 | .18 |
Abbreviation: IQR, interquartile range.
Includes 218 infants and excludes 2 infants with only positive urine culture results. There were no isolates susceptible to ampicillin and nonsusceptible to gentamicin. Unless otherwise indicated, data are expressed as number (percentage) of isolates. Percentages were rounded and may not total 100.
Calculated using unadjusted analysis comparing all 3 groups separately.
Calculated using unadjusted analysis comparing the ampicillin and gentamicin susceptible group and ampicillin nonsusceptible and gentamicin susceptible group with the ampicillin and gentamicin nonsusceptible group.
Pharmacy data were not available for all infants, leading to different denominators for each group (62 for ampicillin and gentamicin susceptible, 114 for ampicillin nonsusceptible and gentamicin susceptible, and 21 for ampicillin and gentamicin nonsusceptible).
Timing of death based on days after birth.
Discussion
We examined the epidemiologic characteristics and antibiotic susceptibility patterns of E coli among a large sample of newborns admitted to NICUs across the US from 2009 to 2017. We found substantial rates of resistance to commonly used drugs; a mean (SD) of 66.8% (1.5%) of isolates were nonsusceptible to ampicillin, 16.8% (4.5%) were nonsusceptible to aminoglycosides, and 5.0% (3.7%) of isolates fulfilled criteria for the ESBL phenotype. We observed no resistance to carbapenems. Similar patterns of resistance were observed among isolates from blood, cerebrospinal fluid, and urine specimens in newborns with EOS and in those with LOS, and among both very low-birth-weight and larger-birth-weight newborns. We observed no significant temporal change in these findings during the study period, with the exception of a small decrease in aminoglycoside nonsusceptibility of E coli causing LOS. Among isolates causing EOS, we observed that 10.1% were nonsusceptible to both ampicillin and gentamicin, the antibiotics most commonly administered for empirical therapy to infants at risk for EOS.
Our findings emphasize the importance of ongoing surveillance of neonatal antibiotic susceptibility patterns to inform empirical antibiotic therapies for newborn infants.28,29,30 Such surveillance optimally requires longitudinal analysis with sample sizes large enough to detect a significant change over time.16 Changes in the incidence of neonatal infection with E coli resistant to ampicillin were variably reported after widespread implementation of intrapartum antibiotic prophylaxis for women colonized with GBS, particularly among preterm infants.31,32 Some single-site studies reported no change in incidence or increases in the proportion of ampicillin-resistant infections as rates of GBS disease decreased.17,30 In contrast, studies encompassing 21st century data consistently reported increases in the incidence of neonatal infection due to antibiotic-resistant E coli. Increasing ampicillin resistance rates as high as 54% were reported among neonatal E coli infections from 1979 to 2006 at Yale University, but no gentamicin resistance was identified.13 A total of 58% of 258 neonatal E coli bloodstream infections from 77 Pediatrix Medical Group centers in 2012 were caused by ampicillin-resistant isolates.33 Active surveillance by the CDC during 2005 to 2014 found that 68% of neonatal E coli isolates causing EOS were ampicillin resistant and 10% were gentamicin resistant.12 Such increases in resistance to specific antimicrobial agents correlated with increasing rates of resistance among E coli isolates in adults. Data from the SENTRY Antimicrobial Surveillance Program for 2017 showed that among 1831 E coli isolates from adult urinary tract infections, 45.9% were resistant to combined ampicillin and sulbactam and 12.3% to gentamicin.34 The substantial rates of fluoroquinolone and combined trimethoprim-sulfamethoxazole resistance in our study may reflect substantial resistance in the adult uropathogen ecology; although these drugs are rarely used for neonatal patients, such findings suggest the need for ongoing surveillance in pediatric and adult care.
Of the E coli isolates included in our study, 5.0% met the phenotypic definition for ESBL, which is higher than prior neonatal reports, although resistance to individual drugs (ie, cephalosporins) is often reported without ESBL distinction. We found that 17.1% of isolates were nonsusceptible to cefazolin. Conversely, we observed low resistance to third-generation cephalosporins and combined piperacillin-tazobactam. We found no instances of E coli nonsusceptibility to carbapenems, although other Enterobacteriaceae, such as Klebsiella species, may have higher rates of carbapenem resistance.35,36 The longitudinal Yale report identified only 1 case of third-generation cephalosporin-resistant E coli during a 28-year period.15 Single-center studies have also reported minimal or no cases of ESBL among neonatal E coli isolates.37,38 To date, most reports of neonatal ESBL-producing E coli and carbapenem-resistant E coli have come from international sites or single-center outbreaks.20,39,40,41
The observation that 10.1% of E coli isolates causing EOS were nonsusceptible to both ampicillin and gentamicin is highly relevant to perinatal care. Not only are ampicillin and gentamicin frequently administered to newborns for risk of EOS, but they are recommended agents for maternal intrapartum antibiotic treatment for risk of intra-amniotic infection, and ampicillin is often used for intrapartum prophylaxis for women colonized with GBS. National surveillance data from the CDC for 2005 to 2014 found that 44% of all cases of EOS occurring among term infants were caused by GBS, an organism that remains almost universally susceptible to ampicillin, and 25% were caused by E coli.42 Multicenter surveillance data from 2015 to 2017 among infants cared for in Neonatal Research Network sites found that 51% of all cases of EOS occurring among term infants were caused by GBS, and only 15% were caused by E coli.3 The combination of ampicillin and gentamicin should therefore provide adequate empirical therapy to most term infants with suspected EOS.12 Among very preterm infants, in contrast, 46% of early infections recorded in the 2005-2014 CDC surveillance study and 51% of those recorded in the Neonatal Research Network study were caused by E coli.3,12 Taken together, our findings and the 2015-2017 Neonatal Research Network surveillance data suggest that the incidence of infection due to E coli with nonsusceptibility to combined ampicillin-gentamicin may approach 1 in 1000 very low-birth-weight infants.3 Such estimates support the American Academy of Pediatrics recommendation to consider individual patient characteristics (including gestational age at birth, circumstances of birth, and infant clinical condition) when determining the optimal empirical therapy for EOS and to consider the addition of broader-spectrum antibiotic therapy for severely ill preterm infants at highest risk for EOS until culture results are available.2,4 Our observation that only 3% to 4% of E coli isolates causing EOS met the current CDC definition for ESBL (eTable 2 in the Supplement) would support the use of a third- or fourth-generation cephalosporin in high-risk circumstances, but our findings do not support the routine empirical use of these drugs. Decisions regarding empirical antibiotic therapy for EOS should ideally balance the local impact of frequent, indiscriminate use of broad-spectrum antibiotics with the needs of individual patients. At present, no consensus for the threshold resistance prevalence at which empirical agents are no longer recommended for neonatal sepsis exists.
Of note, EOS cases caused by E coli isolates nonsusceptible to both ampicillin and gentamicin were not associated with a higher adjusted odds of mortality. Prior studies have not consistently found an increased risk of death among infants with EOS due to ampicillin-resistant E coli compared with those with nonresistant infections, and adequate initial empirical antibiotic therapy has not been shown to be associated with mortality among those with resistant infections.12,33 Our analysis of the association between the antibiotic susceptibility of E coli causing EOS and mortality was limited by the small sample size and predominantly high-risk preterm infants. Our findings support the need to rigorously assess the association between the content and dosage of initial empirical antibiotic therapy and risk of mortality among infected newborns.
Strengths and Limitations
Strengths of our study include the large and contemporary sample of infants admitted to NICUs across the US, with inclusion of both academic and community hospitals, and the detailed antibiotic susceptibility data for more than 700 neonatal-specific E coli isolates. Our study also has limitations. We only analyzed antibiotic susceptibility patterns of E coli; further analysis of other organisms, namely additional gram-negative bacteria, would more accurately inform empirical antibiotic prescription for the neonatal population. Approximately 30% of centers in the Premier Health Database contribute microbiology data, which may introduce sampling bias, and we only studied inborn infants, which may limit the generalizability of our findings. Information on antibiotic dosage or administration frequency was not available, nor were minimum inhibitory concentrations from the culture results. In addition, granular patient-level data, such as maternal antibiotic exposures, gestational age, and mode of delivery, were not available, and therefore, we could not include those variables in adjusted analyses.
Conclusions
In this cohort study, substantial proportions of neonatal E coli isolates were not susceptible to commonly administered antibiotics, with no significant change from 2009 to 2017. Longitudinal, national surveillance for susceptibility patterns among organisms infecting neonates are needed to continue to optimize empirical antibiotic therapy for this population.
eTable 1. Distribution of Positive Escherichia coli Culture Combinations by First Episode
eTable 2. Nonsusceptibility Counts and Rates Over Time by Patient Category and Antibiotic Category
eTable 3. Estimated Yearly Change in by Patient Category and Antibiotic Category
eTable 4. Multivariable Regression of Isolate Antibiotic Susceptibility Profile and In-Hospital Mortality
eFigure. Neonatal Escherichia coli Nonsusceptibility Trends Over Time by Specimen Source, Birth Weight Category, and Timing of Infection
References
- 1.Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770-1780. doi: 10.1016/S0140-6736(17)31002-4 [DOI] [PubMed] [Google Scholar]
- 2.Puopolo KM, Benitz WE, Zaoutis TE; Committee on Fetus and Newborn; Committee on Infectious Diseases . Management of neonates born at ≤34 6/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics. 2018;142(6):e20182896. doi: 10.1542/peds.2018-2896 [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Puopolo KM, Hansen NI, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Early-onset neonatal sepsis 2015 to 2017, the rise of Escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. 2020;174(7):e200593. doi: 10.1001/jamapediatrics.2020.0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puopolo KM, Benitz WE, Zaoutis TE; Committee on Fetus and Newborn; Committee on Infectious Diseases . Management of neonates born at ≥35 0/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics. 2018;142(6):e20182894. doi: 10.1542/peds.2018-2894 [DOI] [PubMed] [Google Scholar]
- 5.Rubin LG, Sánchez PJ, Siegel J, Levine G, Saiman L, Jarvis WR; Pediatric Prevention Network . Evaluation and treatment of neonates with suspected late-onset sepsis: a survey of neonatologists’ practices. Pediatrics. 2002;110(4):e42. doi: 10.1542/peds.110.4.e42 [DOI] [PubMed] [Google Scholar]
- 6.Patel SJ, Saiman L. Antibiotic resistance in neonatal intensive care unit pathogens: mechanisms, clinical impact, and prevention including antibiotic stewardship. Clin Perinatol. 2010;37(3):547-563. doi: 10.1016/j.clp.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fjalstad JW, Esaiassen E, Juvet LK, van den Anker JN, Klingenberg C. Antibiotic therapy in neonates and impact on gut microbiota and antibiotic resistance development: a systematic review. J Antimicrob Chemother. 2018;73(3):569-580. doi: 10.1093/jac/dkx426 [DOI] [PubMed] [Google Scholar]
- 8.Prevention of Group B Streptococcal Early-Onset Disease in Newborns Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion, number 782. Obstet Gynecol. 2019;134(1):e19-e40. [DOI] [PubMed] [Google Scholar]
- 9.Puopolo KM, Lynfield R, Cummings JJ; Committee on Fetus and Newborn; Committee on Infectious Diseases . Management of infants at risk for group B streptococcal disease. Pediatrics. 2019;144(2):e20191881. doi: 10.1542/peds.2019-1881 [DOI] [PubMed] [Google Scholar]
- 10.Committee on Practice Bulletins-Obstetrics ACOG Practice Bulletin No. 199: use of prophylactic antibiotics in labor and delivery. Obstet Gynecol. 2018;132(3):e103-e119. doi: 10.1097/AOG.0000000000002833 [DOI] [PubMed] [Google Scholar]
- 11.Committee on Obstetric Practice Committee opinion No. 712: intrapartum management of intraamniotic infection. Obstet Gynecol. 2017;130(2):e95-e101. doi: 10.1097/AOG.0000000000002236 [DOI] [PubMed] [Google Scholar]
- 12.Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138(6):e20162013-e20162013. doi: 10.1542/peds.2016-2013 [DOI] [PubMed] [Google Scholar]
- 13.Bizzarro MJ, Dembry L-M, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121(4):689-696. doi: 10.1542/peds.2007-2171 [DOI] [PubMed] [Google Scholar]
- 14.Weissman SJ, Hansen NI, Zaterka-Baxter K, Higgins RD, Stoll BJ. Emergence of antibiotic resistance-associated clones among Escherichia coli recovered from newborns with early-onset sepsis and meningitis in the United States, 2008-2009. J Pediatric Infect Dis Soc. 2016;5(3):269-276. doi: 10.1093/jpids/piv013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diekema DJ, Hsueh PR, Mendes RE, et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 2019;63(7):e00355-19. doi: 10.1128/AAC.00355-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore MR, Schrag SJ, Schuchat A. Effects of intrapartum antimicrobial prophylaxis for prevention of group-B-streptococcal disease on the incidence and ecology of early-onset neonatal sepsis. Lancet Infect Dis. 2003;3(4):201-213. doi: 10.1016/S1473-3099(03)00577-2 [DOI] [PubMed] [Google Scholar]
- 17.Patel SJ, Green N, Clock SA, et al. Gram-negative bacilli in infants hospitalized in the neonatal intensive care unit. J Pediatric Infect Dis Soc. 2017;6(3):227-230. doi: 10.1093/jpids/piw032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wójkowska-Mach J, Chmielarczyk A, Borszewska-Kornacka M, et al. Enterobacteriaceae infections of very low birth weight infants in Polish neonatal intensive care units: resistance and cross-transmission. Pediatr Infect Dis J. 2013;32(6):594-598. doi: 10.1097/INF.0b013e318287fe2a [DOI] [PubMed] [Google Scholar]
- 19.Cadot L, Bruguière H, Jumas-Bilak E, et al. Extended spectrum β-lactamase-producing Klebsiella pneumoniae outbreak reveals incubators as pathogen reservoir in neonatal care center. Eur J Pediatr. 2019;178(4):505-513. doi: 10.1007/s00431-019-03323-w [DOI] [PubMed] [Google Scholar]
- 20.Folgori L, Bielicki J, Heath PT, Sharland M. Antimicrobial-resistant gram-negative infections in neonates: burden of disease and challenges in treatment. Curr Opin Infect Dis. 2017;30(3):281-288. doi: 10.1097/QCO.0000000000000371 [DOI] [PubMed] [Google Scholar]
- 21.Dautzenberg MJD, Haverkate MR, Bonten MJM, Bootsma MCJ. Epidemic potential of Escherichia coli ST131 and Klebsiella pneumoniae ST258: a systematic review and meta-analysis. BMJ Open. 2016;6(3):e009971. doi: 10.1136/bmjopen-2015-009971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNally A, Kallonen T, Connor C, et al. Diversification of colonization factors in a multidrug-resistant Escherichia coli lineage evolving under negative frequency-dependent selection. mBio. 2019;10(2):e00644-19. doi: 10.1128/mBio.00644-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Center for Disease Dynamics Economics & Policy. ResistanceMap: antibiotic resistance. Published 2020. Accessed April 29, 2020. https://resistancemap.cddep.org/index.php
- 24.Thompson G, Barker CI, Folgori L, et al. Global shortage of neonatal and paediatric antibiotic trials: rapid review. BMJ Open. 2017;7(10):e016293. doi: 10.1136/bmjopen-2017-016293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flannery DD, Puopolo KM. Neonatal antibiotic use: what are we doing and where shall we go? Neoreviews. 2018;19(9):e516-e525. doi: 10.1542/neo.19-9-e516 [DOI] [Google Scholar]
- 26.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117(1):67-74. doi: 10.1542/peds.2005-0179 [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Antibiotic/antimicrobial resistance (AR/ARM): biggest threats and data: 2019. AR threats report. Reviewed June 18, 2020. Accessed December 17, 2019. https://www.cdc.gov/DrugResistance/Biggest-Threats.html
- 28.Ting JY, Synnes A, Roberts A, et al. ; Canadian Neonatal Network Investigators . Association between antibiotic use and neonatal mortality and morbidities in very low-birth-weight infants without culture-proven sepsis or necrotizing enterocolitis. JAMA Pediatr. 2016;170(12):1181-1187. doi: 10.1001/jamapediatrics.2016.2132 [DOI] [PubMed] [Google Scholar]
- 29.Grohskopf LA, Huskins WC, Sinkowitz-Cochran RL, Levine GL, Goldmann DA, Jarvis WR; Pediatric Prevention Network . Use of antimicrobial agents in United States neonatal and pediatric intensive care patients. Pediatr Infect Dis J. 2005;24(9):766-773. doi: 10.1097/01.inf.0000178064.55193.1c [DOI] [PubMed] [Google Scholar]
- 30.Flannery DD, Ross RK, Mukhopadhyay S, Tribble AC, Puopolo KM, Gerber JS. Temporal trends and center variation in early antibiotic use among premature infants. JAMA Netw Open. 2018;1(1):e180164. doi: 10.1001/jamanetworkopen.2018.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347(4):240-247. doi: 10.1056/NEJMoa012657 [DOI] [PubMed] [Google Scholar]
- 32.Puopolo KM, Eichenwald EC. No change in the incidence of ampicillin-resistant, neonatal, early-onset sepsis over 18 years. Pediatrics. 2010;125(5):e1031-e1038. doi: 10.1542/peds.2009-1573 [DOI] [PubMed] [Google Scholar]
- 33.Bergin SP, Thaden JT, Ericson JE, et al. ; Antibacterial Resistance Leadership Group . Neonatal Escherichia coli bloodstream infections: clinical outcomes and impact of initial antibiotic therapy. Pediatr Infect Dis J. 2015;34(9):933-936. doi: 10.1097/INF.0000000000000769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Critchley IA, Cotroneo N, Pucci MJ, Mendes R. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PLoS One. 2019;14(12):e0220265. doi: 10.1371/journal.pone.0220265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiotos K, Tamma PD, Flett KB, et al. Multicenter study of the risk factors for colonization or infection with carbapenem-resistant Enterobacteriaceae in children. Antimicrob Agents Chemother. 2017;61(12):1-9. doi: 10.1128/AAC.01440-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiotos K, Han JH, Tamma PD. Carbapenem-resistant Enterobacteriaceae infections in children. Curr Infect Dis Rep. 2016;18(1):2. doi: 10.1007/s11908-015-0510-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole BK, Ilikj M, McCloskey CB, Chavez-Bueno S. Antibiotic resistance and molecular characterization of bacteremia Escherichia coli isolates from newborns in the United States. PLoS One. 2019;14(7):e0219352. doi: 10.1371/journal.pone.0219352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cordero L, Rau R, Taylor D, Ayers LW. Enteric gram-negative bacilli bloodstream infections: 17 years’ experience in a neonatal intensive care unit. Am J Infect Control. 2004;32(4):189-195. doi: 10.1016/j.ajic.2003.07.004 [DOI] [PubMed] [Google Scholar]
- 39.Khalid S, Ahmad N, Ali SM, Khan AU. Outbreak of efficiently transferred carbapenem-resistant blaNDM-producing gram-negative bacilli isolated from neonatal intensive care unit of an Indian hospital. Microb Drug Resist. 2020;26(3):284-289. doi: 10.1089/mdr.2019.0092 [DOI] [PubMed] [Google Scholar]
- 40.Folgori L, Bielicki J. Future challenges in pediatric and neonatal sepsis: emerging pathogens and antimicrobial resistance. J Pediatr Intensive Care. 2019;8(1):17-24. doi: 10.1055/s-0038-1677535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballot DE, Bandini R, Nana T, et al. A review of multidrug-resistant Enterobacteriaceae in a neonatal unit in Johannesburg, South Africa. BMC Pediatr. 2019;19(1):320. doi: 10.1186/s12887-019-1709-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nanduri SA, Petit S, Smelser C, et al. Epidemiology of invasive early-onset and late-onset group B streptococcal disease in the United States, 2006 to 2015: multistate laboratory and population-based surveillance. JAMA Pediatr. 2019;173(3):224-233. doi: 10.1001/jamapediatrics.2018.4826 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Distribution of Positive Escherichia coli Culture Combinations by First Episode
eTable 2. Nonsusceptibility Counts and Rates Over Time by Patient Category and Antibiotic Category
eTable 3. Estimated Yearly Change in by Patient Category and Antibiotic Category
eTable 4. Multivariable Regression of Isolate Antibiotic Susceptibility Profile and In-Hospital Mortality
eFigure. Neonatal Escherichia coli Nonsusceptibility Trends Over Time by Specimen Source, Birth Weight Category, and Timing of Infection