Key Points
Question
For cancer drugs approved by the US Food and Drug Administration in 2018, what is the potential influence on cancer drug spending, and how many patients may receive the new approvals?
Finding
This economic evaluation study determined that if used in all eligible patients, the 2018 oncology drug approvals would add $39.5 billion to US cancer drug spending (>75% increase from total 2017 cancer drug spending). Using the approved drugs in fewer than 20% of eligible patients would be consistent with industry forecasting for drug spending.
Meaning
The US would face an unprecedented increase in cancer drug spending if 2018 oncology drug approvals are used widely, while only limited uptake of the drugs maintains the current spending trajectories.
Abstract
Importance
The growth of cancer drug spending in the US has outpaced spending in nearly all other sectors, and an increasing proportion of the drug development pipeline is devoted to oncology. In 2018, there was a record number of drugs entering the US market.
Objective
To estimate the number of patients with cancer who are eligible for the newly approved drug-indication pairs, and project potential spending and use of the approvals in the US.
Design, Setting, Participants
This is a retrospective review of 2018 US Food and Drug Administration (FDA) oncology drug approvals with estimation of the eligible population. The cost of new therapy was estimated, and savings from displaced therapies were subtracted. Two-way sensitivity analysis explored uncertainty in pricing and market diffusion. Data were collected between March 1, 2019, and September 30, 2019.
Exposures
Data related to the cancer drug approval (ie, indications, approval pathway, basis for approval), cancer incidence, and drug price were extracted from publicly available sources, including the FDA, National Cancer Institute, and American Cancer Society websites, as well as the RED BOOK database.
Main Outcomes and Measures
The primary outcome was the projected net expenditure in the US associated with the new therapies. The secondary outcome described how variable market diffusion and pricing permit expected levels of spending.
Results
A total of 46 oncology approvals were included in the analysis, with 17 novel drugs and 29 new indications. The average price per patient per treatment course was $150 384. From a national perspective and with 100% market diffusion, the projected net expenditure for newly approved drugs was $39.5 billion per year. To maintain the recent trend of cancer drug spending, the 2018 cancer drug approvals need to be used in fewer than 20% of eligible patients.
Conclusions and Relevance
New cancer drugs approved by the FDA in 2018 would drastically increase cancer drug spending in the US if used widely. Alternatively, only low-level use of the new drugs is consistent with market forecasting.
This economic evaluation study estimates the number of patients with cancer who are eligible for the newly approved drug-indication pairs and projects potential spending and use of the approvals in the United States.
Introduction
Rising drug prices and national expenditures are receiving increasing attention after 2018 yielded a record 59 novel drug approvals by the US Food and Drug Administration (FDA). In the US, cancer drug spending has grown 12% to 15% annually and is projected to continue on this trajectory in the coming years.1 Cancer drug spending outpaces spending in other common sectors with growth of 3.3% for all prescription drugs and 4.4% for hospital spending in 2018.2 For 2020 through 2027, US prescription drug spending is projected to grow 6.1% annually. Many factors contribute to the exceptional growth in cancer drug spending, including changing demographics, improved survival, and rising drug prices.3
Currently, 35% of the drug development pipeline is dedicated to cancer drugs—a 30% increase over the past decade.4 The increasing interest in the development of anticancer therapeutics is presumably a product of technologic advances and perceived financial opportunity for manufacturers. Marketing authorization for a new cancer drug can yield a significant financial return irrespective of the clinical benefit demonstrated in pivotal trials with median launch price more than $100 000 per treatment course.5,6,7
Regardless of whether the high prices are justified by efficacy, the need to recoup development costs, or other factors, there are signs that patient access to new treatments may be in jeopardy. New high-cost therapies may be unaffordable for payers balancing short-term budgets. This principle was demonstrated with the approval of direct-acting antivirals for treatment of hepatitis C. The curative therapy was widely considered efficacious and cost effective, yet some Medicaid plans were forced to impose access restrictions to limit the budgetary consequences.8
We sought to explore the following question: if every approved cancer drug were applied to all potentially eligible patients, based on FDA labeling restrictions including biomarkers, clinical characteristics, and line of therapy, what would be the upper bound for projected associated spending? We sought to examine the prices and potential spending in the US for oncology drugs approved by the FDA in 2018, the most recent complete year at the time of the study outset. We also explored how variable discounting and market diffusion may influence spending related to new drugs in comparison with projected oncology drug expenditures.
Methods
Data Set
Novel cancer drugs and new oncologic indications approved in 2018 were identified using approval and safety notifications available on the FDA website (https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm). All oncology approvals from January 2018 through December 2018 were reviewed. Both novel drugs and drugs receiving new or expanded indications were included in the present analysis. Drugs approved for nonmalignant disease, supportive care, and biosimilars were excluded. Two approvals (larotrectinib and iobenguane I 131) were excluded owing to a paucity of data to estimate the eligible population. Two authors (P.C.D. and V.P.) reviewed each approval to confirm appropriateness of inclusion and to identify existing alternative therapies.
For each identified approval, data were extracted from FDA announcement and package insert. Published results from studies leading to approval were used to supplement the analysis. The approved indication, trial design, primary outcome, and approval pathway were collected. Cancer incidence and mortality data were extracted from the publicly available National Cancer Institute and American Cancer Society websites and supplemented with peer-reviewed publications when necessary. Drug price was obtained as the wholesale acquisition cost (WAC) from RED BOOK (IBM Micromedex). The WAC is an estimate of the manufacturers’ list price (the price encountered by wholesale purchases without accounting for rebates or discounts). Data were originally collected between March 1, 2019, and September 30, 2019, while WAC was added in July 2020. No institutional review board approval was required because this retrospective analysis reviewed publicly available data and did not use protected health information.
Data Collection and Estimation of Population and Price
For each oncology drug approval, we first estimated the upper bound number of patients eligible for the approved indication in the US per annum by using 2019 population estimates. The indication per FDA labeling defined eligibility, and the number of individuals meeting this definition were estimated using public and peer-reviewed data. A complete list of approvals, assumptions, and corresponding references are available in the eTable in the Supplement. We used death as a surrogate for incident presentation with advanced and/or metastatic cancer, as has been used in prior investigations.9,10,11 For indications where this was not applicable, annual incidence data was extracted from the American Cancer Society’s Cancer Statistics Center website (http://www.cancerstatisticscenter.cancer.org) or peer-reviewed publications. Where the methods diverged from this prespecified approach, we provided rationale within the eAppendix in the Supplement. Here we describe the example of brentuximab as approved for adults with untreated stage III or IV classical Hodgkin lymphoma. The American Cancer Society estimated 8110 incident cases of Hodgkin lymphoma in 2019. An estimated 12.2% of Hodgkin lymphoma occurs in those younger than 20 years (excluded from analysis), and 6% will have nonclassical Hodgkin lymphoma (also excluded) while 41% will have distant disease (https://www.seer.cancer.gov/statistics). Thus, we estimated 2720 incident cases of stage III or IV classical Hodgkin lymphoma in adults.
There were 3 instances of overlapping indications among the new approvals (enzalutamide/apalutamide for prostate cancer, talazoparib/olaparib for breast cancer, and dacomitinib/afatinib/osimertinib for lung cancer). The market share was divided equally among entrants (ie, 50% talazoparib and 50% olaparib).
Next, the cost of the newly approved therapy per patient was estimated. The dose was extracted from FDA package inserts. The duration of therapy was taken from the duration of exposure on the clinical trial leading to FDA approval. For drugs with fixed courses, we calculated the cost per course. For drugs taken indefinitely, we limited treatment duration to 12 months for the analysis. The dose, duration, and WAC were then used to calculate the estimated price per patient. Only the cost of the drug was examined with no consideration of ancillary costs, change in health care use, drug rebates, other costs, or savings. Returning to the brentuximab example: the pivotal study reported a mean of 10.8 doses received with a dose of 1.2 mg/kg and WAC determined to be $206.208 per mg, yielding a price of $226 828 per patient.
The savings from the displaced alternative therapies were then estimated (ie, the savings from not using bleomycin when it is replaced by brentuximab, for instance, in Hodgkin lymphoma). Some approvals were determined to have no displaced therapy (simply added to existing treatment regimens), including maintenance therapies when the prior standard of care was clinical surveillance. For many approvals, the active control arm in the pivotal clinical trial was deemed an appropriate alternative therapy. Where there was no active comparator in the trial, the authors jointly determined the most likely displaced therapy. The price of treatment with the displaced therapy was estimated using the dose and duration from the pivotal trial (or prior studies). For approvals without an alternative drug being displaced, there was no savings subtracted from the estimated expenditure. Per the pivotal study leading to the brentuximab approval cited previously, bleomycin was the displaced therapy with a mean of 11 doses of bleomycin received on study with a dose of 10 units/m2, and a WAC of $2.045 per mg. The cost of treatment with bleomycin ($427) was subtracted from the brentuximab cost to provide the net cost.
The study assumptions are upper bound owing to the following assumptions: all people with cancer are fit for therapy (based on performance status), all people have access to companion diagnostics or appropriate molecular tests, all patients desire all FDA-approved therapies, there are no cost or cost-sharing barriers, and the duration of treatment is at least as long as pivotal trials.
Statistical Analysis
Descriptive statistics were computed for the extracted data. The main outcome was the projected expenditure for newly approved oncology drugs from the individual patient and national perspectives. This expenditure was estimated with and without projected savings from displaced therapies. The 2-sample t test was used for the comparison of means. A deterministic 2-way sensitivity analysis was performed to explore uncertainty in drug discounting and market diffusion. The Medicaid best-price guarantee of a 23.1% rebate and a 50% discount to approximate oncology drug prices in the European Union12 were used to examine discounting uncertainty. A budget threshold of $7.5 billion was used to demonstrate the anticipated increase in oncology spending per the historic 15% annual growth.1 The analysis was completed using Microsoft Excel (Microsoft Corp) and STATA software, version 15.1 (StataCorp).
Results
A total of 46 oncology drug approvals were included in the analysis, with 17 (37%) novel drugs and 29 (63%) new indications (Table 1). Most approvals were granted priority review (n = 42; 91%), and only 3 (6.5%) approvals used no expedited pathway. Single-arm trials were the sole basis of evidence for 18 (39%) approvals. Progression-free survival and response rate were the most common primary outcomes for pivotal trials (n = 17 [37%] and n = 20 [43%], respectively), whereas overall survival was the primary outcome in 7 (15%) of the approvals. Overall survival improvement was demonstrated in 13 (28%) of the approvals.
Table 1. Characteristics of 2018 US Food and Drug Administration Oncology Approvals.
| Characteristics | No. (%) |
|---|---|
| No. of eligible approvals analyzed | 46 |
| New drug | 17 (37) |
| New indication | 29 (63) |
| Approval pathway | |
| Nonaccelerated approval | 3 (7) |
| Priority review | 42 (91) |
| Fast track | 3 (7) |
| Breakthrough | 16 (35) |
| Orphan | 17 (37) |
| Design of trial(s) leading to approval | |
| Single arma | 18 (39) |
| Primary end point from trial(s) leading to approval | |
| Overall survivalb | 7 (15) |
| Progression-free survival | 17 (37) |
| Response rate | 20 (43) |
| Metastasis-free survival | 2 (4) |
| Approvals | |
| With any overall survival benefit | 13 (28) |
| Displacing alternative therapy | 21 (46) |
| Indication with long-term disease-free intent | |
| Yes | 11 (24) |
| No | 35 (76) |
No trials with more than 1 arm leading to approval.
Any 1 trial using overall survival if more than 1 trial conducted.
An estimated 402 445 individuals were eligible for the approvals in aggregate with a mean of 8750 eligible per drug per year in the US (Table 2). The average price per patient per treatment course for newly approved therapies was $150 384. There was no significant difference in the mean cost of novel drugs compared with new or expanded indications ($172 506 and $137 415, respectively; P = .09). The mean price of displaced therapies ($17 118) was significantly lower than newly approved therapies (150 384; P < .05). The eTable in the Supplement describes population size, displaced therapy, and projected expenditures for each approval.
Table 2. Estimated Eligible US Population and Expenditures for 2018 US Food and Drug Administration Oncology Approvals.
| Variable | 2019, $a |
|---|---|
| Total population eligible for new approvals | 402 445 |
| Annual eligible population for each approval, mean (range) | 8750 (80-79 300) |
| Price per patient per approval, mean | 150 384 |
| Novel drug: average price per patient (95% CI) | 172 506 (104 313-240 700) |
| New indication: average price per patient (95% CI) | 137 415 (104 821-170 009) |
| Average price of existing alternative therapy, n = 21 (95% CI) | 17 118 (7603-26 632) |
| Average net price per patient (displaced alternative therapy subtracted) | 133 638 |
| Analysis for 100% drug diffusion in US | |
| Average US expenditure per new approval | 1 015 171 726 |
| Total gross expenditure for all approvals | 46 697 878 708 |
| Net spending | |
| Displaced therapy subtracted | 39 557 218 376 |
| For all approvals with Medicaid best price rebate of 23.1% | 30 419 500 931 |
Maximum of 12 months of therapy.
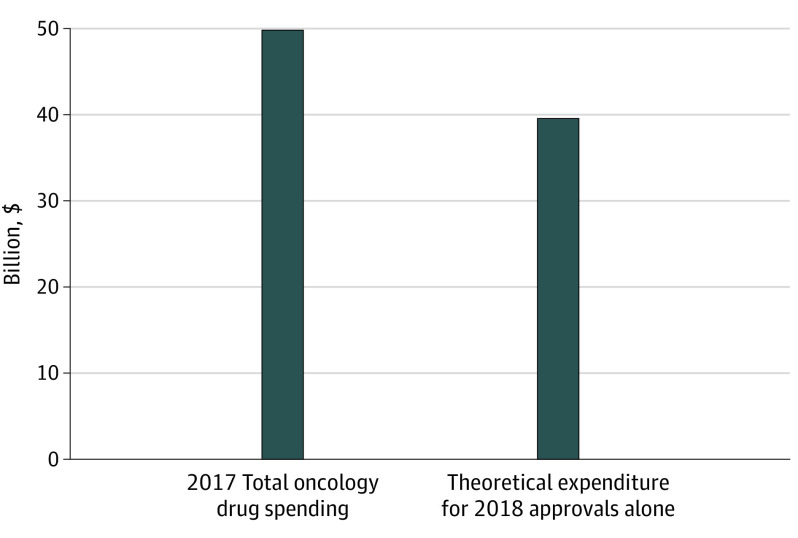
From a national perspective, with 100% market diffusion of the 2018 approvals, the projected total gross spending would be $46.70 billion per year (market diffusion being the proportion of eligible patients receiving the therapy). Accounting for savings from displaced therapies, the projected total net expenditure is $39.56 billion per year ($7.14 billion per year saved on displaced drugs). Figure 1 compares this result with the total 2017 observed national oncology drug expenditures reported by the IQVIA Institute for Human Data Science (all drugs).1 With no discounting (full price) and market diffusion rates of 10%, 20%, 50%, the estimated incremental increase in US oncology drug spending owing to 2018 approvals would be $3.96 billion, $7.91 billion, and $19.78 billion, respectively.
Figure 1. 2017 US Expenditures of Oncology Drug Spending and Theoretical Influence of 2018 Approvals.
The 2017 total oncology spending is reported by the IQVIA Institute for Human Data Science.1
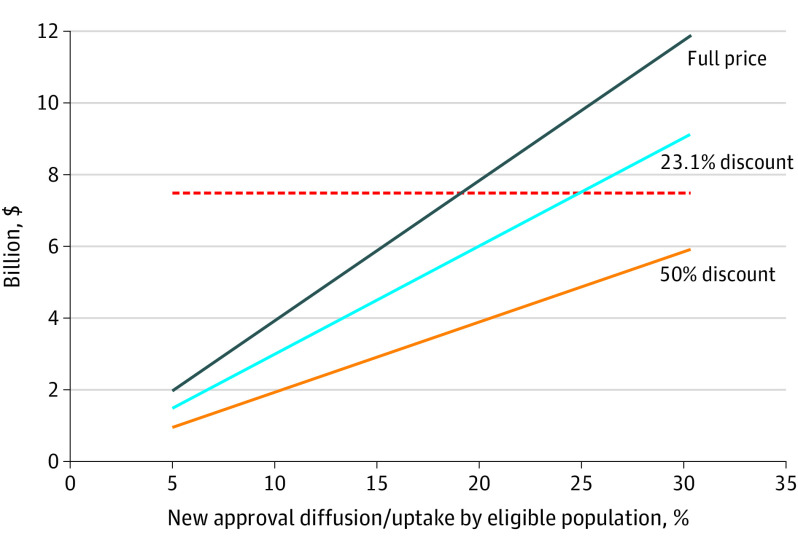
The influence of discounting and market diffusion on projected expenditures are demonstrated in Figure 2 (2-way sensitivity analysis).1 Increasing diffusion results in increased spending from the national perspective (more eligible patients receiving new drugs). The threshold of $7.5 billion (Figure 2) represents the annual increase in oncology spending that would be expected given the historic norm of 15% annual growth.1 To maintain this expected trajectory of US cancer drug spending, the 2018 approvals need to be used in fewer than 20% of eligible patients with no discounting. If a 23.1% discount (the Medicaid best price rebate) is applied to all approvals, fewer than 25% of eligible patients would receive the new drugs to maintain this historic trend. With a 50% discount (approximation of European Union pricing), less than 40% diffusion would be consistent with expected increase in annual spending.
Figure 2. Projected Increase in US Annual Oncology Drug Spending per Degree of Market Diffusion and Discounting.
The 23.1% discount reflects the Medicaid best price rebate; the 50% discount is an estimate of pricing in the European Union. The red dotted line indicates a threshold of $7.5 billion, the forecasted annual increase in cancer drug spending.1
Discussion
The results of this study suggest that the recent trajectory of annual growth in cancer drug spending is modest in comparison with potential expenditures associated with oncology drugs approved by the FDA in 2018. The newly approved drugs would increase annual cancer drug spending by nearly 80% if used in all eligible patients without discounting, which would add $39.56 billion in spending and increase the total national health expenditure by 1.1%.13 Conversely, low level of market diffusion (<25%), shorter durations of treatment, or a combination, along with the Medicaid guaranteed rebate of 23.1% would be needed for the oncology drug–spending trajectory to remain unchanged from recent years (and consistent with industry forecasting). The present results suggest many novel drugs may not be used widely or for as long among eligible patients.
This analysis may not accurately reflect real-world conditions given uncertainty in discounting, diffusion, duration of treatment, or market share, but it demonstrates 2 key points. First, the price of an average treatment course with a newly approved oncology drug is untenable. The mean price of $150 384 per patient is 2.4 times the median household income in the US in 2018.14 If used broadly, a single year of anticancer drug approvals could drastically alter the global cancer drug budget. If a novel therapeutic class (ie, cellular therapy) emerged as a highly effective therapy across many tumor types, the budgetary consequences may be massive.
Second, many of the newly approved cancer drugs are likely to experience limited uptake for the approved indications. Forecasting by the IQVIA Institute for Human Data Science anticipates sustained growth of 12% to 15% per year in US oncology drug spending though 2022.1 This is in contrast with the estimated 79% increase in annual cancer drug spending if the 2018 new approvals were used in all eligible patients (an unlikely but illustrative scenario). Figure 2 demonstrates how varying discounting and use of the 2018 approvals may influence spending. Industry forecasting suggests no more than $7.5 billion of increased oncology drug spending per annum. Only low-level uptake of the new drugs would be consistent with this incremental change in spending.
Of note, the present analysis does not account for increases in oncology drug spending owing to other factors known to be important (eg, more patients because of shifting demographics and increasing prices for existing drugs outpacing inflation15). For this reason, the analysis demonstrated in Figure 2 only provides an upper bound of diffusion compatible with spending forecasts.
While many factors may depress uptake of new drugs (eg, prescriber preference, patient cost sharing, formulary restrictions), we would posit that the limited effectiveness of many new approvals is a primary driver of this finding. Patients or prescribers may not be widely drawn to drugs shown to marginally improve surrogate end points alone. The sheer volume or rapidity of approvals does not necessarily translate into improving the lives of patients in the US.16
We acknowledge that there is no circumstance in which a drug achieves 100% market diffusion. The results based on this assumption provide a frame of reference from a national perspective. While the 15% annual growth in cancer drug spending deserves the attention it has received, we note this rise in spending is likely associated with low-level use of new drugs among eligible patients.
Limitations
Projecting the spending on new approvals was complicated by uncertainty in pricing and market diffusion. The WAC does not reflect the true cost of a given therapy, though is widely used for similar purposes. As detailed in the eAppendix in the Supplement, disease incidence and duration of therapy were estimated, and when possible, we erred toward the conservative estimate of population size, dosing, and duration (lowering the estimated cost of new approvals). Additionally, the present study was limited to 1 year of approvals, and these findings cannot be generalized to other years of approvals.
Prior work demonstrated that the duration of therapy for some anticancer drugs is shorter in the real-world compared with clinic trials,17,18 and shorter courses of therapy would permit a greater degree of use for a given budgetary threshold. Lastly, determining some of the displaced therapies (mostly with single-arm studies) required judgment as practicing adult and pediatric hematologist-oncologists, and we erred toward more expensive comparators, thus lowering the estimated net cost of new approvals. It is also possible that the displaced therapies may still be used in other lines of therapy, which would cause this analysis to underestimate the incremental costs. Others may use alternate displacements strategies. We encourage further investigations of this topic.
Conclusions
If widely used, the new cancer drugs approved by the FDA in 2018 would drastically increase cancer drug spending in the US. Alternatively, only low-level market diffusion of the new drugs allows for maintenance of budgetary trends. Industry forecasting suggests low-level uptake of the new drugs is most likely. While cancer drug pricing and expenditures deserve the attention received, the financial state of affairs would be far worse if newly approved therapies were more effective and used widely. The current drug pricing system is likely contingent on a sizable portion of eligible patients not receiving the latest FDA-approved therapies.
eTable. 2018 FDA oncology approvals
eAppendix. Assumptions and references used for estimating eligible population and prices
References
- 1.Global oncology trends 2018. IQVIA Institute for Human Data Science. May 24, 2018. Accessed September 30, 2019. https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncology-trends-2018
- 2.National health expenditure projections 2018-2027. Centers for Medicare & Medicaid Services. Updated December 17, 2019. Accessed January 13, 2020. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/ForecastSummary.pdf
- 3.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128. doi: 10.1093/jnci/djq495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spending on cancer meds in the U.S. doubled from 2012-2017—expected to double again by 2022 to $100 billion. News release. IQVIA Institute for Human Data Science. May 24, 2018. Accessed December 31, 2019. https://www.iqvia.com/newsroom/2018/05/iqvia-institute-for-human-data-science-study-spending-on-cancer-meds-in-the-us-doubled-from-2012-201
- 5.Mailankody S, Prasad V. Five years of cancer drug approvals: innovation, efficacy, and costs. JAMA Oncol. 2015;1(4):539-540. doi: 10.1001/jamaoncol.2015.0373 [DOI] [PubMed] [Google Scholar]
- 6.Prasad V, McCabe C, Mailankody S. Low-value approvals and high prices might incentivize ineffective drug development. Nat Rev Clin Oncol. 2018;15(7):399-400. doi: 10.1038/s41571-018-0030-2 [DOI] [PubMed] [Google Scholar]
- 7.Prasad V, Mailankody S. Research and development spending to bring a single cancer drug to market and revenues after approval. JAMA Intern Med. 2017;177(11):1569-1575. doi: 10.1001/jamainternmed.2017.3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman E Colorado is the latest state to be sued for restricting access to hepatitis C drugs. STAT News. September 22, 2016. Accessed December 31, 2019. https://www.statnews.com/pharmalot/2016/09/22/colorado-sued-for-restricting-hepatitis-c-medicine/
- 9.Marquart J, Chen EY, Prasad V. Estimation of the percentage of US patients with cancer who benefit from genome-driven oncology. JAMA Oncol. 2018;4(8):1093-1098. doi: 10.1001/jamaoncol.2018.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. doi: 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open. 2020;3(3):e200423-e200423. doi: 10.1001/jamanetworkopen.2020.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vokinger KN, Hwang T, Tibau A, Rosemann TJ, Kesselheim AN. Clinical benefit and prices of cancer drugs in the US and Europe. Ann Oncol. 2019;30(5):v924. doi: 10.1093/annonc/mdz394.086 [DOI] [PubMed] [Google Scholar]
- 13.National health expenditure fact sheet. Centers for Medicare & Medicaid Services. Accessed December 31, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- 14.Guzman G New data show income increased in 14 states and 10 of the largest metros. United States Census Bureau. September 26, 2019. Accessed November 1, 2019. https://www.census.gov/library/stories/2019/09/us-median-household-income-up-in-2018-from-2017.html
- 15.Gordon N, Stemmer SM, Greenberg D, Goldstein DA. Trajectories of injectable cancer drug costs after launch in the United States. J Clin Oncol. 2018;36(4):319-325. doi: 10.1200/JCO.2016.72.2124 [DOI] [PubMed] [Google Scholar]
- 16.Gyawali B, Hey SP, Kesselheim AS. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. JAMA Intern Med. 2019;179(7):906-913. doi: 10.1001/jamainternmed.2019.0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aspinall SL, Zhao X, Geraci MC, et al. ; Targeted Therapies in Veterans with RCC Study Group . Use of targeted therapies for advanced renal cell carcinoma in the Veterans Health Administration. Cancer Med. 2019;8(15):6651-6661. doi: 10.1002/cam4.2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson PG, San Miguel JF, Moreau P, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8(11):109. doi: 10.1038/s41408-018-0141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. 2018 FDA oncology approvals
eAppendix. Assumptions and references used for estimating eligible population and prices