Key Points
Question
Does treatment with hydroxychloroquine improve clinical outcomes of adults hospitalized with coronavirus disease 2019 (COVID-19)?
Findings
In this randomized clinical trial that included 479 hospitalized adults with respiratory symptoms from COVID-19, the distribution of the day 14 clinical status score (measured using a 7-category ordinal scale) was not significantly different for patients randomized to receive hydroxychloroquine compared with placebo (adjusted odds ratio, 1.02).
Meaning
These findings do not support the use of hydroxychloroquine for treatment of COVID-19 among hospitalized adults.
Abstract
Importance
Data on the efficacy of hydroxychloroquine for the treatment of coronavirus disease 2019 (COVID-19) are needed.
Objective
To determine whether hydroxychloroquine is an efficacious treatment for adults hospitalized with COVID-19.
Design, Setting, and Participants
This was a multicenter, blinded, placebo-controlled randomized trial conducted at 34 hospitals in the US. Adults hospitalized with respiratory symptoms from severe acute respiratory syndrome coronavirus 2 infection were enrolled between April 2 and June 19, 2020, with the last outcome assessment on July 17, 2020. The planned sample size was 510 patients, with interim analyses planned after every 102 patients were enrolled. The trial was stopped at the fourth interim analysis for futility with a sample size of 479 patients.
Interventions
Patients were randomly assigned to hydroxychloroquine (400 mg twice daily for 2 doses, then 200 mg twice daily for 8 doses) (n = 242) or placebo (n = 237).
Main Outcomes and Measures
The primary outcome was clinical status 14 days after randomization as assessed with a 7-category ordinal scale ranging from 1 (death) to 7 (discharged from the hospital and able to perform normal activities). The primary outcome was analyzed with a multivariable proportional odds model, with an adjusted odds ratio (aOR) greater than 1.0 indicating more favorable outcomes with hydroxychloroquine than placebo. The trial included 12 secondary outcomes, including 28-day mortality.
Results
Among 479 patients who were randomized (median age, 57 years; 44.3% female; 37.2% Hispanic/Latinx; 23.4% Black; 20.1% in the intensive care unit; 46.8% receiving supplemental oxygen without positive pressure; 11.5% receiving noninvasive ventilation or nasal high-flow oxygen; and 6.7% receiving invasive mechanical ventilation or extracorporeal membrane oxygenation), 433 (90.4%) completed the primary outcome assessment at 14 days and the remainder had clinical status imputed. The median duration of symptoms prior to randomization was 5 days (interquartile range [IQR], 3 to 7 days). Clinical status on the ordinal outcome scale at 14 days did not significantly differ between the hydroxychloroquine and placebo groups (median [IQR] score, 6 [4-7] vs 6 [4-7]; aOR, 1.02 [95% CI, 0.73 to 1.42]). None of the 12 secondary outcomes were significantly different between groups. At 28 days after randomization, 25 of 241 patients (10.4%) in the hydroxychloroquine group and 25 of 236 (10.6%) in the placebo group had died (absolute difference, −0.2% [95% CI, −5.7% to 5.3%]; aOR, 1.07 [95% CI, 0.54 to 2.09]).
Conclusions and Relevance
Among adults hospitalized with respiratory illness from COVID-19, treatment with hydroxychloroquine, compared with placebo, did not significantly improve clinical status at day 14. These findings do not support the use of hydroxychloroquine for treatment of COVID-19 among hospitalized adults.
Trial Registration
ClinicalTrials.gov: NCT04332991
This randomized trial compares the effects of hydroxychloroquine vs placebo on clinical status at 14 days (home, requiring noninvasive or invasive ventilation or extracorporeal membrane oxygenation, hospitalized, died) among adults hospitalized with coronavirus disease 2019 (COVID-19).
Introduction
Through September 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused more than 30 million confirmed cases of coronavirus disease 2019 (COVID-19), resulting in more than 1 million deaths globally.1,2
Hydroxychloroquine has been widely promoted as a potential therapy for COVID-19 due to its anti-inflammatory effects and in vitro studies suggesting antiviral activity.3,4,5,6,7,8,9 Hydroxychloroquine was adopted into routine care for hospitalized adults with COVID-19 at many hospitals.10,11,12 However, lack of evidence on efficacy and safety led multiple groups, including the National Institutes of Health (NIH) and Infectious Diseases Society of America, to recommend clinical trials to evaluate hydroxychloroquine as a potential treatment for patients with COVID-19.13,14,15
This trial—Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among Inpatients With Symptomatic Disease (ORCHID)—was conducted to test the hypothesis that, compared with placebo, hydroxychloroquine improves clinical outcomes for adults hospitalized with COVID-19.
Methods
Trial Design and Oversight
Details of the trial’s rationale and design were previously published16 and are available in the trial protocol and statistical analysis plan included in Supplement 1 and Supplement 2, respectively. We conducted a multicenter, blinded, randomized clinical trial comparing hydroxychloroquine vs placebo among hospitalized adults with respiratory illness from COVID-19. Patients were enrolled between April 2, 2020, and June 19, 2020, at 34 hospitals in the US within the Prevention and Early Treatment of Acute Lung Injury (PETAL) Clinical Trials Network (eTable 1 in Supplement 3). The final outcome assessment was scheduled on July 17, 2020. The trial was funded by the National Heart, Lung, and Blood Institute (NHLBI) of the NIH. A central institutional review board at Vanderbilt University Medical Center approved the study. A data and safety monitoring board (DSMB) appointed by the NHLBI provided trial oversight. The Food and Drug Administration (FDA) issued an investigational new drug exemption (IND No. 149243). Patients or legally authorized representatives provided informed consent for participation, primarily using electronic consent procedures, including electronic consent forms and video conferencing for informed consent discussions, to reduce the risk of spreading the virus and to conserve personal protective equipment.16
Patient Population
Adults (aged ≥18 years) who were hospitalized for less than 48 hours with laboratory-confirmed SARS-CoV-2 infection and symptoms of respiratory illness for less than 10 days were enrolled. The main exclusion criteria were more than 1 dose of hydroxychloroquine or chloroquine in the prior 10 days; QTc interval greater than 500 ms; prior receipt or planned administration of select medications that prolong the QTc interval; and seizure disorder. Full eligibility criteria are listed in eTable 2 in Supplement 3. Race and ethnicity were reported in this study because the efficacy of hydroxychloroquine for COVID-19 might vary by race or ethnicity. Race and ethnicity were reported by the participant or surrogate; categories of race and ethnicity were provided in the trial’s case report form.
Due to delays in SARS-CoV-2 testing early in the pandemic, the trial was initially designed to enroll hospitalized patients with suspected or confirmed SARS-CoV-2 infection, but after testing capacity increased, eligibility criteria were narrowed to include only laboratory-confirmed cases. Prior to this change, 2 patients without laboratory confirmation of SARS-CoV-2 infection were enrolled; these patients were included in the primary analysis.
Randomization
Using a centralized electronic system, we randomly assigned enrolled patients to hydroxychloroquine or placebo in a 1:1 ratio stratified by enrolling hospital using randomization block sizes of 2 and 4. Allocation was concealed. Patients, treating clinicians, trial personnel, and outcome assessors were blinded to group assignment.
Trial Interventions
The first dose of the trial drug was administered within 4 hours of randomization. Patients assigned to the hydroxychloroquine group received 400 mg of hydroxychloroquine sulfate in pill form twice a day for the first 2 doses and then 200 mg in pill form twice a day for the subsequent 8 doses, for a total of 10 doses over 5 days.7 Patients assigned to the placebo group received matching placebo in the same dosing frequency. Patients discharged from the hospital before day 5 continued the trial medication after discharge to complete the 10-dose course.
An important safety consideration for hydroxychloroquine is QTc prolongation.17,18 Hence, trial personnel systematically assessed the QTc interval between 24 and 48 hours after administration of the first dose of trial drug. Additional doses of the trial drug were held for a QTc greater than 500 ms. Study personnel monitored daily for administration of medications with potential interactions with hydroxychloroquine and did not administer the trial drug if the participant received a concomitant medication with a high risk for interaction (eTable 3 in Supplement 3).
Open-label, clinical use of hydroxychloroquine and chloroquine was not allowed during the 5-day course of trial drug. Treating clinicians determined all other aspects of patient care. Concomitant medications were recorded through hospital discharge.
Outcomes
The primary outcome was clinical status 14 days after randomization assessed with a 7-category ordinal scale (the COVID Outcomes Scale) recommended by the World Health Organization.19 The scale consisted of 7 mutually exclusive categories: 1, death; 2, hospitalized, receiving extracorporeal membrane oxygenation (ECMO) or invasive mechanical ventilation; 3, hospitalized, receiving noninvasive mechanical ventilation or nasal high-flow oxygen therapy; 4, hospitalized, receiving supplemental oxygen without positive pressure or high flow; 5, hospitalized, not receiving supplemental oxygen; 6, not hospitalized and unable to perform normal activities; and 7, not hospitalized and able to perform normal activities. To distinguish between category 6 and category 7, study personnel assessed the patient’s performance of usual activities with questions consistent with validated health status measures.20,21 Patients who were discharged from the hospital were contacted by telephone for assessment of the COVID Outcome Scale at 7, 14, and 28 days after randomization.
The trial included 12 secondary outcomes: scores on the COVID Outcomes Scale at 2, 7, and 28 days after randomization; all-cause all-location mortality at 14 and 28 days after randomization; time to recovery, defined as time to reach COVID Outcome Scale category 5, 6, or 7; the composite of death or receipt of ECMO through 28 days; and support-free days through 28 days, including hospital-free, oxygen-free, intensive care unit (ICU)–free, ventilator-free, and vasopressor-free days.22 Data on the occurrence of several safety events with potential mechanistic links to hydroxychloroquine were also systematically collected between randomization and 28 days later, including cytopenia, plasma aspartate aminotransferase or alanine aminotransferase concentration greater than twice the local laboratory upper limit of normal, cardiac arrest treated with cardiopulmonary resuscitation, symptomatic hypoglycemia, ventricular tachyarrhythmia, and seizure. Serious adverse events, defined as untoward medical events leading to death, a life-threatening experience, prolongation of hospitalization, or persistent or significant disability or incapacity in the judgment of the site investigator, were also reported. Definitions for all outcomes are available in the statistical analysis plan (Supplement 2).
Statistical Analysis
The trial was analyzed by comparing patients randomized to hydroxychloroquine vs those randomized to placebo, with the placebo group serving as the referent. The primary outcome was analyzed with a multivariable proportional odds model with the following prespecified covariables: age, sex, baseline (prerandomization) COVID Outcomes Scale category, baseline Sequential Organ Failure Assessment (SOFA) score,23 and duration of acute respiratory symptoms prior to randomization. An adjusted odds ratio (aOR) greater than 1.0 indicated more favorable outcomes on the COVID Outcomes Scale among patients randomized to hydroxychloroquine compared with placebo.
Due to the paucity of information available on COVID-19 at the beginning of the trial for estimation of event rates and treatment effects, we used a bayesian framework to guide serial interim analyses. Interim analyses for the DSMB to evaluate trial data were planned after approximately 102, 204, 306, 408, and 510 patients reached follow-up time for the primary outcome. Enrollment was planned to continue until the DSMB recommended stopping the trial for evidence of efficacy, futility, or harm, based on evaluation of all available data, including data internal and external to the trial. At interim analyses, the DSMB was presented with the probability that the aOR for the primary outcome met each of 3 separate thresholds: greater than 1.0 with a skeptical prior (evidence of efficacy); less than 1.1 with a noninformative prior (evidence of futility); and less than 0.7 with a noninformative prior (evidence of harm) (eTable 4 in Supplement 3). Although there were no mandatory stopping criteria, the investigators suggested and specified in the statistical analysis plan that the DSMB strongly consider stopping the trial if the probability of efficacy (aOR > 1.0) was greater than 95%, the probability of futility (aOR < 1.1) was greater than 90%, or the probability of harm (aOR < 0.7) was greater than 70%. Based on statistical simulation of a range of possible treatment effect sizes, the investigators anticipated that enrolling approximately 510 patients would provide sufficient data for the DSMB to draw conclusions regarding hydroxychloroquine and support recommendations about stopping or continuing the trial.16 The minimal clinically important difference between groups on the COVID Outcomes Scale was unknown. Enrollment of 510 patients would provide 90% power to detect an aOR of 1.82, which the investigators considered a moderate effect size, using a 2-sided significance level of .05.
Sensitivity analyses for the primary outcome included (1) a modified population limited to patients with laboratory-confirmed SARS-CoV-2 infection; (2) a modified population limited to patients who received at least 1 dose of trial drug; and (3) a post hoc analysis including enrolling site as a random effect in the multivariable proportional odds model.
Heterogeneity of treatment effect by prespecified baseline characteristics was evaluated by adding an interaction term between randomized group assignment and the baseline characteristic of interest in the primary model.24 Baseline characteristics evaluated in heterogeneity of treatment effect analyses included baseline COVID Outcomes Scale category, hospital location at randomization (ICU vs outside an ICU), baseline SOFA score, duration of symptoms prior to randomization, age, sex, and race/ethnicity.
Secondary outcomes were analyzed using regression models including the same covariables as the primary model (details are provided in the statistical analysis plan in Supplement 2). Survival and hospital discharge through day 28 were analyzed using proportional hazards regression. For the time-to-hospital discharge model, death was treated as a competing risk, and the subdistribution hazard ratio was reported.25 A treatment × time interaction was used to test the proportional hazards assumption for the survival and time to discharge models; the proportional hazards assumption was determined to be met for both models.
Post hoc analyses included a comparison of persistent symptoms at 14 and 28 days after randomization between the hydroxychloroquine and placebo groups and evaluation of the primary outcome among patients who received each of the following medications during the same hospitalization as trial enrollment: remdesivir, azithromycin, and corticosteroids.
In presentation of final trial results, between-group differences were reported using point estimates and 2-sided 95% CIs. Results with a 95% CI that did not include the null (eg, a 95% CI for an aOR that did not include 1.0) were considered statistically significant. The widths of confidence intervals were not adjusted for multiplicity and thus findings for analyses of secondary outcomes should be interpreted as exploratory. For patients who remained hospitalized 14 days after randomization, primary outcome ascertainment was completed by medical record review. For patients who were discharged prior to 14 days after randomization, primary outcome ascertainment was completed by telephone calls. Patients who could not be reached by telephone for the primary outcome assessment at day 14 had the COVID Outcomes Scale score carried forward from a day 7 follow-up call if such a call was successfully completed or had a category 6 score (not hospitalized and unable to perform normal activities) imputed if no prior follow-up calls were successfully completed. Mortality was not imputed when vital status was unknown. Analyses were performed using SAS version 9.4 (SAS Institute) and R rms package version 6.0 and rmsb package version 0.0.1 (R Project for Statistical Computing).
Stopping the Trial
On June 19, 2020, enrollment was stopped for futility based on recommendations from the DSMB after it reviewed information both internal and external to the trial. Enrollment was stopped at the fourth interim analysis, which included 371 patients with primary outcome data and an additional 108 patients who had not reached 14 days after randomization for primary outcome assessment. At that time, trial data did not meet the prespecified threshold for futility (defined as >90% probability of an aOR < 1.1 for the primary outcome) but demonstrated an 81% probability for an aOR less than 1.1. Furthermore, a post hoc conditional power analysis showed less than 1% probability of the trial reaching the prespecified threshold for efficacy (defined as >95% probability of an aOR > 1.0) if it continued to a sample size of 510 participants (eTable 5 in Supplement 3). At that time, new information about hydroxychloroquine from sources external to the trial reviewed by the DSMB included (1) a June 5, 2020, press release from the Randomized Evaluation of COVID-19 Therapy (RECOVERY) platform trial leadership stating that results from their trial suggested no survival benefit from hydroxychloroquine26; (2) a June 15, 2020, revision to the FDA Emergency Use Authorization for remdesivir recommending against co-administration of remdesivir and hydroxychloroquine due to the potential of hydroxychloroquine reducing the efficacy of remdesivir27; and (3) a June 16, 2020, press release from the Medicines and Healthcare products Regulatory Agency instructing all clinical trials of hydroxychloroquine in the United Kingdom to suspend recruitment.28
Results
Patients
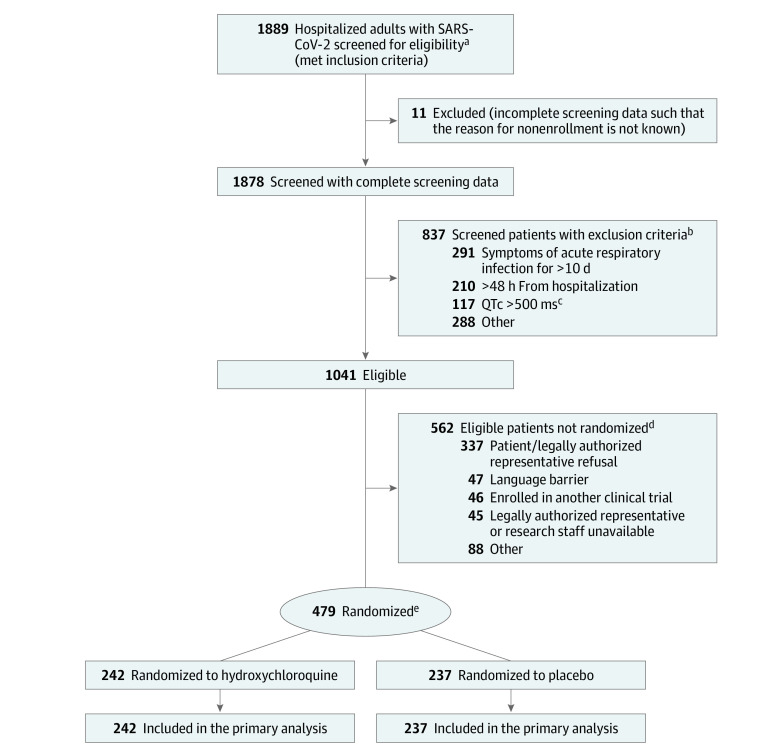
During the 78-day enrollment period, 1889 patients were screened; 1041 patients met eligibility criteria and 479 patients were randomized (Figure 1). The most common reasons for exclusion among screened patients were duration of respiratory symptoms longer than 10 days (34.8% of exclusions), hospitalization for more than 48 hours at the time of screening (25.1%), and QTc greater than 500 ms (14.0%). The most common reason for eligible patients not to be enrolled was the patient or legally authorized representative declining participation (60.0%). Among enrolled patients, the median age was 57 years (interquartile range [IQR], 44 to 68 years), 44.3% were female, 37.2% were Hispanic/Latinx, and 23.4% were Black. The median duration of symptoms prior to randomization was 5 days (IQR, 3 to 7 days). Among 479 enrolled patients, 242 (50.5%) were randomized to hydroxychloroquine and 237 (49.5%) were randomized to placebo. Baseline characteristics of patients randomized to the hydroxychloroquine group and placebo group are presented in Table 1 and eTables 6-11 in Supplement 3.
Figure 1. Participant Flow in a Randomized Clinical Trial of Hydroxychloroquine vs Placebo in Patients Hospitalized With Respiratory Symptoms of Coronavirus Disease 2019 (COVID-19).
aBetween April 2 and April 21, 2020, screened patients included both those with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and those with suspected SARS-CoV-2 infection. Between April 21, 2020, and the end of the trial (June 19, 2020), only patients with laboratory-confirmed SARS-CoV-2 infection were screened.
bExclusion criteria were not mutually exclusive.
cQTc was assessed as a study procedure during the screening process; patients must have had a QTc less than 500 ms at the time of screening to be eligible for the trial.
dReasons for not randomizing were not mutually exclusive.
eRandomization was stratified by enrolling hospital.
Table 1. Baseline Patient Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| Hydroxychloroquine (n = 242) | Placebo (n = 237) | |
| Age, median (IQR), y | 58 (45-69) | 57 (43-68) |
| Sex | ||
| Female | 107 (44.2) | 105 (44.3) |
| Male | 135 (55.8) | 132 (55.7) |
| Race/ethnicity | n = 232 | n = 227 |
| Hispanic/Latinx | 91 (39.2) | 87 (38.3) |
| Non-Hispanic | ||
| White | 72 (31.0) | 65 (28.6) |
| Black | 57 (24.6) | 55 (24.2) |
| American Indian or Alaska Native | 5 (2.2) | 8 (3.5) |
| Asian | 4 (1.7) | 7 (3.1) |
| Native Hawaiian or Other Pacific Islander | 2 (0.9) | 4 (1.8) |
| Multirace | 1 (0.4) | 1 (0.4) |
| Living at home in the community prior to hospitalization | 190 (78.5) | 183 (77.2) |
| Body mass index, median (IQR)a | 31.3 (26.4-37.2) | 31.1 (27.2-36.5) |
| No. | 226 | 219 |
| Chronic conditions | ||
| Hypertension | 136 (56.2) | 117 (49.4) |
| Diabetes | 88 (36.4) | 78 (32.9) |
| Chronic kidney disease | 28 (11.6) | 14 (5.9) |
| Coronary artery disease | 19 (7.9) | 23 (9.7) |
| Chronic obstructive pulmonary disease | 18 (7.4) | 21 (8.9) |
| Location at time of randomization | n = 228 | n = 224 |
| Hospital ward | 157 (68.9) | 132 (58.9) |
| Intensive care unit | 37 (16.2) | 54 (24.1) |
| Emergency department | 34 (14.9) | 38 (17.0) |
| Symptoms of acute respiratory infection | ||
| Shortness of breath | 175 (72.3) | 168 (70.9) |
| Cough | 143 (59.1) | 140 (59.1) |
| Fever (temperature >37.5 °C) | 138 (57.0) | 132 (55.7) |
| Duration of symptoms prior to randomization, median (IQR), d | 5 (3-7) | 5 (3-7) |
| Time between hospital presentation and randomization, median (IQR), hb | 22.2 (14.6-33.1) | 22.7 (14.1-29.9) |
| No. | 240 | 234 |
| COVID Outcomes Scale category at randomizationc | ||
| 2: Hospitalized, receiving ECMO or invasive mechanical ventilation | 13 (5.4) | 19 (8.0) |
| 3: Hospitalized, receiving noninvasive ventilation or nasal high-flow oxygen | 28 (11.6) | 27 (11.4) |
| 4: Hospitalized, receiving supplemental oxygen without positive pressure or high flow | 116 (47.9) | 108 (45.6) |
| 5: Hospitalized, not receiving supplemental oxygen | 85 (35.1) | 83 (35.0) |
| Vasopressor use at enrollment | 8 (3.3) | 20 (8.4) |
| Total SOFA score at enrollment, median (IQR)d | 2 (1-4) | 2 (1-4) |
| Laboratory measurementse | ||
| White blood cell count, median (IQR), ×103/μL (normal range, 3.9-10.7) | 6.0 (4.3-7.9) | 5.9 (4.1-7.7) |
| No. | 224 | 218 |
| Platelet count, median (IQR), ×103/μL (normal range, 135-371) | 199 (151-247) | 200 (147-251) |
| No. | 237 | 230 |
| Creatinine, median (IQR), mg/dL (normal range, 0.57-1.11) | 0.90 (0.75-1.47) | 0.90 (0.75-1.25) |
| No. | 235 | 231 |
| Aspartate aminotransferase, median (IQR), U/L (normal range, 5-40) | 39 (29-62) | 45 (31-70) |
| No. | 173 | 184 |
| Alanine aminotransferase, median (IQR), U/L (normal range, 0-55) | 30 (18-47) | 34 (23-62) |
| No. | 174 | 183 |
| Bilateral infiltrates on chest imagingf | 147/230 (63.9) | 145/230 (63.0) |
| QTc interval, median (IQR), msg | 430 (414-452) | 435 (416-452) |
| No. | 242 | 236 |
Abbreviations: COVID, coronavirus disease; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
SI conversion factors: To convert aspartate aminotransferase and alanine aminotransferase to μkat/L, multiply by 0.0167; creatinine to μmol/L, multiply by 88.4.
Calculated as weight in kilograms divided by height in meters squared.
Defined as the time of the first contact with an acute care hospital during the health care episode that resulted in the hospitalization during which the patient was enrolled. For patients who initially presented to the emergency department, time of hospital presentation was the time of emergency department arrival. For patients directly hospitalized without presenting to the emergency department, time of hospital presentation was the time of arrival at the admission unit.
The COVID Outcomes Scale is a 7-category ordinal scale that classifies a patient’s clinical status.19 Lower scores indicate more severely ill clinical status. Patients in the following categories at baseline were not eligible for enrollment: category 1 (death); category 6 (not hospitalized and unable to perform normal activities); and category 7 (not hospitalized and able to perform normal activities).
The SOFA score23 categorizes illness severity based on organ dysfunction across 6 organ systems: respiratory, coagulation, liver, cardiovascular, central nervous system, and kidney. SOFA scores range from 0 to 24, with higher scores indicating greater illness severity. A SOFA score of 2 indicates moderate dysfunction in 1 organ system or mild dysfunction in 2 organ systems.
Laboratory normal ranges were reported base on the clinical laboratory normal ranges from Vanderbilt University Medical Center. Normal ranges may vary across laboratories.
Reported chest imaging interpretations were based on final interpretation from radiologists.
Reported QTc was based on automated readings.
Primary outcome assessment of the COVID Outcomes Scale 14 days after randomization was completed for 433 (90.4%) of 479 randomized patients; 46 patients who were discharged from the hospital before primary outcome assessment, including 25 in the hydroxychloroquine group and 21 in the placebo group, were not successfully contacted for primary outcome evaluation and had values imputed based on a follow-up call on day 7 or were assigned a score of 6 if no call was completed on day 7. Follow-up information on survival through day 28 was completed for 477 (99.6%) of 479 randomized patients; 1 patient in the hydroxychloroquine group and 1 patient in the placebo group were lost to follow-up for vital status.
Receipt of Trial Drug and Cointerventions
In the hydroxychloroquine group, 242 (100%) of 242 patients received at least 1 dose of the trial drug, and 2149 (88.8%) of 2420 scheduled doses of trial drug were received (eTables 12-13 in Supplement 3). In the placebo group, 231 (97.5%) of 237 patients received at least 1 dose of placebo, and 2038 (86.0%) of 2370 scheduled doses of placebo were received. QTc prolongation greater than 500 ms was the reason for 38 (14.0%) of the missed doses in the hydroxychloroquine group and 21 (6.3%) of the missed doses in the placebo group.
Among the 479 patients in the trial, remdesivir, azithromycin, and corticosteroids were received by 104 (21.7%), 91 (19.0%), and 88 (18.4%) patients, respectively, during the same hospitalization in which they were enrolled in the trial (eTables 14-15 in Supplement 3).
Primary Outcome
At 14 days after randomization, there was no significant difference in the COVID Outcomes Scale score between the hydroxychloroquine group (median [IQR] score, 6 [4-7]) and placebo group (median [IQR] score, 6 [4-7]) (aOR, 1.02 [95% CI, 0.73-1.42) (Table 2; Figure 2). Similarly, there were no significant differences in the primary outcome in sensitivity analyses that limited the population to patients with laboratory-confirmed SARS-CoV-2 infection (n = 477), that limited the population to patients who received at least 1 dose of trial drug (n = 473), and that included enrolling site as a random effect (n = 479) (eTable 16 in Supplement 3). There was no significant difference in the primary outcome between the hydroxychloroquine group and placebo group in any prespecified subgroups, including those based on age, sex, race/ethnicity, baseline illness severity, and duration of symptoms (eFigure in Supplement 3). In post hoc analyses among subgroups of patients treated clinically with open-label remdesivir, azithromycin, and corticosteroids, there were no significant differences in the primary outcome between the hydroxychloroquine group and placebo group (eTable 17 in Supplement 3).
Table 2. Outcomes, Systematically Collected Safety Events, and Serious Adverse Events.
| Outcome | Hydroxychloroquine (n = 242) | Placebo (n = 237) | Unadjusted absolute difference (95% CI)a | Adjusted odds ratio or odds ratio (95% CI)b |
|---|---|---|---|---|
| Primary outcome | ||||
| COVID Outcomes Scale score at 14 d, median (IQR)c | 6 (4 to 7) | 6 (4 to 7) | 0d | 1.02 (0.73 to 1.42) |
| Secondary outcomes | ||||
| COVID Outcomes Scale score, median (IQR)c | ||||
| At 2 d | 4 (3 to 5) | 4 (3 to 5) | 0d | 1.28 (0.90 to 1.81) |
| At 7 d | 5 (4 to 7) | 6 (3 to 6) | −1 (−2 to 0) | 1.16 (0.84 to 1.61) |
| At 28 d | 6 (6 to 7) | 6 (6 to 7) | 0 (−1 to 1) | 0.97 (0.69 to 1.38) |
| All-cause, all-location death, No. (%) | n = 241 | n = 236 | ||
| At 14 d | 18 (7.5) | 14 (5.9) | 1.5 (−2.9 to 6.0) | 1.56 (0.68 to 3.57) |
| At 28 d | 25 (10.4) | 25 (10.6) | −0.2 (−5.7 to 5.3) | 1.07 (0.54 to 2.09) |
| Time to recovery in days, median (IQR) | 5 (1 to 14) | 6 (1 to 15) | −1 (−3 to 1) | 0.97 (0.69 to 1.35) |
| Composite of death or ECMO through 28 d, No./total No. (%) | 29/241 (12.0) | 28/236 (11.9) | 0.2 (−5.6 to 6.0) | 1.13 (0.60 to 2.14) |
| Support-free days through day 28, median (IQR) | ||||
| Hospital-free days | 21 (11 to 24) | 20 (10 to 24) | 1 (−1 to 3) | 1.17 (0.85 to 1.61) |
| Oxygen-free days | 21 (0 to 27) | 20 (0 to 27) | 1 (−2 to 4) | 0.96 (0.68 to 1.34) |
| ICU-free days | 28 (21 to 28) | 28 (18 to 28) | 0 (0 to 0) | 1.26 (0.84 to 1.88) |
| Ventilator-free days | 28 (28 to 28) | 28 (28 to 28) | 0d | 1.26 (0.76 to 2.08) |
| Vasopressor-free days | 28 (28 to 28) | 28 (28 to 28) | 0d | 1.03 (0.61 to 1.72) |
| Systematically collected safety events, No. (%)e | ||||
| Cytopeniaf | 92 (38.0) | 87 (36.7) | 1.3 (−7.4 to 10.0) | 1.06 (0.73 to 1.53) |
| AST or ALT ≥2 times upper limit of normal | 50 (20.7) | 65 (27.4) | −6.8 (−14.4 to 0.9) | 0.69 (0.45 to 1.05) |
| Cardiac arrest treated with CPRg | 10 (4.1) | 4 (1.7) | 2.5 (−0.8 to 5.6) | 2.51 (0.78 to 8.12) |
| Symptomatic hypoglycemiah | 10 (4.1) | 8 (3.4) | 0.8 (−2.8 to 4.3) | 1.23 (0.48 to 3.18) |
| Ventricular tachyarrhythmiai | 5 (2.1) | 6 (2.5) | −0.5 (−3.4 to 2.4) | 0.81 (0.24 to 2.70) |
| Seizure | 1 (0.4) | 0 | 0.4 (−1.0 to 1.8) | |
| Patients with ≥1 SAEs reportedj | 14 (5.8) | 11 (4.6) | 1.1 (−3.0 to 5.2) | 1.26 (0.56 to 2.84) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID, coronavirus disease; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range; SAE, serious adverse event.
For multilevel ordinal variables (COVID Outcomes Scale and support-free outcomes), the unadjusted absolute difference was calculated as the median value for the hydroxychloroquine group minus the median value for the placebo group; CIs were computed based on quantile regression using the proc quantreg procedure. For dichotomous variables, the unadjusted absolute difference was calculated as the percentage of participants with the outcome in the hydroxychloroquine group minus the percentage of participants with the outcome in the placebo group; CIs for binomial risk differences were computed using a Wald or Agresti-Coull method.
Models for the primary and secondary outcomes were constructed with trial group assignment (hydroxychloroquine vs placebo) as the independent variable, the outcome as the dependent variable, and the following covariables: age, sex, baseline COVID Outcome Scale category, baseline Sequential Organ Failure Assessment score, and duration of acute respiratory infection symptoms prior to randomization. Multivariable proportional odds models were used for the COVID Outcomes Scale outcomes and support-free outcomes. Multivariable logistic regression models were used for death outcomes. Systematically collected safety events and SAEs were analyzed with simple logistic regression models without covariable adjustment. Odds ratios (ORs) greater than 1.0 indicated more favorable outcomes for patients in the hydroxychloroquine group compared with the placebo group for the following outcomes: COVID Outcomes Scale score (adjusted OR [aOR] >1.0 indicated higher score on the scale) and support-free days (aOR >1.0 indicated more support-free days). ORs greater than 1.0 indicated less favorable outcomes for patients in the hydroxychloroquine group compared with the placebo group for the following outcomes: death (aOR >1.0 indicated more death), systematically collected safety events (OR >1.0 indicated more safety events), and SAEs (OR >1.0 indicated more SAEs).
The COVID Outcomes Scale is a 7-category ordinal scale that classifies a patient’s clinical status.19 The 7 categories are 1: death; 2: hospitalized, receiving ECMO or invasive mechanical ventilation; 3: hospitalized, receiving noninvasive mechanical ventilation or nasal high-flow oxygen therapy; 4: hospitalized, receiving supplemental oxygen; 5: hospitalized, not receiving supplemental oxygen; 6: not hospitalized and unable to perform normal activities; and 7: not hospitalized and able to perform normal activities.
CIs for the absolute difference were not calculated for ordinal variables with identical medians and IQRs in the hydroxychloroquine and placebo groups.
Variables collected based on known potential toxicities of hydroxychloroquine were collected for every participant. Adverse event and serious adverse event reporting was based on the judgement of site investigators.
Defined as any of the following values on a clinically obtained laboratory test between randomization and 28 days later: absolute neutrophil count less than 1000 cells/μL; absolute lymphocyte count less than 1000 cells/μL; hemoglobin less than 12.0 g/dL; and platelet count less than 50 000/μL.
Defined as loss of a palpable pulse treated as a cardiac arrest with resuscitative efforts between randomization and 28 days later. Expected cardiac arrest that occurred as part of the dying process for patients on comfort measures was not classified as cardiac arrest treated with CPR.
Defined as a clinically reported low blood glucose level (no specific threshold provided) that led to treatment for reversal of hypoglycemia between randomization and 28 days later.
Ventricular tachyarrhythmia was defined as ventricular fibrillation or ventricular tachycardia treated with a medication or electrical cardioversion or defibrillation between randomization and 28 days later.
Serious adverse event was defined as an untoward medical event leading to death, a life-threatening experience, prolongation of hospitalization, or persistent or significant disability or incapacity. A detailed report of adverse events and serious adverse events is provided in eTable 24 in Supplement 3.
Figure 2. Clinical Status on the Coronavirus Disease (COVID) Outcomes Scale 14 Days and 28 Days After Randomization.
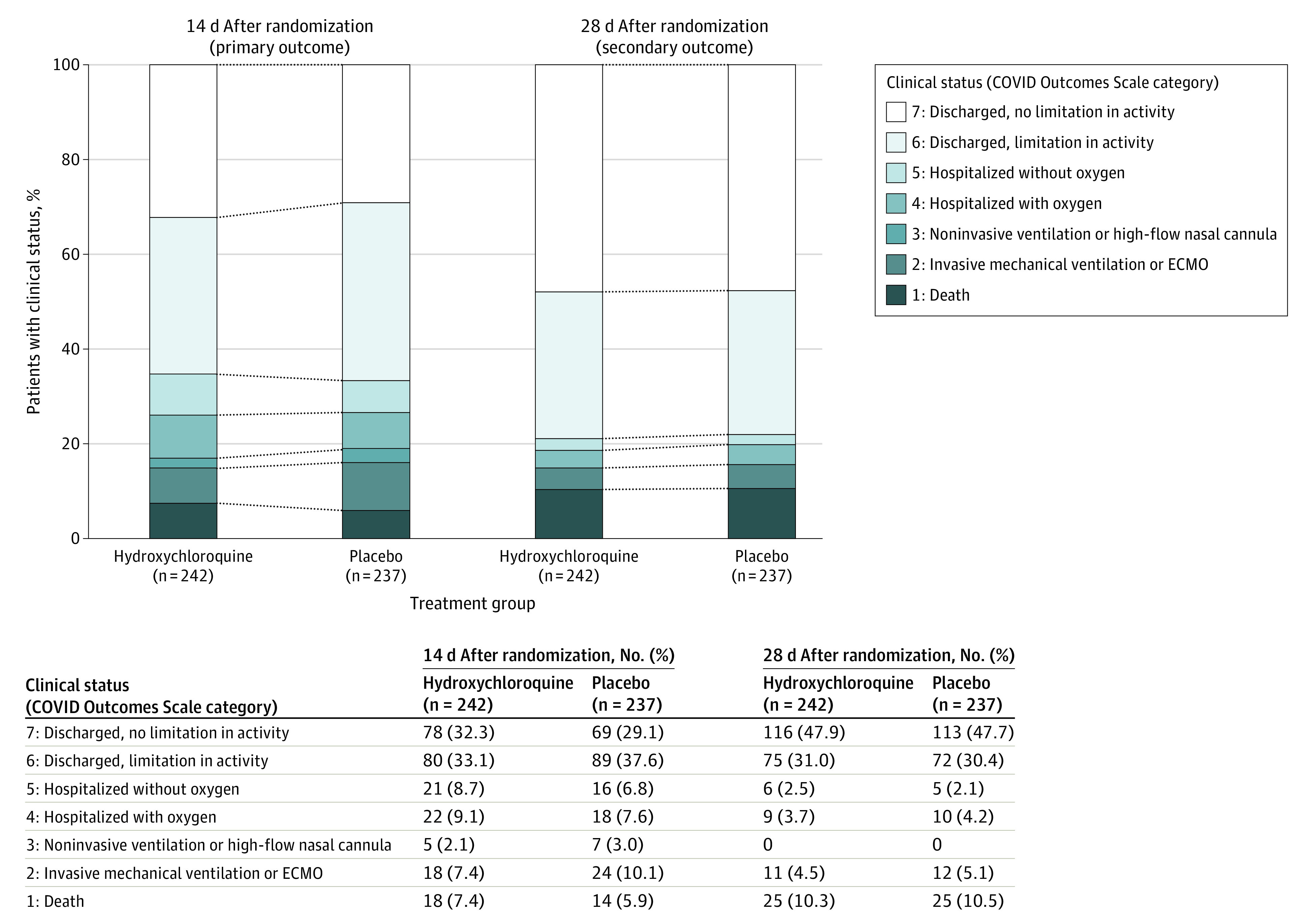
ECMO indicates extracorporeal membrane oxygenation. There was no significant difference between the hydroxychloroquine group and placebo group in the overall distribution of scores at 14 days (adjusted odds ratio, 1.02 [95% CI, 0.73-1.42]) or 28 days (adjusted odds ratio, 0.97 [95% CI, 0.69-1.38]).
Secondary Outcomes
There was no significant difference in any of the 12 secondary outcomes between the hydroxychloroquine and placebo groups (Table 2; eTables 18-19 in Supplement 3). At 28 days after randomization, 25 (10.4%) of 241 patients with confirmed vital status in the hydroxychloroquine group and 25 (10.6%) of 236 patients with confirmed vital status in the placebo group had died (aOR, 1.07 [95% CI, 0.54-2.09]) (Figure 3). In a post hoc analysis, persistent symptoms of COVID-19 were common in both the hydroxychloroquine and placebo groups at 14 days (34.7% vs 32.9%) and 28 days (28.5% vs 30.4%) after randomization (eTable 20 in Supplement 3).
Figure 3. Survival and Hospital Discharge Through 28 Days Following Randomization.
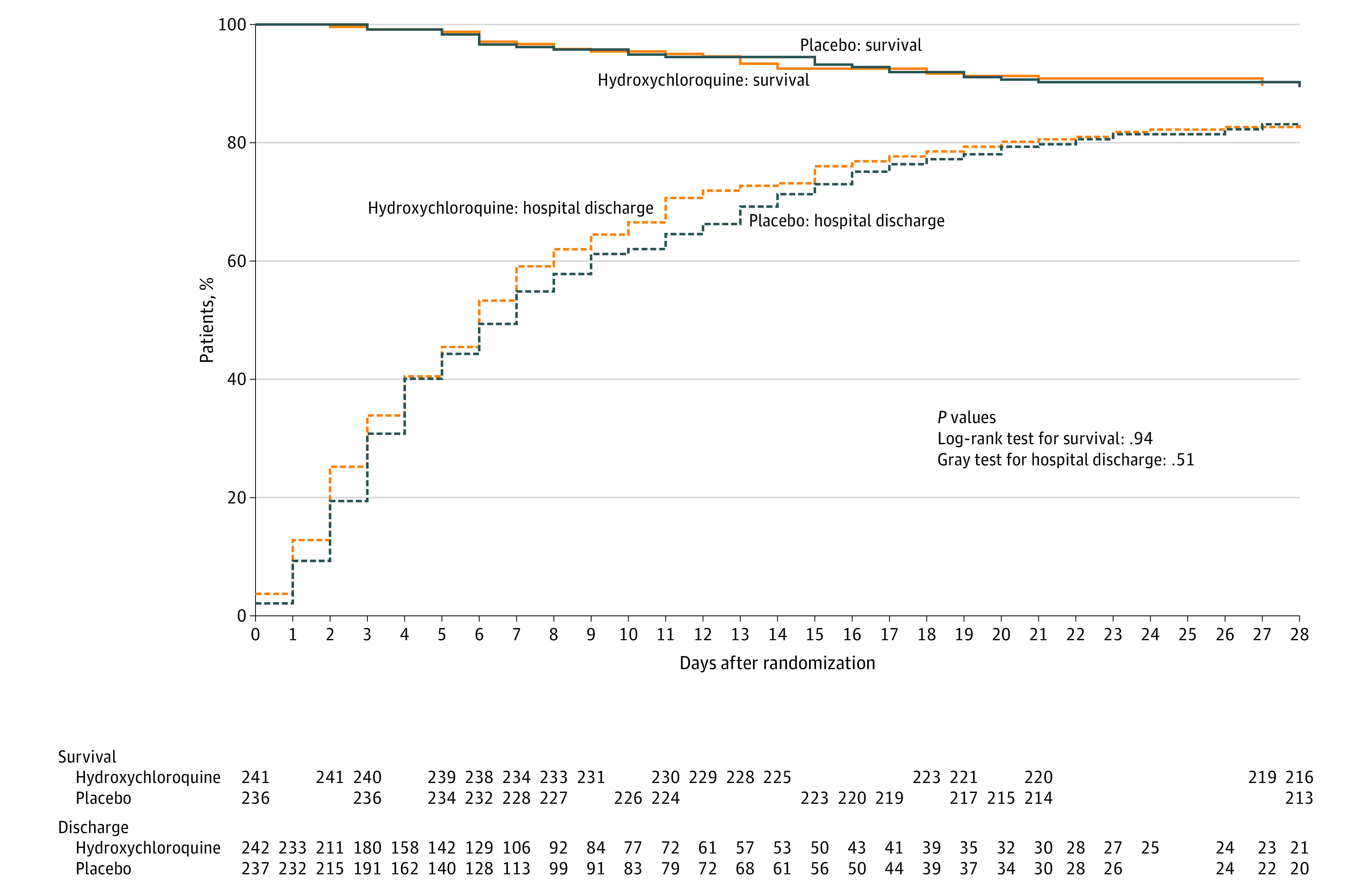
The survival curves are survival function (Kaplan-Meier) curves with a P value calculated by the log-rank test. Patients were followed up for death until 28 days following randomization using in-hospital records and telephone follow-up. Two patients had unknown vital status at 28 days and were not included in this analysis. The hospital discharge curves are cumulative incidence curves of hospital discharge accounting for the competing risk of death with a P value calculated by Gray test. For hospital discharge, all patients were followed up to discharge or 28 days after randomization. A patient was considered discharged from the hospital once discharged from the index hospitalization; rehospitalizations were not considered in this analysis. There was no difference between the hydroxychloroquine group and placebo group in survival (adjusted hazard ratio, 1.05 [95% CI, 0.60-1.85]) or time to discharge (adjusted hazard ratio, 1.09 [95% CI, 0.89-1.32]).
Systematically Collected Safety Events and Adverse Events
Data on systematically collected safety events and adverse events are presented in eTables 21 to 24 in Supplement 3. In the 5 days following randomization, 13 patients (5.9% of 221 patients with QTc assessed) in the hydroxychloroquine group and 7 patients (3.3% of 214 patients with QTc assessed) in the placebo group had a recorded QTc interval greater than 500 ms. A total of 30 serious adverse events were reported, including 18 serious adverse events from 14 patients (5.8%) in the hydroxychloroquine group and 12 serious adverse events from 11 patients (4.6%) in the placebo group.
Discussion
In this multicenter, blinded, placebo-controlled randomized clinical trial conducted at 34 US hospitals, treatment with hydroxychloroquine did not improve or worsen clinical outcomes for adults hospitalized for respiratory illness from COVID-19. These findings were consistent in all subgroups and for all outcomes evaluated, including an ordinal scale of clinical status, mortality, organ failures, duration of oxygen use, and hospital length of stay.
Enthusiasm for hydroxychloroquine as a potential therapy for COVID-19 was sparked by in vitro studies that suggested it limited entry of SARS-CoV-2 into human cells by inhibiting glycosylation of cell receptors targeted by coronaviruses and increasing endosomal pH, thereby reducing endosome-mediated viral entry.6,7,8 Additionally, hydroxychloroquine reduces the production of several proinflammatory cytokines involved in the development of acute respiratory distress syndrome, a severe manifestation of COVID-19.3,4,5 These factors, combined with broad availability, oral administration, and perceived safety based on historical use in the treatment of malaria and rheumatologic diseases,4 led to widespread clinical use of hydroxychloroquine for COVID-19.10,15 On March 28, 2020, the FDA issued an Emergency Use Authorization for hydroxychloroquine to treat adults hospitalized with COVID-19,29 which was later revoked on June 15, 2020.30
The finding of this clinical trial that hydroxychloroquine was not efficacious for the treatment of COVID-19 is consistent with results from recent in vitro studies suggesting no antiviral activity for hydroxychloroquine against SARS-CoV-231,32 and open-label pragmatic trials in the United Kingdom33 and Brazil34 suggesting no clinical benefit. Interpreted along with these prior studies, the results of this trial provide strong evidence that hydroxychloroquine is not beneficial for adults hospitalized with COVID-19.
Strengths of this trial included its blinded, placebo-controlled design, high adherence to the study protocol, rigorous monitoring for safety events and adverse events, and rapid recruitment from geographically diverse hospitals serving ethnically and racially diverse populations within the US. Additionally, the primary outcome was a patient-centered, clinically meaningful ordinal scale that captured mortality and morbidity related to COVID-19.
Limitations
This trial had several limitations. First, the trial only included hospitalized adults, and findings may not be generalizable to other populations.
Second, patients with respiratory symptoms for up to 10 days prior to randomization were included. Some trials of antiviral medications limit enrollment to patients with symptoms for a shorter duration in an effort to enrich the population for patients most likely to benefit; however, notably, no evidence to suggest efficacy of hydroxychloroquine among patients with shorter duration of symptoms was found in this trial.
Third, outcome ascertainment was limited to 28 days after randomization to accelerate dissemination of findings in the context of an ongoing pandemic; reporting long-term outcomes of trial participants is planned for the future.
Fourth, the minimal clinically important difference in scores on the COVID Outcomes Scale is unknown. While the 95% CI for the aOR for the primary outcome in this trial (0.73-1.42) did not include point estimates that have been considered clinically meaningful in prior trials of COVID-19 therapies,35,36 moderate sample size in this trial may mean that it had inadequate power to exclude small, yet clinically meaningful, differences between groups.
Fifth, the trial did not include collection of information on serum hydroxychloroquine concentrations, viral shedding, or biomarkers of inflammation.
Sixth, only 1 dosing regimen of hydroxychloroquine was studied in the trial; this regimen was selected based on guidance from the FDA, in vitro studies of hydroxychloroquine lung concentrations,7 and doses commonly used in US hospitals for COVID-19. Other trials that evaluated higher doses of hydroxychloroquine also demonstrated no clinical benefit.33,34
Seventh, this trial evaluated hydroxychloroquine as monotherapy for COVID-19 and did not systematically study co-administration with azithromycin,9 zinc,37 remdesivir,35,36 or other agents.
Conclusions
Among adults hospitalized with respiratory illness from COVID-19, treatment with hydroxychloroquine, compared with placebo, did not significantly improve clinical status at day 14. These findings do not support the use of hydroxychloroquine for treatment of COVID-19 among hospitalized adults.
Trial Protocol
Statistical Analysis Plan
eAppendix. Writing Committee Members and Collaborators
eTable 1. Enrolling Hospital Characteristics
eTable 2. ORCHID Trial Eligibility Criteria
eTable 3. Protocol Guidance to Prevent Drug-Drug Interactions With the Trial Drug (Hydroxychloroquine)
eTable 4. Recommendations for Stopping the Trial
eTable 5. Interim Analyses
eTable 6. Chronic Comorbidities
eTable 7. Medications Prior to Hospital Presentation
eTable 8. Physiologic Measurements in the 12 Hours Prior to Randomization
eTable 9. Sequential Organ Failure Assessment (SOFA) Prior to Randomization
eTable 10. Laboratory Values in the 24 Hours Prior to Randomization
eTable 11. Medications Received Between Hospital Presentation and Randomization
eTable 12. Receipt of Trial Drug
eTable 13. Reasons Trial Drug Not Administered
eTable 14. Receipt of Concomitant Medications After Randomization During Hospitalization
eTable 15. Receipt of Respiratory Support After Randomization During Hospitalization
eTable 16. Sensitivity Analyses for the Primary Outcome
eTable 17. Post Hoc Subgroup Analyses of the Primary Outcome
eTable 18. COVID Outcomes Scale Over Time by Study Group
eTable 19. Additional Clinical Outcomes by Study Group
eTable 20. Symptoms of Respiratory Infection by Study Group
eTable 21. Laboratory Values Through Day 5 by Study Group
eTable 22. Pathogen Testing Data
eTable 23. Systematically Collected Safety Events
eTable 24. Adverse Events by Organ System
eFigure. Subgroup Analyses of the Primary Outcome
Data Sharing Statement
References
- 1.Johns Hopkins University Coronavirus resource center. Accessed July 28, 2020. https://coronavirus.jhu.edu/map.html
- 2.World Health Organization WHO coronavirus disease (COVID-19) dashboard. Accessed September 22, 2020. https://covid19.who.int/
- 3.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020;55(6):105982. doi: 10.1016/j.ijantimicag.2020.105982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155-166. doi: 10.1038/s41584-020-0372-x [DOI] [PubMed] [Google Scholar]
- 5.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824-1836. doi: 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi: 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71(15):732-739. doi: 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Azoulay E, de Waele J, Ferrer R, et al. International variation in the management of severe COVID-19 patients. Crit Care. 2020;24(1):486. doi: 10.1186/s13054-020-03194-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massachusetts General Hospital Massachusetts General Hospital COVID-19 treatment guidance. Accessed May 8, 2020. https://web.archive.org/web/20200410013441/https://www.massgeneral.org/assets/MGH/pdf/news/coronavirus/mass-general-COVID-19-treatment-guidance.pdf
- 12.Vanderbilt University Medical Center Clinical recommendations for treatment of COVID-19 adult patients. Accessed March 13, 2020. https://www.vumc.org/coronavirus/sites/default/files/COVID%20Documents/VUMC%20interim%20recommendations%20for%20clinical%20care%20of%20COVID%20patients%203.14.2020%20final.pdf
- 13.National Institutes of Health (NIH) Coronavirus disease 2019 (COVID-19) treatment guidelines. Accessed June 30, 2020. https://www.covid19treatmentguidelines.nih.gov [PubMed]
- 14.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. Published online April 27, 2020. doi: 10.1093/cid/ciaa478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin EJ, Harrington DP, Hogan JW, Gatsonis C, Baden LR, Hamel MB. The urgency of care during the COVID-19 pandemic: learning as we go. N Engl J Med. 2020;382(25):2461-2462. doi: 10.1056/NEJMe2015903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey JD, Johnson NJ, Semler MW, et al. Rationale and design of ORCHID: a randomized placebo-controlled clinical trial of hydroxychloroquine for adults hospitalized with COVID-19. Ann Am Thorac Soc. 2020;17(9):1144-1153. doi: 10.1513/AnnalsATS.202005-478SD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for drug interactions on QTc in exploratory COVID-19 treatment. Circulation. 2020;141(24):e906-e907. doi: 10.1161/CIRCULATIONAHA.120.047521 [DOI] [PubMed] [Google Scholar]
- 18.Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(9):1036-1041. doi: 10.1001/jamacardio.2020.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization WHO R&D blueprint: novel coronavirus: COVID-19 therapeutic trial synopsis. Accessed June 28, 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf
- 20.EuroQol Group EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 21.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200(7):828-836. doi: 10.1164/rccm.201810-2050CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine: reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189-2194. doi: 10.1056/NEJMsr077003 [DOI] [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 26.RECOVERY Investigators No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19: statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on hydroxychloroquine, 5 June 2020. Accessed June 28, 2020. https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19
- 27.Food and Drug Administration (FDA) Fact sheet for healthcare providers: emergency use authorization (EUA) of Veklury (remdesivir) for hospitalized pediatric patients weighing 3.5 kg to less than 40 kg or hospitalized pediatric patients less than 12 years of age weighing at least 3.5 kg. Accessed October 8, 2020. https://www.fda.gov/media/137566/download
- 28.Medicines and Healthcare Products Regulatory Agency. MHRA suspends recruitment to COVID-19 hydroxychloroquine trials. Accessed October 10, 2020. https://www.gov.uk/government/news/mhra-suspends-recruitment-to-covid-19-hydroxychloroquine-trials
- 29.Food and Drug Administration (FDA) Request for emergency use authorization for use of chloroquine phosphate or hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of 2019 coronavirus disease. Accessed July 2, 2020. https://www.fda.gov/media/136534/download
- 30.Food and Drug Administration (FDA) Letter revoking EUA for chloroquine phosphate and hydroxychloroquine sulfate. Accessed July 2, 2020. https://www.fda.gov/media/138945/download
- 31.Maisonnasse P, Guedj J, Contreras V, et al. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585(7826):584-587. doi: 10.1038/s41586-020-2558-4 [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann M, Mösbauer K, Hofmann-Winkler H, et al. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020;585(7826):588-590. doi: 10.1038/s41586-020-2575-3 [DOI] [PubMed] [Google Scholar]
- 33.Horby P, Mafham M, Linsell L, et al. ; RECOVERY Collaborative Group . Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020. doi: 10.1056/NEJMoa2022926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavalcanti AB, Zampieri FG, Rosa RG, et al. ; Coalition Covid-19 Brazil I Investigators . Hydroxychloroquine with or without azithromycin in mild-to-moderate COVID-19. N Engl J Med. 2020. doi: 10.1056/NEJMoa2019014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spinner CD, Gottlieb RL, Criner GJ, et al. ; GS-US-540-5774 Investigators . Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048-1057. doi: 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of COVID-19: preliminary report: reply. N Engl J Med. 2020;383(10):994. doi: 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 37.Derwand R, Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today’s battle against COVID-19? Med Hypotheses. 2020;142:109815. doi: 10.1016/j.mehy.2020.109815 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix. Writing Committee Members and Collaborators
eTable 1. Enrolling Hospital Characteristics
eTable 2. ORCHID Trial Eligibility Criteria
eTable 3. Protocol Guidance to Prevent Drug-Drug Interactions With the Trial Drug (Hydroxychloroquine)
eTable 4. Recommendations for Stopping the Trial
eTable 5. Interim Analyses
eTable 6. Chronic Comorbidities
eTable 7. Medications Prior to Hospital Presentation
eTable 8. Physiologic Measurements in the 12 Hours Prior to Randomization
eTable 9. Sequential Organ Failure Assessment (SOFA) Prior to Randomization
eTable 10. Laboratory Values in the 24 Hours Prior to Randomization
eTable 11. Medications Received Between Hospital Presentation and Randomization
eTable 12. Receipt of Trial Drug
eTable 13. Reasons Trial Drug Not Administered
eTable 14. Receipt of Concomitant Medications After Randomization During Hospitalization
eTable 15. Receipt of Respiratory Support After Randomization During Hospitalization
eTable 16. Sensitivity Analyses for the Primary Outcome
eTable 17. Post Hoc Subgroup Analyses of the Primary Outcome
eTable 18. COVID Outcomes Scale Over Time by Study Group
eTable 19. Additional Clinical Outcomes by Study Group
eTable 20. Symptoms of Respiratory Infection by Study Group
eTable 21. Laboratory Values Through Day 5 by Study Group
eTable 22. Pathogen Testing Data
eTable 23. Systematically Collected Safety Events
eTable 24. Adverse Events by Organ System
eFigure. Subgroup Analyses of the Primary Outcome
Data Sharing Statement