Abstract
Background: Muscle-invasive bladder cancer (MIBC) is a lethal disease with poor treatment response and a high death rate. Immune cells infiltrating the tumor tissues have been shown to play a vital role in tumorigenesis and tumor progression, but their prognostic significance in MIBC remains unclear. Objectives: To explore the landscape and prognostic significance of tumor-infiltrating immune cells (TIICs) in MIBC, and to develop a model to improve the prognostic predictions of MIBC. Methods and Materials: The gene expression profile and clinical data of MIBC patients were downloaded from the Gene Expression Omnibus and The Cancer Genome Atlas portal. The fractions of 22 TIIC subtypes were calculated using the Cell Type Identification by Estimating Relative Subsets of RNA Transcripts (CIBERSORT) algorithm. A TIICs-based model was constructed using least absolute shrinkage and selection operator (LASSO) Cox regression in a training cohort and validated in the validation cohort. Results: Ten types of TIICs demonstrated different infiltration abundance between MIBC and normal tissue. We also found 11 types of TIICs that were significantly associated with overall survival (OS). A TIICs-based model was established, consisting of 15 types of immune cells, and an immunoscore was calculated. Significant differences in OS were found between the high and low immunoscore groups, in both training (n = 343) and validation (n = 146) cohorts. The model could identify patients who would have worse OS despite having similar clinical characteristics. Furthermore, multivariate analysis identified the immunoscore as an independent risk factor (hazard ratio, 3.23; 95% confidence interval; 2.22-4.70) for OS in MIBC patients. Conclusion: The landscape of immune infiltration is different between MIBC and normal tissue. The TIICs-based model could provide promising predictive value to complement the existing staging system for predicting the OS of MIBC patients.
Keywords: Muscle-invasive bladder cancer, immune cell, tumor microenvironment, immunoscore, CIBERSORT
Introduction
Bladder cancer is the most common urinary tract cancer, with an estimated 549,000 new cases and 200,000 deaths in 2018, globally [1,2]. About 25% of patients who have bladder cancer are diagnosed with muscle-invasive bladder cancer (MIBC) [3], for which radical cystectomy combined with pelvic lymphadenectomy is the standard treatment [4]. Despite improvements in surgical techniques and therapeutic regimens, the clinical outcome of MIBC patients remains dismal, with a 5-year survival rate of 40%-60% [4-8]. Traditionally, the disease progression of MIBC is mainly estimated using the Union for International Cancer Control tumor-node-metastasis classification system [9]. However, in clinical practice, the anatomy-based system provides useful but incomplete prognostic information. Therefore, novel biomarkers that could improve the prognostication of MIBC and facilitate patient management are needed.
Tumor-infiltrating immune cells (TIICs) are a component of the tumor microenvironment and act as a trigger for determining the sensitivity of cancer cells to immune system attack [10]. Their distribution has been shown to influence the prognosis of cancer patients [11]. However, their prognostic significance seems to vary in different types of tumors or in patients with the same tumor type but different clinical characteristics [12,13]. Several studies have demonstrated the clinical importance of infiltrating immune cells in bladder cancer. It has been reported that high M2 macrophage infiltration is associated with poor survival in bladder cancer patients [14]. A low density of CD3+ and CD8+ T cells at the tumor center and the invasive margin was correlated strongly with a poor overall survival (OS) in MIBC patients who underwent radical cystectomy [15]. Miyake et al. from Japan reported that a high level of regulatory T cells (Tregs) or tumor-associated macrophages was associated with a poor progression-free survival in 71 non muscle-invasive bladder cancer (NMIBC) patients [16]. However, these studies have been limited by the small number of investigated cases or focus on only a few types of immune cells. Thus, a comprehensive analysis of TIICs in MIBC with larger samples is needed.
The Cell Type Identification by Estimating Relative Subsets of RNA Transcripts (CIBERSORT) algorithm is a novel bioinformatic tool that can accurately evaluate immune cell subsets using RNA specimens from numerous solid tumors. It has surpassed other methods by eliminating noise, unknown mixed components, and closely related cell types [17]. It has been successfully used to assess the composition of TIICs and evaluate the prognosis significance in breast, lung, and gastric cancer [18-20].
In this present study, CIBERSORT was implemented to assess the fractions of 22 types of TIICs in MIBC tissues, and a comprehensive landscape of TIICs in MIBC was constructed. Least absolute shrinkage selection operator (LASSO) [21] Cox regression analysis was performed to establish a TIICs-based model to predict the prognosis of patients with MIBC. An immunoscore calculated by the model could identify patients with worse OS expectancy despite having similar clinical characteristics. Therefore, this immunoscore could be a significant complement to the existing staging system for predicting the OS of MIBC patients.
Methods and materials
Gene expression and corresponding clinical data in the GEO database
To obtain the gene expression data with relevant clinical information on MIBC, systematic searches were conducted in the Gene Expression Omnibus (GEO) datasets (https://www.ncbi.nlm.nih.gov/geo/). The series GSE13507, GSE31684, GSE32894, GSE48075, GSE48276, GSE70691, and GSE69795 were selected, normalized matrix files of these series were downloaded directly, and the relevant clinical data were manually organized.
Gene expression and corresponding clinical data in the TCGA database
Bladder cancer gene expression and corresponding clinical information data were downloaded, with no exclusion criteria applied, from The Cancer Genome Atlas (TCGA, https://cancergenome.nih.gov/) bladder cancer cohort. The dataset included 411 bladder cancer samples and 19 normal samples.
Evaluation of TIIC fractions
To evaluate the relative fractions of TIIC types in each bladder cancer sample, the CIBERSORT method was utilized, with the algorithm run using the LM22 signature matrix at 100 permutations. The types of TIICs were distinguished using the LM22 signature matrix, which includes 547 genes and possesses highly sensitive and specific discernment of 22 types of TIICs, i.e., macrophages M1, macrophages M2, macrophages M0, follicular helper T cells, resting memory CD4+ T cells, activated memory CD4+ T cells, gamma delta T cells, CD8+ T cells, Tregs, naive CD4+ T cells, resting natural killer (NK) cells, activated NK cells, resting mast cells, activated mast cells, memory B cells, resting dendritic cells, activated dendritic cells, naive B cells, monocytes, plasma cells, neutrophils, and eosinophils [17]. For each sample, the sum of the infiltrating fractions of the 22 subtypes of immune cells equaled to 1. Assessment of the fractions of TIICs was performed in all samples downloaded, which included 1366 bladder cancer tissues and 78 normal tissues. The statistical significance of the deconvolution results of each sample was reflected by the CIBERSORT P-value, which provides a measure of confidence in the obtained results. At a threshold of P < 0.05, the results calculated by CIBERSORT were considered accurate and eligible for further analysis.
Statistical analysis
The two-tailed Student’s t test was performed to assess the statistical relevance between groups. The Kaplan-Meier method and log-rank test were performed to analyze the association between variables and OS. Hazard ratios (HRs) for univariate analyses were calculated using a univariate Cox proportional hazards regression model. For the univariate analysis and the construction of the prediction model, immune cell fractions were all divided into high and low levels according to the optimal cut points, which were evaluated based on the association between OS and TIIC fractions in all MIBC samples and based on the principle of maximum standard log-rank statistics using the survminer package (https://CRAN.R-project.org/package=survminer). The patients were randomly separated into training and validation cohorts in a ratio of 7:3. The LASSO Cox regression method was employed to build the TIICs-based model in the training cohort, and then an immunoscore was calculated on the basis of the model. To assess the accuracy of the prediction model, time-dependent receiver operating characteristic (ROC) curves for OS by immunoscore were generated, and then the areas under the curves (AUCs), optimal cut-off value (selected by the Youden index), sensitivity (SE), specificity (SP), positive predictive value (PPV), and negative predictive value (NPV) at the predicting times of 3 years and 5 years were calculated in the training cohort, validation cohort, and entire cohort. Moreover, survival curves were generated in these three cohorts; in this part, immunoscore was considered as a binary variable, and patients were divided into low and high immunoscore group, with a cut-off immunoscore value. The cut-off value was calculated on the basis of (i) the association between OS and immunoscore in the training cohort, using the survminer package in R software, and (ii) the principle of maximum standard log-rank statistics. To verify the repeatability of our model, we treated the cut-off value as a fixed value and did not change it in the subsequent analysis. Furthermore, multivariable Cox regression analysis with the enter method was used to determine independent prognostic factors. R software, version 3.6.1, was used for all statistical analyses. P-values < 0.05 were considered statistically significant.
Results
Characteristics of included samples
Patients with NMIBC, lacking survival information, or receiving neoadjuvant chemotherapy were excluded, as well as patients with a CIBERSORT P-value > 0.05. By applying these exclusion criteria, 489 MIBC and 59 normal samples were selected for further analysis. The median follow-up time of MIBC patients was 22.3 months (range from 1 to 250 months). Their baseline characteristics are shown in Table 1. Of note, all MIBC tissues were obtained from radical cystectomy, and a total of 143 patients received platinum-based adjuvant chemotherapy.
Table 1.
Baseline characteristics of patients
| Characteristics | No. of patients (n = 489) |
|---|---|
| Age (years) | |
| ≤ 65 | 166 |
| > 65 | 268 |
| Unknown | 55 |
| Primary tumor stage | |
| T2 | 166 |
| T3 | 210 |
| T4 | 62 |
| Unknown | 51 |
| Regional lymph node metastasis | |
| N0 | 260 |
| N+ | 145 |
| Unknown | 84 |
| Chemotherapy | |
| Yes | 143 |
| No | 210 |
| Unknown | 136 |
Profile of immune infiltration in normal and MIBC samples
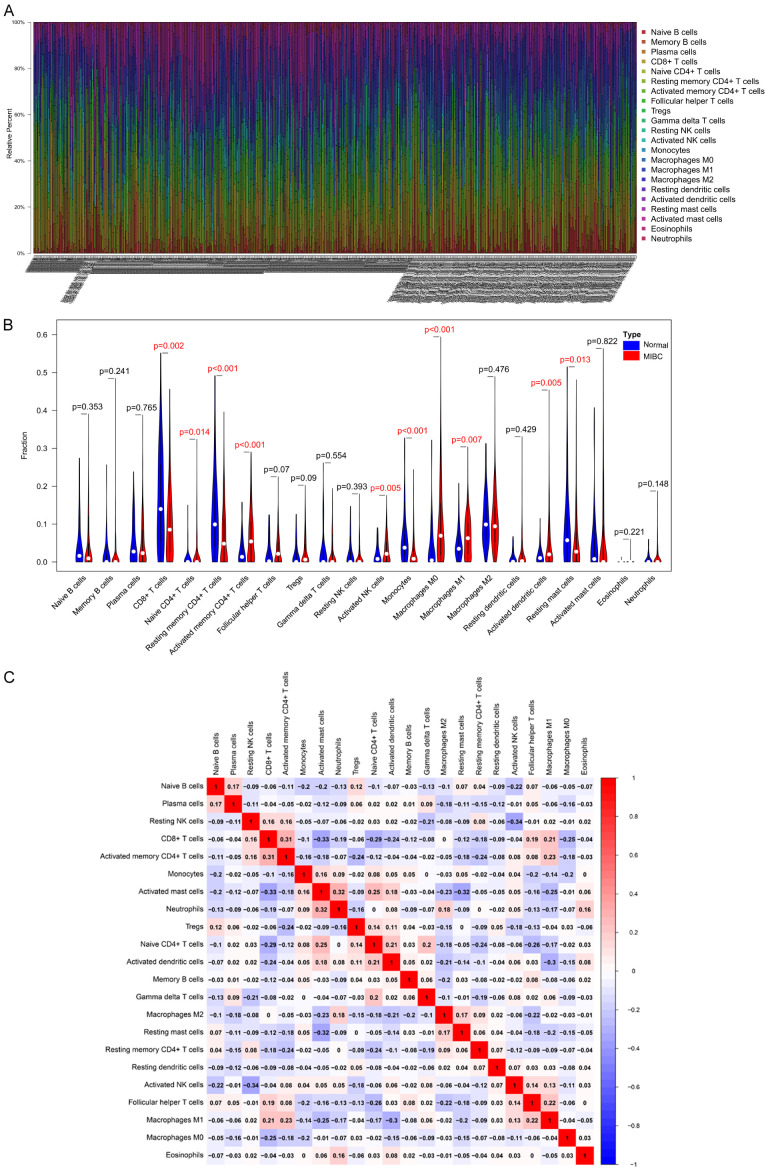
The profiles of immune infiltration of 59 normal samples and 489 MIBC samples are illustrated as bar plot in Figure 1A. The proportions of immune cells were variable in each sample. The differences of each type of TIIC between the normal and MIBC samples were further analyzed (Figure 1B). We identified 10 types of immune cells that were significantly different between normal and MIBC samples; the proportions of infiltrating activated memory CD4+ T cells, naive CD4+ T cells, activated NK cells, macrophages M0, macrophages M1, and activated dendritic cells were significantly higher in MIBC samples than in normal samples, whereas the infiltrating fractions of CD8+ T cells, resting memory CD4+ T cells, monocytes, and resting mast cells were lower in MIBC tissues compared to those in normal tissues. These results demonstrated a different landscape of TIICs between MIBC and normal samples.
Figure 1.
Landscape of immune infiltration in MIBC and normal samples. A. Stacked bar chart illustrating deviations in immune infiltration in each sample, 59 normal samples are shown on the left, and 489 MIBC samples are shown on the right. B. Difference in proportion of each TIIC type in normal and MIBC tissues. P < 0.05 represents statistical significance. C. Correlation matrix of TIIC proportions. The red color represents a positive correlation, and the blue color represents a negative correlation. MIBC, muscle-invasive bladder cancer. TIICs, tumor-infiltrating immune cells.
Additionally, correlation analysis suggested that CD8+ T cells had the strongest positive correlation with activated memory CD4+ T cells, whereas resting NK cells had the strongest negative correlation with activated NK cells. The proportions of other immune cell subtypes presented weak correlations (Figure 1C).
Prognostic value of TIICs in MIBC patients
The associations between the prognosis of MIBC patients and each subtype of TIICs were investigated. The optimal cut-off points for the fraction of each immune cell subtype were calculated (Table S1), and Kaplan-Meier survival curves of the 22 subtypes of TIICs were generated (Figure S1). Higher proportions of infiltrating Tregs, resting memory CD4+ T cells, neutrophils, monocytes, and resting mast cells were found to be associated with shorter OS. Furthermore, patients with higher infiltrating levels of naive B cells, gamma delta T cells, follicular helper T cells, CD8+ T cells, activated memory CD4+ T cells, and plasma cells had longer OS (the HRs of 22 subtypes of TIICs for OS are shown with 95% confidence intervals [CIs] in Figure S2). Additionally, multivariate Cox regression analysis indicated that monocytes, macrophages M2, resting mast cells, and neutrophils were independent risk factors for shorter OS, whereas naive B cells and resting dendritic cells were independent predictors for favorable OS in MIBC patients (Figure S3).
Construction and validation of the TIICs-based model
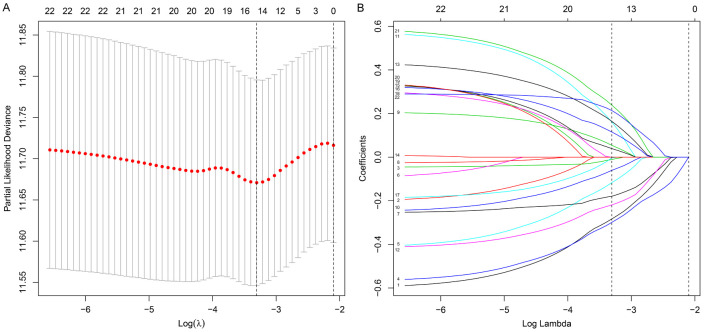
The TIICs-based model was constructed using LASSO Cox regression analysis in the training cohort (Figure 2A, 2B), and for each patient, a TIICs-related immunoscore was calculated as follows: Immunoscore = (0.0542 × fraction of Tregs) - (0.2832 × fraction of naive B cells) - (0.0106 × fraction of plasma cells) - (0.2989 × fraction of CD8+ T cells) - (0.1168 × fraction of naive CD4+ T cells) - (0.1792 × fraction of activated memory CD4+ T cells) - (0.0605 × fraction of gamma delta T cells) + (0.1610 × fraction of resting NK cells) - (0.2190 × fraction of activated NK cells) + (0.1642 × fraction of monocytes) + (0.1152 × fraction of macrophages M2) - (0.0031 × fraction of resting dendritic cells) + (0.0392 × fraction of resting mast cells) + (0.2386 × fraction of eosinophils) + (0.2138 × fraction of neutrophils). In this formula, the TIIC fraction was valued as 1 or 0. A value of 0 was assigned if the fraction of the type of TIIC was less than the corresponding cut-off point; otherwise, a value of 1 was assigned (the cut-off points of each TIIC subtype are shown in Table S1).
Figure 2.
Fifteen TIIC subtypes selected by LASSO Cox regression analysis. A. Tenfold cross-validation for tuning parameter selection in the LASSO model. The partial likelihood deviance is plotted against log(λ), where λ is the tuning parameter. Partial likelihood deviance values are shown, with error bars representing standard error. The two dotted vertical lines are drawn at the optimal values by minimum criteria (left) and 1 standard error criteria (right). B. LASSO coefficient profiles of the 22 TIIC subtypes. The left vertical line is drawn at the optimal value by minimum criteria and results in 15 non-zero coefficients. TIICs, tumor-infiltrating immune cells.
To evaluate the prognostic accuracy of the model, time-dependent ROC curves for the immunscore and OS were generated. The AUCs were 0.710, 0.684, and 0.703 at 3 years and 0.714, 0.718, and 0.717 at 5 years in the training cohort, validation cohort, and entire cohort, respectively. The optimal cut-off values, SE, SP, PPV, and NPV at predicting OS for 3 years and 5 years are shown in Figure 3A-C. Patients in all three cohorts were then divided into a high and low immunoscore group, based on a cut-off value of 0.9189, as calculated by the survminer package in the training cohort. Compared with patients in the low immunoscore group, patients in the high immunoscore group had significantly shorter OS, with an HR of 2.69 (95% CI, 2.00-3.63), 2.56 (95% CI, 1.58-4.14), and 2.65 (95% CI, 2.06-3.42) in the training cohort, validation cohort, and entire cohort, respectively (Figure 3D-F).
Figure 3.
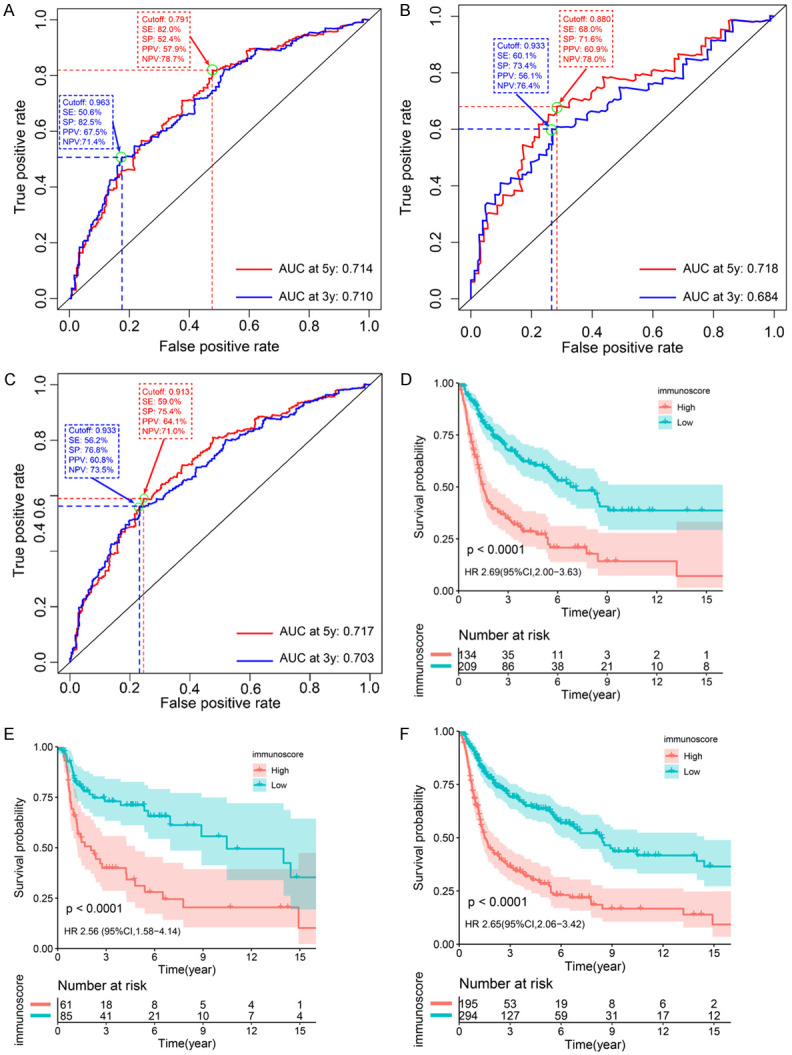
Validation of the TIICs-based model. (A-C) Time-dependent ROC curves and AUCs. The AUCs for immunoscore and OS are 0.710, 0.684, and 0.703 at 3 years and 0.714, 0.718, and 0.717 at 5 years in the training cohort, validation cohort, and entire cohort, respectively. (D-F) Kaplan-Meier curves for OS by the immunoscore group, in the training cohort (D), validation cohort (E), and entire cohort (F). Patients in all three cohorts were divided into high and low immunoscore groups on the basis of a cut-off value of 0.9189. A high immunoscore is associated with a shorter OS in the three cohorts. P < 0.05 represents statistical significance. ROC, receiver operating characteristic. AUC, area under the ROC curve. MIBC, muscle-invasive bladder cancer. HR, hazard ratio. CI, confidence interval. OS, overall survival. SE, sensitivity. SP, specificity. PPV, positive predictive value. NPV, negative predictive value.
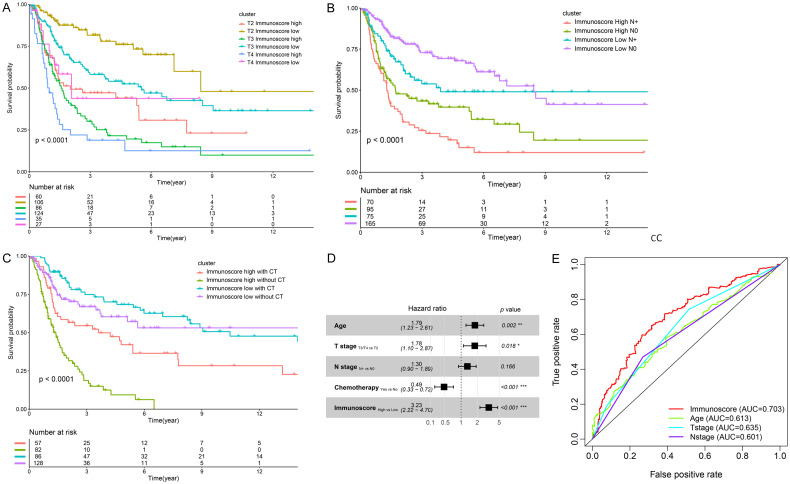
To ascertain whether the clinical factors (primary tumor stage, regional lymph node metastasis, and chemotherapy) could influence the predictive accuracy of our model, stratified analysis was performed. Again, patients were divided into a high immunoscore and low immunoscore group, with an immunoscore of 0.9189 as the cut-off value. Compared with patients in the low immunescore group, patients in the high immunoscore group had a worse OS, which revealed that the TIICs-based model was still a clinically and statistically significant prognostic tool for OS prediction even when stratified by primary tumor stage (Figure 4A), regional lymph node metastasis (Figure 4B), or chemotherapy (Figure 4C). Indeed, the OS of the patients with T2 disease and a high immunoscore was significantly shorter than the OS of patients with T3 disease and a low immunoscore (HR, 1.59; 95% CI, 1.03-2.43). Moreover, patients without regional lymph node metastasis in the high immunoscore group had shorter OS than those with regional lymph node metastasis in the low immunoscore group (HR, 1.65; 95% CI, 1.06-2.56). Of note, although chemotherapy was identified as beneficial for patients with high immunoscore (HR, 0.46; 95% CI, 0.28-0.74), patients who had a high immunoscore and received chemotherapy had a worse OS than those with a low immunoscore and did not undergo chemotherapy (HR, 1.63; 95% CI, 1.02-2.60). Meanwhile, in the low immunoscore group, no significant differences in OS were found irrespective of chemotherapy use (P > 0.05). Similar results were found in patients with T3/T4 disease or regional lymph node metastasis (Figures S4 and S5). In addition, for the patients who had complete clinical information (with respect to age, primary tumor stage, regional lymph node status, and chemotherapy), multivariate Cox regression analysis was performed. After adjusting for clinical variables, the immunoscore remained an independent prognostic factor for predicting OS (HR, 3.23; 95% CI, 2.22-4.70), and age > 65 and a high tumor stage (T3/T4) were independent risk factors (Figure 4D). Moreover, the prognostic value of the immunoscore was compared with established prognostic factors using time-dependent ROC analysis. The AUC was 0.703 at 5 years, which is superior to that of tumor stage (0.635), N stage (0.601), and age (0.613) in predicting the OS of MIBC patients (Figure 4E). These results revealed a promising prediction efficiency of the TIICs-based model.
Figure 4.
The association between immunoscore and clinical factors. A high immunoscore was associated with a worse OS when stratified by primary tumor stage (A), regional lymph node metastasis (B), and chemotherapy (C). (A) The overall survival of the patients with T2 disease and a high immunoscore was significantly shorter than those with T3 disease and a low immunoscore (HR, 1.59; 95% CI, 1.03-2.43). (B) Patients without regional lymph node metastasis in the high immunoscore group had shorter overall survival than those with regional lymph node metastasis in the low immunoscore group (HR, 1.65; 95% CI, 1.06-2.56). (C) Patients in the high immunoscore group could benefit from chemotherapy (HR, 0.46; 95% CI, 0.28-0.74), however, in the low immunoscore group, no significant difference in overall survival was found irrespective of chemotherapy use (P > 0.05). Patients who received chemotherapy with a high immunoscore had a worse OS than the patients with a low immunoscore without chemotherapy (HR, 1.63; 95% CI, 1.02-2.60). (D) Multivariate Cox regression analysis of the immunoscore with overall survival in 276 patients. The immunoscore is an independent risk factor for OS (HR, 3.23; 95% CI, 2.22-4.70). (E) Time-dependent ROC curves and AUCs at 5 years of the immunoscore, compared with age, T stage, and N stage. P < 0.05 represents statistical significance. CT, chemotherapy. OS, overall survival. TIICs, tumor-infiltrating immune cells. HR, hazard ratio. CI, confidence interval.
Discussion
A hypothesis was advanced by Paul Ehrlich that the immune system can detect and kill tumor cells before clinical signs appear [22,23]. TIICs are the constituents of the immune system infiltrating in tumor tissue, where they exert important anti-tumor or pro-tumor effects [24]. Intravesical BCG immunotherapy is one of the earliest immunotherapies and is still recommended for high-risk NMIBC [25], and a growing body of evidence highlights the key role of TIICs in bladder cancer [26,27]. However, the functions and prognostic significance of TIICs in MIBC patients remain ambiguous and deserve further analysis [26].
In this study, an overall landscape of immune cell infiltration in MIBC samples was constructed, and significant differences in TIIC composition between MIBC and normal tissues were observed. These findings support that the patterns of immune infiltration in tumor and normal tissues are different [18,22,28,29]. Additionally, the correlation between each TIIC subtype and the OS of patients with MIBC was investigated, and we found that patients with higher levels of infiltrating Tregs, resting memory CD4+ T cells, neutrophils, monocytes, and resting mast cells had poor prognoses, whereas patients with higher levels of infiltrating naive B cells, gamma delta T cells, follicular helper T cells, CD8+ T cells, activated memory CD4+ T cells, and plasma cells had longer OS.
In previous studies, the functions and prognostic significance of several subtypes of TIICs in multiple types of cancers have been reported, but some of the results have been inconsistent. For instance, infiltrating CD8+ T cells are commonly regarded as tumor inhibitors that are associated with a favorable prognosis in most types of cancers [30-33]; however, in renal cell carcinoma [34] and prostate cancer [35], CD8+ T cells were reported to be associated with poor clinical outcomes. In addition, Hald et al. [36] reported CD8+ T cells as a predictor of good prognosis in non-small cell lung cancer, which was contradicted by the findings of Tian et al. [37], who found CD8+ T cells serve as a predictor of poor prognosis. Similar contradictory findings have also been found in macrophages, NK cells, and dendritic cells [11,13]. Considering the specialized functions and prognostic significance of TIICs, additional samples with rigorously designed research works are needed to confirm the prognostic significance of these cells. In this study, 489 patients from the TCGA and GEO databases were included, and the CIBERSORT algorithm was utilized, which is a significant supplement to the present situation.
We then developed a TIICs-based model to predict the OS of patients with MIBC. The calculated immunoscore was validated to be an independent prognostic factor with a significant accuracy for MIBC. Several prognostic tools based on immune contexture have been reported and provided a statistically significant predictive value for prognosis in patients with multiple types of solid tumors [38]. However, in most of these research works, especially in bladder cancer [39,40], immune contexture was evaluated mainly by immunohistochemistry, and only a limited number of biomarkers could be analyzed due to insufficient biopsy specimens. Therefore, most of these studies included smaller sample sizes and/or mainly focused on only a few cell types or biomarkers. Additionally, the measurement of the intensity of staining may be (i) subjective to a certain level of bias or (ii) non-reproducible as these mainly depend on the pathologists’ experience. In this study, the fractions of TIICs were calculated using the CIBERSORT algorithm, which has been verified by fluorescence-activated cell sorting [17], and the results were filtered by the CIBERSORT P-value to ensure a high credibility.
Tumor stage and regional lymph node metastasis were previously identified as crucial independent variables affecting survival outcomes in bladder cancer [8]. However, different clinical outcomes among bladder cancer patients with the same primary tumor stage or regional lymph node metastasis status [41,42] have shown that the use of existing prognostic systems may be insufficient. In our study, the difference in OS between patients with the same primary tumor stage or regional lymph node metastasis status could be distinguished using the proposed immunoscore. Notably, the European Association of Urology Guidelines on muscle-invasive and metastatic bladder cancer defined pathological T3/T4 and regional lymph node metastasis as high-risk factors, and adjuvant chemotherapy is recommended for these patients [4,43]. However, according to our results, T2 stage tumor patients with high immunoscore had a poorer clinical outcome compared with T3 stage patients with low immunoscore, and patients in the high immunoscore group without regional lymph node metastasis had shorter OS than those with regional lymph node metastasis in the low immunoscore group. These results suggest that patients with T2 disease or patients without regional lymph node metastasis, who were traditionally considered as low risk, may need additional and individualized treatments. These results therefore suggest that the immunoscore could be used as a complement to existing staging systems for scheduling patient follow-up.
Chemotherapy is the first-line regimen for advanced or metastatic bladder cancer [43,44]. According to our study, patients with a high immunoscore could benefit from chemotherapy. Particularly, in the cluster of high immunoscore without regional lymph node metastasis, we found that patients who received chemotherapy had a favorable OS compared with patients without chemotherapy. Considering that patients with high immunoscore had a worse OS, chemotherapy could be important for such MIBC patients. Meanwhile, in the low immunoscore group, as no significant difference in OS was found for patients with T3/T4 disease or regional lymph node metastasis, irrespective of chemotherapy usage, this suggests that for those with low immunoscore, chemotherapy could have limited advantages. Therefore, for patients in the low immunoscore group with T3/T4 disease or regional lymph node metastasis, systematic chemotherapy seems not to be the optimal treatment regimen. Considering the limited number of cases in our study, further studies are required to validate these findings. Furthermore, the patients who received chemotherapy with a high immunoscore had a worse prognosis than those with a low immunoscore without chemotherapy, revealing that a high immunoscore is a strong risk factor, and additional treatment strategies are in need.
There were several limitations to this study. First, the patients’ information was downloaded from public databases, and clinical information for each patient was incomplete. Second, it is desirable to assess the immune status in different regions of a sample, for instance in the core of the tumor and the invasive margin; however, the CIBERSORT algorithm is based on the gene expression profiles, and information about the exact location of the infiltrating cells is not contained. Finally, although a total of 489 MIBC patients were included in our study for the retrospective observations regarding the associations between the immunoscore and chemotherapy, further prospective studies are required to validate these findings.
In conclusion, our study revealed the comprehensive landscape of TIICs in MIBC tissue and investigated the prognostic significance of 22 subtypes of TIICs in patients with MIBC. This study improves our understanding of the effects of TIICs in bladder cancer and will help in the design of novel diagnostic and prognostic tools as well as treatments. More importantly, the TIICs-based model was validated to be a practical and reliable prognostic tool for MIBC; it could distinguish the difference in survival between patients with the same primary tumor stage or regional lymph node metastasis status, indicating that the model is a promising complement for the existing staging system of MIBC. Finally, the model has the potential to guide physicians to make more informed treatment decisions for patients after radical cystectomy.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos. 81872091, 81972382, 81672530, 81772716, and 81802553) and the National Natural Science Foundation of Guangdong Province (nos. 2016A030310213 and 2018A030310235) and the Key Cultivation Project for Young Teachers of Sun Yat sen University (no. 19ykzd46). Funding was used only for data collection and analysis. None of the authors received payments or services, either directly or indirectly, that could be perceived to influence or have the potential to influence what is written in this work. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure of conflict of interest
None.
Abbreviations
- BCG
Bacillus Calmette-Guerin
- CIBERSORT
Cell Type Identification by Estimating Relative Subsets of RNA Transcripts
- HR
hazard ratio
- LASSO
least absolute shrinkage selection operator
- MIBC
muscle-invasive bladder cancer
- NMIBC
non-muscle-invasive bladder cancer
- OS
overall survival
- TIIC
tumor-infiltrating immune cell
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;39:22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence-SEER 18 Regs Research Data 1 Hurricane Katrina Impacted Louisiana Cases, Nov 2014 Sub (1973-2012 varying)-Linked To County Attributes Total US, 1969-2013 Counties. Bethesda, MD: National Cancer Institute, Department of Cancer Control and Population Sciences, Surveillance Research Program, Surveillance Systems Branch; 2015. [Google Scholar]
- 4.Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG, Sherif A European Association of Urology. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65:778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 5.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 6.Diao X, Cai J, Zheng J, Kong J, Wu S, Yu H, Huang H, Xie W, Chen X, Huang C, Huang L, Qin H, Huang J, Lin T. Association of chromosome 7 aneuploidy measured by fluorescence in situ hybridization assay with muscular invasion in bladder cancer. Cancer Commun (Lond) 2020;40:167–180. doi: 10.1002/cac2.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, Raghavan D, Skinner DG. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 8.Hautmann RE, de Petriconi RC, Pfeiffer C, Volkmer BG. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol. 2012;61:1039–1047. doi: 10.1016/j.eururo.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Brierley JD GM, Wittekind C. UICC International Union Against Cancer. 8th edition. Chichester, UK: Wiley-Blackwell; 2017. TNM classification of malignant tumors. [Google Scholar]
- 10.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 11.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 12.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 13.Becht E, Giraldo NA, Germain C, de Reyniès A, Laurent-Puig P, Zucman-Rossi J, Dieu-Nosjean MC, Sautès-Fridman C, Fridman WH. Immune contexture, immunoscore, and malignant cell molecular subgroups for prognostic and theranostic classifications of cancers. Adv Immunol. 2016;130:95–190. doi: 10.1016/bs.ai.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Xue Y, Tong L, LiuAnwei Liu F, Liu A, Zeng S, Xiong Q, Yang Z, He X, Sun Y, Xu C. Tumorinfiltrating M2 macrophages driven by specific genomic alterations are associated with prognosis in bladder cancer. Oncol Rep. 2019;42:581–594. doi: 10.3892/or.2019.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li XD, Huang CW, Liu ZF, Jiang LJ, Chen JW, Xie D, Zhou FJ, Lu HM, Liu ZW. Prognostic role of the immunoscore for patients with urothelial carcinoma of the bladder who underwent radical cystectomy. Ann Surg Oncol. 2019;26:4148–4156. doi: 10.1245/s10434-019-07529-y. [DOI] [PubMed] [Google Scholar]
- 16.Miyake M, Tatsumi Y, Gotoh D, Ohnishi S, Owari T, Iida K, Ohnishi K, Hori S, Morizawa Y, Itami Y, Nakai Y, Inoue T, Anai S, Torimoto K, Aoki K, Shimada K, Konishi N, Tanaka N, Fujimoto K. Regulatory T cells and tumor-associated macrophages in the tumor microenvironment in non-muscle invasive bladder cancer treated with intravesical bacille calmette-guerin: a long-term follow-up study of a japanese cohort. Int J Mol Sci. 2017;18:2186–2197. doi: 10.3390/ijms18102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali HR, Chlon L, Pharoah PD, Markowetz F, Caldas C. Patterns of immune infiltration in breast cancer and their clinical implications: a gene-expression-based retrospective study. PLoS Med. 2016;13:e1002194. doi: 10.1371/journal.pmed.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng D, Zhou R, Yu Y, Luo Y, Zhang J, Sun H, Bin J, Liao Y, Rao J, Zhang Y, Liao W. Gene expression profiles for a prognostic immunoscore in gastric cancer. Br J Surg. 2018;105:1338–1348. doi: 10.1002/bjs.10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, Hackl H, Trajanoski Z. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18:248–262. doi: 10.1016/j.celrep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16:385–395. doi: 10.1002/(sici)1097-0258(19970228)16:4<385::aid-sim380>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Babjuk M, Burger M, Compérat EM, Gontero P, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF, Sylvester R, Zigeuner R, Capoun O, Cohen D, Escrig JLD, Hernández V, Peyronnet B, Seisen T, Soukup V. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and Carcinoma In Situ) - 2019 update. Eur Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Schneider AK, Chevalier MF, Derre L. The multifaceted immune regulation of bladder cancer. Nat Rev Urol. 2019;16:613–630. doi: 10.1038/s41585-019-0226-y. [DOI] [PubMed] [Google Scholar]
- 27.Borcoman E, De La Rochere P, Richer W, Vacher S, Chemlali W, Krucker C, Sirab N, Radvanyi F, Allory Y, Pignot G, Barry de Longchamps N, Damotte D, Meseure D, Sedlik C, Bieche I, Piaggio E. Inhibition of PI3K pathway increases immune infiltrate in muscle-invasive bladder cancer. Oncoimmunology. 2019;8:e1581556. doi: 10.1080/2162402X.2019.1581556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao J, Yang X, Li J, Wu H, Li P, Yao Z, Dong Z, Tian J. Screening and identifying immune-related cells and genes in the tumor microenvironment of bladder urothelial carcinoma: based on TCGA database and bioinformatics. Front Oncol. 2019;9:1533. doi: 10.3389/fonc.2019.01533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aggen DH, Drake CG. Biomarkers for immunotherapy in bladder cancer: a moving target. J Immunother Cancer. 2017;5:94. doi: 10.1186/s40425-017-0299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallis SP, Stafford ND, Greenman J. Clinical relevance of immune parameters in the tumor microenvironment of head and neck cancers. Head Neck. 2015;37:449–459. doi: 10.1002/hed.23736. [DOI] [PubMed] [Google Scholar]
- 31.West NR, Kost SE, Martin SD, Milne K, Deleeuw RJ, Nelson BH, Watson PH. Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2013;108:155–162. doi: 10.1038/bjc.2012.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingels A, Sanchez Salas RE, Ravery V, Fromont-Hankard G, Validire P, Patard JJ, Pignot G, Prapotnich D, Olivier F, Galiano M, Barret E, Rozet F, Weber N, Cathelineau X. T-helper 1 immunoreaction influences survival in muscle-invasive bladder cancer: proof of concept. Ecancermedicalscience. 2014;8:486. doi: 10.3332/ecancer.2014.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y, Bae JM, Li G, Cho NY, Kang GH. Image analyzer-based assessment of tumor-infiltrating T cell subsets and their prognostic values in colorectal carcinomas. PLoS One. 2015;10:e0122183. doi: 10.1371/journal.pone.0122183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becht E, Giraldo NA, Beuselinck B, Job S, Marisa L, Vano Y, Oudard S, Zucman-Rossi J, Laurent-Puig P, Sautes-Fridman C, de Reynies A, Fridman WH. Prognostic and theranostic impact of molecular subtypes and immune classifications in renal cell cancer (RCC) and colorectal cancer (CRC) Oncoimmunology. 2015;4:e1049804. doi: 10.1080/2162402X.2015.1049804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ness N, Andersen S, Valkov A, Nordby Y, Donnem T, Al-Saad S, Busund LT, Bremnes RM, Richardsen E. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate. 2014;74:1452–1461. doi: 10.1002/pros.22862. [DOI] [PubMed] [Google Scholar]
- 36.Hald SM, Bremnes RM, Al-Shibli K, Al-Saad S, Andersen S, Stenvold H, Busund LT, Donnem T. CD4/CD8 co-expression shows independent prognostic impact in resected non-small cell lung cancer patients treated with adjuvant radiotherapy. Lung Cancer. 2013;80:209–215. doi: 10.1016/j.lungcan.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 37.Tian C, Lu S, Fan Q, Zhang W, Jiao S, Zhao X, Wu Z, Sun L, Wang L. Prognostic significance of tumor-infiltrating CD8(+) or CD3(+) T lymphocytes and interleukin-2 expression in radically resected non-small cell lung cancer. Chin Med J (Engl) 2015;128:105–10. doi: 10.4103/0366-6999.147828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angell H, Galon J. From the immune contexture to the immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Fu H, Zhu Y, Wang Y, Liu Z, Zhang J, Xie H, Fu Q, Dai B, Ye D, Xu J. Identification and validation of stromal immunotype predict survival and benefit from adjuvant chemotherapy in patients with muscle-invasive bladder cancer. Clin Cancer Res. 2018;24:3069–3078. doi: 10.1158/1078-0432.CCR-17-2687. [DOI] [PubMed] [Google Scholar]
- 40.Efstathiou JA, Mouw KW, Gibb EA, Liu Y, Wu CL, Drumm MR, da Costa JB, du Plessis M, Wang NQ, Davicioni E, Feng FY, Seiler R, Black PC, Shipley WU, Miyamoto DT. Impact of immune and stromal infiltration on outcomes following bladder-sparing trimodality therapy for muscle-invasive bladder cancer. Eur Urol. 2019;76:59–68. doi: 10.1016/j.eururo.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghoneim MA, Abdel-Latif M, el-Mekresh M, Abol-Enein H, Mosbah A, Ashamallah A, el-Baz MA. Radical cystectomy for carcinoma of the bladder: 2,720 consecutive cases 5 years later. J Urol. 2008;180:121–127. doi: 10.1016/j.juro.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 42.Yafi FA, Aprikian AG, Chin JL, Fradet Y, Izawa J, Estey E, Fairey A, Rendon R, Cagiannos I, Lacombe L, Lattouf JB, Bell D, Drachenberg D, Kassouf W. Contemporary outcomes of 2287 patients with bladder cancer who were treated with radical cystectomy: a Canadian multicentre experience. BJU Int. 2011;108:539–545. doi: 10.1111/j.1464-410X.2010.09912.x. [DOI] [PubMed] [Google Scholar]
- 43.Alfred Witjes J, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, Hernandez V, Espinos EL, Dunn J, Rouanne M, Neuzillet Y, Veskimae E, van der Heijden AG, Gakis G, Ribal MJ. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71:462–475. doi: 10.1016/j.eururo.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 44.Leow JJ, Martin-Doyle W, Rajagopal PS, Patel CG, Anderson EM, Rothman AT, Cote RJ, Urun Y, Chang SL, Choueiri TK, Bellmunt J. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66:42–54. doi: 10.1016/j.eururo.2013.08.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.