Abstract
This study aimed to compare the efficacy and safety of apatinib plus drug-eluting bead (DEB) transarterial chemoembolization (TACE), apatinib plus conventional TACE (cTACE) and apatinib alone in advanced intrahepatic cholangiocarcinoma (ICC) patients. We analyzed 35 advanced ICC patients retrospectively, including the apatinib plus DEB-TACE group (n=10), the apatinib plus cTACE group (n=12) and the apatinib group (n=13). Treatment response, survival data (including progression-free survival (PFS) and overall survival (OS)) and adverse events were assessed during the follow-up. Both the objective response rate (ORR) and the disease control rate (DCR) showed trends to be the highest in the apatinib plus DEB-TACE group (ORR: 84.6%/DCR: 100.0%), followed by the apatinib plus cTACE group (ORR: 75.0%/DCR: 91.7%) and then the apatinib group (ORR: 40.0%/DCR: 80.0%). PFS and OS were both the highest in the apatinib plus DEB-TACE group, followed by the apatinib plus cTACE group, and the shortest in the apatinib group, which was also confirmed by a multivariate Cox regression analysis. The incidences of adverse events were similar between the apatinib plus DEB-TACE group and the apatinib plus cTACE group but were higher in the apatinib plus DEB-TACE group and the apatinib plus cTACE than in the apatinib group; however, all of the adverse events were tolerable in the three groups. In conclusion, apatinib plus DEB-TACE is a promising therapeutic strategy for the treatment of advanced ICC.
Keywords: Intrahepatic cholangiocarcinoma, conventional transarterial chemoembolization, drug-eluting beads transarterial chemoembolization, apatinib, efficacy, adverse events
Introduction
Intrahepatic cholangiocarcinoma (ICC) represents the second most common primary liver cancer and its incidence is increasing globally [1,2]. Surgical resection is the only curative treatment option, but surgical therapy is only suitable for 30% of ICC cases, and the five-year survival rates of ICC patients who undergo surgical resection vary from 14%-40% due to the high risk of cancer recurrence and metastasis [2,3]. For the vast majority of patients with advanced ICC, the effective treatments are limited and the median overall survival (OS) with the current standard chemotherapy regimen is less than 1 year [4,5]. Therefore, it is essential to explore novel and effective treatment strategies that are able to improve the treatment response and prolong life in patients with advanced ICC.
Transarterial chemoembolization (TACE), including conventional TACE (cTACE) and drug-eluting bead (DEB)-TACE, applies minimally invasive techniques to selectively insert a catheter into the artery supplying blood to the tumor. It is used to inject chemotherapeutic drugs and embolic agents, which is a commonly used intra-arterial therapy for unresectable ICC patients [4,6]. Compared with cTACE, DEB-TACE has a more sustained drug release, results in increased concentrations of drugs within the targeted tumor, lower systemic drug concentrations, and notably, some clinical studies have demonstrated that DEB-TACE has a superior effect on improving treatment responses and survival profiles compared with cTACE [7,8]. However, TACE treatments have some disadvantages since TACE causes ischemia and hypoxia in the embolized tissues, which triggers the production of proangiogenic factors, such as vascular endothelial growth factor (VEGF), and further promotes tumor angiogenesis [9]. Therefore, the application of combined TACE treatments and anti-neovascularization drugs might achieve an enhanced therapeutic efficacy in advanced ICC treatment.
Apatinib, as a small molecule-targeted drug, inhibits VEGF-mediated migration and invasion by targeting the VEGF receptor-2 (VEGFR-2), sequentially decreasing tumor angiogenesis and suppressing tumor growth [10]. Several recent studies established a role of apatinib as an antiangiogenic agent in the treatment of advanced ICC [11-13]. For example, one prospective study reveals that apatinib has efficacy in improving treatment responses and survival profiles in advanced ICC patients, and the findings of another study suggest that apatinib has the potential to be an additional option for the treatment of advanced ICC patients [11,12]. According to the aforementioned evidence, we hypothesized that a combination of apatinib and TACE might have a favorable therapeutic efficacy and a tolerable safety profile for the treatment of advanced ICC patients. This combination has not been studied before. Hence, we performed the present study to compare the efficacy and safety of apatinib plus DEB-TACE, apatinib plus cTACE, and apatinib alone in the management of advanced ICC.
Materials and methods
Patients
The present study was an exploratory study, and a total of 35 advanced ICC patients treated in our hospital from October 2015 to December 2019 were analyzed in this study. The inclusion criteria for all patients were as follows: (i) pathologically diagnosed with ICC; (ii) TNM stage III~IV according to the American Joint Committee on Cancer (AJCC) 7th Edition Cancer Staging System; (iii) confirmed as unresectable disease, or identified as progressed disease after treatment; (iv) age ≥18 years; (v) Eastern Cooperative Oncology Group (ECOG) score ≤2; (vi) adequate bone marrow function (leukocyte count ≥3000 cells/μL, white blood cell ≥3000 cells/mm3, absolute neutrophils ≥1500 cells/μL, platelet count ≥50,000 cells/μL, hemoglobin concentration ≥9.0 g/dL); (vii) adequate liver function (total bilirubin ≤2 mg/dL, aspartate aminotransferase and alanine aminotransferase ≤5 up to the limit of normal), and patients with biliary tract obstruction should have serum bilirubin levels at <2.0 mg/dL after treatment by percutaneous hepatic puncture biliary drainage; (viii) adequate renal function (creatinine ≤2 mg/dL). The exclusion criteria were as follows: (i) Child-Pugh stage C; (ii) heart dysfunction or severe lung dysfunction; (iv) active infection; (v) concurrent with pregnancy or lactation (females); (vi) allergic to any of the research drugs or reagents. This study was approved by the Institutional Review Board of our hospital, and all patients or their families provided written informed consent.
Baseline data collection
Baseline data of the patients were documented in the case report form, which mainly included age, sex, weight, hepatitis B virus (HBV) infection status, ECOG score, Child-Pugh stage, number of intrahepatic tumors, macroscopic vascular invasion status, tumor size, lymph node metastasis status, distant metastasis, TNM stage, serum carbohydrate antigen 199 (CA199) level, and previous treatments.
Grouping and treatment
Patients were divided into three groups according to the treatment they received: the apatinib plus DEB-TACE group (N=13), the apatinib plus cTACE group (N=12), and the apatinib group (N=10).
(1) In the apatinib plus DEB-TACE group, patients began oral administration of apatinib (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, Jiangsu, China) at a dose of 500 mg within 1 week before the DEB-TACE operation, then DEB-TACE was performed. In brief, after successfully puncturing the femoral artery, a 4F catheter was placed in the common hepatic artery for angiography, which was performed to identify the location of the artery supplying blood to the tumor. Thereafter, gemcitabine (Jiangsu Haosen Pharmaceutical Co. Ltd., Lianyungang, Jiangsu, China) and cisplatin (Jiangsu Haosen Pharmaceutical Co. Ltd., Lianyungang, Jiangsu, China) were infused into the common hepatic artery with an infusion time of more than 20 min. Following that, using the superselection technique, a microcatheter was inserted into the artery supplying blood to the tumor, then 1 g gemcitabine-loaded CalliSpheres beads (containing 0.8 g gemcitabine) (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, Jiangsu, China) were infused for chemoembolization until the blood flow in the artery supplying the tumor had stopped. The total doses of gemcitabine and cisplatin were 1 g/m2 and 65 mg/m2, respectively. After DEB-TACE, the patients continued to receive apatinib at a dose of 500 mg until disease progression, intolerable adverse effects, or death. If necessary, repeated DEB-TACE treatments were administered.
(2) In the apatinib plus cTACE group, patients initiated oral administration of apatinib (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, Jiangsu, China) at a dose of 500 mg within 1 week before the cTACE operation, then cTACE was performed. In brief, after successful puncture of the femoral artery, a 4F catheter was placed in the common hepatic artery for angiography, which was performed to identify the location of the artery supplying blood to the tumor. Thereafter, gemcitabine (Jiangsu Haosen Pharmaceutical Co. Ltd., Lianyungang, Jiangsu, China) and cisplatin (Jiangsu Haosen Pharmaceutical Co. Ltd., Lianyungang, Jiangsu, China) were infused into the common hepatic artery with an infusion time of more than 20 min. Following that, using a superselection technique, the microcatheter was inserted into the artery supplying blood to the tumor, then 10 ml lipiodol (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, Jiangsu, China) mixed with 0.8 g gemcitabine was infused for chemoembolization until the blood flow to the tumor stopped. The total doses of gemcitabine and cisplatin were 1 g/m2 and 65 mg/m2, respectively. After cTACE, patients continued to receive apatinib at a dose of 500 mg until disease progression, intolerable side effects or death. If necessary, repeated cTACE treatments were administered.
(3) In the apatinib group, patients were only treated with apatinib (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, Jiangsu, China) at 500 mg daily (orally) until disease progression, intolerable side effects, or death, without any other anticancer therapy.
Patients in the apatinib plus DEB-TACE group and the apatinib plus cTACE group were only treated by DEB-TACE combined with apatinib, or cTACE combined with apatinib, without the use of any other anticancer therapy during the study period. If the intrahepatic lesions progressed, repeated TACE treatments were performed. If any extrahepatic lesions stabilized or improved, the patients continued to take apatinib orally, but if any extrahepatic lesions progressed, the apatinib was stopped and the patients were dropped from study. For all patients in all three groups, at the discretion of the physician adjustment of the apatinib dose, including interruption and reduction, was allowed according to the occurrence of adverse events.
Follow-up and assessment
Surveillance and assessment of treatment response was performed monthly after the initiation of treatment until the patients’ death. Treatment response was evaluated by chest computed tomography (CT), abdominal pelvic CT, or magnetic resonance imaging according to the Response Evaluation Criteria in Solid Tumours (RECIST) 1.1 criteria, which are classified as a complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The objective response rate (ORR) was defined as the proportion of patients who achieved a CR or PR; the disease control rate (DCR) was defined as the proportion of patients who achieved a CR, PR or SD. Progression-free survival (PFS) was defined as the time interval from initiation of study treatment to disease progression or death, whichever occurred first. Overall survival (OS) was defined as the time interval from the initiation of the study treatment to death. All adverse events were continuously monitored during the study, which were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTCAE) version 4.03. No patients were lost to follow-up during the study period.
Statistical analysis
We analyzed data collected up to the deadline of December 31, 2019. SPSS 22.0 (IBM, Chicago, IL, USA) was used for data analysis, and GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA) was used for making graphics. Data were described as the mean and standard deviation (SD), or count (percentage) and 95% confidence interval (CI). Comparisons among the three groups were made with one way analysis of variance (ANOVA) or Chi-square tests followed by post hoc multiple comparisons. PFS and OS were described using Kaplan-Meier curves, and the between/among-group comparisons were determined by the log-rank test. Factors related to ORR at the first month were analyzed by univariate and forward stepwise multivariate logistic regression model analyses. Factors affecting the PFS or OS were analyzed by univariable and forward stepwise multivariable Cox’s proportional hazard regression model analyses. P value <0.05 was considered as statistically significant.
Results
Clinical characteristics among the three groups
The mean ages of the apatinib group, the apatinib plus cTACE group and the apatinib plus DEB-TACE group were 58.7±7.8 years, 56.9±9.7 years and 55.9±14.3 years, respectively (Table 1). The number of women and men was respectively 2 (20.0%) and 8 (80.0%) in the apatinib group, 3 (25.0%) and 9 (75.0%) in the apatinib plus cTACE group, 7 (53.8%) and 6 (46.2%) in the apatinib plus DEB-TACE group. For Child-Pugh stage, the number of patients at stage A and B were respectively 3 (30.0%) and 7 (70.0%) in the apatinib group, 4 (33.3%) and 8 (66.7%) in the apatinib plus cTACE group, and 4 (30.8%) and 9 (69.2%) in the apatinib plus DEB-TACE group. Regarding TNM stage, the number of patients in III and IV TNM stages was 3 (30.0%) and 7 (70.0%) in the apatinib group, 4 (33.3%) and 8 (66.7%) in the apatinib plus cTACE group, 6 (46.2%) and 7 (53.8%) in the apatinib plus DEB-TACE group, respectively. More detailed clinical characteristics of these three groups were presented in Table 1. Notably, further analysis indicated that there was no differences in clinical characteristics among the three groups (all P>0.05).
Table 1.
Clinical characteristics
| Items | Apatinib (N=10) | Apatinib plus cTACE (N=12) | Apatinib plus DEB-TACE (N=13) | P value |
|---|---|---|---|---|
| Age (years), mean ± SD | 58.7±7.8 | 56.9±9.7 | 55.9±14.3 | 0.833 |
| ≤60 years, No. (%) | 6 (60.0) | 7 (58.3) | 7 (53.8) | 0.952 |
| >60 years, No. (%) | 4 (40.0) | 5 (41.7) | 6 (46.2) | |
| Gender, No. (%) | 0.168 | |||
| Female | 2 (20.0) | 3 (25.0) | 7 (53.8) | |
| Male | 8 (80.0) | 9 (75.0) | 6 (46.2) | |
| Weight, No. (%) | 0.976 | |||
| ≤60 kg | 5 (50.0) | 6 (50.0) | 7 (53.8) | |
| >60 kg | 5 (50.0) | 6 (50.0) | 6 (46.2) | |
| HBV status, No. (%) | 0.186 | |||
| Negative | 8 (80.0) | 5 (41.7) | 7 (53.8) | |
| Positive | 2 (20.0) | 7 (58.3) | 6 (46.2) | |
| ECOG score, No. (%) | 0.944 | |||
| 1 | 7 (70.0) | 9 (75.0) | 9 (69.2) | |
| 2 | 3 (30.0) | 3 (25.0) | 4 (30.8) | |
| Child-Pugh stage, No. (%) | 0.984 | |||
| A | 3 (30.0) | 4 (33.3) | 4 (30.8) | |
| B | 7 (70.0) | 8 (66.7) | 9 (69.2) | |
| Number of intrahepatic tumors, No. (%) | 0.154 | |||
| ≤3 | 4 (40.0) | 2 (16.7) | 7 (53.8) | |
| >3 | 6 (60.0) | 10 (83.3) | 6 (46.2) | |
| Macroscopic vascular invasion, No. (%) | 0.602 | |||
| No | 7 (70.0) | 7 (58.3) | 10 (76.9) | |
| Yes | 3 (30.0) | 5 (41.7) | 3 (23.1) | |
| Tumor size (cm), mean ± SD | 6.8±4.4 | 6.8±4.6 | 7.9±2.7 | 0.724 |
| ≤5.0 cm, No. (%) | 3 (30.0) | 5 (41.7) | 1 (7.7) | 0.142 |
| >5.0 cm, No. (%) | 7 (70.0) | 7 (58.3) | 12 (92.3) | |
| Lymph node metastasis, No. (%) | 0.152 | |||
| No | 0 (0.0) | 2 (16.7) | 4 (30.8) | |
| Yes | 10 (100.0) | 10 (83.3) | 9 (69.2) | |
| Distant metastasis, No. (%) | 0.764 | |||
| No | 3 (30.0) | 3 (25.0) | 5 (38.5) | |
| Yes | 7 (70.0) | 9 (75.0) | 8 (61.5) | |
| TNM stage, No. (%) | 0.689 | |||
| III | 3 (30.0) | 4 (33.3) | 6 (46.2) | |
| IV | 7 (70.0) | 8 (66.7) | 7 (53.8) | |
| CA199£, No. (%) | 0.808 | |||
| Normal | 4 (40.0) | 4 (33.3) | 6 (46.2) | |
| Abnormal | 6 (60.0) | 8 (66.7) | 7 (53.8) | |
| Bile duct dilatation, No. (%) | 0.952 | |||
| No | 6 (60.0) | 7 (58.3) | 7 (53.8) | |
| Yes | 4 (40.0) | 5 (41.7) | 6 (46.2) | |
| Biliary drainage, No. (%) | 0.962 | |||
| No | 8 (80.0) | 9 (75.0) | 10 (76.9) | |
| Yes | 2 (20.0) | 3 (25.0) | 3 (23.1) | |
| Previous therapy, No. (%) | 0.877 | |||
| No | 6 (60.0) | 8 (66.7) | 10 (76.9) | |
| Chemotherapy | 1 (10.0) | 1 (8.3) | 0 (0.0) | |
| Radiotherapy | 1 (10.0) | 1 (8.3) | 2 (15.4) | |
| Surgery and chemoradiotherapy | 2 (20.0) | 2 (16.7) | 1 (7.7) |
Comparison was determined by one-way analysis of variance (ANOVA) or Chi-square test.
normal: CA199 level ≤27.0 U/mL, abnormal: CA199 level >27.0 U/mL.
cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting bead transarterial chemoembolization. SD, standard deviation; HBV, hepatitis B virus; ECOG, Eastern Co-operative Oncology Group; CA199, carbohydrate antigen 199.
Comparison of treatment response among the three groups
At the first month post treatment, ORR and DCR were 40.0% and 80.0% in the apatinib group, 75.0% and 91.7% in the apatinib plus cTACE group, and 84.6% and 100.0% in the apatinib plus DEB-TACE group, respectively (Table 2). Three-group analysis indicated that there was no difference of ORR among the three groups (P=0.062). Furthermore, subsequent two-group analysis revealed that ORR was increased in the apatinib plus DEB-TACE group compared with the apatinib group (P=0.039), while there was no difference between the apatinib plus DEB-TACE group and the apatinib plus cTACE group (P=0.645), or between the apatinib plus cTACE group and the apatinib group (P=0.192). Meanwhile, there was no difference of DCR among the apatinib group, apatinib plus cTACE group and the apatinib plus DEB-TACE group (all P>0.05). Furthermore, treatment response was assessed monthly after initiation of treatment until the patients’ death. In general, the three groups all presented with a downward trend of ORR (Figure 1A) and DCR (Figure 1B) over time. As for the differences of treatment response among the three groups, the apatinib plus DEB-TACE group exhibited the best ORR and DCR, followed by the apatinib plus cTACE group, and the worst was the apatinib group.
Table 2.
Treatment response at first month among 3 groups
| Items | Apatinib (N=10) | Apatinib plus cTACE (N=12) | Apatinib plus DEB-TACE (N=13) | P value* | P value# | P value& | P value† |
|---|---|---|---|---|---|---|---|
| Response | 0.185 | 0.559 | 0.060 | 0.251 | |||
| CR, No. (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | - | - | - |
| PR, No. (%) | 4 (40.0) | 9 (75.0) | 11 (84.6) | - | - | - | - |
| SD, No. (%) | 4 (40.0) | 2 (16.7) | 2 (15.4) | - | - | - | - |
| PD, No. (%) | 2 (20.0) | 1 (8.3) | 0 (0.0) | - | - | - | - |
| ORR, No. (%) | 4 (40.0) | 9 (75.0) | 11 (84.6) | 0.062 | 0.645 | 0.039 | 0.192 |
| DCR, No. (%) | 8 (80.0) | 11 (91.7) | 13 (100.0) | 0.236 | 0.480 | 0.178 | 0.571 |
Comparison was determined by Chi-square test or Fisher’s exact test.
Comparison among 3 groups.
Apatinib plus DEB-TACE vs. Apatinib plus cTACE.
Apatinib plus DEB-TACE vs. Apatinib.
Apatinib plus cTACE vs. Apatinib.
cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting bead transarterial chemoembolization. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Figure 1.
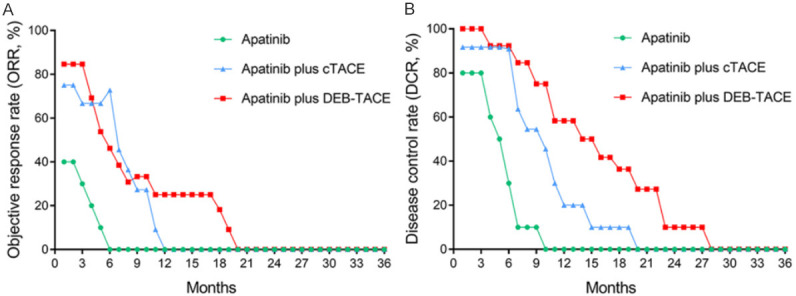
Longitudinal change of treatment response among three groups. A decreased trend of ORR (A) and DCR (B) for the apatinib group, apatinib plus cTACE group and apatinib plus DEB-TACE group. ORR, objective response rate; DCR, disease control rate; ICC, intrahepatic cholangiocarcinoma; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting beads transarterial chemoembolization.
Factors affecting ORR at the first month in advanced ICC patients
Univariate logistic regression indicated that apatinib plus DEB-TACE (vs. apatinib) (OR=8.250, P=0.036) was associated with a higher ORR at the first month, while ECOG score (2 vs. 1) (OR=0.167, P=0.028) was correlated with a lower ORR at the first month in advanced ICC patients (Table 3). Forward stepwise multivariate logistic regression exhibited that apatinib plus DEB-TACE (vs. apatinib) (OR=14.963, P=0.027) was an independent predictive factor for a higher ORR at the first month, while ECOG score (2 vs. 1) (OR=0.100, P=0.023) was an independent predictive factor for a lower ORR at the first month in advanced ICC patients.
Table 3.
Factors related to ORR at first month
| Items | logistic regression analysis | |||
|---|---|---|---|---|
|
| ||||
| P value | OR | 95% CI | ||
|
| ||||
| Lower | Higher | |||
| Univariate logistic regression | ||||
| Therapy | ||||
| Apatinib | Ref | |||
| Apatinib plus cTACE | 0.105 | 4.500 | 0.730 | 27.739 |
| Apatinib plus DEB-TACE | 0.036 | 8.250 | 1.154 | 59.003 |
| Age (>60 years vs. ≤60 years) | 0.600 | 1.481 | 0.341 | 6.425 |
| Gender (male vs. female) | 0.556 | 0.625 | 0.131 | 2.984 |
| Wight (>60 kg vs. ≤60 kg) | 0.633 | 0.705 | 0.168 | 2.955 |
| HBV (positive vs. negative) | 0.600 | 1.481 | 0.341 | 6.425 |
| ECOG score (2 vs. 1) | 0.028 | 0.167 | 0.034 | 0.826 |
| Child-Pugh stage (B vs. A) | 0.232 | 2.500 | 0.556 | 11.250 |
| Number of intrahepatic tumors (>3 vs. ≤3) | 0.130 | 0.263 | 0.047 | 1.481 |
| Macroscopic vascular invasion (yes vs. no) | 0.263 | 2.700 | 0.474 | 15.396 |
| Tumor size (>5 cm vs. ≤5 cm) | 0.494 | 0.540 | 0.092 | 3.159 |
| Lymph node metastasis (yes vs. no) | 0.999 | 0.000 | 0.000 | - |
| Distant metastasis (yes vs. no) | 0.263 | 0.370 | 0.065 | 2.112 |
| TNM stage (IV vs. III) | 0.417 | 0.525 | 0.111 | 2.487 |
| CA199£ (abnormal vs. normal) | 0.766 | 0.800 | 0.184 | 3.487 |
| Bile duct dilatation (yes vs. no) | 0.215 | 2.667 | 0.566 | 12.557 |
| Biliary drainage (yes vs. no) | 0.657 | 1.500 | 0.251 | 8.977 |
| Previous therapy (yes vs. no) | 0.671 | 0.721 | 0.159 | 3.266 |
| Forward stepwise multivariate logistic regression | ||||
| Therapy | ||||
| Apatinib | Ref | |||
| Apatinib plus cTACE | 0.098 | 5.861 | 0.720 | 47.713 |
| Apatinib plus DEB-TACE | 0.027 | 14.963 | 1.371 | 163.356 |
| ECOG score (2 vs. 1) | 0.023 | 0.100 | 0.014 | 0.729 |
ORR, objective response; OR, odds ratio; CI, confidence interval; Ref, reference; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting bead transarterial chemoembolization; HBV, hepatitis B virus; ECOG, Eastern Co-operative Oncology Group; CA199, carbohydrate antigen 199.
normal: CA199 level ≤27.0 U/mL, abnormal: CA199 level >27.0 U/mL.
Comparison of PFS among the three groups
Three-group comparisons found that PFS was the highest in the apatinib plus DEB-TACE group (median: 17.0 months (95% CI: 9.6-24.4 months)), followed by the apatinib plus cTACE group (median: 10.3 months (95% CI: 6.7-13.9 months)) and the shortest in the apatinib group (median: 4.5 months (95% CI: 3.2-5.8 months)) (P<0.001, χ2=23.550) (Figure 2A). Furthermore, subsequent two-group comparisons observed that the apatinib plus DEB-TACE group had a prolonged PFS compared with the apatinib plus cTACE group (P=0.026, χ2=4.933) (Figure 2B) and the apatinib group (P<0.001, χ2=15.938) (Figure 2C); besides, the apatinib plus cTACE group exhibited a longer PFS compared with the apatinib group (P=0.003, χ2=8.835) (Figure 2D).
Figure 2.
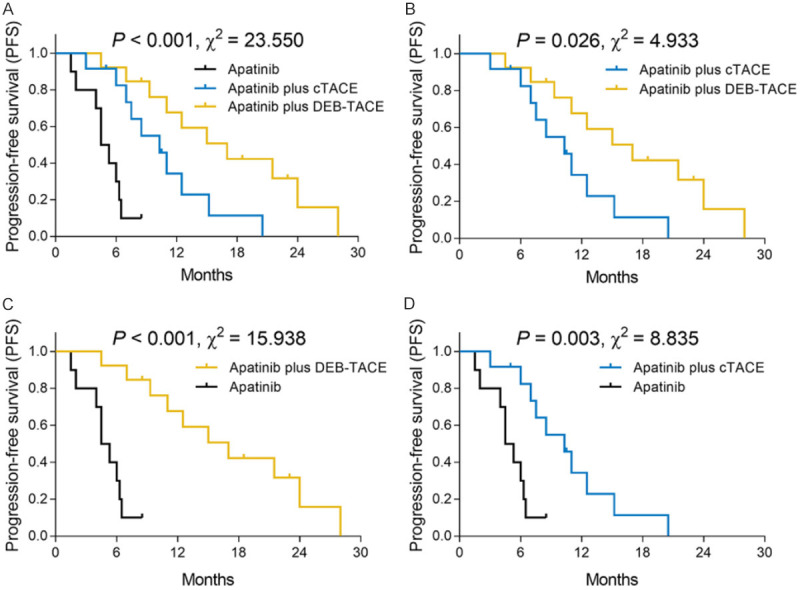
PFS among the three groups. PFS was the highest in the apatinib plus DEB-TACE group, followed by the apatinib plus cTACE group and it was the shortest in the apatinib group (A). Furthermore, PFS was longer in the apatinib plus DEB-TACE group compared with the apatinib plus cTACE group (B) and the apatinib group (C). In addition, PFS was increased in the apatinib plus cTACE group compared with the apatinib group (D). PFS, progression-free survival; ICC, intrahepatic cholangiocarcinoma; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting beads transarterial chemoembolization.
Factors affecting PFS in advanced ICC patients
Univariate Cox’s regression analysis indicated that apatinib plus DEB-TACE (vs. apatinib) (HR=0.068, P<0.001) and apatinib plus cTACE (vs. apatinib) (HR=0.196, P=0.004) were associated with increased PFS, while ECOG score (2 vs. 1) (HR=2.310, P=0.045), number of intrahepatic tumors (>3 vs. ≤3) (HR=2.959, P=0.015), distant metastasis (yes vs. no) (HR=2.845, P=0.029) and TNM stage (IV vs. III) (HR=2.526, P=0.040) were correlated with decreased PFS in advanced ICC patients (Table 4). Forward stepwise multivariate Cox’s regression found that apatinib plus DEB-TACE (vs. apatinib) (HR=0.025, P<0.001) and apatinib plus cTACE (vs. apatinib) (HR=0.090, P<0.001) were independent predictive factors for increased PFS, while ECOG score (2 vs. 1) (HR=6.213, P=0.001), distant metastasis (yes vs. no) (HR=5.417, P=0.002) and CA199 (abnormal vs. normal) (HR=3.012, P=0.017) were independent predictive factors for decreased PFS in advanced ICC patients.
Table 4.
Analysis of factors affecting PFS
| Items | Cox’s proportional hazard regression model | |||
|---|---|---|---|---|
|
| ||||
| P value | HR | 95% CI | ||
|
| ||||
| Lower | Higher | |||
| Univariate Cox’s regression | ||||
| Therapy | ||||
| Apatinib | Reference | - | - | - |
| Apatinib plus cTACE | 0.004 | 0.196 | 0.066 | 0.587 |
| Apatinib plus DEB-TACE | <0.001 | 0.068 | 0.019 | 0.240 |
| Age (>60 years vs. ≤60 years) | 0.763 | 1.124 | 0.526 | 2.400 |
| Gender (male vs. female) | 0.249 | 1.592 | 0.722 | 3.509 |
| Wight (>60 kg vs. ≤60 kg) | 0.804 | 1.099 | 0.523 | 2.309 |
| HBV (positive vs. negative) | 0.862 | 1.068 | 0.508 | 2.247 |
| ECOG score (2 vs. 1) | 0.045 | 2.310 | 1.018 | 5.244 |
| Child-Pugh stage (B vs. A) | 0.677 | 1.207 | 0.498 | 2.924 |
| Number of intrahepatic tumors (>3 vs. ≤3) | 0.015 | 2.959 | 1.232 | 7.106 |
| Macroscopic vascular invasion (yes vs. no) | 0.462 | 1.347 | 0.609 | 2.980 |
| Tumor size (>5 cm vs. ≤5 cm) | 0.676 | 1.202 | 0.507 | 2.848 |
| Lymph node metastasis (yes vs. no) | 0.470 | 1.402 | 0.560 | 3.511 |
| Distant metastasis (yes vs. no) | 0.029 | 2.845 | 1.112 | 7.277 |
| TNM stage (IV vs. III) | 0.040 | 2.526 | 1.043 | 6.118 |
| CA199£ (abnormal vs. normal) | 0.119 | 1.942 | 0.843 | 4.478 |
| Bile duct dilatation (yes vs. no) | 0.709 | 1.162 | 0.528 | 2.557 |
| Biliary drainage (yes vs. no) | 0.694 | 0.820 | 0.306 | 2.200 |
| Previous therapy (yes vs. no) | 0.993 | 0.996 | 0.431 | 2.302 |
| Forward stepwise multivariate Cox’s regression | ||||
| Therapy | ||||
| Apatinib | Reference | - | - | - |
| Apatinib plus cTACE | <0.001 | 0.090 | 0.025 | 0.325 |
| Apatinib plus DEB-TACE | <0.001 | 0.025 | 0.005 | 0.116 |
| ECOG score (2 vs. 1) | 0.001 | 6.213 | 2.221 | 17.382 |
| Distant metastasis (yes vs. no) | 0.002 | 5.417 | 1.863 | 15.750 |
| CA199£ (abnormal vs. normal) | 0.017 | 3.012 | 1.218 | 7.449 |
Factors affecting PFS were analyzed by univariate and forward stepwise multivariate Cox’s proportional hazard regression model.
normal: CA199 level ≤27.0 U/mL, abnormal: CA199 level >27.0 U/mL.
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting bead transarterial chemoembolization; HBV, hepatitis B virus; ECOG, Eastern Co-operative Oncology Group; CA199, carbohydrate antigen 199.
Comparison of OS among the three groups
Three-group comparison found that OS was the longest in the apatinib plus DEB-TACE group (median: 19.3 months (95% CI: 12.6-26.0 months)), followed by the apatinib plus cTACE group (median: 14.0 months (95% CI: 10.2-17.8 months)) and the shortest in the apatinib group (median: 6.5 months (95% CI: 4.7-8.3 months)) (P<0.001, χ2=25.634) (Figure 3A). In addition, the following two-group comparisons found that the apatinib plus DEB-TACE group presented a similar OS compared with the apatinib plus cTACE group (P=0.076, χ2=3.139) (Figure 3B), while it had an elevated OS compared with the apatinib group (P<0.001, χ2=19.245) (Figure 3C); furthermore, the apatinib plus cTACE group exhibited increased OS compared with the apatinib group (P=0.001, χ2=10.085) (Figure 3D).
Figure 3.
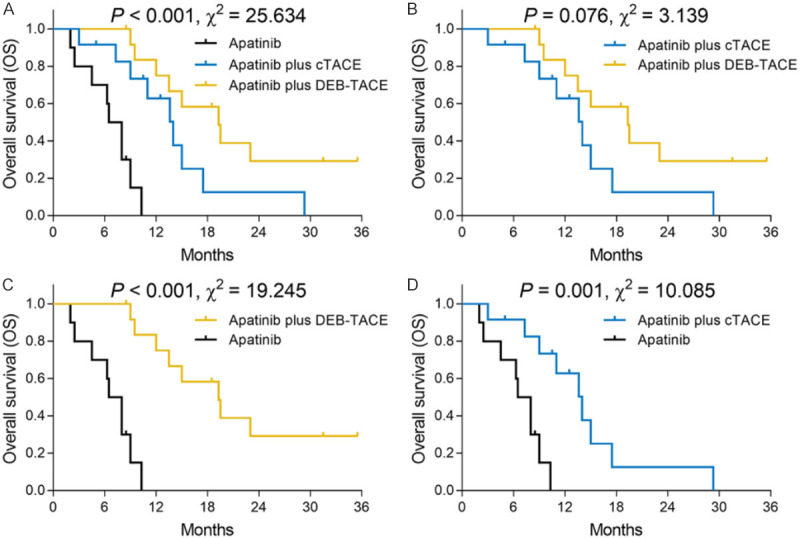
OS among the three groups. OS was the longest in the apatinib plus DEB-TACE group, followed by the apatinib plus cTACE group, and the shortest in the apatinib group (A). Furthermore, OS was similar between the apatinib plus DEB-TACE group and the apatinib plus cTACE group (B) but was increased in the apatinib plus DEB-TACE group (C) and the apatinib plus cTACE group (D) compared with the apatinib group. OS, overall survival; ICC, intrahepatic cholangiocarcinoma; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting beads transarterial chemoembolization.
Factors affecting OS in advanced ICC patients
Univariate Cox’s regression analysis indicated that apatinib plus DEB-TACE (vs. apatinib) (HR=0.059, P<0.001) and apatinib plus cTACE (vs. apatinib) (HR=0.139, P=0.001) were associated with increased OS, while the number of intrahepatic tumors (>3 vs. ≤3) (HR=2.677, P=0.028) and distant metastasis (yes vs. no) (HR=3.226, P=0.024) were correlated with decreased OS in advanced ICC patients (Table 5). Forward stepwise multivariate Cox’s regression revealed that apatinib plus DEB-TACE (vs. apatinib) (HR=0.005, P<0.001) and apatinib plus cTACE (vs. apatinib) (HR=0.013, P<0.001) were independent predictive factors for increased OS, while ECOG score (2 vs. 1) (HR=13.185, P<0.001), Child-Pugh stage (B vs. A) (HR=5.747, P=0.004), number of intrahepatic tumors (>3 vs. ≤3) (HR=13.463, P<0.001), bile duct dilatation (yes vs. no) (HR=3.116, P=0.025) were independent predictive factors for decreased OS in advanced ICC patients.
Table 5.
Analysis of factors affecting OS
| Items | Cox’s proportional hazard regression model | |||
|---|---|---|---|---|
|
| ||||
| P value | HR | 95% CI | ||
|
| ||||
| Lower | Higher | |||
| Univariate Cox’s regression | ||||
| Therapy | ||||
| Apatinib | Reference | - | - | - |
| Apatinib plus cTACE | 0.001 | 0.139 | 0.042 | 0.465 |
| Apatinib plus DEB-TACE | <0.001 | 0.059 | 0.016 | 0.220 |
| Age (>60 years vs. ≤60 years) | 0.815 | 1.098 | 0.500 | 2.415 |
| Gender (male vs. female) | 0.143 | 1.847 | 0.812 | 4.202 |
| Wight (>60 kg vs. ≤60 kg) | 0.810 | 1.100 | 0.507 | 2.382 |
| HBV (positive vs. negative) | 0.978 | 1.011 | 0.466 | 2.192 |
| ECOG score (2 vs. 1) | 0.080 | 2.137 | 0.912 | 5.008 |
| Child-Pugh stage (B vs. A) | 0.355 | 1.522 | 0.625 | 3.706 |
| Number of intrahepatic tumors (>3 vs. ≤3) | 0.028 | 2.677 | 1.115 | 6.426 |
| Macroscopic vascular invasion (yes vs. no) | 0.578 | 1.253 | 0.567 | 2.770 |
| Tumor size (>5 cm vs. ≤5 cm) | 0.676 | 1.215 | 0.486 | 3.039 |
| Lymph node metastasis (yes vs. no) | 0.353 | 1.595 | 0.596 | 4.269 |
| Distant metastasis (yes vs. no) | 0.024 | 3.226 | 1.168 | 8.914 |
| TNM stage (IV vs. III) | 0.070 | 2.216 | 0.938 | 5.230 |
| CA199£ (abnormal vs. normal) | 0.083 | 2.080 | 0.910 | 4.756 |
| Bile duct dilatation (yes vs. no) | 0.432 | 1.383 | 0.616 | 3.104 |
| Biliary drainage (yes vs. no) | 0.989 | 0.993 | 0.365 | 2.700 |
| Previous therapy (yes vs. no) | 0.780 | 0.882 | 0.365 | 2.132 |
| Forward stepwise multivariate Cox’s regression | ||||
| Therapy | ||||
| Apatinib | Reference | - | - | - |
| Apatinib plus cTACE | <0.001 | 0.013 | 0.002 | 0.089 |
| Apatinib plus DEB-TACE | <0.001 | 0.005 | 0.001 | 0.043 |
| ECOG score (2 vs. 1) | <0.001 | 13.185 | 3.368 | 51.625 |
| Child-Pugh stage (B vs. A) | 0.004 | 5.747 | 1.747 | 18.906 |
| Number of intrahepatic tumors (>3 vs. ≤3) | <0.001 | 13.463 | 3.321 | 54.579 |
| Bile duct dilatation (yes vs. no) | 0.025 | 3.116 | 1.152 | 8.429 |
Factors affecting OS were analyzed by univariate and forward stepwise multivariate Cox’s proportional hazard regression model.
normal: CA199 level ≤27.0 U/mL, abnormal: CA199 level >27.0 U/mL.
OS, overall survival; HR, hazard ratio; CI, confidence interval; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting bead transarterial chemoembolization; HBV, hepatitis B virus; ECOG, Eastern Co-operative Oncology Group; CA199, carbohydrate antigen 199.
Comparison of treatment-related adverse events among the three groups
According to the treatment-related adverse events data, three-group comparison observed that the incidences of vomiting (P=0.022), aspartate transaminase (AST) increase (P=0.017), anemia (P=0.001) and neutropenia (P=0.036) were different among the three groups, while there were no differences for the incidences of fatigue, anorexia, diarrhea, hoarseness, hypertension, hand-foot syndrome, mucositis, proteinuria, hypoproteinemia, hyperbilirubinemia, alanine transaminase (ALT) increase, or thrombocytopenia among the three groups (all P>0.05) (Table 6). Furthermore, subsequent two-group comparisons showed that the incidences of vomiting, AST increase, anemia, and neutropenia were decreased in the apatinib group compared with the apatinib plus DEB-TACE group or the apatinib plus cTACE group (all P<0.05). In addition, notably, most of the treatment-related adverse events were at grade I and II, suggesting that the treatments in the three groups were all tolerable. More detailed information about the treatment-related adverse events are shown in Table 6.
Table 6.
Treatment-related adverse events
| Items | Apatinib | Apatinib plus cTACE | Apatinib plus DEB-TACE | P value* | P value# | P value& | P value† | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||||||
| Total | I | II | III | IV | Total | I | II | III | IV | Total | I | II | III | IV | |||||
| Fatigue, No. (%) | 6 (60.0) | 4 (40.0) | 1 (10.0) | 1 (10.0) | 0 (0.0) | 7 (58.3) | 4 (33.3) | 2 (16.7) | 1 (8.3) | 0 (0.0) | 8 (61.5) | 5 (38.5) | 2 (15.4) | 1 (7.7) | 0 (0.0) | 0.987 | 0.870 | 0.940 | 0.937 |
| Anorexia, No. (%) | 3 (30.0) | 2 (20.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 5 (41.7) | 3 (25.0) | 2 (16.7) | 0 (0.0) | 0 (0.0) | 5 (38.5) | 4 (30.8) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0.846 | 0.870 | 0.673 | 0.571 |
| Vomiting, No. (%) | 1 (10.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (66.7) | 4 (33.3) | 3 (25.0) | 1 (8.3) | 0 (0.0) | 7 (53.8) | 3 (23.1) | 3 (23.1) | 1 (7.7) | 0 (0.0) | 0.022 | 0.513 | 0.029 | 0.011 |
| Diarrhea, No. (%) | 1 (10.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (15.4) | 2 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.846 | 0.588 | 0.704 | 0.892 |
| Hoarseness, No. (%) | 3 (30.0) | 3 (30.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (25.0) | 3 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (23.1) | 2 (15.4) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0.929 | 0.910 | 0.708 | 0.793 |
| Hypertension, No. (%) | 8 (80.0) | 2 (20.0) | 4 (40.0) | 2 (20.0) | 0 (0.0) | 11 (91.7) | 3 (25.0) | 4 (33.3) | 4 (33.3) | 0 (0.0) | 12 (92.3) | 3 (23.1) | 5 (38.5) | 4 (30.8) | 0 (0.0) | 0.601 | 0.953 | 0.385 | 0.427 |
| Hand-foot syndrome, No. (%) | 8 (80.0) | 4 (40.0) | 2 (20.0) | 2 (20.0) | 0 (0.0) | 8 (66.7) | 3 (25.0) | 2 (16.7) | 3 (25.0) | 0 (0.0) | 10 (76.9) | 4 (30.8) | 3 (23.1) | 3 (23.1) | 0 (0.0) | 0.747 | 0.568 | 0.859 | 0.484 |
| Mucositis, No. (%) | 4 (40.0) | 3 (30.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 4 (33.3) | 3 (25.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 3 (23.1) | 2 (15.4) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0.676 | 0.568 | 0.382 | 0.746 |
| Proteinuria, No. (%) | 6 (60.0) | 4 (40.0) | 1 (10.0) | 1 (10.0) | 0 (0.0) | 7 (58.3) | 6 (50.0) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 12 (92.3) | 10 (76.9) | 2 (15.4) | 0 (0.0) | 0 (0.0) | 0.109 | 0.073 | 0.127 | 0.937 |
| Hypoproteinemia, No. (%) | 8 (80.0) | 6 (60.0) | 2 (20.0) | 0 (0.0) | 0 (0.0) | 11 (91.7) | 6 (50.0) | 5 (41.7) | 0 (0.0) | 0 (0.0) | 13 (100.0) | 10 (76.9) | 3 (23.1) | 0 (0.0) | 0 (0.0) | 0.236 | 0.288 | 0.178 | 0.427 |
| Hyperbilirubinemia, No. (%) | 5 (50.0) | 2 (20.0) | 1 (10.0) | 2 (20.0) | 0 (0.0) | 11 (91.7) | 3 (25.0) | 6 (50.0) | 2 (16.7) | 0 (0.0) | 10 (76.9) | 7 (53.8) | 0 (0.0) | 3 (23.1) | 0 (0.0) | 0.081 | 0.315 | 0.179 | 0.056 |
| ALT increased, No. (%) | 7 (70.0) | 5 (50.0) | 1 (10.0) | 1 (10.0) | 0 (0.0) | 12 (100.0) | 4 (33.3) | 5 (41.7) | 3 (25.0) | 0 (0.0) | 12 (92.3) | 4 (30.8) | 4 (30.8) | 4 (30.8) | 0 (0.0) | 0.077 | 0.327 | 0.162 | 0.078 |
| AST increased, No. (%) | 7 (70.0) | 4 (40.0) | 2 (20.0) | 1 (10.0) | 0 (0.0) | 12 (100.0) | 4 (33.3) | 4 (33.3) | 4 (33.3) | 0 (0.0) | 13 (100.0) | 2 (15.4) | 6 (46.2) | 4 (30.8) | 1 (7.7) | 0.017 | 1.000 | 0.034 | 0.041 |
| Anemia, No. (%) | 3 (30.0) | 2 (20.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 12 (100.0) | 7 (58.3) | 4 (33.3) | 1 (8.3) | 0 (0.0) | 11 (84.6) | 6 (46.2) | 5 (38.5) | 0 (0.0) | 0 (0.0) | 0.001 | 0.157 | 0.013 | 0.001 |
| Neutropenia, No. (%) | 2 (20.0) | 0 (0.0) | 1 (10.0) | 1 (10.0) | 0 (0.0) | 9 (75.0) | 2 (16.7) | 4 (33.3) | 1 (8.3) | 2 (16.7) | 7 (53.8) | 4 (30.8) | 2 (15.4) | 1 (7.7) | 0 (0.0) | 0.036 | 0.271 | 0.099 | 0.030 |
| Thrombocytopenia, No. (%) | 3 (30.0) | 1 (10.0) | 0 (0.0) | 2 (20.0) | 0 (0.0) | 9 (75.0) | 3 (25.0) | 4 (33.3) | 2 (16.7) | 0 (0.0) | 7 (53.8) | 4 (30.8) | 0 (0.0) | 3 (23.1) | 0 (0.0) | 0.108 | 0.271 | 0.253 | 0.084 |
Comparison was determined by Chi-square test or Fisher’s exact test.
total adverse events comparison among 3 groups.
total adverse events: Apatinib plus DEB-TACE vs. Apatinib plus cTACE.
total adverse events: Apatinib plus DEB-TACE vs. Apatinib.
total adverse events: Apatinib plus cTACE vs. Apatinib.
cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting bead transarterial chemoembolization; ALT, alanine transaminase; AST, aspartate transaminase.
Discussion
In the present study, we found that in advanced ICC patients, (1) Apatinib plus DEB-TACE treatment seemed to have the best treatment response, followed by apatinib plus cTACE and then apatinib alone. (2) Both PFS and OS were the longest under the apatinib plus DEB-TACE treatment, followed by apatinib plus cTACE and the shortest with apatinib alone. (3) The incidence of treatment-related adverse events was increased under the apatinib plus DEB-TACE treatment and the apatinib plus cTACE treatment compared with apatinib alone, but these treatment-related adverse events were all tolerable.
ICC, which accounts for approximately 10% of all primary liver cancers, represents the second most common primary liver cancer following HCC [4]. Surgical resection is considered to be the only curative therapy for ICC, but unfortunately, less than 30% of ICC cases are eligible for surgical resection, and up to two-thirds of ICC patients who undergo surgical resection suffer from disease relapse, with 5-year overall survival rates ranging from 15% to 40% [14,15]. TACE, as one of the intra-arterial therapies, improves survival profiles and relieves tumor-related clinical presentation, and it is increasingly accepted by ICC patients at advanced stages [5,16]. TACE mainly consists of two approaches, lipiodol-based cTACE and DEB-TACE, and the efficacy as well as the safety of these two TACE approaches in ICC management have been reported in some previous studies [8,17,18]. For example, a recent multicenter prospective cohort study of 37 ICC patients indicated that 8.1% and 59.5% patients achieved a CR and PR, respectively, and the ORR was 67.6% after the DEB-TACE treatment [17]. Furthermore, a study involving 26 patients with ICC revealed that patients treated with DEB-TACE had median PFS and OS of 3.9 months and 11.7 months, respectively. Meanwhile, patients treated with cTACE had median PFS and OS of 1.8 months and 5.7 months, respectively, which implied that DEB-TACE presents with a prolongation of PFS and OS superior to cTACE [8]. However, recent research revealed that the anti-tumor effect of TACE alone was not sufficient in terms of the regeneration of tumor angiogenesis caused by the ischemia and hypoxia after TACE treatment [9]. Therefore, a combination of TACE and apatinib (a promising molecule-targeted drug against angiogenic activity) is proposed to improve the therapeutic efficacy of advanced ICC treatment, but it had not previously been studied. However, in the treatment of other tumor types, a combination of TACE and apatinib exhibited a better treatment response and survival profiles compared to TACE alone [7,18-22]. For example, in the treatment of advanced-stage HCC, TACE combined with apatinib (DCR: 95.45%; ORR: 63.64%) has an increased treatment response compared with TACE alone treatment (DCR: 81.82%; ORR: 36.36%); furthermore, the median DFS of TACE combined with apatinib treatment (16.5 months) is increased compared with TACE alone treatment (11.2 months) [20]. In addition, in anther single-center randomized controlled trial, for intermediate and advanced HCC patients, the patients receiving TACE combined with apatinib exhibited an increased long-term curative effect compared with those receiving TACE alone [21]. Therefore, in order to fill the gap that there was no evidence about the therapeutic efficacy of combined apatinib and TACE in the treatment of advanced ICC, we conducted this present study. As far as we know, this is the first study to compare the effectiveness and safety of apatinib plus DEB-TACE, apatinib plus cTACE and apatinib alone for advanced ICC.
In the present study, we recruited 35 advanced ICC patients, and found that, according to the comparisons and tendencies of treatment response, apatinib plus DEB-TACE treatment showed a trend to have the highest treatment response, which was followed by the apatinib plus cTACE treatment and then the apatinib alone treatment. Furthermore, regarding the survival profile, the PFS and OS of apatinib plus DEB-TACE treatment were both the longest, followed by apatinib plus cTACE treatment and apatinib alone treatment, which was also supported by the multivariate Cox’s regression analysis. The possible reasons for this might include that (1) Considering that TACE is an effective treatment approach that delays tumor progression by achieving a high dose of cytotoxic payload within tumor tissue and blocking tumor-feeding arteries, apatinib plus TACE improved the treatment response and survival compared with apatinib alone in advanced ICC management. (2) In addition, apatinib inhibited VEGF-mediated cell proliferation, migration and invasion in ICC cells by inactivating VEGF receptors and its downstream pathways (such as VEGFR2/RAF/MEK/ERK). Meanwhile, the tumor microenvironment after TACE was in a state of ischemia and hypoxia, which promoted tumor angiogenesis via the action of VEGF [10,11,23]. Therefore, when apatinib was combined with TACE in the treatment of advanced ICC, the anti-angiogenesis ability of apatinib could be better reflected by preventing excessive production of VEGF after TACE, contributing to a persistent anti-tumor effect, and further improving the prognosis of advanced ICC patients [23]. (3) For the superior efficacy of DEB-TACE to cTACE, these results might be explained by DEB-TACE increasing the intratumor drug concentration having a more sustained drug release compared with cTACE, thereby leading to a better treatment response and long-term survival of advanced ICC patients. (4) Furthermore, in the apatinib plus DEB-TACE and apatinib plus cTACE groups, they an extra tumor-selective infusion of gemcitabine and cisplatin compared with the apatinib group, which contributed to the efficacy of the chemotherapy in the tumor-feeding arteries, thereby promoting the treatment response in advanced ICC patients.
Regarding adverse events, generally, TACE is tolerated by the majority of ICC patients without major adverse events [4]. The most frequent temporary adverse events after cTACE and DEB-TACE are postembolization syndrome, including nausea, abdominal pain, fever and the increment of liver enzymes [4]. For apatinib in the treatment of ICC, one previous study indicated that the most frequent adverse events consist of liver dysfunction, hand-foot syndrome, hypertension, proteinuria, and symptoms of fatigue, and these adverse events are mild without toxicity-induced death occurring [13]. Although prior evidence suggests the tolerable safety profile of TACE or apatinib alone in the treatment of ICC, the safety of apatinib plus TACE has not been explored before. In the present study, the incidence of most treatment-related adverse events was similar among these three treatments, while the incidences of vomiting, AST increase, and anemia were elevated under the apatinib plus TACE treatment (cTACE and DEB-TACE) compared with the apatinib alone treatment. The majority of adverse events were mild to moderate, and could be relieved through appropriate management, suggesting that all of these three treatments were tolerable. The possible reasons might include that (1) the incidences of bone marrow suppression phenomenon (such as anemia, neutropenia), gastrointestinal symptoms (such as vomiting) and decreased liver function (such as transaminase elevation) are common after treatment with TACE, which are all part of the postembolization syndrome. Therefore, patients receiving apatinib plus TACE had more prevalent TACE-related adverse events compared with patients receiving apatinib alone [24,25]. (2) The mechanisms of action of DEB-TACE and cTACE are quite similar, both of which involve the injection of chemotherapeutic agents and selective vascular embolization, delivered to the artery of the tumor. In addition, the same drugs are used in DEB-TACE and cTACE (gemcitabine and cisplatin) and the adverse events of DEB-TACE and cTACE are mainly caused by the application of chemotherapeutic agents as well as the inflammatory response associated with the embolization itself. Based on the aforementioned information, the treatment-related adverse events would be expected to be quite similar between the apatinib plus DEB-TACE treatment and apatinib plus cTACE treatment [4]; This finding was consistent with the prior studies that DEB-TACE and cTACE had similar adverse side effects in the treatment of other cancers [20,26-28].
There are some limitations in our study. First, as our study was a retrospective cohort study, selection bias and confounding factors might exist, and prospective clinical trials should be conducted in the future. Second, the sample size was only 35 and all of them were from one single center, and hence more patients from multiple centers are needed for validating the results. Third, some confounders, such as the distinctions in the operation skills of the clinicians, were not considered in our study.
In conclusion, apatinib plus DEB-TACE has superior therapeutic efficacy and an equal safety profile compared with apatinib plus cTACE and apatinib alone, suggesting its potential to be a therapeutic strategy in the treatment of advanced ICC.
Acknowledgements
This study was supported by Science and Technology Program of Fujian Province, China, No. 2018Y2003.
Disclosure of conflict of interest
None.
References
- 1.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poultsides GA, Zhu AX, Choti MA, Pawlik TM. Intrahepatic cholangiocarcinoma. Surg Clin North Am. 2010;90:817–837. doi: 10.1016/j.suc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Currie BM, Soulen MC. Decision making: intra-arterial therapies for cholangiocarcinoma-TACE and TARE. Semin Intervent Radiol. 2017;34:92–100. doi: 10.1055/s-0037-1602591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savic LJ, Chapiro J, Geschwind JH. Intra-arterial embolotherapy for intrahepatic cholangiocarcinoma: update and future prospects. Hepatobiliary Surg Nutr. 2017;6:7–21. doi: 10.21037/hbsn.2016.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song JE, Kim DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol. 2017;9:808–814. doi: 10.4254/wjh.v9.i18.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei Y, Liu J, Yan M, Zhao S, Long Y, Zhang W. Effectiveness and safety of combination therapy of transarterial chemoembolization and apatinib for unresectable hepatocellular carcinoma in the chinese population: a meta-analysis. Chemotherapy. 2019;64:94–104. doi: 10.1159/000502510. [DOI] [PubMed] [Google Scholar]
- 8.Kuhlmann JB, Euringer W, Spangenberg HC, Breidert M, Blum HE, Harder J, Fischer R. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. 2012;24:437–443. doi: 10.1097/MEG.0b013e3283502241. [DOI] [PubMed] [Google Scholar]
- 9.Schicho A, Hellerbrand C, Kruger K, Beyer LP, Wohlgemuth W, Niessen C, Hohenstein E, Stroszczynski C, Pereira PL, Wiggermann P. Impact of different embolic agents for transarterial chemoembolization (TACE) procedures on systemic vascular endothelial growth factor (VEGF) levels. J Clin Transl Hepatol. 2016;4:288–292. doi: 10.14218/JCTH.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang M, Huang B, Li G, Zeng S. Apatinib affect VEGF-mediated cell proliferation, migration, invasion via blocking VEGFR2/RAF/MEK/ERK and PI3K/AKT pathways in cholangiocarcinoma cell. BMC Gastroenterol. 2018;18:169. doi: 10.1186/s12876-018-0870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang LY, Gong S, Gao LP, Hou LX, He W. Apatinib for treating advanced intrahepatic cholangiocarcinoma after failed chemotherapy: a case report and literature review. Medicine (Baltimore) 2018;97:e13372. doi: 10.1097/MD.0000000000013372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma FC, Yu Q, Zeng ZM, He RQ, Mo CH, Zhong JC, Ma J, Feng ZB, Chen G, Hu XH. Progression-free survival of up to 8 months of an advanced intrahepatic cholangiocarcinoma patient treated with apatinib: a case report. Onco Targets Ther. 2017;10:5237–5242. doi: 10.2147/OTT.S146051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhen L, Jiali C, Yong F, Han X, Hongming P, Weidong H. The efficacy and safety of apatinib treatment for patients with unresectable or relapsed liver cancer: a retrospective study. J Cancer. 2018;9:2773–2777. doi: 10.7150/jca.26376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol. 2018;7:52. doi: 10.21037/cco.2018.07.03. [DOI] [PubMed] [Google Scholar]
- 15.Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66:322–328. doi: 10.1016/j.crad.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Luo J, Zheng J, Shi C, Fang J, Peng Z, Huang J, Sun J, Zhou G, Li T, Zhu D, Xu H, Hou Q, Ying S, Sun Z, Du H, Xie X, Cao G, Ji W, Han J, Gu W, Guo X, Shao G, Yu Z, Zhou J, Yu W, Zhang X, Li L, Hu H, Hu T, Wu X, Chen Y, Ji J, Hu W. Drug-eluting beads transarterial chemoembolization by CalliSpheres is effective and well tolerated in treating intrahepatic cholangiocarcinoma patients: a preliminary result from CTILC study. Medicine (Baltimore) 2020;99:e19276. doi: 10.1097/MD.0000000000019276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Xie S, Duan X, Chen J, Zhou X, Li Y, Li Z, Han X. Assessment of efficacy and safety of the transcatheter arterial chemoembolization with or without apatinib in the treatment of large hepatocellular carcinoma. Cancer Chemother Pharmacol. 2020;85:69–76. doi: 10.1007/s00280-019-04004-z. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Ke Z, Xiong F, Kan X, Ren Y, Cao Y, Sun T, Yan L, Zhou G, Zheng C. Platelet-to-lymphocyte ratio predicts therapy outcomes of transarterial chemoembolization plus apatinib in the treatment of advanced hepatocellular carcinoma. Anticancer Drugs. 2020;31:966–972. doi: 10.1097/CAD.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Feng B, Mei L, Sun R, Guo C, Zhu J. Clinical efficacy of TACE combined with apatinib in the treatment of advanced hepatocellular carcinoma. J BUON. 2019;24:608–614. [PubMed] [Google Scholar]
- 21.Lu W, Jin XL, Yang C, Du P, Jiang FQ, Ma JP, Yang J, Xie P, Zhang Z. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: a single-center randomized controlled trial. Cancer Biol Ther. 2017;18:433–438. doi: 10.1080/15384047.2017.1323589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan W, Yuan G, Fan H, Li F, Wu Y, Zhao Y, Yao W, Wang Y, Xue M, Yang J, Li J. Apatinib combined with transarterial chemoembolization in patients with hepatocellular carcinoma and portal vein tumor thrombus: a multicenter retrospective study. Clin Ther. 2019;41:1463–1476. doi: 10.1016/j.clinthera.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Xu J, Zhang W, Chen J, Zhou X, Li Z, Han X. Safety and efficacy of drug-eluting bead transarterial chemoembolization combined with apatinib in patients with advanced hepatocellular carcinoma. Acad Radiol. 2019;27:704–709. doi: 10.1016/j.acra.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Miksad RA, Ogasawara S, Xia F, Fellous M, Piscaglia F. Liver function changes after transarterial chemoembolization in US hepatocellular carcinoma patients: the LiverT study. BMC Cancer. 2019;19:795. doi: 10.1186/s12885-019-5989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai Y, Gao S, Liu X, Gao Q, Zhang L, Fan X, Zhu J. Effect of aidi injection plus TACE on hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2018;2018:9196409. doi: 10.1155/2018/9196409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang K, Zhou Q, Wang R, Cheng D, Ma Y. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:920–925. doi: 10.1111/jgh.12439. [DOI] [PubMed] [Google Scholar]
- 27.Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D, Cucchetti A, Bolondi L, Trevisani F PRECISION ITALIA STUDY GROUP. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255–264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Wu F, Duan M, Zhang G. Drug-eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history: a comparison of efficacy and safety. Medicine (Baltimore) 2019;98:e15314. doi: 10.1097/MD.0000000000015314. [DOI] [PMC free article] [PubMed] [Google Scholar]


