Abstract
Background: The incidence of thyroid cancer continues to rise all over the world. Thus, it is urgent to find a novel strategy for the treatment of thyroid cancer. Previous reports have confirmed that lncRNA CASC2 is involved in the pathogenesis of thyroid cancer. However, the mechanism by which CASC2 mediates the tumorigenesis of thyroid cancer remains unclear. Methods: Gene and protein expressions in tissues or cells were detected by q-PCR and Western blot, respectively. Cell proliferation was tested by MTT assay. Flow cytometry was used to test cell apoptosis. Cell migration and invasion in thyroid cancer cells was detected by transwell assay. In addition, the correlation between CASC2 and miR-24-3p were investigated by Targetscan and dual-luciferase reporter assay. Finally, xenograft mice model was established to detect the effect of CASC2 on thyroid cancer in vivo. Results: CASC2 was significantly downregulated in thyroid cancer. Overexpression of CASC2 inhibited the proliferation, migration, and invasion of thyroid cancer cells. In addition, upregulation of CASC2 could inhibit the tumorigenesis of TC via sponging miR-24-3p. Furthermore, overexpression of CASC2 significantly suppressed the growth of thyroid cancer in vivo. Conclusion: Overexpression of CCASC2 inhibits the tumorigenesis of thyroid cancer in vitro and in vivo. Thus, CASC2 may serve as a novel target for the treatment of thyroid cancer.
Keywords: CASC2, miR-24, thyroid cancer
Introduction
Thyroid cancer (TC) is a commonly diagnosed malignancy with high incidence rate which is regarded as one of the leading causes of cancer-related death worldwide [1-3]. The pathogenesis of TC remains unclear, leading to difficulties for the early diagnosis and treatment of the disease. In addition, the long-term prognosis of patient with TC is poor [4,5]. The combination of surgery, radiotherapy and chemotherapy are the most commonly used methods for the treatment of TC [1,5,6]. However, metastasis and recurrence usually happen in many cases even after therapies. Thus, it is of great significance to find the novel methods for the treatment of TC.
Long non coding RNAs (lncRNAs) are a group of non-protein-coding transcripts that have been verified to play key roles in physiological processes related with the metabolic and nervous systems [5]. In addition, lncRNAs have been also considered as important mediators in tumorigenesis of multiple malignancies, including TC [7-10]. Meanwhile, lncRNA CASC2 (CASC2) has been verified to be involved in many cancers, including TC [11-13]. However, the biological role of CASC2 in tumorigenesis of TC remains unclear.
In the present study, we aimed to investigate the effect of CASC2 on the progression of TC. Our results proved that CASC2 exerted inhibitory effect on tumorigenesis of TC via sponging miR-24-3p. Thus, our findings may provide a potential novel therapeutic strategy for the treatment of TC.
Methods
Patients and clinical samples
A total number of 90 of TC tissue samples and adjacent tissue samples were obtained from patients with TC in Department of Thyroid and Breast Surgery, The Second Hospital of Hebei Medical University between Jan 2017 and Feb 2018. All patients were diagnosed as TC pathologically, and patients with the history of radio and/or chemotherapies were excluded from this study. The tissue samples were stored immediately in liquid nitrogen after surgery. The informed consent was obtained from each patient. This study was approved by the Ethical Committee of The Second Hospital of Hebei Medical University. The clinical information of the patients was showed in Table 1.
Table 1.
The expression of CASC2 and miR-24 in tissues of patients with TC
| Factors | Case | CASC2 (mean) | P value | miR-24 (mean) | P value |
|---|---|---|---|---|---|
| Gender | 0.629 | 0.881 | |||
| Male | 54 | 0.605±0.131 | 1.843±0.235 | ||
| Female | 36 | 0.513±0.124 | 1.794±0.186 | ||
| Age (years) | 0.535 | 0.843 | |||
| <60 | 63 | 0.662±0.179 | 1.903±0.239 | ||
| ≥60 | 27 | 0.486±0.105 | 1.825±0.221 | ||
| Histological grade | 0.018* | 0.049* | |||
| Well-intermediately differentiation | 19 | 0.606±0.089 | 1.476±0.162 | ||
| Poor Differentiation | 71 | 0.373±0.044 | 2.134±0.165 |
P<0.05.
Cell culture
Human normal thyroid cell line (Nthy-ori 3-1), and TC cell lines (SW579, B-CPAP, BHT101) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in the RPMI-1640 Medium (Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific, Waltham, MA, USA), 100 units/ml penicillin (Sigma-Aldrich, St. Louis, MO, USA) and 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C.
CASC2 overexpression
CASC2 overexpression plasmid was obtained from GenePharma (Shanghai, China). B-CPAP cells were transfected with CASC2 overexpression plasmid (pcDNA3.1-CASC2, Genepharma, Shanghai, China) or empty vector (pcDNA3.1, Genepharma, Shanghai, China) by using lipofectamine 2000 (Invitrogen) in accordance with the manufacturer’s instructions. The transfection efficiency was determined by qRT-PCR.
MiR-24-3p transfection
B-CPAP cells were transfected with miR-24-3p mimics or miR-NC mimics according to the previous reference [14]. MiR-24-3p mimics and negative control RNAs were purchased from GenePharma (Shanghai, China).
Quantitative real time polymerase chain reaction (qRT-PCR)
Total RNAs were extracted from tissues or cell lines with TRIZOL reagent (Invitrogen, Carlsbad, CA, USA). We carried out reverse transcription and real-time PCR assays by means of PrimeScript RT reagent Kit (Takara, Tokyo, Japan) and SYBR premix Ex Taq II kit (Takara, Tokyo, Japan) severally. U6 or GAPDH was used as the internal control. The primers for LncRNA CASC2 were: forward: 5’-GCACATTGGACGGTGTTTCC-3’ and reverse: 5’-CCCAGTCCTTCACAGGTCAC-3’; the primers for miR-24-3p were: forward: 5’-GACCCAGGCCTCTTCACC-3’ and reverse: 5’-AGTCCTTCATGCGACTCAGG-3’; the primers for U6 were forward: 5’-CTCGCTTCGGCAGCACATATACT-3’ and reverse: 5’-ACGCTTCACGAATTTGCGTGTC-3’ and the primers for GAPDH were: forward: 5’-AATGGGCAGCCGTTAGGAAA-3’ and reverse: 5’-TGAAGGGGTCATTGATGGCA-3’. 2-ΔΔCT method was utilized to measure the relative expression.
MTT assay
The 5-diphenyltetrazolium bromide (MTT) assay was performed to examine the proliferation of the TC cells. Briefly, cells were seeded on 96-well plates (5 × 103/well) and cultured overnight. Then, cells were treated with vector-control (NC) or lncRNA CASC2 overexpression (CASC2 OE) for 0, 12, 24 and 48 h, respectively. After that, cells were incubated with 100 μl 0.5 mg/ml MTT for another 4 h at 37°C and supplemented with 150 μl dimethylsulfoxide (DMSO) per well. Finally, the optical density value of each well at 570 nm was examined by microplate reader (Thermo Fisher Scientific).
Cell apoptosis
TC cells were seeded in 6-well plate. Cells were harvested by centrifuging at 1000 rpm/min for 5 min and the residue was resuspended with 100 μl binding buffer. Then, 5 μl Annexin V-FITC and 5 μl propidium (PI) were added in the cell suspension for 15 min. The cell apoptosis rate was measured by flow-cytometer (BD, Franklin Lake, NJ, USA) and the results were analyzed using the software WinMDI 2.9 (Invitrogen).
Wound healing
TC cells were transfected with vector-control (NC) or lncRNA CASC2 overexpression (CASC2 OE) for 48 h. When the cells reached 100% confluence, sterile pipette tips were used to scratch the wound uniformly. Cell motility was assessed by measuring the movement of cells into a scraped wound. The speed of wound closure was monitored at 24 h by measuring the distance of the wound from 0 h. Each experiment was conducted in triplicate.
Transwell assay
For cell invasion analysis, transwell assay was performed in this study. The upper chamber is pre-treated with 100 μl of Matrigel. TC cells were seeded into the upper chamber in media with 1% FBS, and the density was adjusted to about 1.0 × 104 cells per chamber. RPMI1640 medium with 10% FBS was added in the lower chamber. After 24 h of incubation at 37°C, the transwell chamber was rinsed twice with PBS (5 min per time). The cells in the lower chamber were fixed by 5% glutaraldehyde at 4°C and stained with 0.1% crystal violet for 30 minutes. The stained cells were observed under a microscope.
Western blotting
TC cells were lysed using RIPA lysis buffer. The concentration of protein was detected with a BCA protein kit (Thermo Fisher Scientific). Then, proteins (40 μg per lane) were separated with 10% SDS-PAGE gel and then transferred into polyvinylidene fluoride (PVDF, Thermo Fisher Scientific) membranes. After that, the membranes were blocked with 5% skim milk in TBST for 1 h at room temperature, and incubated with the primary antibodies against Bax (Abcam, 1:1000), Bcl-2 (Abcam, 1:1000), cleaved caspase 3 (Abcam, 1:1000), MMP2 (Abcam, 1:1000), MMP9 (Abcam, 1:1000) and β-actin (Abcam, 1:1000) overnight at 4°C. Then, the membranes were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. Finally, the membranes were detected by Enhanced Chemiluminescence (ECL) kit (Thermo Fisher Scientific). β-actin was used as an internal control.
Luciferase activity assay
CASC2 cDNA fragment including microRNA binding sites was cloned into the pmirGLO plasmids (Promega, Madison, USA). Mutant CASC2 (pmirGLO-DGCR5-MUT) was also generated as the control. Luciferase reporter plasmid, miR-24-3p mimics and NC mimics were co-transfected into B-CPAP cells by using Lipofectamine 2000. After 48 h of transfection, the relative luciferase activity was examined by luminometer by Dual-Luciferase Reporter Assay System (Promega).
Xenograft mice model
6 BALB/C nude mice (4-6 weeks) were purchased from the animal center of Nanjing Medical University (Nanjing, China) and kept in specific pathogen-free (SPF) room supplied with food and water adlibitum. The nude mice were subcutaneously injected with B-CPAP cells (5 × 106 cells, in 100 μl of PBS) according to the previous reference [15]. Next, mice were injected with B-CPAP cells transfected with lncRNA CASC2 overexpression (CASC2 OE) or vector-control (NC). Tumor size was measured weekly for four weeks according to the equation: (length × width^2)/2. At the end of the experiments, the mice were sacrificed for the collection of tumors. Then, each tumor was weighed. All in vivo experiments were performed in accordance with National Institutes of Health guide for the care and use of laboratory animals, following a protocol approved by the Ethics Committees of the Second Hospital of Hebei Medical University.
Statistical analysis
Each group were performed at least three independent experiments and all data were expressed as the mean ± standard deviation (SD). The comparison between two groups was analyzed by Student’s t-test. The comparisons among multiple groups were made with one-way analysis of variance (ANOVA) followed by Tukey’s test (Graphpad Prism7). P<0.05 was considered to indicate a statistically significant difference.
Results
CASC2 is significantly downregulated in TC tissues
To detect the gene expression in TC cells, q-PCR was used firstly. As indicated in Figure 1A, the expression of CASC2 in TC tumor samples was significantly downregulated compared with that in normal tissues. Similarly, the expression of CASC2 in TC cells was notably inhibited, compared to Nthy-ori 3-1 cells (Figure 1B). Since B-CPAP cells were more sensitive to CASC2 than other two cell lines, this cell line was used in the following experiments. In addition, the level of CASC2 in TC tissue was negatively associated with the tumor size and metathesis (Table 1). All these results showed that CASC2 played a role of anti-oncogene during the tumorigenesis of TC.
Figure 1.
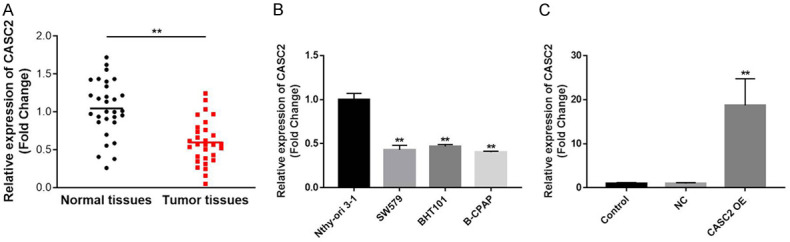
CASC2 is significantly downregulated in TC tissues. A. Expressions of CASC2 in TC tissues and the adjacent normal tissues were detected by qRT-PCR. B. Expressions of CASC2 in TC cell lines and normal cells were investigated by qRT-PCR. C. TC cells were transfected with CASC2 overexpression plasmid. Then, transfection efficiency was obtained by qRT-PCR. GAPDH was used as an internal control. **P<0.01 compared to normal tissues or control.
Next, the expression of CASC2 in B-CPAP cells was forcedly increased using plasmid transfection (Figure 1C). This data indicated that CASC2 overexpression plasmid was stably transfected into TC cells.
Overexpression of CASC2 inhibits TC cell proliferation via inducing apoptosis
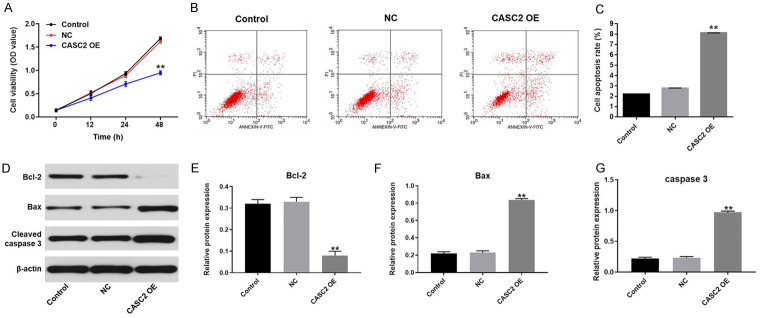
To investigate the cell viability, MTT assay was performed. As demonstrated in Figure 2A, overexpression of CASC2 notably inhibited the viability of B-CPAP cells. In addition, CASC2 overexpression notably induced TC cell apoptosis (Figure 2B and 2C). To verify these results, Western blot was performed. As indicated in Figure 2D-G, overexpression of CASC2 significantly decreased the expression of anti-apoptotic protein (Bcl-2). In contrast, the expression of pro-apoptotic protein (BAX and cleaved caspase-3) was increased CASC2 overexpression. Taken together, overexpression of CASC2 notably inhibited TC cell growth.
Figure 2.
Overexpression of CASC2 induces the inhibition of TC cell growth. A. B-CPAP cells were treated with vector-control (NC) or CASC2 expression vector for 0, 12, 24, or 48 h. Then, the biological function of CASC2 on the viability of B-CPAP cells was assessed by MTT assay. B, C. Cell apoptosis was detected with Annexin V/PI staining. The rate of apoptotic cells was detected by FACS. X axis: the level of Annexin-V FITC fluorescence; Y axis: the PI fluorescence. D. The expressions of Bax, Bcl-2 and cleaved caspase3 in B-CPAP cells were detected by Western blot. β-actin was used as an internal control. E. The relative expression of Bcl-2 was quantified via normalizing to β-actin. F. The relative expression of Bax was quantified via normalizing to β-actin. G. The relative expression of cleaved caspase3 was quantified via normalizing to β-actin. **P<0.01 compared to control.
Upregulation of CASC2 inhibits the migration and invasion of TC in vitro
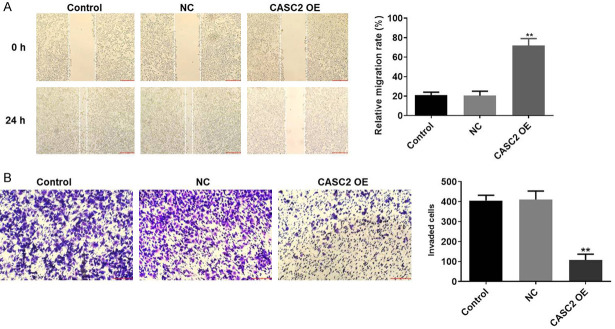
Next, to verify the effect of CASC2 on migration and invasion of TC cells, wound healing and transwell assay were performed. As showed in Figure 3A, relative migration rate of TC cells was significantly increased by CASC2 overexpression. Consistently, the invasion number of TC cells was notably decreased by overexpression of CASC2 (Figure 3B). All these results showed that upregulation of CASC2 inhibited the migration and invasion of TC in vitro.
Figure 3.
Upregulation of CASC2 inhibits the migration and invasion of TC in vitro. A. After 48 h of incubation, relative migration rate of B-CPAP cells was detected by wound healing. B. After 48 h of incubation, the invasion of B-CPAP cells was detected by transwell assay. **P<0.01 compared to control.
Upregulation of CASC2 inhibits the migration and invasion of TC cells via downregulation of MMP2 and MMP9
For the purpose of exploring the mechanism by which CASC2 regulated the migration and invasion of TC cells, Western blot was used. As illustrated in Figure 4A-C, the expressions of migration and invasion related proteins (MMP2 and MMP9) in TC cells were obviously decreased by CASC2 overexpression. All these data suggested that upregulation of CASC2 inhibited the migration and invasion of TC cells via downregulation of MMP2 and MMP9.
Figure 4.
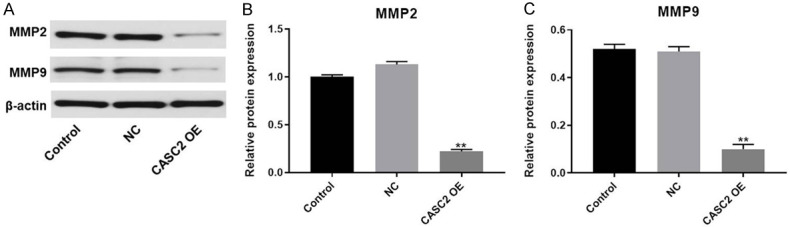
Upregulation of CASC2 inhibits the migration and invasion of TC cells via downregulation of MMP2 and MMP9. A. The expression of MMP2 and MMP9 in B-CPAP cells was detected by Western blot. β-actin was used as an internal control. B. The relative expression of MMP2 was quantified via normalizing to β-actin. C. The relative expression of MMP9 was quantified via normalizing to β-actin. **P<0.01 compared to control.
MiR-24-3p is the downstream target gene of CASC2
To investigate the potential mechanism by which CASC2 modulated the progression of TC in vitro, targetscan and miRDB database were used to search the downstream target gene of CASC2. As indicated in Figure 5A, CASC2 had a putative miR-24-3p targeting site. In addition, Luciferase reporter assay was performed to determine whether miR-24-3p could directly interact with CASC2 in TC cells. The result indicated that co-transfection of the wild-type lncRNA CASC2 vector (WT CASC2) with miR-24-3p mimics significantly reduced luciferase activities compared with mutant CASC2 vector (MT CASC2) (Figure 5B). In addition, the level of miR-24 in TC tissue was negatively correlated with CASC2 expression (Figure 5C). Moreover, the overexpression of miR-24-3p was notably decreased by CASC2 overexpression (Figure 5D). All these data showed that miR-24-3p was the downstream target gene of CASC2.
Figure 5.
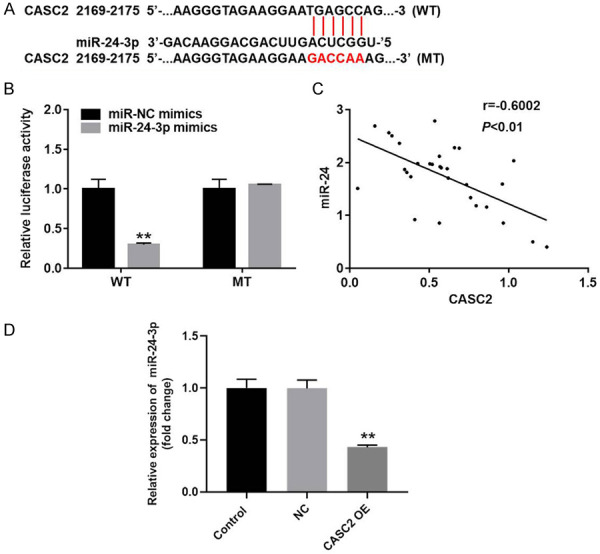
MiR-24-3p is the downstream target gene of CASC2. A. Gene structure of CASC2 at the position of 2169-2175 indicated the predicted target site of miR-24-3p in its 3’UTR. B. The luciferase activity was measured in B-CPAP cells following co-transfecting with WT/MT CASC2 3’-UTR plasmid and miR-24-3p with the dual luciferase reporter assay. C. Equation of linear regression was performed to detect the correction with CASC2 and miR-24-3p. D. The expression of miR-24-3p in TC cells was detected by qRT-PCR. **P<0.01 compared to control.
Overexpression of CASC2 inhibits the progression of TC via sponging miR-24-3p
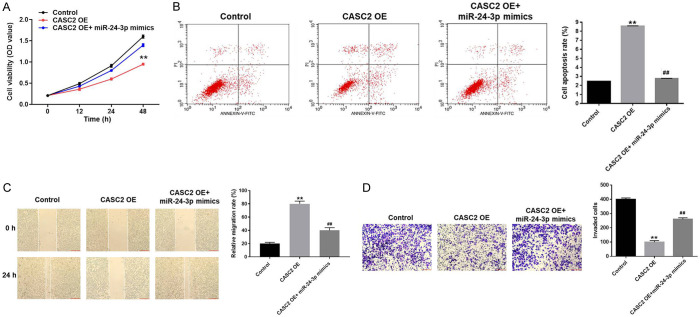
Then, to further investigate the mechanism by which CASC2 modulated the progression of TC, MTT assay was used. As showed in Figure 6A, OD value of TC cells was notably decreased by overexpression of CASC2, while the anti-proliferative effect of CASC2 OE on TC cells was partially reversed by miR-24-3p mimics. Similarly, miR-24-3p mimics significantly suppressed the apoptotic effect of CASC2 overexpression on TC cells (Figure 6B). Next, wound healing and transwell assay were performed. As demonstrated in Figure 6C, relative migration rate of TC cells was significantly increased by overexpression of CASC2. However, anti-migration effect of CASC2 OE on TC was partially reversed by miR-24-3p mimics. Consistently, the invasion of TC cells was significantly inhibited by upregulation of CASC2, which was partially reversed by miR-24-3p mimics (Figure 6D). All these data revealed that upregulation of CASC2 inhibited the progression of TC via sponging miR-24-3p.
Figure 6.
CASC2 inhibits the proliferation and migration of TC cells via targeting miR-24-3p. A. B-CPAP cells were treated vector-control (NC), CASC2 OE or CASC2 OE+miR-24-3p for 0, 12, 24, or 48 h. Then, the biological function of CASC2 on the viability of B-CPAP cells was assessed by MTT assay. B. Cell apoptosis was detected with Annexin V/PI staining. The rate of apoptotic cells was detected by FACS. X axis: the level of Annexin-V FITC fluorescence; Y axis: the PI fluorescence. C. After 48 h of incubation, relative migration rate of B-CPAP cells was detected by wound healing. D. After 48 h of incubation, the invasion of B-CPAP cells was detected by transwell assay. **P<0.01 compared to control. ##P<0.01 compared to CASC2 OE.
Upregulation of CASC2 inhibits the growth TC in vivo
Finally, to investigate the effect of CASC2 on TC in vivo, xenograft mice model was established. As showed in Figure 7A and 7B, the tumor sizes of mice were obviously decreased by CASC2 overexpression. In consistent, tumor weight in CASC2 OE group was significantly decreased compared with that in control group (Figure 7C). Moreover, the expression of CASC2 in tumor tissues of mice was significantly upregulated by CASC2 OE (Figure 7D). All these data suggested that upregulation of CASC2 could inhibit the growth of TC in vivo.
Figure 7.
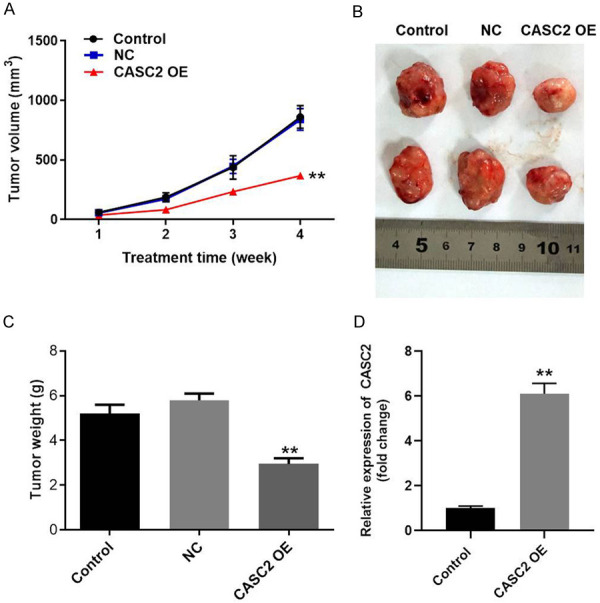
CASC2 inhibits the progression of TC in vivo. B-CPAP cells transfected with CASC2 overexpression plasmid or vector control were subcutaneously injected into nude mice to establish tumor xenograft model. A. Tumor volumes of mice were measured weekly. B. At the end of the study, tumor tissues of mice were collected and pictured. C. Tumor weights in each group of mice were calculated. D. The expression of CASC2 in tumor tissues of mice was detected by qRT-PCR. **P<0.01 compared to control.
Discussion
The roles of CASC2 in different type of cancers, either as tumor suppressor or oncogene, have been discussed previously. For example, Li et al. suggested that down-regulation of CASC2 may contribute to the cisplatin resistance in gastric cancer [16]. Xue et al. proved that CASC2 may inhibit the progression of epithelial ovarian cancer [17]. In addition, Gao et al. suggested that CASC2 can regulate breast cancer cell growth and metastasis through targeting the miR-96-5p [18]. On the other hand, Zheng et al. reported that CASC2 promotes paclitaxel resistance of breast cancer cells via regulation of miR-18a-5p/CDK19 [19]. In TC, Zhou et al. reported that CASC2 expression is down-regulated, and it may promote the cell invasion by affecting epithelial-mesenchymal transition (EMT). In this study, we also observed the decreased of CASC2 in TC tumor tissue and cell lines, which was consistent with Zhou et al. The above results proved that CASC2 was down-regulated in TC and may sever as a tumor suppressor. Our study firstly found CASC2 could bind to miR-24-3p, supplementing the underlying mechanism by which CASC2 mediates the tumorigenesis of TC.
Tumor cells were known as a group of cells with the characteristics of un-controlled growth and increased migration and invasion ability [20,21]. In the present study, we found that overexpression of CASC2 inhibited the proliferation, migration, invasion of TC cells by promoting the apoptosis. Consistently, we also proved that CASC2 could inhibit the growth of TC tumors in xenograft animal models. These results suggested that CASC2 could exert anti-tumor function in vitro and in vivo, suggesting that CASC2 may serve as potential novel target for treating TC.
In previous studies, lncRNAs mediated the progression of diseases via sponging miRNAs [22-24]. With the development of the bioinformatics, the target miRNAs of lncRNAs can be predicted by various methods. In the current study, miR-24-3p has been identified as a target miRNA of CASC2 base on online tools targetscan and miRDB. By literate research, we found that miR-24-3p function as an oncogene in different type of cancers. Our finding was consistent with the previous researches [25-28]. Therefore, we hypothesized that CASC2 may exert its anti-tumor function via downregulating the expression of miR-24-3p. To verify this hypothesis, the relationship between CASC2 and miR-24-3p was also confirmed by dual luciferase reporter assay. We also found the negative correlation relationship between the levels of miR-24-3p and CASC2 in TC tumor tissue. As expected, miR-24-3p mimics partially abrogated the anti-tumor effects of CASC2 on TC cells. Taken together, these results demonstrated that overexpression of CASC2 may inhibit the development of TC by regulation of miR-24-3p.
To sum up, CASC2 may function as a suppressor to inhibit proliferation, migration, and invasion of thyroid cancer cells through sponging miR-24-3p. Our data suggests that overexpression of CASC2 inhibits the progression of TC in vitro and in vivo. Thus, CASC2 may serve as a novel target for treatment of TC.
Disclosure of conflict of interest
None.
References
- 1.Boucai L, Falcone J, Ukena J, Coombs CC, Zehir A, Ptashkin R, Berger MF, Levine RL, Fagin JA. Radioactive iodine-related clonal hematopoiesis in thyroid cancer is common and associated with decreased survival. J Clin Endocrinol Metab. 2018;103:4216–23. doi: 10.1210/jc.2018-00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu W, Zhou Q, Zhao W, Gong Y, Su A, Liu F, Liu Y, Li Z, Zhu J. Ginsenoside Rg3 inhibition of thyroid cancer metastasis is associated with alternation of actin skeleton. Med Food. 2018;21:849–57. doi: 10.1089/jmf.2017.4144. [DOI] [PubMed] [Google Scholar]
- 3.Mousa SA, Glinsky GV, Lin HY, Ashur-Fabian O, Hercbergs A, Keating KA, Davis PJ. Contributions of thyroid hormone to cancer metastasis. Biomedicines. 2018;6:89. doi: 10.3390/biomedicines6030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng X, Xu S, Pan J, Zheng J, Wang X, Yu H, Bao J, Xu Y, Guan H, Zhang L. MKL1 overexpression predicts poor prognosis in patients with papillary thyroid cancer and promotes nodal metastasis. J Cell Sci. 2019;132:jcs231399. doi: 10.1242/jcs.231399. [DOI] [PubMed] [Google Scholar]
- 5.Han CG, Huang Y, Qin L. Long non-coding RNA ZFAS1 as a novel potential biomarker for predicting the prognosis of thyroid cancer. Med Sci Monit. 2019;25:2984–2992. doi: 10.12659/MSM.912921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gul N, Soyluk O, Dogansen SC, Kurtulmus N, Yarman S. Disease activity may not affect the prognosis of coexisting thyroid cancer in acromegalic patients. Exp Clin Endocrinol Diabetes. 2019 doi: 10.1055/a-0915-1982. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Li M, Zhang YY, Shang J, Xu YD. LncRNA SNHG5 promotes cisplatin resistance in gastric cancer via inhibiting cell apoptosis. Eur Rev Med Pharmacol Sci. 2019;23:4185–4191. doi: 10.26355/eurrev_201905_17921. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Hang Y, Xu W, Wu J, Chen L, Chen J, Mao Y, Song J, Song J, Wang H. BLACAT1 predicts poor prognosis and serves as oncogenic lncRNA in small-cell lung cancer. J Cell Biochem. 2018 doi: 10.1002/jcb.27548. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Kang W, Lu X, Ma S, Dong L, Zou B. LncRNA CASC11 promoted gastric cancer cell proliferation, migration and invasion in vitro by regulating cell cycle pathway. Cell Cycle. 2018;17:1886–900. doi: 10.1080/15384101.2018.1502574. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Zhao H, Yu H, Zheng J, Ning N, Tang F, Yang Y, Wang Y. Lowly-expressed lncRNA GAS5 facilitates progression of ovarian cancer through targeting miR-196-5p and thereby regulating HOXA5. Gynecol Oncol. 2018;151:345–55. doi: 10.1016/j.ygyno.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Dai YX, Qiu MK, Wang SQ, Pan C, Wang Y, Ou JM. lncRNA CASC2 suppresses the growth of hemangioma cells by regulating miR-18a-5p/FBXL3 axis. J Biol Regul Homeost Agents. 2020;34:49–56. doi: 10.23812/19-526-A-FULL_ARTICLE. [DOI] [PubMed] [Google Scholar]
- 12.Zou J, Su H, Zou C, Liang X, Fei Z. Ginsenoside Rg3 suppresses the growth of gemcitabine-resistant pancreatic cancer cells by upregulating lncRNA-CASC2 and activating PTEN signaling. J Biochem Mol Toxicol. 2020;34:e22480. doi: 10.1002/jbt.22480. [DOI] [PubMed] [Google Scholar]
- 13.Xiong X, Zhu H, Chen X. Low expression of long noncoding RNA CASC2 indicates a poor prognosis and promotes tumorigenesis in thyroid carcinoma. Biomed Pharmacother. 2017;93:391–7. doi: 10.1016/j.biopha.2017.06.063. [DOI] [PubMed] [Google Scholar]
- 14.Rippa E, La Monica G, Allocca R, Romano MF, De Palma M, Arcari P. Overexpression of gastrokine 1 in gastric cancer cells induces Fas-mediated apoptosis. J Cell Physiol. 2011;226:2571–8. doi: 10.1002/jcp.22601. [DOI] [PubMed] [Google Scholar]
- 15.Luo Y, Hao T, Zhang J, Zhang M, Sun P, Wu L. MicroRNA-592 suppresses the malignant phenotypes of thyroid cancer by regulating lncRNA NEAT1 and downregulating NOVA1. Int J Mol Med. 2019;44:1172–82. doi: 10.3892/ijmm.2019.4278. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Lv S, Ning H, Li K, Zhou X, Xv H, Wen H. Down-regulation of CASC2 contributes to cisplatin resistance in gastric cancer by sponging miR-19a. Biomed Pharmacother. 2018;108:1775–82. doi: 10.1016/j.biopha.2018.09.181. [DOI] [PubMed] [Google Scholar]
- 17.Xue Z, Zhu X, Teng Y. Long noncoding RNA CASC2 inhibits progression and predicts favorable prognosis in epithelial ovarian cancer. Mol Med Rep. 2018;18:5173–81. doi: 10.3892/mmr.2018.9550. [DOI] [PubMed] [Google Scholar]
- 18.Gao Z, Wang H, Li H, Li M, Wang J, Zhang W, Liang X, Su D, Tang J. Long non-coding RNA CASC2 inhibits breast cancer cell growth and metastasis through the regulation of the miR-96-5p/SYVN1 pathway. Int J Oncol. 2018;53:2081–90. doi: 10.3892/ijo.2018.4522. [DOI] [PubMed] [Google Scholar]
- 19.Zheng P, Dong L, Zhang B, Dai J, Zhang Y, Wang Y, Zhang W, Liang X, Su D, Tang J. Long noncoding RNA CASC2 promotes paclitaxel resistance in breast cancer through regulation of miR-18a-5p/CDK19. Int J Oncol. 2018;53:2081–2090. [Google Scholar]
- 20.Zhu K, Ren Q, Zhao Y. lncRNA MALAT1 overexpression promotes proliferation, migration and invasion of gastric cancer by activating the PI3K/AKT pathway. Oncol Lett. 2019;17:5335–42. doi: 10.3892/ol.2019.10253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin YC, Lin CC, Chen TT, Chen JY, Tsai HJ, Wang CY, Tsai HJ, Wang CY, Chen SY. Clozapine induces autophagic cell death in non-small cell lung cancer cells. Cell Physiol Biochem. 2015;35:945–56. doi: 10.1159/000369751. [DOI] [PubMed] [Google Scholar]
- 22.Zhong Y, Wang J, Lv W, Xu J, Mei S, Shan A. LncRNA TTN-AS1 drives invasion and migration of lung adenocarcinoma cells via modulation of miR-4677-3p/ZEB1 axis. J Cell Biochem. 2019;120:17131–17141. doi: 10.1002/jcb.28973. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y, Zhou Y, Han H, Li P, Wei W, Lin N. lncRNA NEAT1 facilitates melanoma cell proliferation, migration, and invasion via regulating miR-495-3p and E2F3. J Cell Physiol. 2019;234:19592–601. doi: 10.1002/jcp.28559. [DOI] [PubMed] [Google Scholar]
- 24.Chu J, Li H, Xing Y, Jia J, Sheng J, Yang L, Sun K, Qu Y, Zhang Y, Yin H, Wan J, He F. LncRNA MNX1-AS1 promotes progression of esophageal squamous cell carcinoma by regulating miR-34a/SIRT1 axis. Biomed Pharmacother. 2019;116:109029. doi: 10.1016/j.biopha.2019.109029. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Jiang L, Zhang G, Li S, Jiang X. MiR-24 promotes migration and invasion of non-small cell lung cancer by targeting ZNF367. J BUON. 2018;23:1413–1419. [PubMed] [Google Scholar]
- 26.Yan L, Ma J, Zhu Y, Zan J, Wang Z, Ling L, Li Q, Lv J, Qi S, Cao Y, Liu Y, Cao L, Zhang Y, Qi Z, Nie L. miR-24-3p promotes cell migration and proliferation in lung cancer by targeting SOX7. J Cell Biochem. 2018;119:3989–98. doi: 10.1002/jcb.26553. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Duan J, Qu Y, Deng T, Liu R, Zhang L, Bai M, Li J, Ning T, Ge S, Wang X, Wang Z, Fan Q, Li H, Ying G, Huang D, Ba Y. Onco-miR-24 regulates cell growth and apoptosis by targeting BCL2L11 in gastric cancer. Protein Cell. 2016;7:141–51. doi: 10.1007/s13238-015-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan B, Chen Y, Song H, Xu Y, Wang R, Chen L. Mir-24-3p downregulation contributes to VP16-DDP resistance in small-cell lung cancer by targeting ATG4A. Oncotarget. 2015;6:317–31. doi: 10.18632/oncotarget.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]