Abstract
Purpose: Inflammatory microenvironment is critical in the transmission of advanced cancer pain. This paper will study how morphine ameliorates advanced cancer pain through inflammatory microenvironment. Methods: Fifty female healthy rats were selected and divided into control group, sham group, model group, morphine group and morphine + 740YP group by random number table. At the left tibia, rats in the model, morphine and morphine + 740YP groups received Walker256 cells injection, while those in the sham group received an equal amount of Hank solution. The control group received no treatment. After modeling, the rats’ spontaneous pain behavior, paw withdrawal mechanical threshold (PWMT) and paw withdrawal thermal latency (PWTL) were measured and statistically analyzed. The protein levels of PI3K, Akt, NF-κB and pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a) in rats were detected. Rat left dorsal root ganglion (DRG) cells were extracted and treated with 10, 20 and 30 μmol/L morphine to observe their effects on the cells. Results: Compared with the control group, the model group presented increased spontaneous pain behavior and PWTL, decreased PWMT, and reduced mechanical pain threshold, as well as enhanced levels of PI3K, Akt, NF-κB and pro-inflammatory factors in vivo as compared to the control group. While the morphine group showed less spontaneous pain behavior and PWTL, increased PWMT, and down-regulated PI3K, Akt, NF-κB and pro-inflammatory factors in vivo in comparison with the model group. After morphine treatment, the apoptosis of DRG cells decreased and the cell activity increased, while PI3K, Akt, NF-κB and pro-inflammatory factors levels decreased. Morphine affected DRG cells in a dose-dependent manner. Up-regulation of PI3K could counteract the inhibitory effect of morphine on chronic tibial cancer pain. Conclusions: Morphine inhibits the promotion of inflammatory microenvironment on chronic tibial cancer pain via the PI3K/Akt/NF-κB pathway, and the regulation of the PI3K/Akt/NF-κB pathway can improve the therapeutic effect of morphine on chronic tibial cancer pain.
Keywords: Chronic tibia cancer pain, morphine, PI3K-Akt-NF-κB pathway, inflammation microenvironment
Introduction
Most of the advanced cancer patients suffer from cancer pain stem from the invasion of tumor cells into bone [1], which is called bone cancer pain (BCP). BCP not only triggers depression in patients, but also seriously damages their quality of life [2,3]. As we know, bone homeostasis is an important basis for the maintenance of essential functions and structures in the human body. However, microenvironment disorders caused by cancer cell invasion excessively promotes osteoclast differentiation and bone resorption activity [4], and this change ignites pain. BCP involves nociceptive pain and neuropathic pain [5]. Pain treatment strategies should be part of the treatment route for cancer patients [6], while unfortunately, due to the incompleteness of the research on the mechanism of BCP, the current treatment strategies cannot fully improve the pain symptoms of cancer patients [7-9].
BCP can be classified into acute, chronic, breakthrough, and refractory cancer pain according to the duration [5], among which the chronic cancer pain is inextricably associated with inflammation microenvironment [10]. Inflammatory factors such as TNF-α, IL-6, and IL-1β secreted by tumor cells are likely to cause chronic cancer pain [11-13]. The inflammatory response disrupts the normal metabolism and biological functions of neurons and non-neurons, thereby inducing or promoting the occurrence of BCP [14-16]. The PI3K-Akt pathway has become a vital tumor-related pathway by regulating cell life processes such as apoptosis and proliferation [17-19]. NF-κB is located downstream of the PI3K/Akt pathway, and its activity is regulated of this pathway [20]. Recently, it is shown that NF-κB is related to the generation and transmission of BCP [21]. The association between NF-κB and BCP may come from its regulation of downstream inflammatory responses [22-24], so NF-κB as a key mediator links the PI3K-Akt pathway to inflammatory responses. However, studies on the effect of PI3K-Akt-NF-κB pathway on BCP remain scanty.
Morphine is a common opioid analgesic that can be used to improve cancer induced BCP [25]. The current research on the mechanism of morphine in treating BCP is not perfect, so in-depth research is still needed. In this paper, the chronic tibial cancer pain model was established by injecting Walker256 cells into the tibia of rats, and then the rats with tibial cancer pain were treated with morphine to observe the effect of morphine on pain behavioral and biological effects of rats. Surprisingly, morphine not only effectively alleviated the pain in rats with tibia cancer pain, but also caused changes in the PI3K-Akt-NF-κB pathway and pro-inflammatory factors. Inspired by the results, we could not help wondering whether morphine could achieve its inhibitory effect on BCP through PI3K-Akt-NF-κB and pro-inflammatory factors. Therefore, this study was carried out to explore a new way of treating BCP with morphine.
Materials and methods
Chronic tibia cancer pain model [26]
The SD rats were anesthetized and placed supine on the operating table, with the left hind limb hair removed and the tibia carefully exposed. Walker256 cells were then injected into the left tibia to construct a chronic tibia cancer pain model. After the injection, the wound was sealed with bone wax and treated with antibiotics to prevent infection. The model group, morphine group, and morphine + 740YP group received Walker256 cell injection, the sham group was injected with the same amount of Hank solution in the left tibia, while the control group received no treatment. In the morphine group, 3 mg/kg morphine was intravenously injected into the tail of rats on the 3rd day after operation, and then 3 mg/kg morphine was injected every two days until the 14th day. In the morphine + 740YP group, 1 mol/L 740YP (single dose) and 3 mg/kg morphine were intravenously injected into the tail of rats on the 3rd day after operation, and then 3 mg/kg morphine was injected every two days until the 14th day.
Pain behavior assessment
Before pain behavior assessment, rats in each group were familiarized with the environment for 2 days. The pain behavior assessment items, including PWMT, PWTL, number of flinches, were tested 3 times before operation, 1 day, 3 days, 7 days and 14 days after operation, and the average value was recorded as the result of the day. The lower the degree of pain, the higher the PWMT and PWTL, and the lower the number of flinches.
ELISA
After evaluating all the pain behaviors of rats 14 days after operation, 1 mL of rat tail vein blood samples were collected and placed in an anticoagulant tube for 30 min of centrifugation at 3 × 103 g and 4°C to collect the supernatant. Pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a) levels in the supernatant were detected by corresponding kits, strictly following the kit operation guide.
Extraction of DRG cells
Fourteen days after operation, euthanasia was performed on rats after evaluating all pain behaviors, and DRG cells, which were from the fourth lumbar segment (L4) and the fifth lumbar vertebra (L5) on both sides, were extracted from the model group. The isolation and culture methods of DRG cells were referenced from Ying [26]. The medium was DMEM containing 10% fetal bovine serum (FBS) + 1% streptomycin-penicillin. DRG cells were cultured at 37°C with 5% CO2. In addition, the brain tissues of rats in each group was separated and cleaned for subsequent research. DRG cells were divided into DMSO group, 0-Mor group, 10-Mor group, 20-Mor group and 30-Mor group. 0-Mor group was not treated with morphine, while 10-Mor group, 20-Mor group and 30-Mor group were given 10 mol/L morphine, 20 mol/L morphine, and 30 mol/L morphine accordingly. Morphine was administered as a single dose, and subsequent detection experiments were performed 36 hours later.
qPCR
Trizol (1 mL) was used to extract total RNA from DRG cells. The total RNA samples with OD260/OD280 > 1.8 were chosen for RT-qPCR. Reverse transcription and amplification of the total RNA samples was performed by TaqMan One Step RT-qPCR Kit (Solarbio, China). Primers for pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a) were designed and synthesized by Tiangen Biotech (Beijing) Co., Ltd. The qPCR reaction system (25 μL) was as follows: upstream primer: 2.5 μL, downstream primer: 2.5 μL, probe: 1.0 μL, RNA template: 10 pg/μg, 25 × One Step RT-qPCR RTase mix: 1 μL, 5 × One Step RT-qPCR Buffer: 5 μL, and RNase-Free ddH2O in a final volume of 25 μL. Reaction conditions: reverse transcription at 50°C for 20 min; denaturation at 95°C for 3 min; denaturation at 95°C for 10-20 s and annealing at 60°C for 20-60 s, cycled 35-50 times. The internal reference genes were GAPDH, and 2-ΔΔCt was used for standardization.
Western blot (WB)
DRG cells or rat DRG tissue samples of each group were prepared as suspensions, and then RIPA lysis buffer was added to lyse the cells. Then the lysate was centrifuged at 1.6104 g and 4°C for 20 min. After discarding the precipitation, 50 μL supernatant was collected for protein concentration determination by bicinchoninic acid (BCA) protein assay kit (Abcam, UK). Next, the proteins were separated by SDS-PAGE electrophoresis with a loading of 20-30 μg. Then the proteins were moved to a polyvinylidene fluoride (PVDF) membrane (EMD millipore Co., Ltd.) and sealed at room temperature with 5% skimmed milk-PBS buffer for 1 h. Thereafter, the proteins to be tested were added with the β-actin antibody and left to stand overnight at 4°C. After that, the PVDF membrane was washed with PBS for three times, added with goat anti-rabbit secondary antibody (HRP cross-linking), and let stand at room temperature for 1 h. Finally, the PVDF membrane was washed with PBS and visualized using enhanced chemiluminescence (ECL) solution. The internal reference protein was β-actin, and the relative expression level of the protein to be measured was equal to the gray value of the band to be measured/the gray value of the GAPDH band. Protein (PI3K, p-PI3K, Akt, p-Akt, NF-κB, TNF-α, IL-1β, IL-6 and IL17a) primary antibodies and goat anti-rabbit secondary antibodies were purchased from Abcam.
MTT method
Cells were inoculated in 3 wells at 3 × 103 cells/well on 96-well plates, and cultured at 37°C with 5% CO2 for 24 h. Then the plate was removed, and 5 mg/ml MTT solution (10 μL) was added for 4 h of incubation at 37°C with 5% CO2. After that, the culture medium was removed and 100 μL of dimethyl sulfoxide (Solarbio company) was added. The OD value at 570 nm was determined.
Flow cytometry
A cell suspension with cell number of 1 × 106 was prepared. Then the cells were treated with 70% ethanol at 4°C for 30 min. Thereafter, the ethanol was removed and the mixed solution of Annexin V-FITC/PI was added to incubate the cells for 30 min at room temperature. The apoptosis rate was determined by FACScan flow cytometer (Becton Dickinson, USA).
Statistics and analysis
The experiment was repeated 3 times, and the measurement results were described as Mean ± SEM (standard error of the mean). SPSS 20.0 (USA) was used for difference analysis between data, and Graphpad 8.0 was applied for picture rendering of the collected data. Differences between the two groups were compared using independent sample T tests, while differences among multiple groups were analyzed by analysis of variance. All comparisons were two-tailed with 95% as the confidence interval. A statistically significant difference was assumed at P<0.05.
Results
Morphine inhibited chronic tibial cancer pain
In this paper, PWMT, PWTL and number of flinches were used to evaluate the chronic tibial cancer pain triggered pain degree and the analgesic effect of morphine on rats with chronic tibial cancer pain. As shown in Figure 1, within 14 days, the PWMT and PWTL in the cancer pain group were statistically lower, while the number of flinches was statistically increased compared with the control group, indicating that the chronic tibia cancer pain model gave rise to obvious pain in rats; compared with the cancer pain group, the PWMT and PWTL in the morphine group were statistically increased, while the number of flinches was statistically decreased within 14 days. The above results suggested that morphine inhibited chronic tibial cancer pain in rats (Figure 1).
Figure 1.
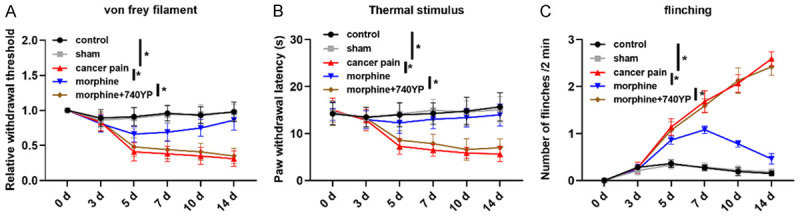
Analgesic effect of morphine on rats with chronic tibia cancer pain. A: Changes of PWMT of rats in each group within 14 days, the value was relative to the control group; B: Changes of PWTL of rats in each group within 14 days; C: Changes of number of flinches in each group within 14 days; *indicates P<0.05.
Effects of morphine on the PI3K-Akt-NF-κB pathway
Results 2.1 identified that morphine could inhibit pain in rats with chronic tibial cancer pain, so this part would discuss the specific molecular mechanism of morphine analgesia. DRG tissues of rats in each group were extracted, and the PI3K-Akt-NF-κB pathway protein (PI3K, Akt, p65, IκB) levels and phosphorylation levels were detected by WB (Figure 2). Compared with the control group, the total protein levels of PI3K, Akt, p65 and IκB in the cancer pain group did not change statistically, but the protein phosphorylation levels of PI3K, Akt, p65 and IκB in the cancer pain group enhanced statistically. Compared with the cancer pain group, PI3K, Akt, p65 and IκB protein levels in the morphine group did not alter statistically, but their protein phosphorylation levels reduced statistically. 740YP is a PI3K activator, which can increase the degree of PI3K phosphorylation. Compared with the morphine group, the PI3K, Akt, p65, and IκB protein levels in the morphine + 740YP group presented no statistical changes, but their protein phosphorylation levels elevated statistically. In addition, result 2.1 showed that the pain degree of the morphine + 740YP group was noticeably stronger than that of the morphine group. The above results revealed that the total protein level of PI3K/Akt/NF-κB pathway did not vary significantly in chronic tibia cancer pain, but the pathway phosphorylation was significantly enhanced, which suggested that morphine inhibited BCP via inhibiting phosphorylation of PI3K/Akt/NF-κB pathway (Figure 2).
Figure 2.
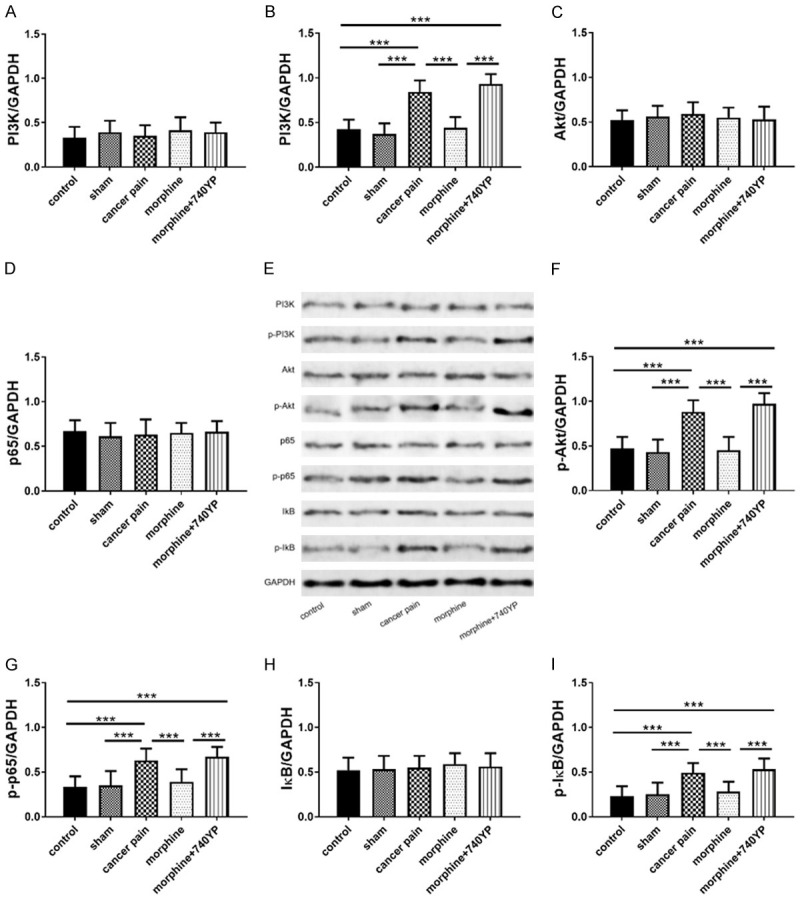
Effects of morphine on PI3K-Akt-NF-κB pathway in DRG tissues of rats with chronic tibial cancer pain. A, C, D, H: Changes of morphine on total protein levels of PI3K, Akt, p65 and IκB; E: Western blot; B, F, G, I: Changes of morphine on the phosphorylation levels of PI3K, Akt, p65, IκB; ***indicates P<0.001.
Effects of morphine on pro-inflammatory factors
Inflammatory microenvironment has a certain influence on cancer pain, while the PI3K/Akt/NF-κB pathway is also involved in regulating the inflammatory microenvironment, so the analgesic effect of morphine may also be related to the inflammatory microenvironment. In this section, pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a) in serum and DRG tissues of each group of rats were measured. As shown in Figure 3, serum and DRG tissue pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a) were statistically increased in the cancer pain group compared with the control group, while those in the morphine group decreased compared with the cancer pain group, and those in the morphine group increased as compared to the morphine group. All these suggested that morphine may inhibit pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a) through the PI3K-Akt-NF-κB pathway (Figure 3).
Figure 3.
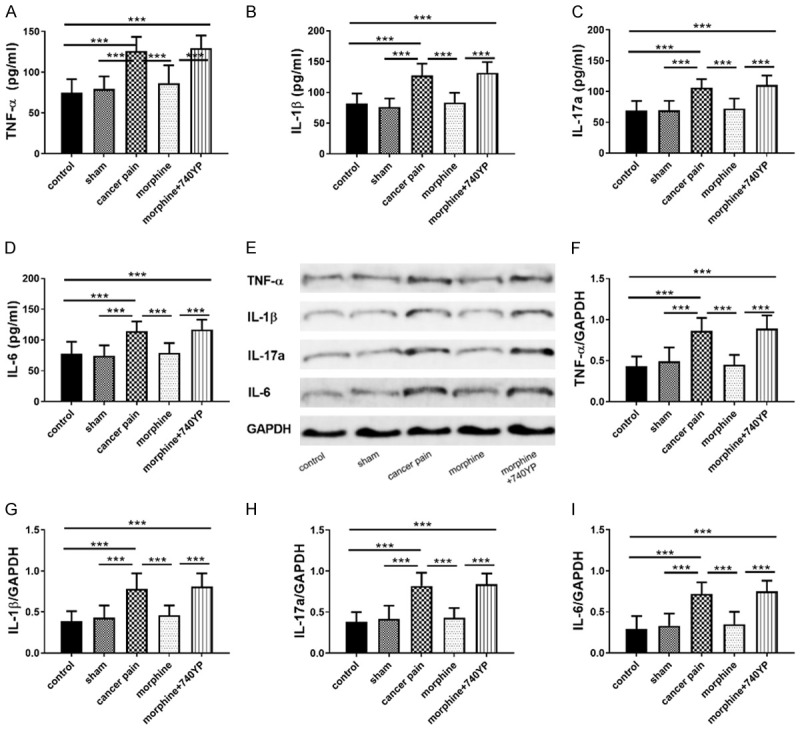
Effects of morphine on inflammatory factors in rats with chronic tibia cancer pain. A-D: Effects of morphine on serum pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a); E: WB diagram; F-I: Effects of morphine on pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a) in DRG tissues; ***indicates P<0.001, 740YP is a PI3K agonist.
Effects of 0-30 μmol/L morphine on DRG cells
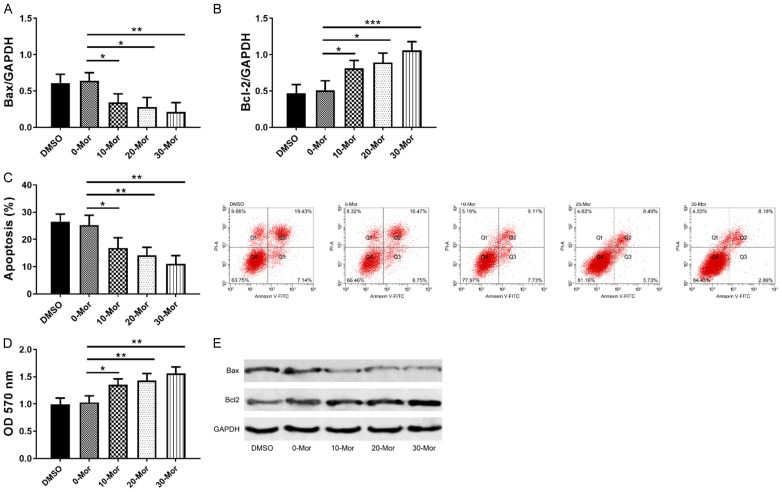
DRG cells play an important role in the transmission and production of BCP, so studying the effects of morphine on DRG cells will also help understand the mechanism by which morphine inhibits cancer pain. In this paper, MTT assay was used to determine the effects of 0-30 μmol/L morphine on DRG cell activity, flow cytometry was used to detect the effects of 0-30 μmol/L morphine on DRG apoptosis, and WB to test apoptosis-related proteins (Bax/Bcl2). As shown in Figure 4, morphine statistically decreased Bax, increased Bcl2 (Figure 4A and 4B), and reduced apoptosis rate (Figure 4C), indicating that morphine inhibited apoptosis by down-regulating Bax and up-regulating Bcl2, and this regulation was dose-dependent. Figure 4E showed a dose-dependent increase in cell activity with morphine. The above results indicated that morphine could inhibit apoptosis and increase cell activity in a dose-dependent manner (Figure 4).
Figure 4.
Effects of 0-30 µmol/L morphine on DRG cells. A, B: Effects of 0-30 µmol/L morphine on Bax, Bcl-2; C: Effects of 0-30 µmol/L morphine on apoptosis; D: Western blot; E: Effects of 0-30 µmol/L morphine on cell activity; *indicates P<0.05, and **indicates P<0.01.
Effects of 0-30 μmol/L morphine on PI3K/Akt/NF-κB pathway in DRG cells
As shown in Figure 5, 0-30 μmol/L morphine did not cause changes in PI3K, Akt, p65, and IκB protein levels, but dramatically reduced p-PI3K, p-Akt, p-p65, and p-IκB levels. These findings revealed that morphine only participated in the regulation of PI3K/Akt/NF/κB pathway activity in DRG, and this regulation was dose-dependent (Figure 5).
Figure 5.
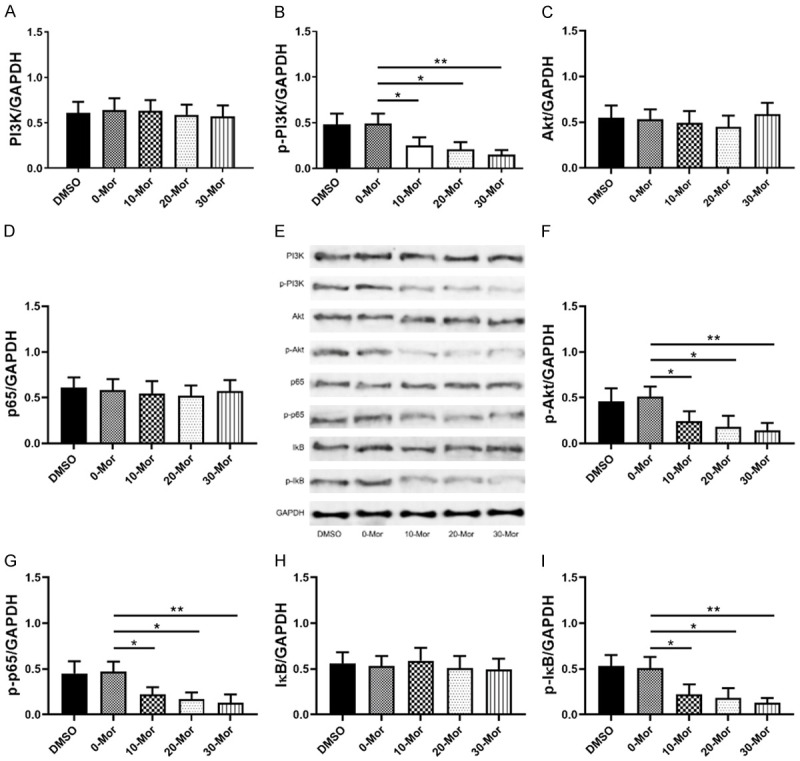
Effects of 0-30 µmol/L morphine on PI3K-Akt-NF-κB pathway in DRG cells. A, B: Effects of 0-30 µmol/L morphine on PI3K and phosphorylated PI3K (p-PI3K); C, F: Effects of 0-30 µmol/L morphine on Akt and phosphorylated Akt (p-Akt); D, G: Effects of 0-30 µmol/L morphine on p65 and phosphorylated p65 (p-65); E: WB diagram; H, I: Effects of 0-30 µmol/L morphine on IκB and phosphorylated IκB (p-IκB); *indicates P<0.05, and **indicates P<0.01.
Effects of 0-30 μmol/L morphine on pro-inflammatory factors in DRG cells
As shown in Figure 6, 0-30 μmol/L morphine could down-regulate mRNA and protein levels of pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a). It suggested that in DRG cells, morphine regulated the inflammatory microenvironment by inhibiting pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a) in DRG cells in a dose-dependent manner (Figure 6).
Figure 6.
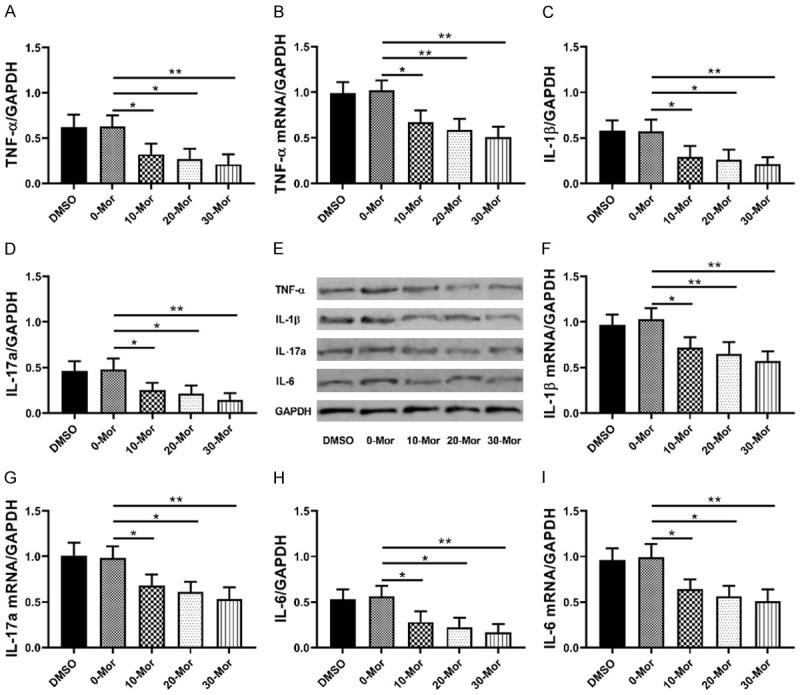
Effects of 0-30 µmol/L morphine on inflammatory factors in DRG cells. A, B: Effects of 0-30 µmol/L morphine on TNF-αprotein and TNF-α mRNA; C, F: Effects of 0-30 µmol/L morphine on IL-1β protein and IL-1β mRNA; D, G: Effects of 0-30 µmol/L morphine on IL-17a protein and IL-17a mRNA; E: WB diagram; H, I: Effects of 0-30 µmol/L morphine on IL-6 protein and IL-6 mRNA; *indicates P<0.05, and **indicates P<0.01.
Discussion
Cancer pain caused by cancer cells invading bone tissues is one of the common complications plaguing cancer patients. The pain not only seriously affects the treatment effect of cancer patients, but also destroys their physical function and mental health. And this is where analgesic drugs, one of the current management strategies for cancer pain, come in. Morphine is a commonly used analgesic. In this paper, the rat tibia was injected with Walker256 cells to establish a model of chronic tibial cancer pain, and the possible ways of treating cancer pain with morphine were explored, so as to expand the application value of morphine.
The following dimensions, including PWMT, PWTL and number of flinches within 14 days were used in this paper to determine the analgesic effect of morphine on chronic tibial cancer pain in rats. Figure 1 shows that morphine effectively restored PWMT and PWTL of rats within 14 days, and reduced number of flinches, indicating that morphine can effectively alleviate the pain in rats with chronic tibia cancer pain. Interestingly, the PI3K/Akt/NF-κB pathway was found to be related to morphine in the subsequent detection of DRG tissue protein levels. As shown in Figure 2, PI3K, Akt, p65 and IκB protein levels in cancer pain rats did not change statistically, while the phosphorylation levels increased evidently, and morphine could markedly inhibit the phosphorylation levels of PI3K, Akt, p65 and IκB, suggesting that rather than directly changing the protein levels, morphine regulated the PI3K-Akt-NF-κB pathway through regulating its phosphorylation levels. The PI3K/Akt pathway is a non-negligible signal pathway in carcinogenesis, and can positively regulate the activity of NF-κB protein through phosphorylation. Inhibition of NF-κB has been shown to alleviate BCP [21]. Morphine inhibits the PI3K/Akt/NF-κB pathway [27], so the inhibitory effect of morphine on BCP may be achieved by down-regulating the activity of the PI3K-Akt-NF-κB pathway.
But how it works? Based on the biological influence of NF-κB, we assumed that the inflammatory microenvironment may be involved in BCP. Figure 3 demonstrates that pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a) were increased in serum and DRG tissues of rats with chronic tibia cancer pain, while morphine effectively reduced pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a), which indicated that NF-κB is a major regulator in inflammatory microenvironment. Morphine down-regulates NF-κB activity through the PI3K/Akt/NF-κB pathway, leading to the down-regulation of downstream levels of pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a). Finally, this change in the inflammatory microenvironment may alleviate BCP by improving endoplasmic reticulum stress [28].
DRG cells are also vital in the transmission and production of BCP. Based on the preceding results, the effects of 0-30 μmol/L morphine on DRG cells were explored. Figures 4, 5 and 6 reveal that DRG cells treated with morphine presented reduced apoptosis, increased cell viability, and down-regulated PI3K, Akt, NF-κB and pro-inflammatory factors (TNF-α/IL-1β/IL-6/IL-17a) levels in a dose-dependent manner. PI3K-Akt-NF-κB pathway is involved in apoptosis and participates in apoptosis by regulating Bad or Caspase-9 phosphorylation. Similarly, NF-κB is an essential regulatory element of cellular inflammatory response and is responsible for regulating the level of downstream inflammatory cytokines such as TNF-α. All these indicate that morphine may also regulate DRG cell apoptosis and inflammatory microenvironment through the PI3K-Akt-NF-κB pathway, thereby mitigating chronic tibia cancer pain.
This paper explores the treatment role of morphine in chronic tibia cancer pain, and holds that morphine regulated the promotion of inflammatory microenvironment on chronic tibial cancer pain through the PI3K-Akt-NF-κB pathway. It is found that in DRG tissues and DRG cells of rats with chronic tibial cancer pain, morphine inactivated the PI3K/Akt/NF-κB pathway, thereby changing pro-inflammatory factors levels in the inflammatory microenvironment, and finally relieving chronic tibial cancer pain. Meanwhile, the analgesic effect of morphine may be dose-dependent. Whereas, the PI3K-Akt-NF-κB pathway is not an independent signaling pathway, whether other mediators are involved in the regulation between morphine and this pathway is also a possible mechanism for future discussion.
Conclusion
In summary, this paper finds that morphine inhibits the promotion of inflammatory microenvironment on chronic tibial cancer pain by inactivating the PI3K/Akt/NF-κB pathway, which may be conducive to expanding the application of morphine in the management of BCP.
Disclosure of conflict of interest
None.
References
- 1.Appel CK, Scheff NN, Viet CT, Schmidt BL, Heegaard AM. Decitabine attenuates nociceptive behavior in a murine model of bone cancer pain. Pain. 2019;160:619–631. doi: 10.1097/j.pain.0000000000001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shueb SS, Erb SJ, Lunzer MM, Speltz R, Harding-Rose C, Akgün E, Simone DA, Portoghese PS. Targeting MOR-mGluR5 heteromers reduces bone cancer pain by activating MOR and inhibiting mGluR5. Neuropharmacology. 2019;160:107690. doi: 10.1016/j.neuropharm.2019.107690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remeniuk B, King T, Sukhtankar D, Nippert A, Li N, Li F, Cheng K, Rice KC, Porreca F. Disease modifying actions of interleukin-6 blockade in a rat model of bone cancer pain. Pain. 2018;159:684–698. doi: 10.1097/j.pain.0000000000001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoneda T, Hiasa M, Okui T. Crosstalk between sensory nerves and cancer in bone. Curr Osteoporos Rep. 2018;16:648–656. doi: 10.1007/s11914-018-0489-x. [DOI] [PubMed] [Google Scholar]
- 5.Russo MM, Sundaramurthi T. An overview of cancer pain: epidemiology and pathophysiology. Semin Oncol Nurs. 2019;35:223–228. doi: 10.1016/j.soncn.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Sindhi V, Erdek M. Interventional treatments for metastatic bone cancer pain. Pain Manag. 2019;9:307–315. doi: 10.2217/pmt-2018-0073. [DOI] [PubMed] [Google Scholar]
- 7.Falk S. Carbenoxolone as a novel therapy for attenuation of cancer-induced bone pain. Pain. 2018;159:1127–1136. doi: 10.1097/j.pain.0000000000001197. [DOI] [PubMed] [Google Scholar]
- 8.Gardner K, Laird BJA, Fallon MT, Sande TA. A systematic review examining clinical markers and biomarkers of analgesic response to radiotherapy for cancer-induced bone pain. Crit Rev Oncol Hematol. 2019;133:33–44. doi: 10.1016/j.critrevonc.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Zhou YQ, Liu DQ, Chen SP, Sun J, Zhou XR, Rittner H, Mei W, Tian YK, Zhang HX, Chen F, Ye DW. Reactive oxygen species scavengers ameliorate mechanical allodynia in a rat model of cancer-induced bone pain. Redox Biol. 2018;14:391–397. doi: 10.1016/j.redox.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sica A, Casale B, Dato MTD, Calogero A, Spada A, Sagnelli C, Santagata M, Buonavolontà P, Fiorelli A, Salzano A, Dodaro CA, Martinelli E, Saracco E, Troiani T, Tammaro D, Ciardiello F, Papa A. Cancer- and non-cancer related chronic pain: from the physiopathological basics to management. Open Med (Wars) 2019;14:761–766. doi: 10.1515/med-2019-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheff NN, Ye Y, Bhattacharya A, MacRae J, Hickman DN, Sharma AK, Dolan JC, Schmidt BL. Tumor necrosis factor alpha secreted from oral squamous cell carcinoma contributes to cancer pain and associated inflammation. Pain. 2017;158:2396–2409. doi: 10.1097/j.pain.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, Ye DW, Tian YK. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. 2016;13:141. doi: 10.1186/s12974-016-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang W, Wang Y, Sun W, Zhang M. Morin suppresses astrocyte activation and regulates cytokine release in bone cancer pain rat models. Phytother Res. 2017;31:1298–1304. doi: 10.1002/ptr.5849. [DOI] [PubMed] [Google Scholar]
- 14.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwekkeboom KL, Tostrud L, Costanzo E, Coe CL, Serlin RC, Ward SE, Zhang Y. The role of inflammation in the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. J Pain Symptom Manage. 2018;55:1286–1295. doi: 10.1016/j.jpainsymman.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veiga-Fernandes H, Artis D. Neuronal-immune system cross-talk in homeostasis. Science. 2018;359:1465–1466. doi: 10.1126/science.aap9598. [DOI] [PubMed] [Google Scholar]
- 17.Spangle JM, Roberts TM, Zhao JJ. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim Biophys Acta Rev Cancer. 2017;1868:123–131. doi: 10.1016/j.bbcan.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danielsen SA, Eide PW, Nesbakken A, Guren T, Leithe E, Lothe RA. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim Biophys Acta. 2015;1855:104–121. doi: 10.1016/j.bbcan.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Singh SS, Yap WN, Arfuso F, Kar S, Wang C, Cai W, Dharmarajan AM, Sethi G, Kumar AP. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: a reality for personalized medicine? World J Gastroenterol. 2015;21:12261–12273. doi: 10.3748/wjg.v21.i43.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham TR, Odero-Marah VA, Chung LW, Agrawal KC, Davis R, Abdel-Mageed AB. PI3K/Akt-dependent transcriptional regulation and activation of BMP-2-Smad signaling by NF-kappaB in metastatic prostate cancer cells. Prostate. 2009;69:168–180. doi: 10.1002/pros.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song ZP, Xiong BR, Guan XH, Cao F, Manyande A, Zhou YQ, Zheng H, Tian YK. Minocycline attenuates bone cancer pain in rats by inhibiting NF-kappaB in spinal astrocytes. Acta Pharmacol Sin. 2016;37:753–762. doi: 10.1038/aps.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somensi N, Rabelo TK, Guimarães AG, Quintans-Junior LJ, de Souza Araújo AA, Moreira JCF, Gelain DP. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int Immunopharmacol. 2019;75:105743. doi: 10.1016/j.intimp.2019.105743. [DOI] [PubMed] [Google Scholar]
- 23.Chamanara M, Abdollahi A, Rezayat SM, Ghazi-Khansari M, Dehpour A, Nassireslami E, Rashidian A. Thymol reduces acetic acid-induced inflammatory response through inhibition of NF-kB signaling pathway in rat colon tissue. Inflammopharmacology. 2019;27:1275–1283. doi: 10.1007/s10787-019-00583-8. [DOI] [PubMed] [Google Scholar]
- 24.Carneiro NVQ, Silva HBFD, Silva RRD, Carneiro TCB, Costa RS, Pires AO, Marques CR, Velozo ES, Conceição AS, Silva TMSD, Silva TMGD, Alcântara-Neves NM, Figueiredo CA. Sambucus australis modulates inflammatory response via inhibition of nuclear factor kappa B (NF-kB) in vitro. An Acad Bras Cienc. 2019;91:e20170831. doi: 10.1590/0001-3765201920170831. [DOI] [PubMed] [Google Scholar]
- 25.Bu H, Xia Y, Liu P, Guo H, Yuan C, Fan X, Huang C, Wen Y, Kong C, Wang Tao, Ma L, Li X, Zhang H, Zhang L, Ma M, Ai Y, Zhang W. The roles of chemokine CXCL13 in the development of bone cancer pain and the regulation of morphine analgesia in rats. Neuroscience. 2019;406:62–72. doi: 10.1016/j.neuroscience.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Han Y, Li Y, Xiao X, Liu J, Meng X, Liu F, Xing G, Wan Y. Formaldehyde up-regulates TRPV1 through MAPK and PI3K signaling pathways in a rat model of bone cancer pain. Neurosci Bull. 2012;28:165–172. doi: 10.1007/s12264-012-1211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin D, Woodruff M, Zhang Y, Whaley S, Miao J, Ferslew K, Zhao J, Stuart C. Morphine promotes Jurkat cell apoptosis through pro-apoptotic FADD/P53 and anti-apoptotic PI3K/Akt/NF-kappaB pathways. J Neuroimmunol. 2006;174:101–107. doi: 10.1016/j.jneuroim.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Mao Y, Wang C, Tian X, Huang Y, Zhang Y, Wu H, Yang S, Xu K, Liu Y, Zhang W, Gu X, Ma Z. Endoplasmic Reticulum stress contributes to nociception via neuroinflammation in a murine bone cancer pain model. Anesthesiology. 2020;132:357–372. doi: 10.1097/ALN.0000000000003078. [DOI] [PubMed] [Google Scholar]