Abstract
Severe acute pancreatitis (SAP) contributes to multiple organ dysfunction and intestine is one of the most susceptible targets. This study aims to explore the role of C3a/C3aR axis in SAP-induced intestinal barrier injury. Adult male Sprague Dawley rats were randomly divided into control, SAP, C3aRA (0.06 mg/kg) and C3aRA (0.12 mg/kg) groups. SAP rat models were established by retrograde injection of 3.5% sodium taurocholate solutions into pancreatic ducts. Histopathological changes and dysfunction in pancreatitis and intestine were measured by hematoxylin and eosin (H&E) staining and detection of amylase (AMY), lipase (LIPA), endotoxins and diamine oxidase (DAO) levels in serum. Cell apoptosis was evaluated by TUNEL assay and western blot analysis. In addition, the expressions of caudin-1, caudin-2, occludin and ZO-1 were detected by western blot assay and immunohistochemical staining. Inflammatory cytokines and oxidative stress levels in SAP rats were determined. The C3a/C3aR expression was increased in pancreatic and intestinal tissues of successfully established SAP rat models. C3a receptor antagonist (C3aRA) alleviated pancreatic and intestinal pathological lesions and dysfunction induced by SAP. C3aRA inhibited cell apoptosis and promoted the expressions of caudin-1, caudin-2, occludin and ZO-1 in intestinal tissues. Moreover, C3aRA repressed inflammatory cytokines by reduction of TNF-α, IL-1β, IL-6 and MCP-1 levels, and ameliorated oxidative stress through regulation of ROS, MPO and SOD activity in rats with SAP-induced intestinal barrier injury. Our findings suggested that inhibition of C3a/C3aR axis diminished pancreatic damage and SAP-induced intestinal barrier injury in vivo, which may provide a new therapeutic strategy for SAP-induced intestinal injury.
Keywords: Complement system, C3a/C3Ar, C3a receptor antagonist, severe acute pancreatitis, intestinal barrier injury
Introduction
Severe acute pancreatitis (SAP) is an acute abdominal disease that is characterized by pancreatic self-necrosis, contributing to systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) [1]. Internal and external pathogenic factors induce cell injury and disconnect the intercellular junction, which provokes the generation of chemokines, pro-inflammatory cytokines, and adhesion factors. Inflammatory mediators can further result in multiple damage of organs, including intestine, lung, and kidney [2,3]. It has been shown that intestine is one of the target organs damaged in SAP-triggered MODS and intestinal barrier damage is intimately associated with the pathogenesis and pathophysiology of SAP [4]. Nevertheless, the underlying mechanisms by which intestinal barrier injury is induced by SAP are still elusive.
Complement system is an indispensable supportive part of the innate immunity. It is widely shared that C3 is the central complement component that modulates cascade activation of complement molecules. The reactions at the point of C3 cleavage lead to generation of bioactive fragments C3a and C3b [5]. C3a has been indicated to be a type of anaphylatoxin that gives rise to the extravasation of host immune cells and the formation of epithelial mesenchymal transition (EMT) by binding to its receptor C3aR, a member of the rhodopsin family [6,7]. A previous study has revealed that C3a and C3aR exert pathogenic effects on aristolochic acid nephropathy (AAN), while an inhibitor of C3aR (C3aRA) can suppress the development of AAN via preventing the coupling of C3a to its receptor [8]. However, the role of C3a and C3aR in SAP and SAP-induced intestinal barrier injury remains underexplored.
In the current study, we hypothesized that C3a/C3aR axis was associated with the pathogenesis and progress of SAP-induced intestinal barrier injury. To test this hypothesis, we established rat models of SAP-induced intestinal barrier injury and detected the effects of C3a/C3aR on pathological changes, biochemical index, cell apoptosis, inflammatory responses, and oxidative stress levels, firstly revealing the molecular mechanism of C3a/C3aR in intestinal barrier injury.
Materials and methods
Animal care and experimental design
Adult male Sprague Dawley rats (weighing 250 ± 50 g) were purchased from the Laboratory Animal Center of China. All animals were individually caged in a temperature-controlled environment (12 hours light-dark cycle) with free access to food and water. All experimental procedures were approved by the Animal Care and Use Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and all animal experiments were performed according to the regulations of the Chinese guidelines for the care and use of laboratory animals.
After one-week acclimation, the rats were randomly divided into four groups (n=15 per group): control, SAP, C3a receptor antagonist (C3aRA; 0.06 mg/kg) and C3aRA (0.12 mg/kg). C3aRA [No. (M04496); Purity (99.45%); Chemical formula (C24H29F3N4O6)] was purchased from Beijing Baiolaibo Technology Co. LTD. After fasting for 12 h before the surgery, rats were anesthetized with 50 mg/kg phenobarbital and were subjected to a midline laparotomy. According to the previous study [9], rats in the SAP group was induced by retrograde infusion of 3.5% sodium taurocholate (0.1 mL/100 g body weight) into the pancreatic duct. Rats in the control group underwent retrograde infusion of normal saline of equivalent volume. Rats in C3aRA group were administered with 0.06 mg/kg and 0.12 mg/kg C3aRA intragastric 30 minutes before SAP induction. Simultaneously, rats in the control and SAP groups were administered with normal saline solution of equivalent volume intragastric 30 minutes before SAP induction. Rats were anesthetized and sacrificed 24 h after the surgery, and the specimens including blood, pancreas and gut tissues were harvested promptly.
Histopathological examination
Pancreatic and intestinal tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 4 μm-thick sections. Then the sections were stained with hematoxylin and eosin (H&E), and the pathological changes in the pancreas and intestine were checked by using a light microscope (Olympus, Tokyo, Japan). The pathological changes of the pancreatic and intestinal tissues were scored by two experienced pathologists in a blinded manner according to previous literatures [10,11], respectively. In brief, the pancreatic histopathology changes were measured and classified according to a scale from 0 to 4 for the degrees of edema (the extent of diffuse expansion of interlobar septae), inflammatory infiltration (leukocyte infiltration quantity in intralobular or perivascular), hemorrhage, and necrosis (number of foci involved). For intestinal tissue: 0 indicates normal small intestine mucosa villi; 1 indicates Gruenhagen’s space beneath the intestinal mucosal epithelium at the apex of the villus, which often accompanies capillary hyperemia; 2 indicates intestinal mucosa epithelial cells rising from the intrinsic membrane and expansion of the spaces under the intestinal epithelium; 3 indicates most of the intestinal mucosa epithelium is raised, villi are toppled over on both sides, and the top parts of the villi have fallen off; 4 indicates scaling and peeling of the intrinsic membrane, expansion of bare capillaries, and intrinsic membrane components increase; and 5 indicates digestion or metamorphosis of the lamina propria, bleeding, or ulceration.
Detection of serum amylase (AMY), lipase (LIPA), endotoxins and diamine oxidase (DAO) levels
Rat blood samples were harvested and centrifuged at 3500 rpm for 5 min at room temperature. The supernatant was collected and stored at -80°C. Serum AMY and LIPA activity were detected with an automatic biochemical analyzer (Vitros V5600, Johnson, USA). Endotoxin levels and DAO activity were measured by using corresponding ELISA kits (Nanjing Jiancheng Co. Ltd., Nanjing, China).
Analysis of tissue wet/dry weight ratio
The pancreatic and intestinal tissues were weighed to assess the wet weight. After that, these tissues were dried at 60°C for 48 h and weighed to gain the dry weight. Finally, the wet/dry weight ratios of the pancreas and intestine were calculated.
TUNEL assay
Cell apoptosis was estimated by terminal deoxynucleotidyl transferasemediated dUTP nick end-labeling (TUNEL) assay. In brief, pancreatic, and intestinal tissue sections were fixed with 4% paraformaldehyde and incubated with proteinase K for 15 min in 37°C. After being placed in 3% H2O2 for 5 min at room temperature, the sections were treated by TUNEL detection kit. The cell nuclei were stained with hematoxylin with staining with 4’, 6-diamidino-2-phenylindole (DAPI; Sigma) as the staining agent. TUNEL-positive cells were observed under a fluorescence microscopy and an average of five areas from each section were analyzed.
Examination of the intestinal permeability
The intestinal permeability was assessed according to the method described in Yasuda et al. [12]. Briefly, rats subjected to the induction of SAP were anesthetized and the midline laparotomy was performed. About 5 cm-length jejunal tissue was dissected and rinsed with normal saline gently. One end of the jejunum was ligated while the other end was injected with 200 mL 40 mg/mL FITC-Dextran and subsequently ligated. Thereafter, the jejunal segment was immersed in 0.9% saline solution for 1 h in agitation. The concentration of FITC-Dextran was detected to identify the intestinal permeability.
Immunohistochemical staining
Intestinal tissue slides were deparaffinized and incubated with 3% hydrogen peroxide for 10 min. The slides were treated with citrate buffer (0.01 M, pH 6.0) for antigen retrieval. After incubation for 15 min, anti-occludin antibodies (ab216327, 1:200) were added to the sections overnight at 4°C. After washing twice with PBS, slides were incubated with Alexa Fluor conjugated secondary antibodies for 1 h at room temperature. Then the tissues were stained with DAB and counterstained with hematoxylin. Five fields were randomly selected in each sample and captured with a light microscope (Olympus, Tokyo, Japan).
Western blot analysis
Total proteins from pancreatic and intestinal tissues were extracted by RIPA lysis buffer and the concentration of the proteins were detected by BCA protein assay kit. Then proteins from each sample were separated using SDS-PAGE gels and transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, USA). After blockage with 5% fat-free milk at 37°C for 1 h, the membrane was incubated with primary antibodies (C3a, C3aR, Bcl-2, Bax, cleaved caspase3, cleaved caspase7, caudin-1, caudin-2, occludin, ZO-1 and GAPDH) at 4°C overnight. The membrane was washed three times with PBS and incubated in HRP-conjugated secondary antibody. Finally, the bands were visualized by an enhanced chemiluminescence kit (Bio-Rad, USA) and analyzed using Image J software.
Measurements of inflammatory cytokines and oxidative stress levels
The levels of TNF-α, IL-1β, IL-6 and MCP-1 in serum of rats were detected by enzyme-linked immunosorbent assay (ELISA) kits (Lengton Company, Shanghai, China) according to the manufacturer. In addition, the ROS level, MPO level and SOD activity were evaluated by corresponding commercially available kit (R&D, Systems Minneapolis, MN, USA) in accordance with manufacturer’s instructions. Each group was replicated for six times.
Statistical analysis
Values were analyzed by SPSS 18.0 software (SPSS Inc., USA) and were depicted as the mean ± standard deviation (SD). Student’s t-test was used to determine the significance of two group differences and one-way analysis of variance (ANOVA) was performed for multiple comparisons. The significance of differences in histopathological scores was assessed using the Kruskal-Wallis test. P < 0.05 was considered to be statistically significant.
Results
The expressions of C3a and C3aR were increased in rats with SAP-induced intestinal barrier injury
To ascertain the role of C3a/C3aR in SAP-induced intestinal barrier injury, SAP rat models were constructed. As shown in Figure 1A, the protein expressions of C3a and C3aR in pancreatic and intestinal tissues were both significantly increased. In addition, neutrophil infiltration, edema, hemorrhage, and acinar cell necrosis on pancreas mucosa were observed in the pancreas of rats with SAP, compared with the control group (Figure 1B), which indicated that SAP rat models were established successfully.
Figure 1.

The levels of C3a and C3aR in SAP rat model were enhanced. A. Western blot analysis showed that the protein levels of C3a and C3aR were upregulated in pancreatic and intestinal tissues. B. Histological changes of pancreatic tissues of SAP-induced rats were observed by H&E staining. Magnification, ×200. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01 versus control.
C3aRA ameliorated SAP-induced pathological and functional damage in pancreas
Next, we examined the effects of C3aRA on pancreatic pathological and functional injuries induced by SAP. As shown in Figure 2A, SAP treatment induced neutrophil infiltration, edema, and hemorrhage of pancreatic tissues, whereas, C3aRA groups significantly reversed the histological changes of pancreatic tissues in SAP group. In C3aRA (0.12 mg/kg) group, the pancreatic lobule structures were clear with only a small amount of edema, hemorrhage, and inflammatory cell invasion, and necrotic areas were the smallest, compared to the SAP group. The mean histopathologic scores of pancreases in C3aRA groups were noticeably decreased in comparison with the SAP group (Figure 2B). Additionally, serum AMY and LIPA levels in the SAP group were significantly elevated relative to the control group and C3aRA reduced AMY and LIPA levels induced by SAP (Figure 2C and 2D). Furthermore, treatment with 0.06 mg/kg and 0.12 mg/kg C3aRA remarkably reduced the pancreas wet/dry ratio reaching the highest level in SAP group (Figure 2E). These data suggested that C3aRA exerted therapeutic effect on SAP-induced pancreatic pathological and functional injuries.
Figure 2.
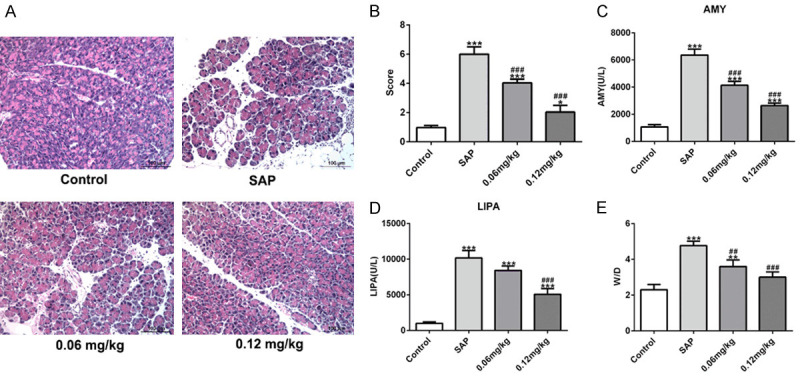
C3aRA alleviated SAP-induced pathological and functional injuries in pancreas. Mice were divided into four groups: control, SAP, C3a receptor antagonist (C3aRA; 0.06 mg/kg) and C3aRA (0.12 mg/kg). Pancreatic tissues and serum were collected at 24 h after the surgery. A. Hematoxylin and eosin (H&E) staining. Magnification, ×200. B. Pathological score of pancreases. C. Serum amylase (AMY) levels. D. Serum lipase (LIPA) levels. E. Pancreas wet/dry ratios. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 versus control; ##P < 0.01, ###P < 0.001 versus SAP groups.
C3aRA alleviated cell apoptosis in SAP-induced pancreatic tissues
To investigate the effect of C3aRA on pancreatic tissues with induction of SAP, TUNEL assay and western blot analysis were performed to assess the cell apoptosis in rats with different treatments. As exhibited in Figure 3A, cell apoptosis rate in the SAP group was extremely higher than that in the control group. Addition of 0.06 mg/kg and 0.12 mg/kg C3aRA markedly reduced the increased apoptosis rate in SAP-induced rats. Moreover, the results from western blot assay revealed that decreased Bcl-2 level and increased levels of Bax, cleaved caspase3 and cleaved caspase7 were observed in the SAP group comparing to the control group. However, C3aRA treatment enhanced the Bcl-2 level and reduced the levels of Bax, cleaved caspase3 and cleaved caspase7 changed by SAP (Figure 3B). The results indicated that C3aRA inhibited cell apoptosis during SAP-induced injury of pancreatic tissues.
Figure 3.
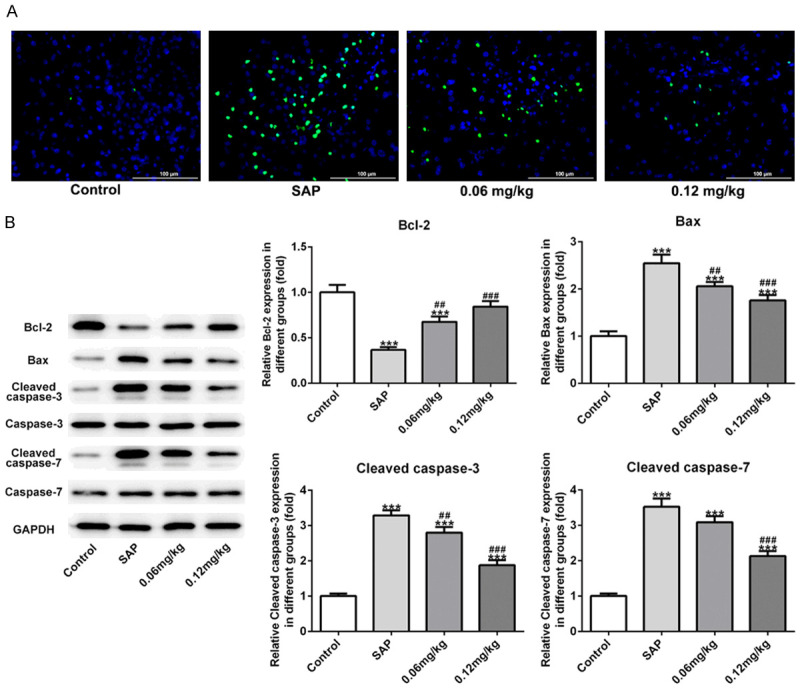
C3aRA inhibited cell apoptosis in pancreatic tissues of SAP-induced rats. A. TUNEL assay was performed to evaluate cell apoptosis rate. Magnification, ×400. B. The levels of Bcl-2, Bax, cleaved caspase3 and cleaved caspase7 were detected by western blot assay. Data are expressed as mean ± SD. ***P < 0.001 versus control; ##P < 0.01, ###P < 0.001 versus SAP groups.
C3aRA attenuated intestinal pathological injury induced by SAP
We further identified the intestinal damage caused by SAP and the effects of C3aRA on SAP-induced intestinal injury. As presented in Figure 4A, the intestinal tissues in the SAP group showed inflammatory infiltration in intestinal mucosa, irregular villi, mucosal necrosis, and intestinal epithelium shedding and necrosis. C3aRA treatment alleviated these intestinal pathological changes under SAP induction. C3aRA (0.12 mg/kg) group exhibited intestinal mucosal epithelial cell swelling, and generally normal morphology when compared with the SAP group. Compared with the SAP group, the scores of intestinal pathological damages were significantly lower in two C3aRA groups (Figure 4B). In addition, the levels of endotoxins and the activity of DAO were greatly decreased in C3aRA groups compared with those in the SAP group (Figure 4C and 4D). And the wet/dry ratios in intestinal tissues with C3aRA treatment were significantly reduced compared with the SAP group (Figure 4E). Besides, SAP distinctly increased intestinal permeability, which was rescued by C3aRA (Figure 4F). These data demonstrated that C3aRA relieved SAP-induced intestinal pathological injury.
Figure 4.
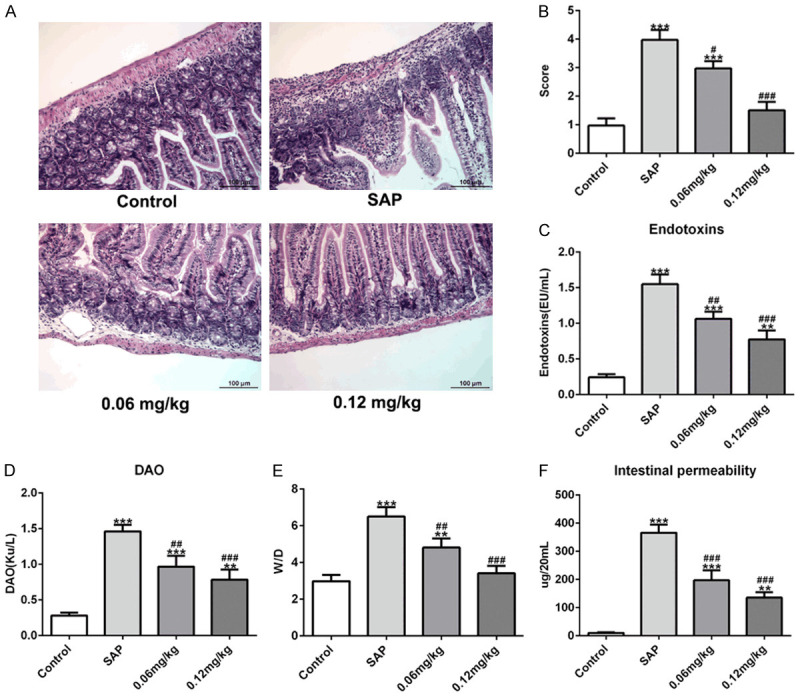
Effects of C3aRA on intestinal pathological injury induced by SAP. A. Hematoxylin and eosin (H&E) staining. Magnification, ×200. B. Pathological score of intestines. C. Endotoxins levels. D. Diamine oxidase (DAO) levels. E. Intestine wet/dry ratios. F. Intestinal permeability. Data are expressed as mean ± SD. **P < 0.01, ***P < 0.001 versus control; #P < 0.05, ##P < 0.01, ###P < 0.001 versus SAP groups.
Effects of C3aRA on tight junctions in SAP-induced intestinal tissues
To explore the influence of C3aRA on intestinal tissues of SAP-induced rats, tight junctions were detected by western blot analysis and immunohistochemistry. As displayed in Figure 5A, the protein levels of caudin-1, caudin-2, occludin and ZO-1 in the SAP group were obviously reduced compared with the control group, but C3aRA abolished the inhibitory effect of SAP on these proteins. In agreement with these results, immunohistochemical staining discovered that SAP-decreased occluding level in intestinal tissues was significantly enhanced by C3aRA treatment (Figure 5B). All the results suggested that the levels of tight junctions in SAP-induced intestinal tissues could be affected by C3aRA.
Figure 5.

C3aRA rescued the tight junctions in intestinal tissues induced by SAP. A. The protein levels of tight junctions were measured by western blot analysis. B. Immunohistochemical staining was employed to determine the occluding level in intestinal tissues. Magnification, ×200. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 versus control; #P < 0.05, ##P < 0.01 versus SAP groups.
C3aRA inactivated cell apoptosis in SAP-induced intestinal tissues
Next, we studied the effect of C3aRA on the apoptosis of intestinal tissues induced by SAP. TUNEL assay was implemented to measure the cell apoptosis, the results of which revealed that the number of apoptotic cells was greatly increased during the induction of SAP while the rate of cell apoptosis was largely reduced after treatment of C3aRA (Figure 6A). Furthermore, western blot results unveiled that C3aRA increased the level of Bcl-2 and reduced the levels of Bax, cleaved caspase3 and cleaved caspase7 caused by SAP (Figure 6B). These results demonstrated that C3aRA played an inhibitory role in intestinal cell apoptosis induced by SAP.
Figure 6.

Effects of C3aRA on cell apoptosis in intestinal tissues of SAP-induced rats. A. TUNEL assay was carried out to elucidate cell apoptosis rate. Magnification, ×400. B. The levels of apoptosis-related proteins were evaluated by western blot analysis. Data are expressed as mean ± SD. **P < 0.01, ***P < 0.001 versus control; #P < 0.05, ##P < 0.01, ###P < 0.001 versus SAP groups.
C3aRA suppressed inflammatory response and oxidative stress in rats with SAP
Finally, we examined the effects of C3aRA on inflammation and oxidative stress in SAP rats. As shown in Figure 7A, the secretion of TNF-α, IL-1β, IL-6 and MCP-1 in serum was obviously increased in the SAP group whereas the increase was reversed after C3aRA treatment. In addition, the elevated levels of ROS and MDA by SAP were significantly reduced and the inhibited SOD activity by SAP was noticeably enhanced in the C3aRA groups (Figure 7B). The results indicated that C3aRA ameliorated SAP-induced inflammatory response and oxidative stress.
Figure 7.

Effects of C3aRA on inflammatory response and oxidative stress in SAP-induced rats. A. The secretions of TNF-α, IL-1β, IL-6 and MCP-1 in serum from SAP-induced rats were detected by ELISA kits. B. The levels of ROS, MDA and SOD were measured after treatment of C3aRA. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 versus control; #P < 0.05, ##P < 0.01, ###P < 0.001 versus SAP groups.
Discussion
SAP is a severe and emergent disease with high-mortality, which are capable of inducing and exacerbating systemic inflammatory response syndrome, even MODS [13,14]. It is well established that SAP contributes to intestinal mucosal barrier injury and leads to a series of bowel diseases [15-17]. The intestinal mucosal barrier, composed of mechanical, immune, chemical, and biological parts, plays a crucial part in preventing toxins, bacteria and inflammatory factors in the enteric cavity entering the blood circulation. The SAP altered intestinal permeability may be the primary cause for the infection complications of the gastrointestinal tract [18,19].
In the present study, the administration of 3.5% sodium taurocholate was implemented to establish the model of SAP with analogous clinical pathological changes [9]. According to the results of H&E staining, the rats treated with sodium taurocholate showed the typical features of pancreatic injury, such as edema, hemorrhage, and inflammatory infiltration, which provided a precondition for the investigation of C3aRA effect on SAP. Thereafter, we found the levels of C3a and C3aR in SAP rats were upregulated and blockade of C3a/C3aR altered pathological and functional damages, cell apoptosis, inflammatory response, and oxidative stress not only in pancreas tissues but also in intestine tissues. These findings innovatively found that C3a/C3aR could be applied as a new intervention target for SAP, providing a new idea for the treatment of SAP-caused diseases.
Previous studies showed that the levels of central complement components of the classical (e.g. C1q, C4) and the alternative (e.g. C3) pathways in plasma of patients with a severe course are obviously decreased, while complement fragments (C3a, C5a) are elevated, indicating that activation of the complement cascade in pancreas may exert detrimental effects [20-23]. For instance, C3a and C5a can induce inflammatory mediators and aggravate renal ischemia-reperfusion injury, deficiency of which attenuates renal ischemia-reperfusion injury [24]. Besides, complement activation has been affirmed to occur in patients with acute pancreatitis [25,26]. Gloor et al. reported that both C3a and sC5b-9 are significantly upregulated in plasma of patients with SAP, which may serve as predictive highly specific parameters for severe acute pancreatitis [27]. In this study, we observed the excessive expressions of C3a and C3aR in both pancreatic and intestinal tissues of rats with SAP, consistent with previous reports.
As the antagonists of complement anaphylatoxin receptors, C3aRA has therapeutic potential in several animal disease models, such as Alzheimer, obesity and metabolic dysfunction [28,29]. In our study, C3aRA reversed SAP-induced pathological and functional damages in pancreas by alleviating histological injury, reducing the excessive release of AMY and LIPA, and relieving pancreatic edema. In addition, intestinal pathological injury resulted from SAP was also ameliorated, which was validated by suppression of endotoxins and DAO level as well as reduction of intestinal permeability after the treatment of C3aRA. Tight junctions, the primary apical structures in epithelium and endothelium, take part in barrier function by forming cell-to-cell contacts and blocking paracellular pathway [11]. It was reported that disruption of tight junctions leads to the leakage of amylase and lipase in acute pancreatitis and the increase generations of pro-inflammatory factors [30]. In the present study, in SAP-induced intestinal tissues, tight junctions including caudin-1, caudin-2, occludin and ZO-1 showed an obvious reduction in protein level but C3aRA rescued the disordered tendency.
Oxidative stress, inflammatory response and cell apoptosis have been proved to play fatal roles in the development of SAP [31-33]. Excessive production of inflammatory cytokines caused by SAP contributes to the pathogenesis of the pancreatic and intestinal barrier dysfunction, further increasing permeability and promoting apoptosis of intestinal mucosal cells [34-36]. Hence, inhibition of the inflammatory cytokines and oxidative stress in SAP may ameliorate gut barrier dysfunction and block the progress of SAP [37,38]. In this study, SAP significantly induced cell apoptosis of pancreatic and intestinal tissues, promoted inflammatory response and oxidative stress. However, C3aRA treatment repressed cell apoptosis in pancreas and intestine, reduced the release of inflammatory factors and alleviated oxidative stress triggered by SAP. These results indicated that C3aRA attenuated SAP-induced pathological and functional injuries in pancreas and intestine and suppressed further damage from inflammatory cytokines and oxidative stress via blocking C3a/C3aR axis.
However the precise mechanism by which C3a/C3aR axis protects against intestinal damage is yet to be comprehensively elucidated. The limitation of the present study, i) Lack of C3a/C3aR knockout or overexpression experiments in vivo, which can intuitively show the role of C3a/C3aR in SAP; ii) Lack of evidence that C3aRA improves other tissue damages in pancreatitis due to SAP, for example lung. These are exactly the direction of our future research.
Conclusions
In conclusion, the present study revealed for the first time that C3aRA protected pancreas and intestine from SAP-induced damage in rats. C3aRA also exerted strong anti-inflammatory, anti-oxidative and anti-apoptosis effects on SAP-induced intestinal injury. These findings suggest that blockage of C3a/C3aR axis may be a novel therapeutic target for pancreatic and intestinal damages induced by SAP.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (No. 81901986). All experimental procedures were approved by the Animal Care and Use Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and all animal experiments were performed according to the regulations of the Chinese guidelines for the care and use of laboratory animals.
Disclosure of conflict of interest
None.
References
- 1.Shen J, Wan R, Shen Z, Gao J, Wang X, Qian L, Lu H, Han W, Wang X. Chemokine receptor CXCR3 is involved in the acute pancreatitis-associated lung injury. Biomed Pharmacother. 2012;66:390–396. doi: 10.1016/j.biopha.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Yang CJ, Chen J, Phillips AR, Windsor JA, Petrov MS. Predictors of severe and critical acute pancreatitis: a systematic review. Dig Liver Dis. 2014;46:446–451. doi: 10.1016/j.dld.2014.01.158. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Li W, Wang X, Li J, Yu W. Early gut mucosal dysfunction in patients with acute pancreatitis. Pancreas. 2008;36:192–196. doi: 10.1097/MPA.0b013e31815a399f. [DOI] [PubMed] [Google Scholar]
- 4.Gurleyik E, Coskun O, Ustundag N, Ozturk E. Prostaglandin E1 maintains structural integrity of intestinal mucosa and prevents bacterial translocation during experimental obstructive jaundice. J Invest Surg. 2006;19:283–289. doi: 10.1080/08941930600889391. [DOI] [PubMed] [Google Scholar]
- 5.Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Complement system part II: role in immunity. Front Immunol. 2015;6:257. doi: 10.3389/fimmu.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawlisch H, Frank R, Hennecke M, Baensch M, Sohns B, Arseniev L, Bautsch W, Kola A, Klos A, Kohl J. Site-directed C3a receptor antibodies from phage display libraries. J Immunol. 1998;160:2947–2958. [PubMed] [Google Scholar]
- 8.Ye J, Qian Z, Xue M, Liu Y, Zhu S, Li Y, Liu X, Cai D, Rui J, Zhang L. Aristolochic acid I aggravates renal injury by activating the C3a/C3aR complement system. Toxicol Lett. 2019;312:118–124. doi: 10.1016/j.toxlet.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Ning JW, Zhang Y, Yu MS, Gu ML, Xu J, Usman A, Ji F. Emodin alleviates intestinal mucosal injury in rats with severe acute pancreatitis via the caspase-1 inhibition. Hepatobiliary Pancreat Dis Int. 2017;16:431–436. doi: 10.1016/S1499-3872(17)60041-9. [DOI] [PubMed] [Google Scholar]
- 10.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 11.Zhao G, Zhuo YZ, Cui LH, Li CX, Chen SY, Li D, Liu JH, Li DH, Cui NQ, Zhang SK. Modified Da-chai-hu decoction regulates the expression of occludin and NF-kappaB to alleviate organ injury in severe acute pancreatitis rats. Chin J Nat Med. 2019;17:355–362. doi: 10.1016/S1875-5364(19)30041-X. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda T, Takeyama Y, Ueda T, Shinzeki M, Sawa H, Nakajima T, Kuroda Y. Breakdown of intestinal mucosa via accelerated apoptosis increases intestinal permeability in experimental severe acute pancreatitis. J Surg Res. 2006;135:18–26. doi: 10.1016/j.jss.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 13.Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol. 2014;20:13879–13892. doi: 10.3748/wjg.v20.i38.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. 2004;291:2865–2868. doi: 10.1001/jama.291.23.2865. [DOI] [PubMed] [Google Scholar]
- 15.Deng W, Abliz A, Xu S, Sun R, Guo W, Shi Q, Yu J, Wang W. Severity of pancreatitisassociated intestinal mucosal barrier injury is reduced following treatment with the NADPH oxidase inhibitor apocynin. Mol Med Rep. 2016;14:3525–3534. doi: 10.3892/mmr.2016.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang XP, Zhang J, Song QL, Chen HQ. Mechanism of acute pancreatitis complicated with injury of intestinal mucosa barrier. J Zhejiang Univ Sci B. 2007;8:888–895. doi: 10.1631/jzus.2007.B0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian R, Tan JT, Wang RL, Xie H, Qian YB, Yu KL. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis. Eur Rev Med Pharmacol Sci. 2013;17:349–355. [PubMed] [Google Scholar]
- 18.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lankisch PG, Koop H, Kaboth U. Serum complement factors in human acute pancreatitis. Hepatogastroenterology. 1981;28:261–263. [PubMed] [Google Scholar]
- 21.Lasson A, Laurell AB, Ohlsson K. Correlation among complement activation, protease inhibitors, and clinical course in acute pancreatitis in man. Scand J Gastroenterol. 1985;20:335–345. doi: 10.3109/00365528509091661. [DOI] [PubMed] [Google Scholar]
- 22.Roxvall L, Bengtson A, Heideman M. Anaphylatoxin generation in acute pancreatitis. J Surg Res. 1989;47:138–143. doi: 10.1016/0022-4804(89)90078-4. [DOI] [PubMed] [Google Scholar]
- 23.Roxvall LI, Bengtson LA, Heideman JM. Anaphylatoxins and terminal complement complexes in pancreatitis. Evidence of complement activation in plasma and ascites fluid of patients with acute pancreatitis. Arch Surg. 1990;125:918–921. doi: 10.1001/archsurg.1990.01410190116019. [DOI] [PubMed] [Google Scholar]
- 24.Peng Q, Li K, Smyth LA, Xing G, Wang N, Meader L, Lu B, Sacks SH, Zhou W. C3a and C5a promote renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23:1474–1485. doi: 10.1681/ASN.2011111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchler M, Malfertheiner P, Schoetensack C, Uhl W, Beger HG. Sensitivity of antiproteases, complement factors and C-reactive protein in detecting pancreatic necrosis. Results of a prospective clinical study. Int J Pancreatol. 1986;1:227–235. doi: 10.1007/BF02795248. [DOI] [PubMed] [Google Scholar]
- 26.Uhl W, Muller C, Buchler MW. [Definition of predictors of a complicated course in acute pancreatitis] . Langenbecks Arch Chir Suppl Kongressbd. 1998;115:427–433. [PubMed] [Google Scholar]
- 27.Gloor B, Stahel PF, Muller CA, Schmidt OI, Buchler MW, Uhl W. Predictive value of complement activation fragments C3a and sC5b-9 for development of severe disease in patients with acute pancreatitis. Scand J Gastroenterol. 2003;38:1078–1082. doi: 10.1080/00365520310005965. [DOI] [PubMed] [Google Scholar]
- 28.Lim J, Iyer A, Suen JY, Seow V, Reid RC, Brown L, Fairlie DP. C5aR and C3aR antagonists each inhibit diet-induced obesity, metabolic dysfunction, and adipocyte and macrophage signaling. FASEB J. 2013;27:822–831. doi: 10.1096/fj.12-220582. [DOI] [PubMed] [Google Scholar]
- 29.Lian H, Yang L, Cole A, Sun L, Chiang AC, Fowler SW, Shim DJ, Rodriguez-Rivera J, Taglialatela G, Jankowsky JL, Lu HC, Zheng H. NFkappaB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron. 2015;85:101–115. doi: 10.1016/j.neuron.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt M, Klonowski-Stumpe H, Eckert M, Luthen R, Haussinger D. Disruption of paracellular sealing is an early event in acute caerulein-pancreatitis. Pancreas. 2004;28:181–190. doi: 10.1097/00006676-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Lopez Martin A, Carrillo Alcaraz A. Oxidative stress and acute pancreatitis. Rev Esp Enferm Dig. 2011;103:559–562. doi: 10.4321/s1130-01082011001100001. [DOI] [PubMed] [Google Scholar]
- 32.Rakonczay Z Jr, Hegyi P, Takacs T, McCarroll J, Saluja AK. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–267. doi: 10.1136/gut.2007.124115. [DOI] [PubMed] [Google Scholar]
- 33.Peng Y, Gallagher SF, Landmann R, Haines K, Murr MM. The role of p65 NF-kappaB/RelA in pancreatitis-induced kupffer cell apoptosis. J Gastrointest Surg. 2006;10:837–847. doi: 10.1016/j.gassur.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Luo Y, Li Z, Liu Y, Liu Z. Effects of erythropoietin pretreatment on pro-and anti-inflammatory balance in rats with severe acute pancreatitis. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:93–96. [PubMed] [Google Scholar]
- 35.Rychter JW, van Minnen LP, Verheem A, Timmerman HM, Rijkers GT, Schipper ME, Gooszen HG, Akkermans LM, Kroese AB. Pretreatment but not treatment with probiotics abolishes mouse intestinal barrier dysfunction in acute pancreatitis. Surgery. 2009;145:157–167. doi: 10.1016/j.surg.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Bai X, Song Z, Zhou Y, Pan S, Wang F, Guo Z, Jiang M, Wang G, Kong R, Sun B. The apoptosis of peripheral blood lymphocytes promoted by hyperbaric oxygen treatment contributes to attenuate the severity of early stage acute pancreatitis in rats. Apoptosis. 2014;19:58–75. doi: 10.1007/s10495-013-0911-x. [DOI] [PubMed] [Google Scholar]
- 37.Wang YL, Zheng YJ, Zhang ZP, Su JY, Lei RQ, Tang YQ, Zhang SD. Effects of gut barrier dysfunction and NF-kappaB activation on aggravating mechanism of severe acute pancreatitis. J Dig Dis. 2009;10:30–40. doi: 10.1111/j.1751-2980.2008.00360.x. [DOI] [PubMed] [Google Scholar]
- 38.Dang S, Shen Y, Yin K, Zhang J. TREM-1 promotes pancreatitis-associated intestinal barrier dysfunction. Gastroenterol Res Pract. 2012;2012:720865. doi: 10.1155/2012/720865. [DOI] [PMC free article] [PubMed] [Google Scholar]


