Abstract
The coronavirus disease-19 (COVID-19) has been a global pandemic and caused thousands of deaths worldwide. So far, although some studies suggested some medications may be helpful, there is no effective treatment for COVID-19. It is critical to find important risk factors that affects the recovery or severity of COVID-19 and guide the treatment for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In this study, we recruited these discharged patients with COVID-19 from hospitals. We collected clinical data and analyzed the time from disease onset to the positive-to-negative transmission (TPNT) of nucleic acid tests and its related clinical variables. TPNT was considered as an important indicator for the recovery of COVID-19 patients from SARS-CoV-2 infection. COVID-19 patients were divided into short TPNT group and long TPNT group. There were significant differences on hypertension, abidol treatment, serum alanine aminotransferase (ALT), lymphocyte counts, and serum triglyceride (TG) between two groups (P<0.05). Patients in low TPNT group had higher serum triglyceride and less proportion of hypertension. Further logistic regression analysis showed that TPNT was highly associated with serum TG level and hypertension that were related to the expression of ACE2, the targeting protein for the invasion of SARS-CoV-2. Therefore, our findings demonstrate that serum triglyceride level and hypertension were important influencing factors for the recovery from SARS-CoV-2 infection. Therefore, diet changes and antihypertensive medications can be translational to the treatment of COVID-19 and promote the recovery of COVID-19 patients.
Keywords: COVID-19, SARS-Cov-2, virus removal, triglyceride, hypertension
Introduction
The coronavirus disease-19 (COVID-19) has been a global pandemic and caused thousands of deaths worldwide. With a sudden outbreak of an unknown-pathogen pneumonia, Chinese scientists firstly reported a coronavirus as the pathogen and announced its RNA sequencing [1], which was officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of viruses on February 11, 2020 [2]. Later, this pneumonia was found to have a potential of person-to-person transmission and a high infectivity [3,4]. Due to the publication of the virus RNA sequences, the detection of viral nucleic acid became the gold standard for the diagnosis of COVID-19. The diagnosis of COVID-19 can be made on the basis of epidemiological data, respiratory system symptoms, and positive real-time fluorescent RT-PCR for samples from patients [5]. Currently, there is no specific drug for antiviral treatment of SARS-CoV-2 infection, although chloroquine and remdesivir were reported to show in some cases of COVID-19 [6,7]. It seemed that COVID-19 is a self-limiting disease in mild-moderate patients [8]. More importantly, a large proportion of patients with COVID-19 were asymptomatic and become the source of disease transmission [9-12]. Therefore, it is critical to find the determinant factors that affects the recovery of COVID-19 or the viral clearance. Several studies have demonstrated that ageing, hypertension, high serum LDH level, serum d-dimer level, and obesity are risk factors that are highly associated with the mortality of severer COVID-19 patients [13-15]. However, the risk factors in the mild and moderate COVID-19 patients were not identified. Current clinical data showed that only about 20% of COVID-19 patients developed severe disease [16]. Considering the large proportion of asymptomatic, mild, and moderate patients, here, we performed a retrospective analysis in COVID-19 patients discharged from hospitals and analyzed the association between the time from disease onset to positive-to-negative transmission (TPNT) and clinical variables.
Materials and methods
Patients
Laboratory-confirmed COVID-19 patients in three cities (Xuzhou, Lianyungang, and Yangzhou) in Jiangsu Province, China from January to March, 2002 were included in this study. The diagnostic criteria of COVID-19 was according to the Guidelines for the Diagnosis and Treatment of Novel Coronavirus Infection by National Health Commission of the People’s Republic of China (Trial Version 6). Types A and B of influenza virus were excluded from all the patients. For SARS-CoV-2-infected patients, before being discharged from hospitals, the disappears of symptoms and two consecutive negative results for viral nucleic acid tests were required. Patients without complete clinical data were removed from this study. This study was approved by the institutional review board from local hospitals. Informed consent was obtained from patients.
Detection of SARS-CoV-2
Samples were collected by using pharyngeal swabs every 1-2 days after admission and sent to the local Center for Disease Control and Prevention for the detection of SARS-CoV-2. The real-time polymerase chain reaction (RT-PCR) method was used to detect SARS-CoV-2 RNA.
The retrospective study designs and data collection
Clinical information was obtained from clinical records. In this study, TPNT was defined as the time from disease onset to the date for the first negative viral RNA test after the diagnosis. According to the median of TPNT, patients were divided into two groups: low TPNT group and high TPNT group. In two groups, we collected the baseline demographic data (i.e. age, gender), clinical risk factors (i.e. hypertension, diabetes mellitus, onset time, TPNT, previous medical history, admission diagnosis, clinical classification, use of antiviral drugs, use of glucocorticoid, use of thymus, use of Aluvia, and use of Interferon). Laboratory examinations included lymphocyte count, neutrophil count, white blood cell (WBC) counts, fasting blood-glucose, serum aspertate aminotransferase (AST), alanine transaminase (ALT), lactate dehydrogenase (LDH), albumin (Alb), C-reactive protein (CRP), ferritin, Interleukin-6 (IL-6), triglycerides (TG), total cholesterol (TC) were measured. NLR was calculated as the ratio of the absolute neutrophil count to the absolute lymphocyte count on admission.
Statistical analysis
Continuous variables were expressed as mean ± SD or median (interquartile range, IQR). The difference between two groups was compared by using student’s t test or Mann-Whitney test as appropriate. The comparison between multiple groups was expressed by LSD-t test. Categorical variables were shown in percentages and compared by chi-squared test or Fisher’s exact test. Univariate logistic regression analysis was performed to find variables that accounted for TPNT. To adjust for confounding factors with P<0.1, multivariate logistic regression analysis was used to assess any independent factors. Data was presented as adjusted OR (aOR) and respective 95% CI. All the data were analyzed by SPSS 22.0 statistical software. The difference was statistically significant (P<0.05).
Results
Total 67 patients (37 males and 30 females) with COVID-19 were included in this study. Most patients are mild-moderate clinical types and 6 cases were severe patients. The average age was 45.2 years old. The median TPNT was 16 days. According to the cut-off value 16 d of TPNT, patients were divided into two groups: short TPNT (≤16 d) group and long TPNT (>16 d) group. The clinical data in two groups were summarized in Table 1. We compared age, gender, clinical types, complications, treatments, and laboratory results in both groups. We found that there were significant differences on hypertension, abidol treatment, serum alanine aminotransferase (ALT), lymphocyte counts, and serum TG between the two groups (P<0.05, Table 1), but there were no differences on age, gender, clinical types, diabetes history, glucocorticoid treatment, serum LDH, serum IL-6 between the two groups (P>0.05, Table 1). We further performed a multivariable logistic analysis to examine the factors that were associated with TPNT. Serum triglyceride level (B=-0.979, OR=0.376, 95.0% CI: 0.159-0.887, P=0.025) and hypertension (B=-2.783, OR=16.174, 95.0% CI: 1.168-161.661, P=0.018) was associated with TPNT.
Table 1.
The relationship between TNPT and Clinical characteristics, treatment, and laboratory findings in patients with COVID-19
| Clinical data | TNPT≤16 | TNPT>16 | t/U χ2 | P |
|---|---|---|---|---|
| Sex (M/F) | 22/13 | 15/17 | 1.727 | 0.189 |
| Age (y) | 41.25±16.25 | 49.56±16.23 | -2.014 | 0.920 |
| Clinical types | 3.807 | 0.237 | ||
| Mild | 14 | 7 | ||
| Moderate | 18 | 22 | ||
| Severe | 3 | 3 | ||
| Complications | ||||
| Diabetes | 3 | 4 | 0.276 | 0.600 |
| Hypertension | 1 | 12 | 12.829 | 0.000* |
| Surgery history | 1 | 1 | ||
| Hepatitis B infection | 2 | 0 | 2.783 | 0.249 |
| Treatment | ||||
| Abidol (Y/N) | 16/19 | 23/9 | 4.703 | 0.03* |
| Aluvia (Y/N) | 31/4 | 30/2 | 0.550 | 0.458 |
| Interferon (Y/N) | 29/6 | 29/3 | 0.867 | 0.352 |
| Thymosin (Y/N) | 8/28 | 8/23 | 1.108 | 0.575 |
| Glucocorticoid (Y/N) | 5/25 | 3/24 | 7.401 | 0.285 |
| Laboratory findings | ||||
| AST (U/L) | 28.24±10.52 | 29.28±21.17 | -0.768 | 0.241 |
| ALT (U/L) | 29.58±19.29 | 45.86±34.46 | -2.44 | 0.000* |
| LDH (U/L) | 192 (111, 420) | 194 (126, 460) | 0.758 | 0.448 |
| Alb (g/L) | 43.78±4.47 | 43.27±6.08 | 0.408 | 0.283 |
| Glu (mmol/L) | 5.83 (4.41, 10.67) | 6.24 (4.51, 8.72) | 0.333 | 0.765 |
| TC (mmol/L) | 3.97 (3, 6) | 3.7 (2, 7) | -0.124 | 0.901 |
| TG (mmol/L) | 1.25 (0.46, 5.22) | 1.03 (0.57, 3.15) | -1.99 | 0.047* |
| WBC (109/L) | 5.56±2.0 | 5.10±1.68 | 0.969 | 0.145 |
| N (109/L) | 1.28±0.63 | 3.69±1.26 | 0.071 | 0.944 |
| L (109/L) | 1.60±0.98 | 1.18±0.52 | 2.187 | 0.048* |
| NLR | 2.81 (1.12, 22.15) | 3.02 (1.45, 9.39) | 0.073 | 0.789 |
| CRP (mg/L) | 6.45 (0, 142.4) | 7.19 (0.2, 174.6) | 0.288 | 0.773 |
| IL-6 (pg/ml) | 6.5 (2, 13) | 10 (2, 62) | 4.035 | 0.058 |
| FER (ng/ml) | 399.6 (72, 1848) | 348.9 (39, 1247) | 0.176 | 0.679 |
Abbreviations: AST, aspertate aminotransferase; ALT, alanine transaminase; Alb, albumin; BMI, body mass index; CRP, C-reactive protein; Glu, Glucose; L, lymphocyte; LDH, lactate dehydrogenase; IL-6, interleukin-6; N, neutrophil; NLR, neutrophil to lymphocyte ratio; TC, total cholesterol; TG, triglyceride; WBC, white blood cells.
P<0.05.
Since serum TG level is negatively associated with TPNT. According to Chinese Guidelines for the Prevention and Treatment of Adult Dyslipidemia (2016 Version), high serum TG was defined as TG≥2.3 mmol/L [17]. Patients was divided into high TG group and normal TG group. The clinical data were listed in Table 2. As shown in Table 2, patients in high TG group had shorter TPNT compared with normal TG group (14.4±4.0 versus 18.9±9.2, P<0.05).
Table 2.
Clinical data of patients in high serum TG group and normal TG group
| Clinical data | TG<2.3 | TG≥2.3 | t/U/χ2 | P |
|---|---|---|---|---|
| Sex (M/F) | 34/25 | 3/5 | 1.154 | 0.283 |
| Age (y) | 45.3±17.6 | 44.4±11.9 | 0.139 | 0.890 |
| TPNT | 18.9±9.2 | 14.4±4.0 | 2.461 | 0.023* |
| Clinical types | 1.830 | 0.608 | ||
| Mild | 20 | 1 | ||
| Moderate | 34 | 6 | ||
| Severe | 5 | 1 | ||
| Complications | ||||
| Bacterial infection | 5/54 | 1/7 | 0.140 | 0.708 |
| Diabetes | 6/53 | 1/7 | 0.041 | 0.840 |
| Hypertension | 12/47 | 1/7 | 0.277 | 0.599 |
| Treatment | ||||
| Abidol (Y/N) | 35/24 | 4/4 | 0.252 | 0.616 |
| Interferon (Y/N) | 51/8 | 7/1 | 0.007 | 0.934 |
| Thymosin (Y/N) | 15/44 | 1/7 | 0.647 | 0.421 |
| Glucocorticoid (Y/N) | 14/45 | 4/4 | 2.475 | 0.116 |
| Laboratory findings | ||||
| AST (U/L) | 28.1±17.8 | 28.0±5.5 | 0.002 | 0.998 |
| ALT (U/L) | 38.2±30.1 | 40.3±21.7 | -0.185 | 0.854 |
| LDH (U/L) | 215.0±79.7 | 209.1±30.1 | 0.687 | 0.492 |
| Alb (g/L) | 43.7±5.6 | 42.0±3.8 | 0.838 | 0.405 |
| Glu (mmol/L) | 6.4±1.7 | 7.5±2.0 | -1.330 | 0.191 |
| TC (mmol/L) | 3.93 (2, 7) | 3.93 (3, 4) | -0.276 | 0.789 |
| TG (mmol/L) | 1.05 (0.57, 2.25) | 2.96 (2.41, 5.22) | 4.556 | -0.000* |
| WBC (109/L) | 5.33±1.9 | 5.9±2.6 | -0.766 | 0.446 |
| N (109/L) | 3.6±1.7 | 3.3±1.2 | 0.357 | 0.723 |
| CRP (mg/L) | 6.8 (0, 174.6) | 9.2 (1.74, 63.8) | 0.619 | 0.536 |
| IL-6 (pg/ml) | 9 (2, 62) | 4.5 (2, 12) | -1.179 | 0.250 |
| Ferritin (ng/ml) | 396.3 (39, 1848) | 422.1 (339, 578) | 0.17 | 0.902 |
| NLR | 2.86 (1.12, 22.15) | 2.85 (1.86, 6.69) | 0.26 | 0.803 |
P<0.05.
In addition, hypertension is also an important factor that influencing the recovery after SARS-CoV-2 infection. In this study, hypertension was also highly associated with TPNT. We examined TPNT level in patients with hypertension or patients without hypertension. The clinical data were summarized in Table 3 in two groups. There were significant differences on age, TPNT, complicated bacterial infection, abidol treatment, serum AST, and serum Alb between the two groups (P<0.05, Table 1), but there were no differences on gender, clinical types, serum LDH, serum TC, and TG between the two groups. Multivariable Logistic multivariate analysis showed that hypertension was associated with age (B=0.097, OR=1.102, 95.0% CI: 1.027-1.183, P=0.007), TPNT (B=0.094, OR=1.099, 95.0% CI: 1.102-1.193, P=0.024), complicated bacterial infection (B=2.288, OR=17.95, 95.0% CI: 1.314-245.125, P=0.030), but not associated with abidol treatment, serum AST, and serum Alb (P>0.05). To further demonstrate the relationship between hypertension and TPNT, cumulative number of patients with positive tests was recorded in Figure 1. We found that cumulative patients with positive tests reduced quickly in non-hypertension group (Figure 1).
Table 3.
Clinical data of patients in hypertension group and non-hypertension group
| Clinical data | HT group | Non-HT group | t/U/χ2 | P |
|---|---|---|---|---|
| Sex (M/F) | 9/4 | 28/26 | 1.28 | 0.258 |
| Age (y) | 61.9±11 | 41.1±15.7 | -4.505 | 0.000* |
| TPNT | 25.5±9.5 | 16.7±7.9 | -3.473 | 0.001* |
| Clinical types | 2.301 | 0.317 | ||
| Mild | 2 | 19 | ||
| Moderate | 9 | 31 | ||
| Severe | 2 | 4 | ||
| Complications | ||||
| Bacterial infection | 4/9 | 2/52 | 9.414 | 0.002* |
| Diabetes | 3/10 | 4/50 | 2.75 | 0.097 |
| Treatment | ||||
| Abidol (Y/N) | 11/2 | 28/26 | 4.623 | 0.032* |
| Interferon (Y/N) | 12/1 | 46/8 | 0.457 | 0.499 |
| Thymosin (Y/N) | 5/8 | 11/43 | 1.886 | 0.17 |
| Glucocorticoid (Y/N) | 4/9 | 14/40 | 0.125 | 0.724 |
| Laboratory findings | ||||
| AST (U/L) | 36.9±32.4 | 25.9±9.5 | -2.174 | 0.033* |
| ALT (U/L) | 51.1±41.3 | 35.4±24.9 | -1.77 | 0.081 |
| LDH (U/L) | 247.6±99.7 | 206.3±67 | -1.804 | 0.076 |
| Alb (g/L) | 40.3±8.2 | 44.3±4.2 | 2.497 | 0.015* |
| Glu (mmol/L) | 7.1±1.7 | 6.4±1.7 | -1.141 | 0.261 |
| TC (mmol/L) | 3.69 (2, 7) | 3.97 (3, 7) | -0.035 | 0.927 |
| TG (mmol/L) | 1.36 (0.85, 3.15) | 1.14 (0.57, 5.22) | 0.048 | 0.962 |
| WBC (109/L) | 5.7±1.7 | 5.3±2.1 | -0.603 | 0.548 |
| N (109/L) | 4.1±1.5 | 3.4±1.7 | -1.098 | 0.279 |
| L (109/L) | 1.1±0.5 | 1.5±0.9 | 1.474 | 0.145 |
| CRP (mg/L) | 16 (0.2, 174.6) | 6.8 (0, 142.4) | 1.237 | 0.216 |
| IL-6 (pg/ml) | 11.5 (3, 62) | 7 (2, 29) | 0.14 | 0.155 |
| Ferritin (ng/ml) | 678.7 (151, 1247) | 346.8 (39, 1848) | 0.338 | 0.367 |
| NLR | 3.02 (1.45, 9.39) | 2.81 (1.12, 22.15) | 0.464 | 0.475 |
P<0.05.
Figure 1.
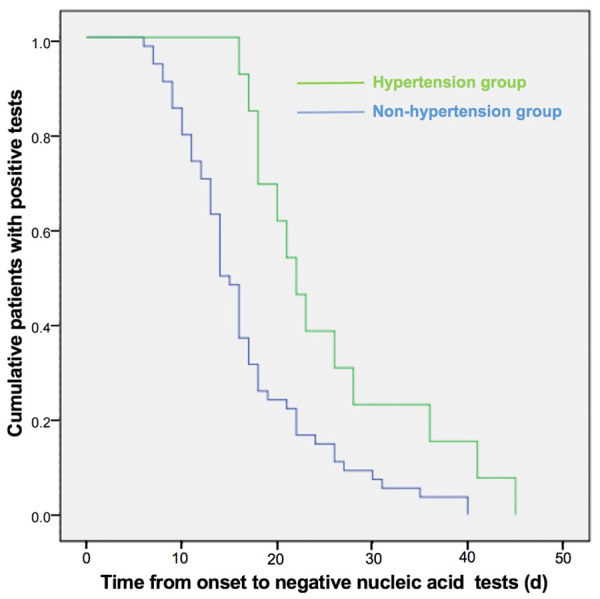
The curve of cumulative COVID-19 patients with positive nucleic acid tests in different groups. COVID-19 patients were divided into two groups: hypertension group and non-hypertension group. The curve of cumulative COVID-19 patients with positive test results was made in two groups.
Discussion
COVID-19 is an emerging infectious disease caused by SARS-CoV-2. COVID-19 mainly has the symptoms of the respiratory system such as fever, cough, shortness of breath, and breathing difficulty, but also involves other systemic symptoms including the nervous system and the digestive system [1,5,18]. SARS-CoV-2 belongs to the β genus of the family of coronavirus and its RNA sequence is closely related to and SARS-CoV and middle east respiratory syndrome coronavirus (MERS-CoV) [1,19]. The understanding of the structure and invasion of SARS-CoV-2 mostly comes from the knowledge of SARS-CoV and MERS-CoV [20]. The lessons from SARS-CoV and MERS-CoV may help the treatment for SARS-CoV-2 infection [21-23]. In 2003, SARS disappeared as the temperature increased. Dose COVID-19 disappear in this summer with increasing temperature? What is the natural course of COVID-19? What factors affect virus positive-to-negative transformation? These issues are still unresolved so far. Although some studies found factors to be highly associated with the mortality of COVID-19 patients [13-15], in this study, we examined the clinical factors that might affect the recovery from the SARS-CoV-2 infection. Our finding showed that serum TG and hypertension were two important factors that were related to TPNT in COVID-19 patients with mild and moderate clinical subtypes, indicating the association of two factors with the viral clearance in non-severe patients.
For the viral clearance from patients, antiviral medication may be important factors. Interferon nebulization therapy was recommended in Chinese guidelines and a potential efficacy of human type I Interferon in suppressing SARS-CoV-2 infection was reported in an in vitro study [24], but this study found that interferon nebulization therapy has no effect on the time for viral removal of SARS-CoV-2 from patients’ throat swab samples. Another broad-spectrum antiviral drug, arbidol, is a non-nucleoside broad-spectrum antiviral drug, which has been approved for the treatment of influenza virus and other viral infections and has also shown good efficacy in the treatment for influenza [25]. In addition, it also showed a significant inhibition on Zika virus in vitro [26]. Although this study found high ratio of abidol use in low TNPT group, the there was no difference of abidol treatment on TPNT between patients taking abidol and those not taking abidol after adjusting the confounder factors. Since antiviral therapy has no specific effect, the modulation on the immune function may be helpful for virus clearance. In this study, we observed that there was no significant difference in virus clearance between patients with and without thymosin.
The application of glucocorticoids has been controversial in SARS patients and a large dose of glucocorticoid may delay the clearance of coronavirus due to its immunosuppression [27], the use of glucocorticoids is unclear for COVID-19 patients. Small doses of glucocorticoids were recommended for COVID-19 patients with severe pneumonia by Chinese guidelines for COVID-19 [28]; however, some clinical evidence was against the use of glucocorticoids for COVID-19 [29]. In this study, there was no difference on TNPT between the group with small dose of glucocorticoid (3-7 days) and the group without glucocorticoid. This also suggests that the short-term application of glucocorticoids has no effect on virus clearance.
Angiotensin-converting enzyme 2 (ACE2) protein of host cells is considered as the targeting protein for the invasion of SARS-CoV-2 and is verified as a potential therapeutic target for antiviral treatment [30]. Therefore, ACE2 expression or its activity is close related to the invasion or removal of SARS-CoV-2. Studies have shown that ACE2 deficiency was associated with increased cellular content of triglyceride while ACE2 activation by diminazene increases serum triglyceride in mice [31,32]. Interestingly, in our patients, the TPNT was highly associated with serum triglyceride. Although no evidence demonstrated that high serum triglyceride might decrease cellular ACE2 protein, our data suggest that the reduced TPNT in high triglyceride group may be related to the reduction of ACE2 activation or expression in human body, which provides a critical clue to how to remove virus in human body. Patients with COVID-19 could be suggested to have high-fat diets to increase serum triglyceride and decrease the TPNT. In addition, hypertension is also associated with the TPNT. On the contrary, previously study indicated that ACE2 levels is up-regulated in patients with cardiovascular diseases including hypertension [33]. In this study, 12 individuals among 13 patients with hypertension had longer TPNT, indicating that hypertension is related to increased TPNT probably by the increase of ACE2 in patients. Paradoxically, because ACE inhibitors (ACEI) are regarded to increase ACE2 level [34], all the patients with hypertension were suggested to replace ACE inhibitors with calcium channel blocker. Based on this evidence, patients with hypertension should continue ACEI anti-hypertensives and may benefit from the use of ACEI as recent recommendations [35]. Therefore, the effect of ACEI therapy on SARS-CoV-2 virus removal awaits more investigations.
In this study, we only found that serum TG and hypertension were highly associated with TPNT in mild and moderate COVID-19 patients, which need to be verified in large populations and to address the limitation of small size of the COVID-19 patients in this study. Through a large population study, more risk factors related to TPNT may be found.
In conclusion, although SARS-CoV-2, SARS-CoV, and MERS-CoV are coronavirus, the pathogenicity, infectivity and clinical characteristics of SARS-CoV-2 infection are different from SARS and MERS. SARS disappears with increasing temperature; MERS has a low infectivity and high mortality, but lasts for several years [36]. The outcome of SARS-CoV-2 infection cannot be predicted as like SARS or MERS. Probably, like MERS-CoV, SARS-CoV-2 may coexist with human kinds for a long time. Therefore, before finding the vaccine for SARS-CoV-2, the risk factors related to the prevention and treatment for SARS-CoV-2 should be further investigated.
Acknowledgements
This work was supported by the grants from National Key R&D Program of China (2017YFE0103700), the National Science Foundation of China (81120108011, 81771454, and 20VYJ068), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Disclosure of conflict of interest
None.
References
- 1.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D’Arminio Monforte A, Ismail S, Kato H, Lapadula G, L’Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldman G, Mayeux R, Claassen J, Agarwal S, Willey J, Anderson E, Punzalan P, Lichtscien R, Bell M, Przedborski S, Ulane C, Roberts K, Williams O, Lassman AB, Lennihan L, Thakur KT. Preparing a neurology department for SARS-CoV-2 (COVID-19): early experiences at columbia university irving medical center and the new york presbyterian hospital in New York city. Neurology. 2020;94:886–891. doi: 10.1212/WNL.0000000000009519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eikenberry SE, Mancuso M, Iboi E, Phan T, Eikenberry K, Kuang Y, Kostelich E, Gumel AB. To mask or not to mask: modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect Dis Model. 2020;5:293–308. doi: 10.1016/j.idm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagashima M, Kumagai R, Yoshida I, Kawakami M, Nagano M, Asakura H, Kaku E, Kitamura Y, Hasegawa M, Hayashi Y, Chiba T, Sadamasu K, Yoshimura K. Characteristics of SARS-CoV-2 isolated from asymptomatic carrier in Tokyo. Jpn J Infect Dis. 2020 doi: 10.7883/yoken.JJID.2020.137. [DOI] [PubMed] [Google Scholar]
- 11.Setti L, Kirienko M, Dalto SC, Bonacina M, Bombardieri E. FDG-PET/CT findings highly suspicious for COVID-19 in an Italian case series of asymptomatic patients. Eur J Nucl Med Mol Imaging. 2020;47:1649–1656. doi: 10.1007/s00259-020-04819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang XL, Zhang XL, Zhao XN, Li CB, Lei J, Kou ZQ, Sun WK, Hang Y, Gao F, Ji SX, Lin CF, Pang B, Yao MX, Anderson BD, Wang GL, Yao L, Duan LJ, Kang DM, Ma MJ. Transmission potential of asymptomatic and paucisymptomatic SARS-CoV-2 infections: a three-family cluster study in China. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa206. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sattar N, McInnes IB, McMurray JJV. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047659. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, Zeng X, Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y, Ni W. Economic evaluation of the 2016 Chinese guideline and alternative risk thresholds of initiating statin therapy for the management of atherosclerotic cardiovascular disease. Pharmacoeconomics. 2019;37:943–952. doi: 10.1007/s40273-019-00791-8. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Xue Q, Xu X. Involvement of the nervous system in SARS-CoV-2 infection. Neurotox Res. 2020;38:1–7. doi: 10.1007/s12640-020-00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Xu W, Hu G, Xia S, Sun Z, Liu Z, Xie Y, Zhang R, Jiang S, Lu L. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol. 2020:1–3. doi: 10.1038/s41423-020-0498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong H, Wang Y, Zhang ZL, Liu YX, Le KJ, Cui M, Yu YT, Gu ZC, Gao Y, Lin HW. Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: a systematic review and meta-analysis. Pharmacol Res. 2020;157:104872. doi: 10.1016/j.phrs.2020.104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padron-Regalado E. Vaccines for SARS-CoV-2: lessons from other coronavirus strains. Infect Dis Ther. 2020;9:1–20. doi: 10.1007/s40121-020-00300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B MZ, Wan B, Xu X. Prevention for posttraumatic stress disorder after the COVID-19 epidemic: lessons from the SARS epidemic. Stress and Brain. 2020 [Google Scholar]
- 24.Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020:104811. doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiselev OI, Maleev VV, Deeva EG, Leneva IA, Selkova EP, Osipova EA, Obukhov AA, Nadorov SA, Kulikova EV. Clinical efficacy of arbidol (umifenovir) in the therapy of influenza in adults: preliminary results of the multicenter double-blind randomized placebo-controlled study ARBITR. Ter Arkh. 2015;87:88–96. doi: 10.17116/terarkh201587188-96. [DOI] [PubMed] [Google Scholar]
- 26.Fink SL, Vojtech L, Wagoner J, Slivinski NSJ, Jackson KJ, Wang R, Khadka S, Luthra P, Basler CF, Polyak SJ. The antiviral drug arbidol inhibits Zika virus. Sci Rep. 2018;8:8989. doi: 10.1038/s41598-018-27224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho JC, Ooi GC, Mok TY, Chan JW, Hung I, Lam B, Wong PC, Li PC, Ho PL, Lam WK, Ng CK, Ip MS, Lai KN, Chan-Yeung M, Tsang KW. High-dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am J Respir Crit Care Med. 2003;168:1449–1456. doi: 10.1164/rccm.200306-766OC. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Shi Y, Xiao T, Fu J, Feng X, Mu D, Feng Q, Hei M, Hu X, Li Z, Lu G, Tang Z, Wang Y, Wang C, Xia S, Xu J, Yang Y, Yang J, Zeng M, Zheng J, Zhou W, Zhou X, Zhou X, Du L, Lee SK, Zhou W Working Committee on Perinatal and Neonatal Management for the Prevention and Control of the 2019 Novel Coronavirus Infection. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition) Ann Transl Med. 2020;8:47. doi: 10.21037/atm.2020.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;18:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao X, Song LN, Zhang YC, Li Q, Shi TT, Yang FY, Yuan MX, Xin Z, Yang JK. Angiotensin-converting enzyme 2 inhibits endoplasmic reticulum stress-associated pathway to preserve nonalcoholic fatty liver disease. Diabetes Metab Res Rev. 2019;35:e3123. doi: 10.1002/dmrr.3123. [DOI] [PubMed] [Google Scholar]
- 32.Fraga-Silva RA, Montecucco F, Costa-Fraga FP, Nencioni A, Caffa I, Bragina ME, Mach F, Raizada MK, Santos RAS, da Silva RF, Stergiopulos N. Diminazene enhances stability of atherosclerotic plaques in ApoE-deficient mice. Vascul Pharmacol. 2015;74:103–113. doi: 10.1016/j.vph.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anguiano L, Riera M, Pascual J, Soler MJ. Circulating ACE2 in cardiovascular and kidney diseases. Curr Med Chem. 2017;24:3231–3241. doi: 10.2174/0929867324666170414162841. [DOI] [PubMed] [Google Scholar]
- 34.Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]


