Abstract
The coronavirus disease 2019 (COVID-19) pandemic has spread to almost all countries. The currently reported epidemiological statistics show that age, gender, and type of comorbidities may be high-risk factors for critically ill patients with COVID-19. However, there is no comprehensive analysis of these risk factors. In the present study, we systematically explored the prognostic value of the clinical factors (gender, age and comorbidities) in 189 COVID-19 patients from Wuhan, China. We discovered that the gender, age and comorbidities were tightly associated with the survival of COVID-19 patients via performing Kaplan-Meier curve analysis. Compared with the female patients, male patients have a lower survival rate. Similarly, the older patients and those with more comorbidities also tended to have an unfavorable survival outcome. In addition, further stratified analysis of COVID-19 patients according to the three risk factors indicated that some laboratory indicators including CRP, IL-6 and lymphocytes showed significant trends in gender, age and comorbidities groups. Together, these result which may provide a certain reference value for the prevention and treatment of COVID-19.
Keywords: COVID-19, risk factors, gender, age, comorbidities
Introduction
At the end of 2019, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) broke out in Wuhan, China [1]. The receptor-binding domain structure of SARS-CoV-2 has been confirmed to be highly similar to that of the severe acute respiratory syndrome Coronavirus (SARS-CoV) [2,3]. Covid-19 is more transmissible than SARS occurred in 2002-03 [4,5]. At present, the COVID-19 has become a pandemic in the world. Since the outbreak, it has caused a large number of casualties and economic losses in a short time. Globally, there have been over 8.06 million confirmed cases of COVID-19, and 440 thousand deaths, reported by WHO on 17 June 2020 [6].
In confirmed cases in China, although the infection rate among men and women is similar, the death rate among men is 4.7% compared with 2.8% for women. Similarly,In Italy, the reported death rate in men (16.6%) is significantly higher than that in women 9.1% [7]. Among the death population of COVID-19 cases, the elderly accounts for a large proportion [8]. Patients with diabetes mellitus, obesity and/or hypertension and COVID-19 have increased mortality and morbidity rate. However, the association of diabetes mellitus hypertension and cardiovascular diseases for COVID-19 is still unknown [9]. Coronary heart disease and other comorbidities are also closely related to the death rate of COVID-19 [10-14]. Although some studies have revealed the correlation between gender and the prevalence, severity and mortality of COVID-19 pneumonia [15,16], there is no detailed analysis on the underlying mechanism. Considering that many patients with COVID-19 are complicated with multiple comorbidities [17-19], classifying comorbidities and paying attention to the impact of different comorbidities on the relevant clinical indicators and prognosis of patients with COVID-19 is of great significance for clinical guidance.
Herein, we grouped 189 COVID-19 inpatients in Tongji Hospital Affiliated to Tongji medical college HUST according to gender, age, and comorbidity types, and analyzed the changes in experimental indicators of patients with COVID-19 under these three factors to find their impact on the severity of the disease. Among them, the patients were divided into those with no comorbidity, one comorbidity, equal or greater than two comorbidities.
Materials and methods
Patients
A total of 189 patient with COVID-19 who were admitted from February 10 to March 28, 2020 in Tongji Hospital Affiliated to Tongji medical college HUST were enrolled. Among all the patients (n=189) tested for SARS-Cov-2, 145 (76.7%) were positive and 44 (23.3%) were clinically diagnosed; They were clinically diagnosed based on the clinical symptoms: 1). fever or respiratory symptoms; 2). early onset of the total number of white blood cells normal or decreased, or decreased lymphocyte count; 3). with typical changes in chest radiology or with a positive result on real-time reverse transcription polymerase chain reaction assay of nasal and pharyngeal swab specimens.
Type of disease
According to the seventh version of the guidelines on the Diagnosis and Treatment of COVID-19 issued by the National Health Commission, COVID-19 severity was classified into below types: 1. Mild type: The clinical symptoms were mild with no abnormal radiological findings. 2. Moderate type: Fever, cough and other symptoms were presented as well as pneumonia on chest computed tomography. 3. Severe type: The disease was classified as severe if one of the following conditions was met: (1) Respiratory distress, respiratory rate ≥30 per min; (2) Oxygen saturation on room air at rest ≤93%; (3) Partial pressure of oxygen in arterial blood/fraction of inspired oxygen ≤300 mmHg. 4. Critical type: The disease was classified as critical if one of the following conditions was met: (1) Respiratory failure occurred and mechanical ventilation was required; (2) Shock; (3) T monitoring treatment.
Statistics analysis
We categorized clinical information into continuous data and categorical data. For the continuous data, we used the One-Way ANOVA test with unpaired parameter. The Chi-square test or Fisher exact test was applied to analyze categorical data as appropriate. P value ≤0.05 was considered as statistically significant. The relationship between clinical characteristics and overall survival of COVID-19 was estimated using the univariate cox regression analysis in the survival R package. Moreover, Kaplan-Meier curve and log-rank test analysis was used to identify survival difference of categorical variables in COVID-19 patients. All analyses were performed with the R package. For all statistical analysis, the P value less than 0.05 was considered as statistically significant.
Results
Landscape of clinical feature
A total of 189 patients (Alive: 164, Dead: 25) were enrolled in our study. According to the clinical types, patients were further classified into common (N=93), severe (N=60) and critical severe types (N=36). The median age in the common, severe and critical severe types were 57 years, 64.5 years and 69.5 years, respectively, suggesting that elderly patients are prone to have critical type of COVID-19 (P<0.001). Additionally, the prevalence of patients with COVID-19 was higher in males (N=102) compared with females (n=87), and the proportion of male cases in the critical severe cases was significantly higher (P=0.042). Most COVID-19 patients presented with at least one symptom in the initial of infection, of which fever was the most common symptoms, accounting for 75.13% of the cases. The expectoration ranked second among the symptoms which accounted for 55.1% of the cases, and dyspnea presented in 33.33% of the cases, following the fever and expectoration (P<0.001). In addition, we also found that hypertension, diabetes and coronary heart disease were the most common comorbidities, which represent 65 (34.39%), 29 (15.34%) and 14 (7.41%) cases, respectively. Moreover, critical severe types had more hypertension cases (P<0.001). We further compared the index level in the three types. As shown in Table 1, the levels of leukocyte, albumin, C-reactive protein (CRP), interleukin 6 (IL-6) and D-dimer presented a clear increasing trend (all P<0.05), while lymphocyte, oxygen saturation and albumin showed a decreasing trend (all P<0.001).
Table 1.
The statistics clinical information of 189 COVID-19 patients
| Items | Sub-items | Case distribution | Common (93) | Severe (60) | Critical severe (36) | df | P |
|---|---|---|---|---|---|---|---|
| Age (years) | Median | 63 (51-71) | 57 (42.5-67.5) | 64.5 (52.5-71.75) | 69.5 (62.25-78.5) | <0.0001 | |
| ≥60 | 58.20% | 42 (45.2%) | 37 (61.7%) | 31 (86.1%) | 18.324 | <0.0001 | |
| ≥70 | 29.70% | 19 (20.4%) | 19 (31.7%) | 18 (50.0%) | 11.059 | 0.0040 | |
| Gender | Male | 102 (53.97%) | 48 (51.61%) | 28 (46.67%) | 26 (72.22%) | 6.324 | 0.0423 |
| Female | 87 (46.03%) | 45 (48.39%) | 32 (53.33%) | 10 (27.78%) | |||
| Symptoms | Fever | 142 (75.13%) | 62 (66.67%) | 47 (78.33%) | 28 (77.78%) | 0.939 | 0.6253 |
| Cough | 55 (29.10%) | 27 (29.03%) | 21 (35.00%) | 7 (19.44%) | 2.639 | 0.2672 | |
| expectoration | 108 (57.14%) | 44 (47.31%) | 40 (66.67%) | 24 (66.67%) | 7.226 | 0.0270 | |
| Fatigue | 43 (22.75%) | 19 (20.43%) | 10 (16.67%) | 14 (38.89%) | 6.883 | 0.0320 | |
| pharyngalgia | 20 (10.58%) | 15 (25.00%) | 5 (8.33%) | 0 (0.00%) | 7.605 | 0.0223 | |
| myalgia | 28 (14.81%) | 16 (17.20%) | 7 (11.67%) | 5 (13.89%) | 0.916 | 0.6324 | |
| Diarrhea | 37 (19.58%) | 23 (24.73%) | 7 (1.67%) | 7 (19.44%) | 3.954 | 0.1384 | |
| Dyspnea | 63 (33.33%) | 24 (25.80%) | 16 (26.67%) | 23 (63.89%) | 18.696 | <0.0001 | |
| Vital signs | Highest temperature (°C) | 38.60±0.68 | 38.55±0.74 | 38.73±0.60 | 38.47±0.67 | 1.480 | 0.2313 |
| Respiratory rate (breaths per min) | 22.60±5.30 | 20.13±3.59 | 25.70±5.80 | 23.51±5.03 | 25.988 | <0.0001 | |
| Oxygen saturation (%) | 94.41±5.08 | 97.04±1.63 | 93.02±4.52 | 90.29±7.33 | 33.034 | <0.0001 | |
| comorbidity | 93 (49.21%) | 29 (31.2%) | 33 (55.00%) | 31 (86.10%) | 32.510 | <0.0001 | |
| High blood pressure | 65 (34.39%) | 18 (19.35%) | 21 (35.00%) | 26 (72.22%) | 32.163 | <0.0001 | |
| Diabetes mellitus | 29 (15.34%) | 9 (9.68%) | 12 (20.00%) | 8 (22.22%) | 4.611 | 0.0997 | |
| Chronic obstructive pulmonary disease | 10 (5.29%) | 3 (3.23%) | 4 (6.67%) | 3 (8.33%) | 1.683 | 0.4310 | |
| Coronary heart disease | 14 (7.41%) | 3 (3.23%) | 6 (10.00%) | 5 (13.89%) | 5.164 | 0.0756 | |
| Cerebrovascular diseases | 6 (2.65%) | 2 (2.15%) | 1 (1.67%) | 3 (8.33%) | 3.878 | 0.1439 | |
| Tumor | 5 (5.38%) | 2 (2.15%) | 2 (3.33%) | 1 (2.78%) | 0.563 | 0.853 | |
| Days of onset (days) | Time from onset to visit | 4.31±5.42 | 4.22±5.00 | 5.22±6.54 | 3.03±4.10 | 1.879 | 0.1556 |
| Primary leukocyte count (×109/L) | 6.84±3.47 | 5.84±1.97 | 6.51±2.30 | 10.06±5.72 | 24.117 | <0.0001 | |
| Primary lymphocyte count (×109/L) | 1.37±0.78 | 1.55±0.789 | 1.31±0.69 | 0.96±0.81 | 7.600 | 0.0007 | |
| Primary albumin (g/L) | 35.45±5.21 | 37.07±3.98 | 35.78±4.89 | 30.63±5.78 | 24.498 | <0.0001 | |
| Primary C-reactive protein (mg/L) | 36.26±52.01 | 14.05±25.62 | 29.05±34.65 | 108.89±65.23 | 76.854 | <0.0001 | |
| Primary IL-6 (pg/ml) | 96.68±499.67 | 8.78±27.04 | 11.01±14.15 | 346.67±949.60 | 5.639 | 0.0046 | |
| Primary D-dimer(ug/mL) | 3.01±5.85 | 0.98±1.66 | 1.90±2.88 | 10.07±9.94 | 42.770 | <0.0001 |
Analysis of risk factors (age, gender and comorbidities) for the COVID-19 patients
Abundant studies have been reported that gender, age and comorbidities were the risk factors for the COVID-19 patients. In our analysis, we found that males showed more death cases than females (P=0.0233). Also, older patients had more death cases (P=0.0005). Moreover, patients with one or more than two comorbidities were at greater risk of death (P=0.0012) (Table 2).
Table 2.
The clinical information for the survival of COVID-19 patients which group by age, gender and comorbidity
| Characteristic | Alive (N=164) | Dead (N=25) | P value | |
|---|---|---|---|---|
| Age | <60 | 77 | 2 | 0.0005 |
| ≥60 | 87 | 23 | ||
| Gender | Female | 83 | 6 | 0.0233 |
| Male | 81 | 19 | ||
| Comorbidities | 0 | 91 | 5 | 0.0012 |
| 1 | 48 | 10 | ||
| ≥2 | 25 | 10 | ||
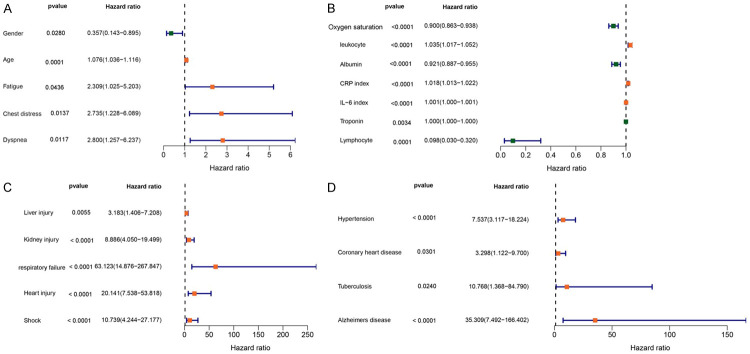
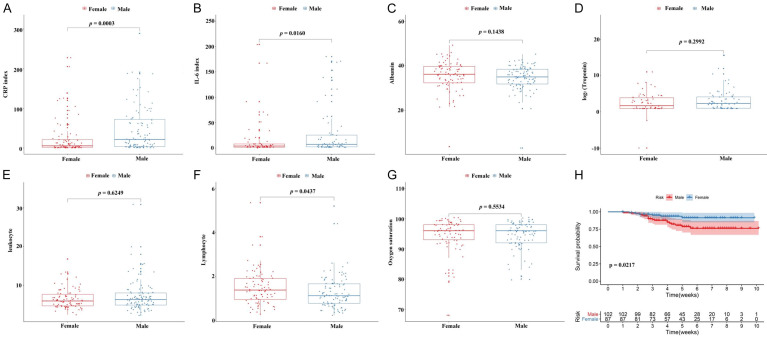
In order to further evaluate the association between overall survival and clinical characteristics, we conducted a univariate cox regression analysis. As shown in Figure 1, we identified 21 risk factors associated with survival of COVID-19 patients. Interestingly, we found that seven factors including oxygen saturation, leukocyte, albumin, CRP index, IL-6 index, troponin and lymphocyte were associated with survival of COVID-19 patients. We further compared the level of the clinical indicators among gender, different age and comorbidities. As showed in the Figure 2, the levels of IL-6 index and CRP index were significantly higher in males compared to females (Figure 2A and 2B), while the level of lymphocyte in males was lower than females (Figure 2F). Kaplan-Meier curve and log-rank test analysis result showed that female patients had prolonged survival time compare to male patients (P<0.05).
Figure 1.
Exploring the relationship between COVID-19 patients survival and clinical trait including clinical signs (A), clinical indicators (B), complications (C) and comorbidity (D) by using the cox regression analysis, the red boxplot represent the protective factors, while the green boxplot represent the risky factors.
Figure 2.
The relationship between laboratory indicators and gender were shown in (A-G), and K-M curve analysis for the survival of COVID-19 patients in gender group (Male VS Female) (H).
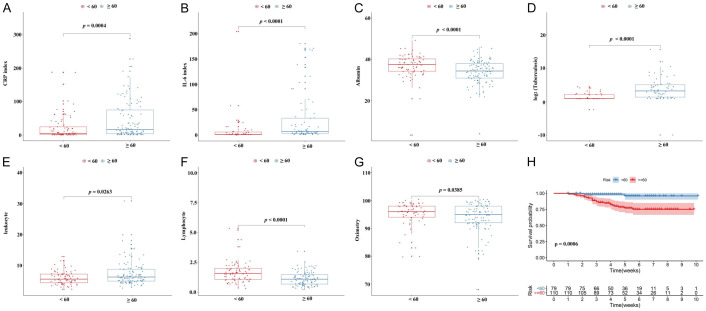
Moreover, we evaluated the level of clinical indicators among different age groups (<60 and ≥60). As shown in Figure 3A-G, the level of IL-6, CRP, tuberculosis and leukocyte presented significantly increasing trend from <60 group to ≥60 group. However, the level of albumin, lymphocyte and oxygen saturation exhibited a declining trend from <60 group to ≥60 group. The Kaplan-Meier curve and log-rank test analysis result indicated that patients in the younger age group (<60) had a better survival outcome than patients in the older age group (≥60) (Figure 3H).
Figure 3.
The relationship between laboratory indicators and gender were shown in the (A-G), and K-M curve analysis for the survival of COVID-19 patients in age group (≤60 VS >60) (H).
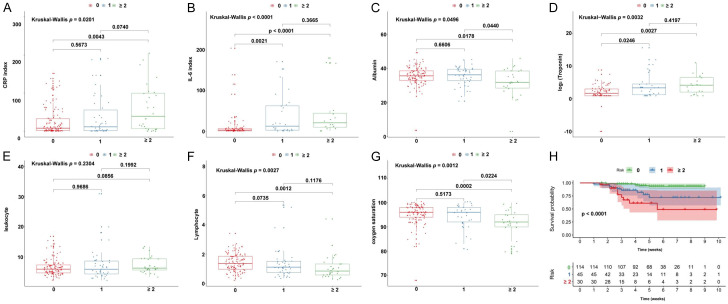
In addition, considering some cases had multiple comorbidities, we further classified the patients into group without comorbidity (0), group with one comorbidity (1) and group with equal to or more than two comorbidities (≥2). As shown in Figure 4, six of seven indexes including IL-6, CRP, tuberculosis, albumin, lymphocyte and oxygen saturation presented a significant difference between 0 comorbidity group and ≥2 comorbidity group. We also observed a significant survival difference among comorbidity groups (P<0.0001). These results indicate that patients in the female group, younger group and no comorbidity group respond better to COVID-19 infection and have a better immune response.
Figure 4.
The relationship between laboratory indicators and gender were shown in (A-G), and K-M curve analysis for the survival of COVID-19 patients in comorbidities group (0 VS 1 VS ≥2) (H).
Discussion
In the present study, a total of 189 hospitalized patients were recruited from a teaching affiliated hospital in Wuhan, China. Among them, about 91 patients (48.15%) developed at least one comorbidity, and 80% death cases were with comorbidity. By performing univariate cox regression analysis, we identified 21 risk factors that associated with overall survival of COVID-19 patients. The age, gender and comorbidity were also identified as risk factors in our study, consistent with most previous studies [14,20,21]. Kaplan-Meier curve analysis results revealed that the elderly patients had a worse survival outcome compared to younger patients. The reason maybe that the nutritional status and immune function of elderly patients are relatively poor, which may lead to the immune defense function damage and develop into severe pneumonia, thereby increasing the risk of death. Moreover, we showed that the risk factors including leukocyte, CRP, IL-6 and tuberculosis were significantly higher in elderly group compared to younger group, while the protective factors albumin and lymphocyte presented opposite trend in younger group and elderly group. Taking into account the immune function is positively correlated with albumin and lymphocyte, it is not surprised that the younger patients have a better survival outcome [22,23].
Although males and females have similar susceptiblility to SARS-CoV-2, males are more likely to have higher severity and mortality, which is similar to the feature of SARS 2003 and Middle East Respiratory Syndrome (MERS) [24-27]. Our study showed that gender was an independent risk factor for COVID-19 deaths. In further COX risk analysis, CRP and IL-6 were significantly higher in ≥60 years patient group than in <60 years patient group, and the survival curve suggested that female was a protective factor for COVID-19 deaths.
So far, the mechanisms underlying the observed gender differences are not entirely clear. Based on the current understanding of gender differences in respiratory virus diseases, some assumptions can be put forward. Some lifestyles, such as smoking, are most likely associated with the negative progression and adverse outcomes of COVID-19 in males [28]. In addition, it is known that, in general, innate and immune responses are more intense and stronger in females than in males. Pro-inflammatory cytokines and chemokines express stronger in males. In particular, the core cytokine storm, IL-6 receptor, is highly expressed in lung epithelial cells in males, suggesting that males are more susceptible to cytokine storm that can lead to the deterioration of COVID-19 [26,29,30], which is consistent with our result. Angiotensin I converting enzyme 2 (ACE2) is crucial for SARS CoV-2 entry into host cells which is similar to SARS-CoV. ACE2 is the main receptor mediating viral attachment to target cells [31]. Cell type-specific expression of the ACE2 receptor in type II alveolar epithelial cells is higher in males than in females [32,33]. Therefore, male respiratory system is more vulnerable. ACE2 provide a key link between infectious agents, immunity, inflammation, and cardiovascular disease, which will be mention in the following discussion.
Comorbidity has also been reported as an important risk factor for mortality in COVID-19 patients. Patients with at least one comorbidity, were associated with poor clinical outcomes. IL-6, CRP, Troponin, lymphocyte count and blood oxygen saturation showed differences among groups of on comorbidity, one comorbidity, and ≥2 comorbidities. Meanwhile, the survival curve indicates that the number of comorbidities is an important risk factor for the prognosis of patients. These findings have provided further evidence for taking into account baseline comorbid diseases in the comprehensive risk assessment of prognosis among patients with COVID-19 [34].
The level of ACE2 receptor is associated with blood pressure level. The patient with hypertension may decrease the level of ACE2 receptors compared with no hypertension patients. SARS-CoV-2 infects cells through ACE2 receptor. ACE2 is highly expressed in the lung, and Imai et al. confirmed the protective effect of ACE2 in acute lung injury. Once SARS-CoV-2 infects the lungs, the amount and function of ACE2 protein in the lungs are reduced, which leads to acute lung failure. Once they are infected with SARS-CoV-2, ACE2 receptor expression will be less. The early manifestations of hypertension patients with COVID-19 are hypertension. In the later stage, COVID-19 patients may present shock and hypotension after respiratory failure and multiple organ failure, thereby entering the severe and critical stage. Diabetic patients are with hyperglycemia, various metabolic disorders. They are susceptible populations to infections of viruses, bacteria and other pathogenic microorganisms. Once diabetic patients are infected, it will not only aggravate basic diseases, but also easily lead to multiple organ failure. Moreover, type 2 diabetes is associated with reduced ACE2 activity [30]. Similarly, myocardial damage is also common in patients with COVID-19. Different reports have used different definitions for acute myocardial injury, including increase in cardiac enzymes (different biomarkers and cut-offs) and/or electrocardiographic abnormalities [17].
Coronary heart disease mainly manifests as acute myocardial injury, accompanied by an increase of leukocyte count, CRP, and troponin. Similar results were obtained in our study. Plasma IL-6 concentrations are elevated in COVID-19 patients with cardiac injury, and abnormalities in a variety of cytokines are prominent in patients with severe COVID-19 disease. Therefore, those patients with underlying conditions (such as hypertension, diabetes, and/or cardiovascular disease, etc.) are at higher risk of a rapid progression to acute respiratory distress syndrome, septic shock, metabolic acidosis, coagulation dysfunction, arrhythmia, kidney damage, heart failure, liver dysfunction, and/or secondary infection, and death [35].
However, several limitations need to elaborated. Firstly, the sample size was small. Secondly, some cases lacked of complete date of the exposure history and laboratory tests. Moreover, some patients are still under treatment in the hospital and their survival outcome is unclear at the time of data collection.
In conclusion, we identified three risk factors including age, gender and comorbidities that associated with the survival of COVID-19 patients. These risk factors may contribute to understanding the underlying mechanism of COVID-19 and provide reference for clinician to personalize management and treatment of patients.
Disclosure of conflict of interest
None.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan-Yeung M, Xu RH. SARS: epidemiology. Respirolog. 2003;8(Suppl 1):S9–14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Gayle AA, Wilder-Smith A, Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27:taaa021. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Coronavirus disease (COVID-19) situation report-149. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200617-covid-19-sitrep-149.pdf. 2020.
- 7.Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. doi: 10.1038/s41420-020-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang WH, Guan WJ, Li CC, Li YM, Liang HR, Zhao Y, Liu XQ, Sang L, Chen RC, Tang CL, Wang T, Wang W, He QH, Chen ZS, Wong SS, Zanin M, Liu J, Xu X, Huang J, Li JF, Ou LM, Cheng B, Xiong S, Xie ZH, Ni ZY, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicentre) and outside Hubei (non-epicentre): a nationwide analysis of China. Eur Respir J. 2020;55:2000562. doi: 10.1183/13993003.00562-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P, D’Acquisto F, Nicklin SA, Marian AJ, Nosalski R, Murray EC, Guzik B, Berry C, Touyz RM, Kreutz R, Wang DW, Bhella D, Sagliocco O, Crea F, Thomson EC, McInnes IB. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tadic M, Cuspidi C, Mancia G, Dell’Oro R, Grassi G. COVID-19, hypertension and cardiovascular diseases: should we change the therapy? Pharmacol Res. 2020;158:104906. doi: 10.1016/j.phrs.2020.104906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen J, She ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Huang X, Yuan Y, Rohit L, Liu PP, Li H. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Zhang Y, Wang F, Liu B, Li H, Tang G, Chang Z, Liu A, Fu C, Gao JJ. Sex differences in clinical findings among patients with coronavirus disease 2019 (COVID-19) and severe condition. medRxiv. 2020 [Google Scholar]
- 16.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A, Uriel N. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 18.Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li S, Biswas A, Choolani M, Mattar C, Su LL. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R. COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun Rev. 2020;19:102523. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LE, Hastie J, Hochman BR, Salazar-Schicchi J, Yip NH, Brodie D, O’Donnell MR. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng X, Yang J, Wang W, Wang X, Zhou J, Chen Z, Li J, Chen Y, Yan H, Zhang J, Zhang Y, Wang Y, Qiu Q, Gong H, Wei X, Wang L, Sun K, Wu P, Ajelli M, Cowling BJ, Viboud C, Yu H. Case fatality risk of novel coronavirus diseases 2019 in China. medRxiv. 2020 [Google Scholar]
- 22.Zhang Y, Ding J, Ren S, Wang W, Yang Y, Li S, Meng M, Wu T, Liu D, Tian S, Tian H, Chen S, Zhou C. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res Ther. 2020;11:207. doi: 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Deng W, Xiong H, Li H, Chen Z, Nie Y, Wang Z, Li K, Li J. Immune-related factors associated with pneumonia in 127 children with coronavirus disease 2019 in Wuhan. Pediatr Pulmonol. 2020 doi: 10.1002/ppul.24907. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, Liu S, Yang JK. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY) 2020;12:10087–10098. doi: 10.18632/aging.103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alghamdi IG, Hussain II, Almalki SS, Alghamdi MS, Alghamdi MM, El-Sheemy MA. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of health. Int J Gen Med. 2014;7:417–423. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vardavas CI, Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V. COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double X-chromosome in females be protective against SARS-CoV-2 compared to the single X-chromosome in males? Int J Mol Sci. 2020;21:3474. doi: 10.3390/ijms21103474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambrosino I, Barbagelata E, Ortona E, Ruggieri A, Massiah G, Giannico OV, Politi C, Moretti AM. Gender differences in patients with COVID-19: a narrative review. Monaldi Arch Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1389. [DOI] [PubMed] [Google Scholar]
- 31.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. medRxiv. 2020 doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai G. Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. medRxiv. 2020 [Google Scholar]
- 34.Yu HQ, Sun BQ, Fang ZF, Zhao JC, Liu XY, Li YM, Sun XZ, Liang HF, Zhong B, Huang ZF, Zheng PY, Tian LF, Qu HQ, Liu DC, Wang EY, Xiao XJ, Li SY, Ye F, Guan L, Hu DS, Hakonarson H, Liu ZG, Zhong NS. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J. 2020;56:2001526. doi: 10.1183/13993003.01526-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]