CONSPECTUS:
In response to water scarcity and an increased recognition of the risks associated with the presence of chemical contaminants, environmental engineers have developed advanced water treatment systems that are capable of converting municipal wastewater effluent into drinking water. This practice, which is referred to as potable water reuse, typically relies upon reverse osmosis (RO) treatment followed by exposure to ultraviolet (UV) light and addition of hydrogen peroxide (H2O2). These two treatment processes individually are capable of controlling many of the chemical and microbial contaminants in wastewater; however, a few chemicals may still be present after treatment at concentrations that affect water quality.
Low-molecular weight (<200 Da), uncharged compounds represent the greatest challenge for RO treatment. For potable water reuse systems, compounds of greatest concern include oxidation products formed during treatment (e.g., N-nitrosodimethylamine, halogenated disinfection byproducts) and compounds present in wastewater effluent (e.g., odorous compounds, organic solvents). Although the concentrations of most of these compounds decrease to levels where they no longer compromise water quality after they encounter the second treatment barrier (i.e., UV/H2O2), low-molecular weight compounds that are resistant to direct photolysis and exhibit low reactivity with hydroxyl radical (·OH) may persist. While attempts to identify the compounds that pass through both barriers have accounted for approximately half of the dissolved organic carbon remaining after treatment, it is unlikely that a significant fraction of the remaining unknowns will ever be identified with current analytical techniques. Nonetheless, the toxicity-weighted concentration of certain known compounds (e.g., disinfection byproducts) is typically lower in RO-UV/H2O2 treated water than conventional drinking water.
To avoid the expense associated with managing the concentrate produced by RO, environmental engineers have begun to employ alternative treatment barriers. The use of alternatives such as nanofiltration, ozonation followed by biological filtration, or activated carbon filtration avoids the problems associated with the production and disposal of RO concentrate, but they may allow a larger number of chemical contaminants to pass through the treatment process. In addition to the transformation products and solvents that pose risks in the RO-UV/H2O2 system, these alternative barriers are challenged by larger, polar compounds that are not amenable to oxidation, such as perfluoroalkyl acids and phosphate-containing flame retardants.
To fully protect consumers who rely upon potable water reuse systems, new policies are needed to prevent chemicals that are difficult to remove during advanced treatment from entering the sewer system. By using knowledge about the composition of municipal wastewater and the mechanisms through which contaminants are removed during treatment, it should be possible to safely reuse municipal wastewater effluent as a drinking water source.
Graphical Abstract

INTRODUCTION
In the early 1900s, environmental engineers invented a means of treating source waters that were contaminated with waterborne pathogens.1 The development of drinking water treatment systems meant that over approximately two decades, outbreaks of waterborne diseases like typhoid fever decreased, as lifespans were extended.2 The system responsible for this public health triumph typically involved a combination of physical removal of microbes by sand filtration followed by disinfection with chlorine. The term “multiple barrier,” which originally referred to watershed protection, water treatment, and addition of a chemical disinfectant after treatment, was adopted by researchers to describe this series of processes commonly employed during drinking water treatment.3
Although drinking water treatment was originally developed to address the risks of waterborne pathogens, it was extended to include removal of chemical compounds that impact aesthetics (e.g., geosmin, a compound with an organoleptic threshold of ~1 ng/L4) and pose potential health risks (e.g., arsenic, which can cause cancer and other diseases5). To address the potential presence of chemical contaminants in drinking water, regulations developed in the 1970s and 1980s specified targets (e.g., maximum contaminant levels, MCLs) for chemicals known to impact human health, including synthetic organic compounds in source waters and toxic compounds formed during disinfection.
During this period, discharges of municipal wastewater effluent (i.e., treated sewage) were viewed as a potential risk to water supplies that could be mitigated through dilution of wastewater-impacted waters with water from cleaner sources. Over the past 50 years, expanding populations and decreasing availability of pristine water sources, coupled with advances in treatment technologies, have led to a gradual shift in attitude and practice.6 Now, blending highly treated municipal wastewater into a source water (i.e., potable water reuse) is seen as a viable means of augmenting drinking water supplies.7
Around the time that potable water reuse systems were expanding, high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) enabled the measurement of polar compounds that had rarely been detected in the aquatic environment. In particular, the discovery that trace concentrations (i.e., <5 ng/L) of steroidal estrogens in wastewater were responsible for feminization of fish in effluent-receiving rivers,8–11 coupled with detection of pharmaceuticals and personal care products in municipal wastewater effluent,10,12,13 raised awareness of the issue of wastewater-derived contaminants.
There are currently ~25 full-scale potable water reuse projects in operation worldwide.7 Orange County (California) Water District’s potable water reuse system was the first system to employ reverse osmosis (RO) and has served as a trendsetter, as the practice has expanded beyond Southern California. The project began in 1977 with the installation of an advanced treatment plant that injected 57 000 m3/d of highly treated wastewater to counteract seawater intrusion caused by overpumping of groundwater. The facility originally treated half of the wastewater by RO to remove salts and chemical contaminants. Because of the high cost of early RO systems, the other half of the flow was subjected to granular activated carbon (GAC) filtration. Researchers studying these parallel physical treatment barriers reported that RO treatment resulted in lower concentrations of contaminants, such as ethylenediaminetetraacetic acid (EDTA) and dissolved organic carbon (DOC; i.e., after GAC and RO, DOC concentrations were 5.4 and 0.8 mg C/L, respectively).14,15
When the capacity of the system was expanded to 265 000 m3/d in 2008, an integrated membrane system with microfiltration and RO treated the entire flow. When the expansion was being designed, the toxic compounds N-nitrosodimethylamine (NDMA) and 1,4-dioxane were discovered in water produced by the first-generation treatment plant at concentrations exceeding levels known to pose chronic health risks. This discovery led to the installation of an additional chemical treatment barrier after RO: an advanced oxidation process (AOP) using UV and H2O2. The resulting advanced treatment plant, referred to as the Groundwater Replenishment System (GWRS), consisted of three sequential barriers to control waterborne pathogens: microfiltration, RO, and high-intensity UV light in the AOP. The system also combined physical (RO) and chemical (UV/H2O2) barriers to chemical contaminants (Figure 1). The GWRS currently operates with a flow of 379 000 m3/d. After its planned expansion to 492 000 m3/d in 2023, essentially all of the wastewater that can be recovered practically will be returned to the water supply. Research on the performance of this system is relevant worldwide, because the advanced treatment plant has operated longer than other projects that employ similar approaches, and it has been well-accepted by the community.16 As a result, Orange County’s multiple barrier treatment system has been replicated in potable water reuse projects in Texas,17 Arizona,7 Singapore,7 and Western Australia.18 Simply put, the GWRS potable reuse project has emerged as the “Gold Standard” to which proposed projects are compared.
Figure 1.
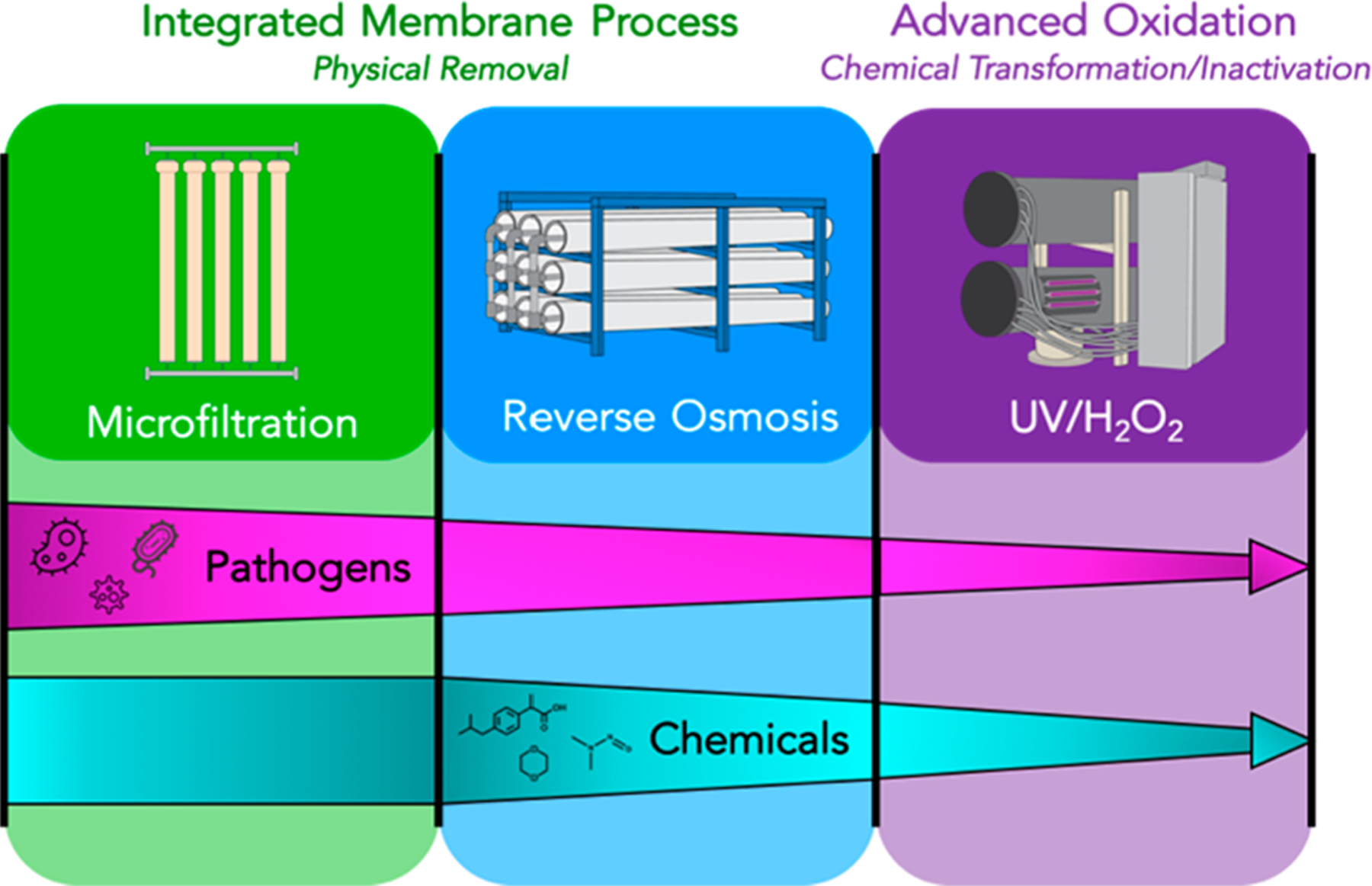
Advanced wastewater treatment for potable reuse employs microfiltration, RO, and UV/H2O2.
Given the array of chemicals potentially present in wastewater, it would be difficult to validate their removal on a chemical-by-chemical basis. A key assumption behind the “dual barrier” system is that physical (RO) and chemical (UV/H2O2) treatment systems will control chemical contaminants with different physical and chemical properties, and few chemicals will be insufficiently treated by the treatment sequence7,19 (Figure 1). The veracity of this assumption is key to the performance of the dual barrier system.
REVERSE OSMOSIS AS A CHEMICAL BARRIER
Modern water reuse systems employ thin-film composite membranes for RO.20 Dissolved solutes are excluded, because they diffuse through the polymer at rates that are much slower than that of the water molecules.21 Other factors, such as electrostatic repulsion and van der Waals interactions, also affect solute removal during RO.22,23 In general, uncharged compounds with molecular weights less than 200 Da are only partially rejected by RO membranes.24 For charged compounds and larger compounds, concentrations typically decrease by over 95% during RO treatment under conditions employed in water reuse.25,26
In contrast, compounds that pose the greatest challenges to the GWRS and similar facilities are uncharged, low-molecular weight compounds. NDMA (molecular weight = 74 g/mol) is a mutagenic and carcinogenic compound27 (e.g., the notification level for NDMA in California is 10 ng/L28) that has been particularly problematic for potable water reuse systems. Early efforts to identify the source of NDMA implicated sewage (e.g., NDMA concentrations as high as 790 ng/L were detected in sewage29). NDMA was also formed when chloramines30,31 or ozone32 were applied upstream of microfiltration to control biological fouling on membrane surfaces. Precursors of NDMA include industrial chemicals33 and chemicals present in domestic discharges, such as the wastewater from clothes washing.34
After NDMA was detected, efforts were made to control its formation and enhance its removal. Researchers discovered that minimizing the concentration of dichloramine—the chloramine species most responsible for NDMA formation—by using preformed monochloramine,35 or by employing multiple introduction points for hypochlorite into ammonia-containing wastewater,36 decreased NDMA formation. They also demonstrated that removal of NDMA during RO was affected by the type of membrane used,37 the water temperature,38 and the membrane age.39
Chloramination upstream of membrane processes can also produce low-molecular weight halogenated disinfection byproducts (DBPs) through reactions with effluent organic matter.31,40 These include a variety of toxic halogenated compounds (e.g., haloacetonitriles, haloacetamides, haloacetaldehydes, haloketones, and halonitromethanes).41,42 Because of their small size (i.e., mostly <200 Da), these uncharged compounds are poorly rejected by RO membranes43,44 (Figure 2).
Figure 2.

Apparent overall rejection of select DBPs during RO, including charged haloacetic acids (HAAs) and uncharged trihalomethanes (THMs), dihaloacetonitriles (DHANs), and bromophenols. Reproduced with permission from ref 43. Copyright 2010 Elsevier.
When ozone is used as an oxidant upstream of microfiltration to reduce fouling,45 the bulk organic matter in the wastewater effluent reacts with ozone and/or ·OH produced during ozone decomposition46 to form aldehydes and ketones, such as formaldehyde and glyoxal.47 Not only are many of these uncharged compounds poorly rejected during RO,48,49 some exhibit high toxicity.50
In addition to oxidation products, there are compounds present in wastewater effluent that are poorly rejected by RO systems. Because most uncharged compounds are difficult to quantify by LC-MS/MS and are not monitored routinely, their presence has only come to the attention of researchers using sensitive gas chromatography-tandem mass spectrometry (GC-MS/MS) methods. For example, compounds with extremely low odor thresholds (i.e., 0.5–10 ng/L) are responsible for most consumer complaints about drinking water.51 Odorous compounds, including 2,4,6-trichloroanisole (molecular weight = 211 g/mol), have been detected at concentrations up to an order of magnitude above their odor threshold after RO treatment.52 Municipal wastewater also contains a variety of uncharged, low-molecular weight organic solvents (e.g., acetone, 1,4-dioxane). Concentrations of solvents in recycled water are usually below values developed for protection of public health.53,54
Despite their low frequency of detection in wastewater samples, solvents sometimes can pose serious problems for potable reuse systems. In 2001, at Orange County’s GWRS, 1,4-dioxane was detected prior to RO at up to 200 μg/L and in supply wells receiving recycled water at concentrations ranging from 4 to 20 μg/L55 (the California notification level for the compound at the time was 3 μg/L). One significant 1,4-dioxane source was a manufacturer of cellulose acetate membranes (ironically, similar to membranes used in the facility).55 In 2013, acetone was detected at the same facility at elevated concentrations for ~1 day56 (Figure 3). Although solvents typically do not pose acute health risks at the concentrations detected after RO, such episodic events could result in taste and odor problems.
Figure 3.

Total organic carbon (TOC) online analyzer data at Orange County Water District GWRS. Immediate investigation as part of a source control program revealed that the increase was attributable to a pulse of acetone. The dashed line represents the TOC regulatory limit in California (0.5 mg C/L) for water produced in potable reuse. Raw data provided with permission from Orange County Water District and available from ref 56.
UV/H2O2 AS A CHEMICAL BARRIER
When H2O2 absorbs UV light, it undergoes homolytic cleavage to form ·OH (eq 1).
| (1) |
This process is particularly effective when it is used after RO due to the low concentrations of dissolved organic carbon (i.e., DOC <0.5 mg C/L), which typically serves as both a sink for·OH and as a source of chromophores that compete for absorption of UV light. The steady-state concentration of ·OH ([·OH]ss) is a function of the rate of formation from photolysis of H2O2 (Rform,·OH,H2O2), which can be reduced due to light screening from DOC and other chromophores, as well as by solutes that consume ·OH (eq 2). Steady-state ·OH concentrations typically range from 1 × 10−10 to 1 × 10−13 M when UV/H2O2 is employed after RO in potable water reuse.57
| (2) |
Under typical operating conditions employed during UV/H2O2 after RO treatment, an increase in DOC from 0.1 to 4 mg C/L will decrease [·OH]ss by approximately an order of magnitude.
The transformation of a contaminant in UV/H2O2 depends on its rate of photolysis and its reactivity with ·OH (kHO·). Only compounds with high molar absorption coefficients and quantum yields (e.g., NDMA) are transformed to a significant extent by photolysis in UV/H2O2 systems, and ·OH reacts with most organic compounds at nearly diffusion-controlled rates (i.e., kHO· = (5–10) × 109 M−1 s−1). The UV/H2O2 process in advanced treatment is typically designed to achieve 1.2-log reduction (i.e., where a 1.2 log reduction corresponds to a 94.7% decrease in initial concentration or [C]/[C]0 = 1 × 10−1.2) in the concentration of NDMA (by direct photolysis) and 0.5-log reduction in the concentration of 1,4-dioxane (by ·OH oxidation). However, some of the same uncharged, low-molecular weight solvents and halogenated DBPs that are poorly rejected by RO also exhibit low reactivity with ·OH or do not undergo direct photolysis. For example, compounds like formaldehyde (kHO· = 1.3 × 108 M−1 s−1) or chloroform (kHO· = 5.4 × 107 M−1 s−1) will not be removed to an appreciable extent (Figure 4).
Figure 4.

Reactivity of compounds in UV/H2O2 as a function of the log of the second-order rate constants of their reaction with ·OH (log kHO·) assuming [·OH]ss = 4 × 10−10 M (calculated based on 0.5-log removal of 1,4-dioxane, kHO· = 2.8 × 109 M−1 s−1). The dashed line represents reactivity with a DOC concentration of 4 mg C/L under similar operating conditions.
Chloroform does not absorb UV light at 254 nm and exhibits low reactivity with ·OH. The UV dose needed for 50% removal of chloroform in a UV/H2O2 system would be 5500 mJ/cm2 (Figure 5).58 The more toxic brominated trihalomethane species are better removed by both direct photolysis and to a minor extent by reaction with ·OH (kHO· = (6–10) × 107 M−1 s−1), yet only 65% removal of bromoform would be expected under conditions used in most advanced treatment facilities (i.e., 750 mJ/cm2 UV dose).
Figure 5.
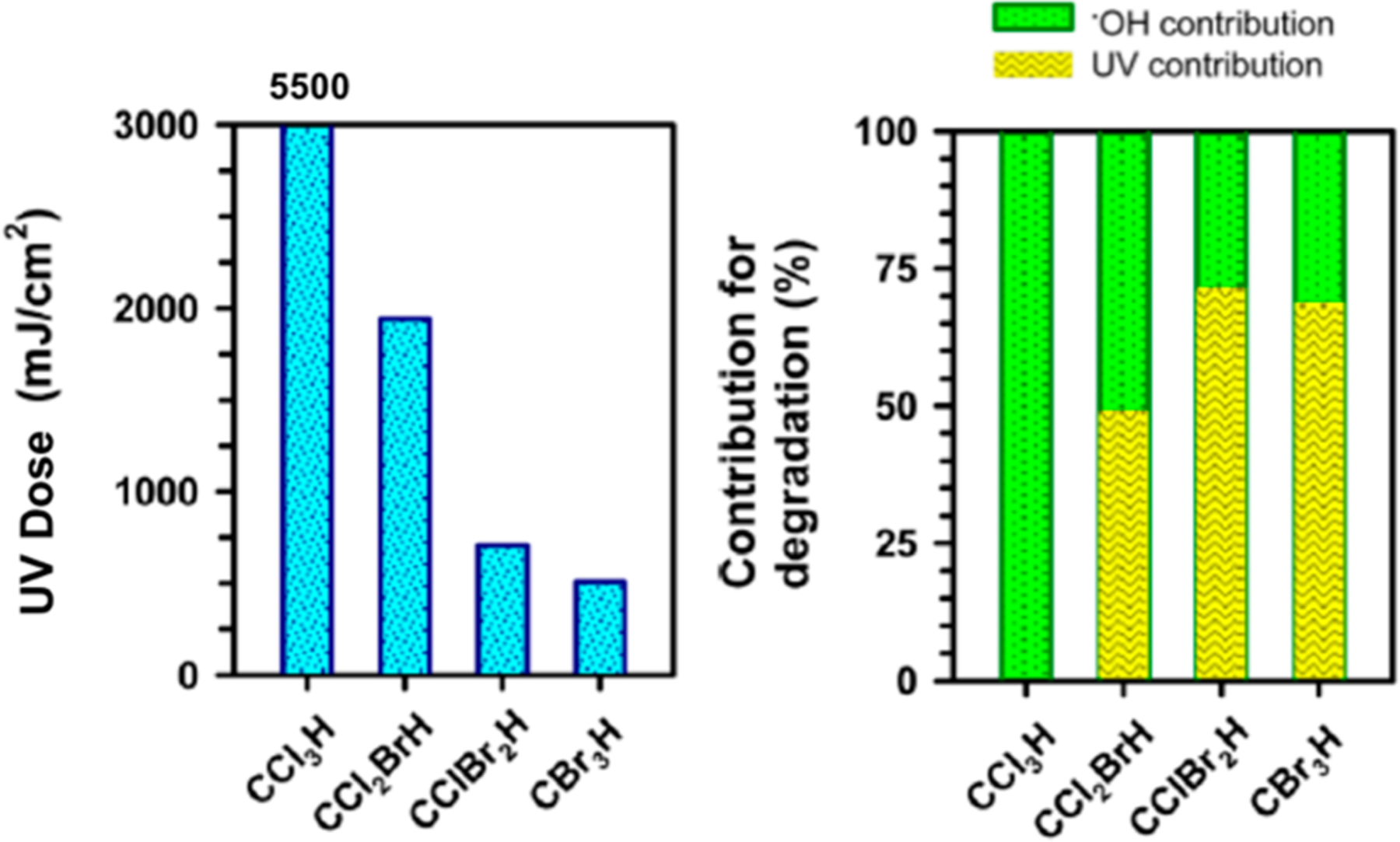
UV dose (254 nm) needed to achieve 50% abatement of trihalomethanes (left) and the percentage contribution of direct photolysis or ·OH oxidation (right) in UV/H2O2 with 3.4 mg/L H2O2. Adapted with permission from ref 58. Copyright 2016 American Chemical Society.
The design constraint of UV/H2O2 in advanced treatment reflects the trade-off between system cost and the efficacy of the barrier. The less reactive, low-molecular weight compounds could be removed by increasing the size of the reactor. For example, 50% removal of chloroform through indirect photolysis could be achieved (Figure 5) but only by increasing the dose (and hence reactor size and energy consumption) by a factor of ~5.58 Thus, oxidation of less-reactive contaminants, while theoretically possible, is too costly in practice.
When UV/H2O2 or any other treatment process that relies upon oxidation is used, it is important to understand the potential formation of toxic byproducts. Although concentrations of target contaminants typically decrease during UV/H2O2 treatment, oxidation processes mostly lead to compound transformation rather than complete mineralization.59 For example, phenolic compounds can be transformed into products that are more toxic than the parent compounds (e.g., benzoquinones60 and α,β-unsaturated enedials and oxoenals61) during UV/H2O2 treatment.
KNOWING THE UNKNOWNS IN THE DUAL BARRIER SYSTEM
After close to three decades of research on chemical compounds in potable water reuse systems, it is evident that only a small subset of contaminants in wastewater effluent passes through RO membranes and resists oxidation by ·OH during UV/H2O2 treatment. Under conditions typically employed in advanced treatment plants, the final product water typically contains ~100 μg C/L.31,49 Known organic compounds (mainly formaldehyde, acetone,49 and halogenated DBPs31,54) account for ~35% of the organic carbon present post-RO treatment (Figure 6). The remainder of the organic carbon (~65 μg/L) likely consists of other uncharged, low-molecular weight compounds.
Figure 6.

Approximate contributions of select chemical contaminants to the DOC (assuming 100 μg C/L) post-RO treatment in potable water reuse. Data averaged and compiled from three different treatment facilities in refs 31, 49, and 54.
Because most of these neutral, low-molecular weight compounds are not amenable to quantification at these low concentrations by either GC-MS/MS or LC-MS/MS, it may be necessary to employ other approaches, such as bioanalytical tools, to assess the potential health risks associated with the residual constituents in product waters. A challenge for the application of bioanalytical tools to the problem is the significant concentration factor needed to obtain bioassay responses. Furthermore, the low-molecular weight, uncharged compounds are often lost during sample concentration due to their high volatility.62 The National Research Council concluded that NDMA and DBPs likely pose the greatest chemical risks for consumers of recycled water.7 However, it is important to note that these contaminants are present in source waters or can be formed during conventional drinking water treatment. When DBP concentrations from different types of waters are weighted by metrics of toxic potency, the total toxicity-weighted byproduct concentration is typically lower in recycled water produced by RO-UV/H2O2 systems relative to conventional drinking water.31,63
POTABLE WATER REUSE WITHOUT REVERSE OSMOSIS TREATMENT
Under conditions typically employed during potable water reuse, ~15% of the wastewater effluent is retained as RO concentrate.64 Most communities that were early adopters of potable water reuse discharged their RO concentrate through existing ocean outfalls. However, discharge of RO concentrate is challenging for many inland communities, because zero-liquid discharge technologies65 are expensive. As a result, many utilities are seeking alternatives to RO treatment.
Nanofiltration has been proposed as an alternative to RO, because tight nanofiltration membranes reject organic matter as well as microbes but allow monovalent salts to pass, resulting in a smaller volume of concentrate.66 Another advantage is that nanofilters operating in place of RO membranes require less pressure, thereby reducing energy consumption and cutting electricity costs by up to 50%.66 However, nanofiltration membranes are not as effective as RO with respect to rejection of uncharged, low-molecular weight compounds (Figure 7). Furthermore, relative to RO, elevated concentrations of DOC after nanofiltration could reduce the efficacy of the UV/H2O2 process by scavenging ·OH and absorbing UV light.
Figure 7.

Percent remaining of NDMA, tris(2-carboxyethyl)-phosphine hydrochloride (TCEP), carbamazepine (CBZ), and perfluorooctanesulfonate (PFOS) after treatment by nanofiltration or RO. Data from refs 66–68.
Another alternative to RO employs the use of either powdered or granular activated carbon (PAC or GAC). For example, the Upper Occoquan Service Authority (Virginia) uses GAC to remove trace organic compounds and DOC prior to reservoir augmentation with recycled water.69 Hydrophobic compounds tend to be well-removed by activated carbon, whereas charged compounds (e.g., naproxen, gemfibrozil, and ibuprofen) or hydrophilic compounds tend to be poorly removed under conditions typically employed during water treatment.26,70 In addition, activated carbon is inefficient with respect to removal of perfluoroalkyl acids (PFAAs).71 Finally, activated carbon must be regenerated more frequently when it is used to treat water with relatively high DOC concentrations, like municipal wastewater effluent.69
Another advanced treatment process involves use of ozone followed by sand filtration or filtration through GAC, which due to the presence of a biofilm is referred to as biologically activated carbon (BAC).72 Combinations of ozone and BAC are currently being used in Switzerland to upgrade wastewater treatment plants discharging to sensitive aquatic ecosystems.73 Compared to RO/AOP, combined ozone/BAC consumes less than half of the energy and costs approximately half as much to operate and maintain.74 This approach does not remove compounds that exhibit low reactivity with ozone or ·OH (e.g., PFAAs). Furthermore, ozonation of wastewater can produce high concentrations of bromate,75,76 a potential human carcinogen (MCL = 10 μg/L). Careful control of ozone doses during ozonation of wastewater can keep bromate concentration within an acceptable range.77 In addition to wastewater treatment plant upgrades, combined ozone/activated carbon systems have also been used in existing and planned potable reuse systems, including surface water augmentation in Gwinnett County (Georgia)7 and direct potable reuse in Windhoek (Namibia).18
THE THIRD BARRIER: CHEMICAL STEWARDSHIP TO SUPPORT POTABLE WATER REUSE
Industrial discharges were important sources of NDMA and 1,4-dioxane at Orange County Water District’s GWRS, despite a relatively modest number of industrial dischargers and a well-funded industrial source control program. Other examples of problematic compounds reported elsewhere include antiyellowing agents discharged by textile industries that produce high concentrations of NDMA upon ozonation78 and resin manufacturing plants that discharge problematic amounts of odorous solvent 1,3-dioxane.79 Consumer products like the insecticide fipronil, which is applied topically to dogs, has been detected in municipal wastewater at concentrations ranging from 13 to 88 ng/L.80
Ultimately, the dual barrier system may not provide adequate protection from all of the chemical contaminants present in wastewater. Rather than rely upon additional treatment processes, a third barrier may be necessary: robust source control programs that employ existing knowledge, as described in the previous sections, to identify and eliminate compounds that are not adequately removed in advanced treatment systems. The third barrier may be particularly important in systems that do not employ RO, where compounds like PFAAs and phosphate-containing flame-retardants like TCEP will not be well removed. Product substitution or better chemical stewardship could be much more cost-effective and sustainable than relying solely on treatment processes. This would also reduce exposure of aquatic ecosystems and downstream water supplies to these contaminants. The implementation of rigorous source control and chemical stewardship measures, coupled with research to identify problematic contaminants, will protect public health and provide confidence in potable water reuse as a viable path for communities worldwide.
ACKNOWLEDGMENTS
This research was supported by the National Science Foundation (NSF) through the Engineering Research Center for Re-Inventing the Nation’s Water Infrastructure (ReNUWIt) EEC-1028968.
Biographies
Emily L. Marron is a Ph.D. candidate in the Department of Civil and Environmental Engineering at the University of California, Berkeley, researching the formation and fate of low-molecular weight compounds during potable water reuse.
William A. Mitch is a Professor in the Department of Civil and Environmental Engineering at Stanford University focusing on chemical contaminants, particularly disinfection byproducts, in potable reuse systems.
Urs von Gunten is a Professor at EPFL, the Swiss Federal Institute of Technology in Lausanne, and a senior researcher at Eawag, the Swiss Federal Institute of Aquatic Science and Technology. His main interests are kinetic and mechanistic studies of disinfection and abatement of micropollutants during oxidative processes.
David L. Sedlak is the Plato Malozemoff Professor in the Department of Civil and Environmental Engineering at the University of California, Berkeley. He is Deputy Director of the U.S. National Science Foundation Engineering Research Center for Reinventing the Nation’s Urban Water Infrastructure, where he conducts research on the fate of chemical contaminants in urban water systems.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Sedlak DL Water 4.0: The Past, Present, and Future of the World’s Most Vital Resource; Yale University Press: New Haven, CT, 2014. [Google Scholar]
- (2).Cutler D; Miller G The Role of Public Health Improvements in Health Advances: The Twentieth-Century United States. Demography 2005, 42, 1–22. [DOI] [PubMed] [Google Scholar]
- (3).AWWA Organisms in Water Committee. Committee Report: Microbiological Considerations for Drinking Water Regulation. J. Am. Water Works Assoc 1987, 79, 81–88 [Google Scholar]
- (4).Young WF; Horth H; Crane R; Ogden T; Arnott M Taste and Odour Threshold Concentrations of Potential Potable Water Contaminants. Water Res. 1996, 30, 331–340. [Google Scholar]
- (5).National Research Council. Arsenic in Drinking Water; National Academies Press: Washington, DC, 1999. [Google Scholar]
- (6).Harris-Lovett S; Sedlak DL The History of Water Reuse in California In Sustainable Water: Challenges and Solutions from California; University of California Press, 2015; pp 220–243. [Google Scholar]
- (7).National Research Council. Water Reuse: Potential for Expanding the Nation’s Water Supply Through Reuse of Municipal Wastewater; National Academies Press: Washington, DC, 2012. [Google Scholar]
- (8).Jobling S; Nolan M; Tyler CR; Sumpter JP; et al. Widespread Sexual Disruption in Wild Fish. Environ. Sci. Technol 1998, 32, 2498–2506. [Google Scholar]
- (9).Huang CH; Sedlak DL Analysis of Estrogenic Hormones in Municipal Wastewater Effluent and Surface Water Using Enzyme-Linked Immunosorbent Assay and Gas Chromatography/Tandem Mass Spectrometry. Environ. Toxicol. Chem 2001, 20, 133–139. [PubMed] [Google Scholar]
- (10).Snyder SA; Westerhoff P; Yoon Y; Sedlak DL Pharmaceuticals, Personal Care Products, and Endocrine Disruptors in Water: Implications for the Water Industry. Environ. Eng. Sci 2003, 20, 449–469. [Google Scholar]
- (11).Snyder EM; Snyder SA; Kelly KL; Gross TS; Villeneuve DL; Fitzgerald SD; Villalobos SA; Giesy JP Reproductive Responses of Common Carp (Cyprinus Carpio) Exposed in Cages to Influent of the Las Vegas Wash in Lake Mead, Nevada, from Late Winter to Early Spring. Environ. Sci. Technol 2004, 38, 6385–6395. [DOI] [PubMed] [Google Scholar]
- (12).Ternes TA Occurrence of Drugs in German Sewage Treatment Plants and Rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar]
- (13).Kolpin DW; Furlong ET; Meyer MT; Thurman EM; Zaugg SD; Barber LB; Buxton HT Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol 2002, 36, 1202–1211. [DOI] [PubMed] [Google Scholar]
- (14).McCarty PL; Argo D; Reinhard M Operational Experiences With Activated Carbon Adsorbers at Water Factory 21. J. - Am. Water Works Assoc 1979, 71, 683–689. [PubMed] [Google Scholar]
- (15).Fujita Y; Ding W-H; Reinhard M Identification of Wastewater Dissolved Organic Carbon Characteristics in Reclaimed Wastewater and Recharged Groundwater. Water Environ. Res 1996, 68, 867–876. [Google Scholar]
- (16).Harris-Lovett SR; Binz C; Sedlak DL; Kiparsky M; Truffer B Beyond User Acceptance: A Legitimacy Framework for Potable Water Reuse in California. Environ. Sci. Technol 2015, 49, 7552–7561. [DOI] [PubMed] [Google Scholar]
- (17).Steinle-Darling E The Many Faces of DPR in Texas. Water Works Assoc. 2015, 107, 16–20. [Google Scholar]
- (18).Gerrity D; Pecson B; Trussell RR; Trussell RS Potable Reuse Treatment Trains throughout the World. Aqua 2013, 62, 321– 338. [Google Scholar]
- (19).Adin A; Asano T The Role of Physical-Chemical Treatment in Wastewater Reclamation and Reuse. Water Sci. Technol 1998, 37, 79–90. [Google Scholar]
- (20).Warsinger DM; Chakraborty S; Tow EW; Plumlee MH; Bellona C; Loutatidou S; Karimi L; Mikelonis AM; Achilli A; Ghassemi A; Padhye LP; Snyder SA; Curcio S; Vecitis CA; Arafat HA; Lienhard JH A Review of Polymeric Membranes and Processes for Potable Water Reuse. Prog. Polym. Sci 2018, 81, 209–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Wijmans JG; Baker RW The Solution-Diffusion Model: A Review. J. Membr. Sci 1995, 107, 1–21. [Google Scholar]
- (22).Reinhard M; Goodman NL; Mccarty PL; Argo DG Removing Trace Organics by Reverse Osmosis Using Cellulose Acetate and Polyamide. J. - Am. Water Works Assoc 1986, 78, 163– 174. [Google Scholar]
- (23).Ozaki H; Li H Rejection of Organic Compounds by Ultra-Low Pressure Reverse Osmosis Membrane. Water Res. 2002, 36, 123–130. [DOI] [PubMed] [Google Scholar]
- (24).Bellona C; Drewes JE; Xu P; Amy G Factors Affecting the Rejection of Organic Solutes during NF/RO Treatment - A Literature Review. Water Res. 2004, 38, 2795–2809. [DOI] [PubMed] [Google Scholar]
- (25).Drewes JE; Bellona C; Oedekoven M; Xu P; Kim TU; Amy G Rejection of Wastewater-Derived Micropollutants in High-Pressure Membrane Applications Leading to Indirect Potable Reuse. Environ. Prog 2005, 24, 400–409. [Google Scholar]
- (26).Snyder SA; Adham S; Redding AM; Cannon FS; DeCarolis J; Oppenheimer J; Wert EC; Yoon Y Role of Membranes and Activated Carbon in the Removal of Endocrine Disruptors and Pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar]
- (27).Mitch WA; Sharp JO; Trussell RR; Valentine RL; Alvarez-Cohen L; Sedlak DL N-Nitrosodimethylamine (NDMA) as a Drinking Water Contaminant: A Review. Environ. Eng. Sci 2003, 20, 389–404. [Google Scholar]
- (28).California Division of Drinking Water State Water Resources Control Board. Drinking Water Notification Levels and Response Levels: An Overview http://www.waterboards.ca.gov/drinking_water/programs/ (accessed March 9, 2018).
- (29).Sedlak DL; Deeb RA; Hawley EL; Mitch WA; Durbin TD; Mowbray S; Carr S Sources and Fate of Nitrosodimethylamine and Its Precursors in Municipal Wastewater Treatment Plants. Water Environ. Res 2005, 77, 32–39. [DOI] [PubMed] [Google Scholar]
- (30).Mitch WA; Sedlak DL Formation of N-Nitrosodimethylamine (NDMA) from Dimethylamine during Chlorination. Environ. Sci. Technol 2002, 36, 588–595. [DOI] [PubMed] [Google Scholar]
- (31).Zeng T; Plewa MJ; Mitch WA N-Nitrosamines and Halogenated Disinfection Byproducts in U.S. Full Advanced Treatment Trains for Potable Reuse. Water Res. 2016, 101, 176–186. [DOI] [PubMed] [Google Scholar]
- (32).Sgroi M; Roccaro P; Oelker GL; Snyder SA N-Nitrosodimethylamine Formation upon Ozonation and Identification of Precursors Source in a Municipal Wastewater Treatment Plant. Environ. Sci. Technol 2014, 48, 10308–10315. [DOI] [PubMed] [Google Scholar]
- (33).von Gunten U; Salhi E; Schmidt CK; Arnold WA Kinetics and Mechanisms of N-Nitrosodimethylamine Formation upon Ozonation of N,N-Dimethylsulfamide-Containing Waters: Bromide Catalysis. Environ. Sci. Technol 2010, 44, 5762–5768. [DOI] [PubMed] [Google Scholar]
- (34).Zeng T; Mitch WA Contribution of N-Nitrosamines and Their Precursors to Domestic Sewage by Greywaters and Blackwaters. Environ. Sci. Technol 2015, 49, 13158–13167.] [DOI] [PubMed] [Google Scholar]
- (35).Mitch WA; Oelker GL; Hawley EL; Deeb RA; Sedlak DL Minimization of NDMA Formation during Chlorine Disinfection of Municipal Wastewater by Application of Pre-Formed Chloramines. Environ. Eng. Sci 2005, 22, 882–890. [Google Scholar]
- (36).Furst KE; Pecson BM; Webber BD; Mitch WA Distributed Chlorine Injection to Minimize NDMA Formation during Chloramination of Wastewater. Environ. Sci. Technol. Lett 2018, 5, 462–466. [Google Scholar]
- (37).Steinle-Darling E; Zedda M; Plumlee MH; Ridgway HF; Reinhard M Evaluating the Impacts of Membrane Type, Coating, Fouling, Chemical Properties and Water Chemistry on Reverse Osmosis Rejection of Seven Nitrosoalklyamines, Including NDMA. Water Res. 2007, 41, 3959–3967 [DOI] [PubMed] [Google Scholar]
- (38).Fujioka T; Nghiem LD; Khan SJ; McDonald JA; Poussade Y; Drewes JE Effects of Feed Solution Characteristics on the Rejection of N-Nitrosamines by Reverse Osmosis Membranes. J. Membr. Sci 2012, 409–410, 66–74. [Google Scholar]
- (39).Fujioka T; Khan SJ; McDonald JA; Henderson RK; Poussade Y; Drewes JE; Nghiem LD Effects of Membrane Fouling on N-Nitrosamine Rejection by Nanofiltration and Reverse Osmosis Membranes. J. Membr. Sci 2013, 427, 311–319. [Google Scholar]
- (40).Linge KL; Blythe JW; Busetti F; Blair P; Rodriguez C; Heitz A Formation of Halogenated Disinfection By-Products during Microfiltration and Reverse Osmosis Treatment: Implications for Water Recycling. Sep. Purif. Technol 2013, 104, 221–228. [Google Scholar]
- (41).Plewa MJ; Wagner ED; Jazwierska P; Richardson SD; Chen PH; McKague AB Halonitromethane Drinking Water Disinfection Byproducts: Chemical Characterization and Mammalian Cell Cytotoxicity and Genotoxicity. Environ. Sci. Technol 2004, 38, 62–68. [DOI] [PubMed] [Google Scholar]
- (42).Richardson SD; Plewa MJ; Wagner ED; Schoeny R; DeMarini DM Occurrence, Genotoxicity, and Carcinogenicity of Regulated and Emerging Disinfection by-Products in Drinking Water: A Review and Roadmap for Research. Mutat. Res., Rev. Mutat. Res 2007, 636, 178–242. [DOI] [PubMed] [Google Scholar]
- (43).Agus E; Sedlak DL Formation and Fate of Chlorination By-Products in Reverse Osmosis Desalination Systems. Water Res. 2010, 44, 1616–1626. [DOI] [PubMed] [Google Scholar]
- (44).Doederer K; Farŕe MJ; Pidou M; Weinberg HS; Gernjak W Rejection of Disinfection By-Products by RO and NF Membranes: Influence of Solute Properties and Operational Parameters. J. Membr. Sci 2014, 467, 195–205. [Google Scholar]
- (45).Wang H; Park M; Liang H; Wu S; Lopez IJ; Ji W; Li G; Snyder SA Reducing Ultrafiltration Membrane Fouling during Potable Water Reuse Using Pre-Ozonation. Water Res. 2017, 125, 42–51. [DOI] [PubMed] [Google Scholar]
- (46).von Sonntag C; von Gunten U Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications, 1st ed.; IWA Publishing: London, UK, 2012. [Google Scholar]
- (47).Wert EC; Rosario-Ortiz FL; Drury DD; Snyder SA Formation of Oxidation Byproducts from Ozonation of Wastewater. Water Res. 2007, 41, 1481–1490. [DOI] [PubMed] [Google Scholar]
- (48).Madireddi K; Babcock RW Jr.; Levine B; Huo TL; Khan E; Ye QF; Neethling JB; Suffet IH; Stenstrom MK Wastewater Reclamation at Lake Arrowhead, California: An Overview. Water Environ. Res 1997, 69, 350–362. [Google Scholar]
- (49).Orange County Water District. Groundwater Replenishment System 2017 Annual Report; Orang County: Irvine, CA, 2017. [Google Scholar]
- (50).Lopachin RM; Gavin T Molecular Mechanisms of Aldehyde Toxicity: A Chemical Perspective. Chem. Res. Toxicol 2014, 27, 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Suffet IHM; Khiari D; Bruchet A The Drinking Water Taste and Odor Wheel for the Millennium: Beyond Geosmin and 2-Methylisoborneol. Water Sci. Technol 1999, 40, 1–13. [Google Scholar]
- (52).Agus E; Lim MH; Zhang L; Sedlak DL Odorous Compounds in Municipal Wastewater Effluent and Potable Water Reuse Systems. Environ. Sci. Technol 2011, 45, 9347–9355. [DOI] [PubMed] [Google Scholar]
- (53).Rodriguez C; Linge K; Blair P; Busetti F; Devine B; Van Buynder P; Weinstein P; Cook A Recycled Water: Potential Health Risks from Volatile Organic Compounds and Use of 1,4-Dichlorobenzene as Treatment Performance Indicator. Water Res. 2012, 46, 93–106. [DOI] [PubMed] [Google Scholar]
- (54).Linge KL; Blair P; Busetti F; Rodriguez C; Heitz A Chemicals in Reverse Osmosis-Treated Wastewater: Occurrence, Health Risk, and Contribution to Residual Dissolved Organic Carbon. Aqua 2012, 61, 494–505. [Google Scholar]
- (55).Woodside G; Talebi M Source Control Enhancements to Foster Indirect Potable Reuse. Proc. Water Environ. Fed 2003, 807– 817. [Google Scholar]
- (56).Orange County Water District. Groundwater Replenishment System 2013 Annual Report; Irvine, CA, 2013. https://www.ocwd.com/media/3485/2013_gwrs_annual-report_final.pdf (Accessed Jan 30, 2019). [Google Scholar]
- (57).Chuang YH; Chen S; Chinn CJ; Mitch WA Comparing the UV/Monochloramine and UV/Free Chlorine Advanced Oxidation Processes (AOPs) to the UV/Hydrogen Peroxide AOP under Scenarios Relevant to Potable Reuse. Environ. Sci. Technol 2017, 51, 13859–13868. [DOI] [PubMed] [Google Scholar]
- (58).Chuang YH; Parker KM; Mitch WA Development of Predictive Models for the Degradation of Halogenated Disinfection Byproducts during the UV/H2O2 Advanced Oxidation Process. Environ. Sci. Technol 2016, 50, 11209–11217. [DOI] [PubMed] [Google Scholar]
- (59).Von Gunten U Oxidation Processes in Water Treatment: Are We on Track? Environ. Sci. Technol 2018, 52, 5062–5075. [DOI] [PubMed] [Google Scholar]
- (60).Alnaizy R; Akgerman A Advanced Oxidation of Phenolic Compounds. Adv. Environ. Res 2000, 4, 233–244. [Google Scholar]
- (61).Prasse C; Ford B; Nomura DK; Sedlak DL Unexpected Transformation of Dissolved Phenols to Toxic Dicarbonyls by Hydroxyl Radicals and UV Light. Proc. Natl. Acad. Sci. U. S. A 2018, 115, 2311–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Stalter D; Peters LI; O’Malley E; Tang JYM; Revalor M; Farŕe MJ; Watson K; Von Gunten U; Escher BI Sample Enrichment for Bioanalytical Assessment of Disinfected Drinking Water: Concentrating the Polar, the Volatiles, and the Unknowns. Environ. Sci. Technol 2016, 50, 6495–6505. [DOI] [PubMed] [Google Scholar]
- (63).Szczuka A; Parker KM; Harvey C; Hayes E; Vengosh A; Mitch WA Regulated and Unregulated Halogenated Disinfection Byproduct Formation from Chlorination of Saline Groundwater. Water Res. 2017, 122, 633–644. [DOI] [PubMed] [Google Scholar]
- (64).Pérez-Gonzàlez A; Urtiaga AM; Ibàñez R; Ortiz I State of the Art and Review on the Treatment Technologies of Water Reverse Osmosis Concentrates. Water Res. 2012, 46, 267–283. [DOI] [PubMed] [Google Scholar]
- (65).Van Der Bruggen B; Lejon L; Vandecasteele C Reuse, Treatment, and Discharge of the Concentrate of Pressure-Driven Membrane Processes. Environ. Sci. Technol 2003, 37, 3733–3738. [DOI] [PubMed] [Google Scholar]
- (66).Bellona C; Heil D; Yu C; Fu P; Drewes JE The Pros and Cons of Using Nanofiltration in Lieu of Reverse Osmosis for Indirect Potable Reuse Applications. Sep. Purif. Technol 2012, 85, 69–76. [Google Scholar]
- (67).Fujioka T; Khan SJ; McDonald JA; Roux A; Poussade Y; Drewes JE; Nghiem LD N-Nitrosamine Rejection by Nanofiltration and Reverse Osmosis Membranes: The Importance of Membrane Characteristics. Desalination 2013, 316, 67–75. [Google Scholar]
- (68).Tang CY; Fu QS; Criddle CS; Leckie JO Effect of Flux (Transmembrane Pressure) and Membrane Properties on Fouling and Rejection of Reverse Osmosis and Nanofiltration Membranes Treating Perfluorooctane Sulfonate Containing Wastewater. Environ. Sci. Technol 2007, 41, 2008–2014. [DOI] [PubMed] [Google Scholar]
- (69).Sebastiani EG; Snoeyink VL; Angelotti RW Thermal Regeneration of Spent and Acidwashed GAC from the Upper Occoquan Sewage Authority. Water Environ. Res 1994, 66, 199–205. [Google Scholar]
- (70).Westerhoff P; Yoon Y; Snyder S; Wert E Fate of Endocrine-Disruptor, Pharmaceutical, and Personal Care Product Chemicals during Simulated Drinking Water Treatment Processes. Environ. Sci. Technol 2005, 39, 6649–6663. [DOI] [PubMed] [Google Scholar]
- (71).Inyang M; Dickenson ERV The Use of Carbon Adsorbents for the Removal of Perfluoroalkyl Acids from Potable Reuse Systems. Chemosphere 2017, 184, 168–175. [DOI] [PubMed] [Google Scholar]
- (72).Simpson DR Biofilm Processes in Biologically Active Carbon Water Purification. Water Res. 2008, 42, 2839–2848. [DOI] [PubMed] [Google Scholar]
- (73).Eggen RIL; Hollender J; Joss A; Schärer M; Stamm C Reducing the Discharge of Micropollutants in the Aquatic Environment: The Benefits of Upgrading Wastewater Treatment Plants. Environ. Sci. Technol 2014, 48, 7683–7689. [DOI] [PubMed] [Google Scholar]
- (74).Stanford BD; Dickenson ERV; Wert E; Inyang M Controlling Trace Organic Compounds Using Alternative, Non-FAT Technology for Potable Water Reuse; Water Environment and Research Foundation: Alexandria, VA, 2017. [Google Scholar]
- (75).Soltermann F; Abegglen C; Götz C; Von Gunten U Bromide Sources and Loads in Swiss Surface Waters and Their Relevance for Bromate Formation during Wastewater Ozonation. Environ. Sci. Technol 2016, 50, 9825–9834. [DOI] [PubMed] [Google Scholar]
- (76).Gerrity D; Snyder S Review of Ozone for Water Reuse Applications: Toxicity, Regulations, and Trace Organic Contaminant Oxidation. Ozone: Sci. Eng 2011, 33, 253–266. [Google Scholar]
- (77).Soltermann F; Abegglen C; Tschui M; Stahel S; von Gunten U Options and Limitations for Bromate Control during Ozonation of Wastewater. Water Res. 2017, 116, 76–85. [DOI] [PubMed] [Google Scholar]
- (78).Kosaka K; Asami M; Konno Y; Oya M; Kunikane S Identification of Antiyellowing Agents as Precursors of N-Nitrosodimethylamine Production on Ozonation from Sewage Treatment Plant Influent. Environ. Sci. Technol 2009, 43, 5236–5241. [DOI] [PubMed] [Google Scholar]
- (79).Quintana J; Vegué L; Martín-Alonso J; Paraira M; Boleda MR; Ventura F Odor Events in Surface and Treated Water: The Case of 1,3-Dioxane Related Compounds. Environ. Sci. Technol 2016, 50, 62–69. [DOI] [PubMed] [Google Scholar]
- (80).Sadaria AM; Sutton R; Moran KD; Teerlink J; Brown JV; Halden RU Passage of Fiproles and Imidacloprid from Urban Pest Control Uses through Wastewater Treatment Plants in Northern California, USA. Environ. Toxicol. Chem 2017, 36, 1473–1482. [DOI] [PubMed] [Google Scholar]


