ABSTRACT
Syphilis is a public health problem, especially in pregnant women, due to the risk of transmission to the fetus and the involvement of the central nervous system, causing neurosyphilis. A case-control study was carried out to analyze the variables associated with neurosyphilis in Brazilian newborns of pregnant women with syphilis admitted for childbirth. Newborns were submitted to treponemal and non-treponemal tests, cerebrospinal fluid analysis, and long bone radiography. Newborns diagnosed with neurosyphilis and congenital syphilis were defined as cases and controls, respectively. The length of hospitalization and mean cost of neurosyphilis treatment were also evaluated. Twenty-one cases of newborns with neurosyphilis and 42 controls with congenital syphilis were included in the study. Out of 63 pregnant women with syphilis, 95.2% (60/63) received prenatal care, 74.6% (47/63) were diagnosed with syphilis during this period, 31.9% (15/47) underwent treponemic tests, 80.8% (38/47) were treated with penicillin and only 46.8% (22/47) of the partners received the treatment. Clinical complications such as low birth weight were observed in 12.7% (8/63) of the newborns. About 50.8% (32/63) of the newborns were hospitalized due to syphilis complications and each case of neurosyphilis spent at least US$ 881.48 on treatment and hospitalization. The results showed that the prenatal coverage is not sufficient to prevent neurosyphilis. The late diagnosis of syphilis in pregnant women and inadequate follow-up of sexual partners may favor the vertical transmission of T. pallidum in pregnant Brazilian women. Thus, improving the quality of health services is important for a more effective control of neurosyphilis.
Keywords: Syphilis, Neurosyphilis, Risk factors, Newborns, Pregnant women, Congenital infections, Congenital syphilis
INTRODUCTION
Syphilis, an infectious, sexually transmitted disease, remains a major public health problem in the world with several clinical manifestations 1 . The occurrence of syphilis in its different forms can be a predictor of important health services failures and its increase has raised national concern in recent years 2 . The World Health Organization (WHO) estimates more than 11 million new cases every year, and more than 90% of them occur in developing countries, such as Brazil 1 , 3 , 4 . Treponema pallidum is the etiologic agent of syphilis, and in pregnant women the infection has a potential risk of vertical transmission 5 .
Gestational syphilis affects approximately 1.5 million pregnant women per year worldwide, and nearly half of the untreated pregnant women present with adverse outcomes 3 . A meta-analysis showed that 25.6% of the pregnant women infected with T. pallidum evolved with abortions or stillbirths, 15.5% had clinical evidence of syphilis, 12.3% of the newborns died and 12.1% were born preterm or with low birth weight 6 . In Brazil 7 , the prevalence of T. pallidum infection in pregnant women is 1.6%. In 2017, about 24,666 cases of congenital syphilis were reported, corresponding to a 17% increase compared with the previous year. In this period, the rate of death from congenital syphilis in children under one year of age was 7.2 per 100,000 live births, showing an insufficient control of the disease throughout the national territory 8 .
During pregnancy, T. pallidum is transmitted to the fetus through the transplacental route. It can affect the central nervous system (CNS), causing neurosyphilis, with varied clinical manifestations, ranging from cerebrospinal fluid changes in asymptomatic newborns to more serious symptomatology such as progressive general paralysis. More than 50% of children infected with T. pallidum are asymptomatic at birth. Moreover, about 23% of infants born to mothers with syphilis have neurosyphilis. Thus, one of the major concerns of this congenital disease, even if asymptomatic, is the possibility of involvement of the CNS 9 , 10 . The diagnosis of neurosyphilis is complex, with the cerebrospinal fluid (CSF) evaluation as the only way to diagnose asymptomatic neurosyphilis. Although a positive VDRL in CSF is considered specific for neurosyphilis, it has limited sensitivity 11 - 13 .
Interventions aimed at preventing and timely diagnosing syphilis during the prenatal care are recommended to control this disease 14 , as behavioral, socio-demographic and health care factors are considered facilitators of syphilis in pregnant women 15 . Despite the increase in new cases of syphilis in Brazil, neurosyphilis is not sufficiently studied. The purpose of this study was to analyze the variables associated with neurosyphilis in Brazilian newborns and assist in implementing public health strategies to control the disease.
METHODS
Study design
This is a case control study carried out in a University Hospital (HU) from the Midwest region of Brazil. Mato Grosso do Sul (MS) is a state in the Midwest of Brazil bordering Paraguay and Bolivia, with a population of 2.5 million people. The study was conducted from July to December 2017 with parturients admitted to a maternity ward of the HU of Dourados, Midwest of Brazil. The HU is a public hospital with 237 beds that provides medium to high complex assistance in various medical specialties. It is the only referral hospital for attending high-risk pregnancies in 34 municipalities, with approximately 900,000 inhabitants, with an average of 1,000 visits and 300 births per month.
Study population
The study included newborns of parturient who were 18 years of age or older, with the diagnosis of syphilis, admitted to the HU maternity ward. They should be mentally competent to understand the research and agree to participate. The parturients were screened using treponemal and non-treponemal serological tests. Those who tested positive, irrespective of the titer, had their newborns screened following the protocol of the Ministry of Health, which included treponemal and nontreponemal serological tests, cerebrospinal fluid and long bones radiography. Newborns with altered cerebrospinal fluid (CSF) analysis and diagnosed with neurosyphilis, were considered a case. Newborns without CSF changes and diagnosed with congenital syphilis, were defined as a control. Each case was further randomly matched with two paired controls.
Data collection
Data collection was performed during admission for childbirth in two stages. Firstly, each parturient was interviewed using a standardized questionnaire. The variables obtained included age, marital status, education level, drug use, sexual history, diagnosis of sexually transmitted infections (STI), obstetric data, prenatal care, diagnosis and treatment of syphilis. The skin color of the participant (white and non-white) was self-reported. Secondly, medical charts and prenatal records of the participants were Newborns data were obtained from their medical records. A team of previously trained health professionals performed the interviews and data collection.
Serological tests
For serological tests, 10 mL and 3 mL of peripheral venous blood were collected by a team of previously trained health professionals from the parturients and newborns, respectively. Parturients were screened during admission using the rapid treponemal test (ABON Biopharm, Hangzhou, China) and the enzyme immunoassay (ELISA) (ICE * Syphilis, DiaSorin, Saluggia, Italy) for the detection of IgG and IgM anti- T. pallidum . Reagent (positive) samples were serially diluted and titrated with respect to anti-cardiolipin antibodies by the Venereal Disease Research Laboratory (VDRL) test (Abbott Murex, Dartford, UK). VDRL titers were used to determine treatment insufficiency or immunological memory in pregnant women. Blood samples of newborns from mothers with reactive serological results were tested using the treponemal and the VDRL tests.
Lumbar puncture
The newborns were immobilized in the fetal position, and disinfection of the puncture site was performed with iodine solution (1% to 2% iodine dye or 10% povidone-iodine). The CSF sample was obtained by lumbar puncture using a hypodermic needle and syringe with a minimum volume of 2 mL. The CSF collected was stored in a sterile flask until the analysis. The collection was performed by a pediatrician using an aseptic technique.
Cerebrospinal fluid analysis
The CSF samples of the newborns were tested using the VDRL test. A case of neurosyphilis was considered if the newborn presented with one or more of the following: any titer in the VDRL test, high protein levels (> 150 mg/dL), and leukocyte count (> 25 leukocytes/mm 3 of CSF) (6.14).
Data analysis
The results of the interview and serological tests were recorded in the Electronic Data Capture program (REDCap) and analyzed by the statistical software SAS (version 9.2, SAS Institute, Cary, NC, USA). The descriptive measures, the absolute frequencies and percentages were calculated in the univariate analysis. The Chi-squared test was used to compare the characteristics of the cases and the control group. The univariate analysis verified the association between the independent and dependent variables. The odds ratio (OR) and the confidence interval of 95% (95% CI) were calculated. The level of statistical significance adopted was p ≤ 0.05.
Ethical considerations
This study was carried out after the approval of the research ethics committee from the Federal University of Grande Dourados (Nº 1.402.529). All eligible participants were provided with an Informed Consent Form, as noted in Resolution 466 of December 12, 2012, from the Brazilian National Health Council.
RESULTS
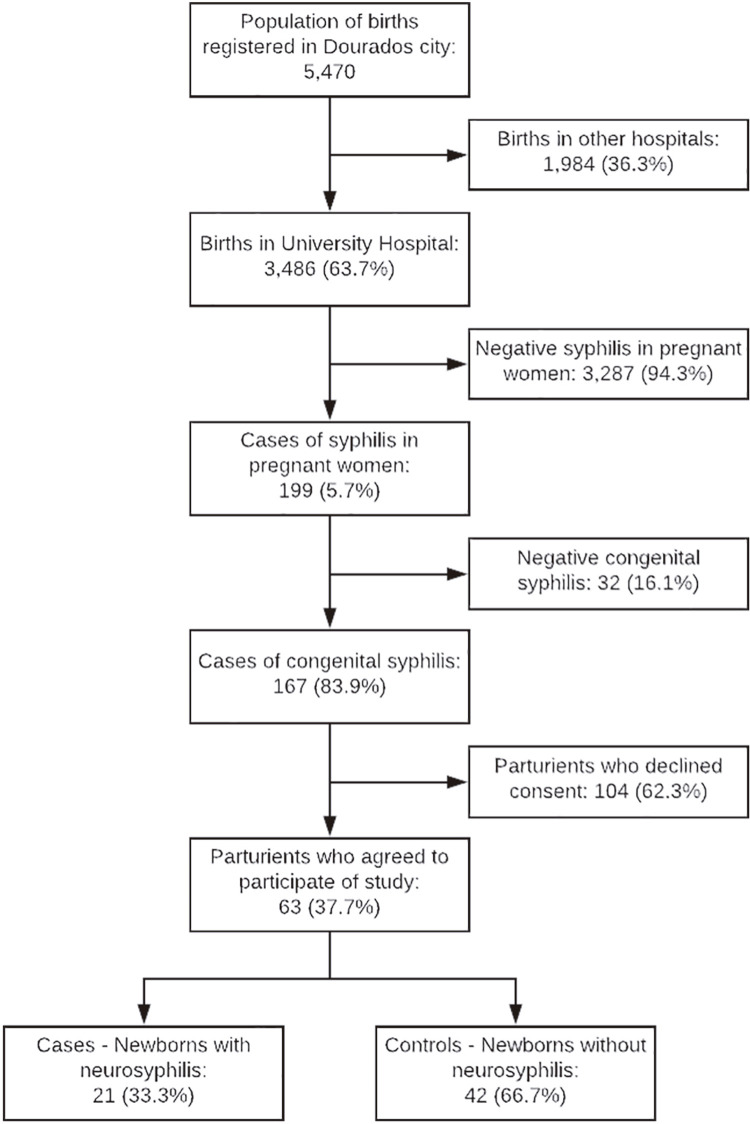
In 2017, 5,470 births were registered in Dourados, of which 3,486 were in the HU, which represents 63.7% of the total births. In the HU, 199 cases of pregnant women with syphilis and 167 cases of congenital syphilis were registered during the study period. The incidence rate of congenital syphilis was 4,790 per 100,000 live births. Sixty-three pregnant women with syphilis that agreed to participate in this study were included ( Figure 1 ).
Figure 1. Flowchart of cases of congenital syphilis in the University Hospital, Dourados, Mato Grosso do Sul State, Brazil, from July to December, 2017.
From the total of 63 women with syphilis admitted for childbirth, 33.3% (21/63) were over 30 years of age, 63.5% (40/63) did not complete the primary education, 82.5% (52/63) declared themselves as non-white, 66.7% (42/63) were married, 65% (41/63) were unemployed, and 71.4% (45/63) lived in Dourados. Seventy-three percent (46/63) had income less than one minimum wage, 55.6% (35/63) had their first sexual experience at ≥ 15 years of age, and 90.5% (57/63) reported not using condoms during sexual intercourses. Table 1 shows the distribution of the main socio-demographic and behavioral variables of pregnant women.
Table 1. Sociodemographic characteristics and risk behaviors of pregnant women admitted for delivery at University Hospital of Dourados, Mato Grosso do Sul State.
| Variable | Total n = 63 | Case n = 21 | Control n = 42 | OR | 95% CI | p value |
|---|---|---|---|---|---|---|
| Age (years) | 0.201 | |||||
| < 20 | 11 (17.5) | 4 (19) | 7 (16.7) | 1 | ||
| 20-25 | 18 (28.6) | 4 (19) | 14 (33.3) | 0.50 | 0.09-2.62 | |
| 25-30 | 13 (20.6) | 2 (9.5) | 11 (26.2) | 0.31 | 0.04-2.22 | |
| 30 | 21 (33.3) | 11 (52.5) | 10 (23.8) | 1.92 | 0.43-8.60 | |
| Education level | 1.10 | 0.37-3.27 | 0.854 | |||
| Elementary school | 40 (63.5) | 13 (62) | 27 (64) | |||
| High school | 23 (36.5) | 8 (38) | 15 (36) | |||
| Skin color | 0.85 | 0.21-3.30 | 0.815 | |||
| White | 11 (17.5) | 4 (19) | 7 (16.7) | |||
| Non-white | 52 (82.5) | 17 (81) | 35 (83.3) | |||
| Marital status | 1.00 | 0.32-3.03 | 1.00 | |||
| Married | 42 (66.7) | 14 (66.7) | 28 (66.7) | |||
| Single | 21 (33.3) | 7 (33.3) | 14 (33.3) | |||
| Occupation | 0.65 | 0.20-2.01 | 0.458 | |||
| Unemployed | 41 (65) | 15 (71.4) | 26 (61.9) | |||
| Other occupation | 22 (35) | 6 (28.6) | 16 (38.1) | |||
| City | 3.33 | 0.84-13.19 | 0.086 | |||
| Dourados | 45 (71.4) | 18 (85.7) | 27 (42.3) | |||
| Others | 18 (28.6) | 3 (14.3) | 15 (57.7) | |||
| Wage | 1.12 | 0.34-3.63 | 0.842 | |||
| < 1 minimum wage | 46 (73) | 15 (71.4) | 31 (73.9) | |||
| ≥ 1 minimum wage | 17 (27) | 6 (28.6) | 11 (26.1) | |||
| Age of first sexual experience | 1.10 | 0.38-3.16 | 0.858 | |||
| ≤ 14 years | 28 (44.4) | 9 (43) | 19 (45.2) | |||
| ≥ 15 years | 35 (55.6) | 12 (57) | 23 (54.8) | |||
| Condom use | 1.00 | 0.16-5.95 | 1.000 | |||
| Yes | 6 (9.5) | 2 (9.5) | 4 (9.5) | |||
| No | 57 (90.5) | 19 (90.5) | 38 (90.5) | |||
| Sexual partners in the last two years | 1.00 | 0.32–3.03 | 1.000 | |||
| 1 | 42 (66.7) | 14 (66.7) | 28 (66.7) | |||
| ≥ 2 | 21 (33.3) | 7 (33.3) | 14 (33.3) | |||
| Sexual activity with inmate | 1.41 | 0.33-5.98 | 0.641 | |||
| Yes | 11 (17.5) | 3 (14.3) | 8 (19.5) | |||
| No | 52 (82.5) | 18 (85.7) | 34 (80.5) | |||
| Tattoo | 5.14 | 1.47-17.90 | 0.010 | |||
| Yes | 27 (42.9) | 4 (19.5) | 23 (54.7) | |||
| No | 36 (57.1) | 17 (80.5) | 19 (45.3) | |||
| Piercing | 2.12 | 0.60-7.52 | 0.240 | |||
| Yes | 18 (28.6) | 4 (19.5) | 14 (33.3) | |||
| No | 45 (71.4) | 17 (80.5) | 28 (66.7) | |||
| Smoking | 2.12 | 0.52-8.65 | 0.287 | |||
| Yes | 14 (22.2) | 3 (14.3) | 11 (26.2) | |||
| No | 49 (77.8) | 18 (85.7) | 31 (73.8) | |||
| Alcohol use | 2.50 | 0.74-8.44 | 0.140 | |||
| Yes | 49 (77.8) | 14 (66.7) | 35 (83.3) | |||
| No | 14 (22.2) | 7 (33.3) | 7 (16.7) | |||
| Illicit drug use | 5.02 | 0.2597.86 | 0.974 | |||
| Yes | 4 (6.4) | 0 (0) | 4 (9.5) | |||
| No | 59 (93.6) | 21 (100) | 38 (90.5) |
In addition, 95.2% (60/63) of pregnant women received prenatal care, and only 60% (36/60) started it in the first trimester of pregnancy. Among these, 74.6% (47/63) were diagnosed with syphilis during the prenatal care, 31.9% (15/47) underwent treponemic tests, and 80.8% were treated with penicillin (38/47). Table 2 shows the variables related to prenatal and maternity care (clinical and obstetrical) for pregnant women. Table 2 shows the variables related to the clinical and the obstetric history and health care in the prenatal and maternity periods for the cases and controls.
Table 2. Obstetric, prenatal and maternity health care for syphilis in pregnant women at the University Hospital of Dourados, Mato Grosso do Sul State.
| Variable | Total n = 63 | Case n = 21 | Control n = 42 | OR | 95% CI | p value |
|---|---|---|---|---|---|---|
| Prenatal care | 0.26 | 0.01-5.32 | 0.213 | |||
| Yes | 60 (95.2) | 21 (100) | 39 (92.8) | |||
| No | 3 (4.8) | 0 (0) | 3 (7.2) | |||
| Place of prenatal care | 1.55 | 0.06-39.79 | 0.479 | |||
| Public | 59 (98.3) | 21 (100) | 41 (97.6) | |||
| Private | 1 (1.7) | 0 (0) | 1 (2.7) | |||
| Beginning of prenatal care | 1.20 | 0.40-3.52 | 0.742 | |||
| 1º trimester | 36 (60) | 12 (57.1) | 24 (61.5) | |||
| 2º or 3º trimester | 24 (40) | 9 (42.9) | 15 (38.5) | |||
| Medical visits | 0.88 | 0.28-2.76 | 0.840 | |||
| ≤ 5 | 41 (68.3) | 14 (66.7) | 27 (69.2) | |||
| ≥ 6 | 19 (31.7) | 7 (33.3) | 12 (30.8) | |||
| History of abortion | 0.70 | 0.22-2.21 | 0.557 | |||
| Yes | 18 (28.6) | 7 (33.3) | 11 (26.1) | |||
| No | 45 (71.4) | 14 (66.7) | 31 (73.9) | |||
| History of stillbirth | 2.10 | 0.22-20.11 | 0.513 | |||
| Yes | 5 (8) | 1 (4.7) | 4 (9.5) | |||
| No | 58 (92) | 20 (95.3) | 38 (90.5) | |||
| History of preterm birth < 37 weeks | 0.89 | 0.29-2.74 | 0.849 | |||
| Yes | 20 (31.8) | 7 (33.3) | 13 (30.9) | |||
| No | 43 (68.2) | 14 (66.7) | 29 (69.1) | |||
| History of neonatal death | 1.53 | 0.15-15.75 | 0.717 | |||
| Yes | 4 (6.4) | 1 (4.7) | 3 (7.1) | |||
| No | 59 (93.6) | 20 (95.3) | 39 (92.9) | |||
| History of STI in the last year | 0.61 | 0.21-178 | 0.376 | |||
| Yes | 31 (49.2) | 12 (57.1) | 19 (45.2) | |||
| No | 32 (50.8) | 9 (42.9) | 23 (54.8) | |||
| Genital injury | 1.14 | 0.32-3.98 | 0.831 | |||
| Yes | 14 (22.2) | 5 (23.8) | 9 (21.4) | |||
| No | 49 (77.8) | 16 (76.2) | 33 (78.6) | |||
| Vaginal discharge | 1.47 | 0.51-4.25 | 0.472 | |||
| Yes | 37 (58.7) | 11 (52.3) | 26 (61.9) | |||
| No | 26 (41.3) | 10 (47.7) | 16 (38.1) | |||
| VDRL test during prenatal care | 2.28 | 0.67-7.75 | 0.182 | |||
| Yes | 47 (78.3) | 14 (66.7) | 33 (84.6) | |||
| No | 13 (21.7) | 7 (33.3) | 6 (15.4) | |||
| VDRL titer | 0.58 | 0.19-1.79 | 0.352 | |||
| ≤1:4 | 27 (57.4) | 10 (52.6) | 17 (60.7) | |||
| ≥1:8 | 20 (42.6) | 9 (47.4) | 11 (39.3) | |||
| Rapid test during prenatal care | 0.50 | 0.15-1.64 | 0.254 | |||
| Yes | 40 (66.7) | 16 (76.2) | 24 (61.5) | |||
| No | 20 (33.3) | 5 (23.8) | 15 (38.5) | |||
| HIV serological test during prenatal care | 0.34 | 0.03-3.12 | 0.325 | |||
| Yes | 54 (90) | 20 (95.2) | 34 (87.2) | |||
| No | 6 (10) | 1 (4.8) | 5 (12.8) | |||
| HIV serology | 1.18 | 0.10-13.99 | 0.892 | |||
| Reactive | 3 (5.6) | 1 (5) | 2 (5.9) | |||
| Non-reactive | 51 (94.4) | 19 (95) | 32 (94.1) | |||
| Treponemal test during prenatal care | 5.05 | 0.97-26.17 | 0.053 | |||
| Yes | 15 (31.9) | 2 (12.5) | 13(41.9) | |||
| No | 32 (68.1) | 14 (87.5) | 18 (58.1) | |||
| Number of children | 1.62 | 0.55-4.73 | 0.375 | |||
| ≤2 | 29 (46) | 8 (38.1) | 21 (50) | |||
| ≥3 | 34 (54) | 13 (61.9) | 21 (50) | |||
| Type of delivery | 2.64 | 0.89-7.76 | 0.077 | |||
| VB | 34 (54) | 8 (38.1) | 26 (61.9) | |||
| CS | 29 (46) | 13 (61.9) | 16 (38.1) | |||
| Diagnosis of syphilis during pregnancy | 0.88 | 0.26-2.97 | 0.839 | |||
| Yes | 47 (74.6) | 16 (76.1) | 31 (73.8) | |||
| No | 16 (25.4) | 5 (23.9) | 11 (26.2) | |||
| Treatment with penicillin | 0.96 | 0.20–4.48 | 0.960 | |||
| Yes | 38 (80.8) | 13 (81.2) | 25 (80.6) | |||
| No | 9 (19.2) | 3 (18.8) | 6 (19.4) | |||
| VDRL test (monthly) | 0.330 | |||||
| Yes | 4 (8.6) | 0 (0) | 4 (13.4) | |||
| No | 43 (91.4) | 17 (100) | 26 (86.6) | |||
| Sexual partner treated | 0.76 | 0.19-2.97 | 0.702 | |||
| Yes | 22 (46.8) | 7 (50) | 15 (45.5) | |||
| No | 25 (53.2) | 7 (50) | 18 (54.5) |
CS = cesarean section; VB = vaginal birth; STI = sexually transmitted infections.
No newborns showed alterations in the X-rays of long bones. The CSF analyses showed that 42.8% (9/21) of neurosyphilis cases had high protein (> 150 mg/dL), 28.6% (6/21) had a high number of leukocytes (>25 leukocytes/mm 3 CSF) and 28.6% (6/21) showed a positive VDRL test ( Table 3 ). The clinical complications in newborns were 12.7% (8/63) of low birth weight and 34.9% (22/63) of prematurity. In addition, 95.2% (20/21) of the cases and 28.5% (12/42) of the controls were hospitalized. The newborns with neurosyphilis were hospitalized for treatment for a minimum of ten days. Each case of neurosyphilis spent at least US$ 64.21 with treatment (Brazilian reals were converted to U.S. dollars using the quotation of 09/28/2018, being R$ 4.03 = 1.00 US$), and R$ 81.72 per day with hospitalization. Thus, the health system spent around US$ 881,48 on each patient with neurosyphilis.
Table 3. Complications in the newborns evaluated in this study.
| Variables | Total n = 63 | Case n = 21 | Control n = 42 | OR | 95% CI | p value |
|---|---|---|---|---|---|---|
| VDRL titer | 0.58 | 0.20-1.71 | 0.330 | |||
| ≤ 1:4 | 33 (52.4) | 13 (61.9) | 20 (47.6) | |||
| ≥ 1:8 | 30 (47.6) | 8 (38.1) | 22 (52.4) | |||
| Hospitalization | 49.99 | 6.02-415.29 | 0.003 | |||
| Yes | 32 (50.8) | 20 (95.2) | 12 (28.6) | |||
| No | 31 (49.2) | 1 (4.8) | 30 (71.4) | |||
| Symptomatic syphilis | 1.36 | 0.21-8.89 | 0.743 | |||
| Yes | 5 (7.9) | 2 (9.5) | 3 (7.1) | |||
| No | 58 (92.1) | 19 (90.5) | 39 (92.9) | |||
| Low birth weight | 4.06 | 0.86-19.04 | 0.075 | |||
| Yes | 8 (12.7) | 5 (23.8) | 3 (7.1) | |||
| No | 55 (87.3) | 16 (76.2) | 39 (92.9) | |||
| Prematurity | 2.27 | 0.76-6.73 | 0.138 | |||
| Yes | 22 (34.9) | 10 (47.6) | 12 (28.6) | |||
| No | 41 (65.1) | 11 (52.4) | 30 (71.4) | |||
| CSF abnormality | ||||||
| Protein (> 150 mg/dL) | 9 (42.8) | 9 (42.8) | 0 | |||
| Leukocytes (> 25 white blood cells/mm 3 ) | 6 (28.6) | 6 (28.6) | 0 | |||
| Reagent VDRL | 6 (28.6) | 6 (28.6) | 0 |
DISCUSSION
In this study, we evaluated cases of neurosyphilis in Brazilian newborns. The results showed that most pregnant women received prenatal care and were diagnosed with syphilis during this period (treponemal test during prenatal care, p = 0.053). Although 80.8% pregnant women were treated with penicillin, only 46.8% of their partners received the treatment. Thus, inadequate treatment, mainly of sexual partners, may favor the vertical transmission of T. pallidum in pregnant Brazilian women. The alarming rise in syphilis cases in Brazil has become a serious public health problem, mainly due to severe complications resulting from the transmission of the infection to the fetus. However, improving the quality of health services to prevent the vertical transmission of T. pallidum in Brazilian pregnant women is still a challenge 16 . Our study identified a higher incidence rate of congenital syphilis than reported in other studies 17 - 21 . In addition, we showed that socio-economic variables, such as low income and poor education were frequent in this population, in agreement with those reported in the USA, China and Argentina 22 - 24 , possibly reflecting inequalities in self-care, as well as difficulties to access and perform follow-ups in health services.
Although health services adopt disease control measures for vulnerable groups such as the one of pregnant women 14 , 15 , they are insufficient to eliminate neurosyphilis. An untreated sexual partner is also associated with neurosyphilis, so that the exclusive treatment of pregnant women seems to be insufficient in reducing the incidence of congenital syphilis and neurosyphilis, thus reflecting a flaw in the follow-up. The treatment of sexual partners is vital for the interruption of the transmission chain and to avoid reinfection of pregnant women, considering that the use of condoms is uncommon 25 , 26 . Therefore, among the efforts to reduce the neurosyphilis cases, the treatment of the sexual partners cannot be neglected during the process of follow-up of pregnant women. Besides, condom use during prenatal care needs to be reinforced by health services as a way to identify strategies of negotiation with sexual partners to avoid permanent reinfection.
From the total of 21 reported cases of neurosyphilis, only 13 pregnant women and seven sexual partners were treated with penicillin. The exposure time of the fetus to T. pallidum may be responsible for the adverse outcomes in the newborns, as prenatal health and maternal vulnerability significantly impact the transplacental transmission of T. pallidum to the fetus. The delay in initiating treatment increases the exposure time of the fetus to T. pallidum and this is one of the critical factors determining the severity of the complications in syphilis 27 . Although the disease has a simple diagnosis and treatment 14 , our results indicate that the control of the transmission of this agent remains an unresolved challenge.
Most cases of neurosyphilis were hospitalized for treatment during a minimum of ten days, increasing the risks of exposure of the newborns to nosocomial infections. In addition, hospitalization represents high costs of at least US$ 881,48 per case to the health system. However, these expenses could be reduced with proper prenatal care including the diagnosis and appropriate treatment of pregnant women and their sexual partners. In this study, 19 of 21 cases of neurosyphilis were asymptomatic. The most frequent alteration in the CSF was high protein levels. Moreover, the majority of cases of neurosyphilis in newborns were asymptomatic; the symptoms generally appeared later and were related to impaired neuropsychomotor development, which may lead to irreversible sequelae 28 . Due to the severe complications of neurosyphilis, maternal screening, and appropriate treatment during prenatal care are extremely important to reduce the number of T. pallidum infections in the fetus. Despite these results, there were some limitations in this study. Firstly, the total number of pregnant women with syphilis treated at the University Hospital in 2017 was 199 cases. However, only 63 pregnant women who gave birth to newborns infected with T. pallidum accepted to participate in the study.
The results of this study indicate that the control of the neurosyphilis remains an unresolved challenge. Late diagnosis of maternal syphilis and inadequate treatment and follow-up of sexual partners showed failures to provide proper prenatal care. Moreover, the high number of newborns hospitalized due to syphilis complications, increased, generating more spending to the Brazilian unified health system. For a more effective control of syphilis in pregnant women, treatment should be prioritized by health managers and professionals. Although the treatment of syphilis is inexpensive, some challenges need to be overcome to implement the guidelines of the Ministry of Health and the WHO guidelines to provide adequate treatment to pregnant women and their sexual partners. In addition, more studies are needed to identify possible obstacles faced by public health service professionals toward the effective management and control of syphilis in pregnant women.
ACKNOWLEDGMENTS
We are grateful to the University Hospital of Dourados for their support and to the participants, without whom this study could not have been performed. Our appreciation is also extended to the staff of the GPBMM/UFGD study group for their support.
Footnotes
FINANCIAL SUPPORT
This work was partially supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq grant Nº 440245/2018-4), Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT grants Nº 092/2015 and 041/2017), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, grant 001) and Universidade Federal da Grande Dourados (UFGD).
REFERENCES
- 1.Avelleira JC, Bottino G. Sífilis: diagnóstico, tratamento e controle. An Bras Dermatol. 2006;81:111–126. [Google Scholar]
- 2.Santos MM, Lopes AK, Roncalli AG, Lima KC. Trends of syphilis in Brazil: a growth portrait of the treponemic epidemic. PLoS One. 2000;15:e0231029. doi: 10.1371/journal.pone.0231029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Department of Reproductive Health and Research . Investment case for eliminating mother-to-child transmission of syphilis: promoting better maternal and child health and stronger health systems. Geneva: WHO; 2012. [cited 2020 Sep 3]. https://www.who.int/reproductivehealth/publications/rtis/9789241504348/en/ [Google Scholar]
- 4.World Health Organization. Department of Reproductive Health and Research . Global incidence and prevalence of selected curable sexually transmitted infections - 2008. Geneva: WHO; 2012. [cited 2020 Sep 3]. https://www.who.int/reproductivehealth/publications/rtis/stisestimates/en/ [Google Scholar]
- 5.World Health Organization . Global guidance on criteria and processes for validation: elimination of mother-to-child transmission of HIV and syphilis. 2. Geneva: WHO; 2017. [cited 2020 Sep 3]. http://www.who.int/reproductivehealth/publications/emtct-hiv-syphilis/en/ [Google Scholar]
- 6.Gomez GB, Kamb ML, Newman LM, Mark J, Broutet N, Hawkes SJ. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217–226. doi: 10.2471/BLT.12.107623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica . Doenças infecciosas e parasitárias: guia de bolso. 8. Brasília: Ministério da Saúde; 2010. [cited 2020 Sep 3]. http://vigilancia.saude.mg.gov.br/index.php/download/doencas-infecciosas-e-parasitarias-guia-de-bolso/ [Google Scholar]
- 8.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde Sífilis 2018. [cited 2020 Sep 3];Bol Epidemiol. 2018 49:1–43. http://www.aids.gov.br/pt-br/pub/2018/boletim-epidemiologico-de-sifilis–2018 [Google Scholar]
- 9.Menezes ML, Marques CA, Leal TM, Melo MC, Lima PR. Neurossífilis congênita: ainda um grave problema de saúde pública. J Bras Doenças Sex Transm. 2007;19:134–138. [Google Scholar]
- 10.Woods CR. Syphilis in children: congenital and acquired. Semin Pediatr Infect Dis. 2005;16:245–257. doi: 10.1053/j.spid.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Kingston M, French P, Higgins S, McQuillan O, Sukthankar A, Stott C, et al. UK national guidelines on the management of syphilis 2015. Int J STD AIDS. 2016;27:421–446. doi: 10.1177/0956462415624059. [DOI] [PubMed] [Google Scholar]
- 12.Ghanem KG, Workowski KA. Management of adult syphilis. Clin Infect Dis. 2011;53(Suppl 3):S110–S128. doi: 10.1093/cid/cir701. [DOI] [PubMed] [Google Scholar]
- 13.Sparling PF. Diagnosis of neurosyphilis: new tools. Sex Transm Dis. 2010;37:288–289. [PubMed] [Google Scholar]
- 14.Macêdo VC, Lira PI, Frias PG, Romaguera LM, Caires SF, Ximenes RA. Risk factors for syphilis in women: case-control study. 78Rev Saude Publica. 2017;51 doi: 10.11606/S1518-8787.2017051007066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lago EG, Rodrigues LC, Fiori RM, Stein AT. Congenital syphilis: identification of two distinct profiles of maternal characteristics associated with risk. Sex Transm Dis. 2004;31:33–37. doi: 10.1097/01.OLQ.0000105003.72411.FB. [DOI] [PubMed] [Google Scholar]
- 16.Viellas EF, Domingues RM, Dias MA, Gama SG, Theme Filha MM, Costa JV, et al. Prenatal care in Brazil. Cad Saude Publica. 2014;30(Suppl 1):S85–100. doi: 10.1590/0102-311x00126013. [DOI] [PubMed] [Google Scholar]
- 17.Bezerra ML, Fernandes FE, Nunes JP, Baltar SL, Randau KP. Congenital syphilis as a measure of maternal and child healthcare, Brazil. Emerg Infect Dis. 2019;25:1469–1476. doi: 10.3201/eid2508.180298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde Sífilis 2019. [cited 2020 Sep 3];Bol Epidemiol. 2019 (Esp) http://www.aids.gov.br/pt-br/pub/2019/boletim-epidemiologico-sifilis-2019 [Google Scholar]
- 19.Wang Y, Wu M, Gong X, Zhao L, Zhao J, Zhu C, et al. Risk factors for congenital syphilis transmitted from mother to infant – Suzhou, China, 2011–2014. MMWR Morb Mortal Wkly Rep. 2019;68:247–250. doi: 10.15585/mmwr.mm6810a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slutsker JS, Hennessy RR, Schillinger JA. Factors contributing to congenital syphilis cases – New York City, 2010–2016. MMWR Morb Mortal Wkly Rep. 2018;67:1088–1093. doi: 10.15585/mmwr.mm6739a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong FC, Wu XB, Yang F, Lan LN, Guan Y, Zhang CL, et al. Risk of congenital syphilis (CS) following treatment of maternal syphilis: results of a CS control program in China. Clin Infect Dis. 2017;65:588–594. doi: 10.1093/cid/cix371. [DOI] [PubMed] [Google Scholar]
- 22.Parker LA, Deschutter EJ, Bornay-Llinares FJ, Hernandez-Aguado I, Silva G, Piragine RD, et al. Clinical and socioeconomic determinants of congenital syphilis in Posadas, Argentina. Int J Infect Dis. 2012;16:256–261. doi: 10.1016/j.ijid.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Umapathi KK, Thavamani A, Chotikanatis K. Incidence trends, risk factors, mortality and healthcare utilization in congenital syphilis-related hospitalizations in the United States: a nationwide population analysis. Pediatr Infect Dis J. 2019;38:1126–1130. doi: 10.1097/INF.0000000000002445. [DOI] [PubMed] [Google Scholar]
- 24.Qin JB, Feng TJ, Yang TB, Hong FC, Lan LN, Zhang CL, et al. Risk factors for congenital syphilis and adverse pregnancy outcomes in offspring of women with syphilis in Shenzhen, China: a prospective nested case-control study. Sex Transm Dis. 2014;41:13–23. doi: 10.1097/OLQ.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 25.Dallé J, Baumgarten VZ, Ramos MC, Jimenez MF, Acosta L, Bumaguin DB, et al. Maternal syphilis and accomplishing sexual partner treatment: still a huge gap. Int J STD AIDS. 2017;28:876–880. doi: 10.1177/0956462416678710. [DOI] [PubMed] [Google Scholar]
- 26.Carreno I, Costa JS. Use of condoms during sexual intercourse: a population-based study. Rev Saude Publica. 2006;40:720–726. doi: 10.1590/s0034-89102006000500024. [DOI] [PubMed] [Google Scholar]
- 27.Berman SM. Maternal syphilis: pathophysiology and treatment. Bull World Health Organ. 2004;82:433–438. [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. [cited 2020 Sep 3]. https://www.cdc.gov/std/tg2015/default.htm