Abstract
The DeKAF study was developed to better understand the causes of late allograft loss. Preliminary findings from the DeKAF cross-sectional cohort (with follow-up < 20 months) have been published. Herein, we present long-term outcomes in those recipients (mean follow-up ± SD, 6.6 ± 0.7 years). Eligibility included being transplanted prior to October 1, 2005; serum creatinine ≤ 2.0 mg/dL on January 1, 2006; and subsequently developing new-onset graft dysfunction leading to a biopsy. Mean time from transplant to biopsy was 7.5 ± 6.1 years. Histologic findings and DSA were studied in relation to postbiopsy outcomes. Long-term follow-up confirms and expands the preliminary results of each of 3 studies: (1) increasing inflammation in area of atrophy (irrespective of inflammation in nonscarred areas [Banff i]) was associated with increasingly worse postbiopsy death-censored graft survival; (2) hierarchical analysis based on Banff scores defined clusters (entities) that differed in long-term death-censored graft survival; and (3) C4d−/DSA− recipients had significantly better (and C4d+/DSA+ worse) death-censored graft survival than other groups. C4d+/DSA− and C4d−/DSA+ had similar intermediate death-censored graft survival. Clinical and histologic findings at the time of new-onset graft dysfunction define high- vs low-risk groups for long-term death-censored graft survival, even years posttransplant. These findings can help differentiate groups for potential intervention studies.
Keywords: antibody biology, chronic allograft nephropathy, classification systems: Banff classification, clinical research/practice, clinical trial, graft survival, kidney transplantation/nephrology
1 |. INTRODUCTION
In the last 2 decades, there has been improvement in the short-term outcomes after kidney transplantation but little commensurate improvement in long-term outcomes.1,2 A significant proportion of recipients with 1-year graft survival are thought to experience a slow and steady decline in graft function leading to graft loss. Initially, this process was with 1-year graft survival are thought to experience a slow and steady decline in graft function leading to graft loss. Initially, this process was attributed to an inevitable immune response associated with allogeneic transplantation and was termed “chronic rejection.”3,4 Recognition that nonimmunologic injury was also associated with late deterioration of function led to use of the term “chronic allograft nephropathy” (CAN) to encompass both immune and nonimmune injury.5 Given that high calcineurin inhibitor (CNI) levels early posttransplant could cause acute nephrotoxicity, one hypothesis that became popular was that late graft dysfunction was due to chronic CNI nephrotoxicity.6,7
The Deterioration of Kidney Allograft Function (DeKAF) study was developed to better understand the causes of late allograft loss. Specifically, its 2 hypotheses were that (1) chronic graft dysfunction was not the result of past events but was due to new ongoing active injury, and (2) discrete, definable entities were responsible for injury leading to chronic graft deterioration.8 The hope was that defining different entities could lead to individual trials on preventing and/or treating such entities. Seven transplant centersa in the United States and Canada participated in the study, which also included a central pathology core,b a central lab for anti-HLA donor-specific antibody (DSA)c determination, a central database, and a central biostatistical core.d
The DeKAF study included a cross-sectional cohort of previously transplanted recipients who, at the time of study initiation, had a serum creatinine ≤ 2 mg/dL (irrespective of when transplanted) and subsequently developed new-onset graft dysfunction leading to a graft biopsy. This cohort provided an opportunity to assess grafts that had survived as long as several decades posttransplant before developing dysfunction but there was no a comparable control group to determine clinical and demographic factors leading to late dysfunction.
Three preliminary findings of the cross-sectional cohort — (1) the impact of inflammation in areas of atrophy on graft survival;9 (2) hierarchical cluster analysis of Banff scores to define individual “entities” affecting graft outcome10 and (3) the impact of C4d staining and/or circulating DSA on graft outcome11— have been published. Mean follow-up in these analyses was < 20 months. Herein, we update these 3 analyses with a mean (SD) follow-up of 6.6 (0.7) years.
2 |. METHODS
2.1 |. Study population
Enrollment criteria and patient characteristics have been described in detail.8,10 Recipients were eligible for enrollment into the cross-sectional cohort if they had undergone a kidney or kidney/pancreas transplant before October 1, 2005, had a serum creatinine ≤ 2.0 mg/dL before January 1, 2006, and subsequently developed deterioration of graft function resulting in an allograft biopsy (“index biopsy”). Participants were enrolled at the time of biopsy and followed until graft loss, death, or September 1, 2013. The research was approved by the University of Minnesota institutional review board (IRB; #0407M62262) and the IRB at each participating clinical center.
2.2 |. Central pathology and donor-specific antibody determination
All index biopsies were read by a central pathologist (JG) masked to clinical information, local pathology findings including C4d staining, and DSA results. In addition to traditional Banff scoring (e.g., “i”: 0 = inflammation in < 10% of unscarred parenchyma; 1 = 10% to 25% parenchyma inflamed; 2 = 26% to 50% parenchyma inflamed; 3 = > 50% parenchyma inflamed), the central pathologist also made specific histologic observations that, at the time the biopsies were obtained, were not included in Banff scoring. Those observations (which were scored in a manner similar to Banff scoring) included inflammation in areas of atrophy (termed “iatr”) and tubulitis in areas of atrophy (termed “tatr”). The iatr score was assessed as the percentage of atrophic cortex with inflammatory infiltrates: 0 = inflammation in < 10% of atrophic regions; 1 = inflammation in 10% to 25% of atrophic regions; 2 = inflammation in 26% to 50% of atrophic regions; 3 = inflammation in > 50% of atrophic regions. The tatr score was derived similarly.
The histologic sections were stained for C4d by immunoperoxidase and interpreted as positive if more than 10% of peritubular capillaries stained positively.12 Local primary and secondary pathology diagnoses were also recorded. Blood samples collected at the time of the biopsy, recipient and donor HLA types, and recipient pretransplant sensitization status were sent to the central lab (FMC) for DSA determination. DSA was considered positive if antibody were detected against donor HLA-A, B, DR, or DQ. The central lab used a mean fluorescent intensity (MFI) cutoff of 1000 to classify a DSA as positive.
2.3 |. Three analyses with extended follow-up
The original methodology for the 3 DeKAF analyses that were updated for this report is briefly described here:
2.3.1 |. Inflammation in areas of atrophy (iatr)
Using index biopsies where both C4d and DSA determinations were available, we studied the difference in time to death-censored graft failure from index biopsy by iatr score alone and by iatr in combination with i score. Groups were defined as no inflammation (i = 0; iatr = 0), iatr only (iatr > 0; i = 0), and presence of i (i > 0; iatr ≥ 0).
2.3.2 |. Hierarchical cluster analysis
A full description of the clustering method has previously been reported.10 Briefly, clusters were formed using hierarchical clustering by centroids using L_1 norm distance on the vector of Banff scores for inflammation (i), glomerulitis (g), tubular atrophy (ct), vascular fibrous intimal thickening (cv), hyalinosis (ah), and mesangial matrix (mm), plus a scoring of tubulitis in areas of atrophy (tatr). Recipients with a local diagnosis explaining new-onset graft dysfunction (recurrent disease, BK nephropathy) were excluded. Because iatr and tatr were highly correlated, only one of the scores (tatr) was included in clustering. Of the 13 clusters created by the algorithm, 7 were very small and the original publication focused on death-censored graft failure in the 6 larger clusters.
2.3.3 |. Impact of DSA and positive C4d staining
Index biopsies (where both C4d and DSA were available) were classified by C4d and DSA into 4 groups: C4d−/DSA−, C4d−/DSA+, C4d+/DSA−, and C4d+/DSA+. Recipients with a local diagnosis explaining new-onset graft dysfunction (recurrent disease, BK nephropathy) were excluded. In our original publication, the primary endpoint was time from biopsy to death or graft failure. To be consistent with the other analyses in this report, the endpoint studied herein is time from index biopsy to death-censored graft failure.
With additional data cleaning since the initial analyses, it was discovered that 3 of the biopsies included in these initial analyses (1 in the iatr analysis, 3 in the cluster analysis, and 1 in the DSA/C4d analysis) were incorrectly labeled as long-term cohort index biopsies in preliminary results and are excluded from our updated analyses.
2.4 |. Statistical analysis
Mean baseline characteristics were compared between clusters using chi-square tests for categorical variables and ANOVA or Kruskal-Wallis tests for continuous variables. Survival analysis methods were used to compare groups for time to death-censored graft failure, defined as the return to dialysis or retransplantation. Kaplan-Meier plots were used to summarize graft failure times and the log-rank test was used to compare graft survival curves. Cox proportional hazards regression was used to compare graft survival between groups, adjusted for covariates and stratified by clinical center. All hazard ratios (HRs) are presented with their 95% confidence interval (CI). Multivariable analyses were adjusted for the following, as was done in the original publications: creatinine at biopsy and central i, t, ci, and ct scores (iatr score analysis); creatinine at biopsy and central t, ci, and ct scores (iatr and i groups); time from transplant to biopsy and creatinine at biopsy (clusters); and age at biopsy, race (black or nonblack), prior transplant status, creatinine at biopsy and central i, t, g, and ptc scores (C4d and DSA). Treatment with steroids, antibodies, intravenous immunoglobulin (IVIG), plasmapheresis, rituxumab, or another drug for an acute rejection event diagnosed at index biopsy was included as a binary covariate in the updated analyses. Banff scores (scaled 0-3) were fit as categorical covariates. The updated Cox proportional hazards regression analyses correct for multiple comparisons via the Tukey-Kramer method. A Tukey-Kramer adjusted P < .05 was used as the threshold for significance that ensures an overall Type I error rate of 5% for all pairwise comparisons.
3 |. RESULTS
There were 336 cross-sectional cohort participants included in the iatr update, 237 in the cluster update, and 172 in the C4d and DSA update (Table 1). The iatr cohort was 53% female, 78% Caucasian and 14% black, with a mean (SD) age at index biopsy of 48 (14) years, and a mean biopsy creatinine at the time of graft dysfunction of 2.6 (1.5) mg/dL. The participants analyzed in the cluster paper and the C4d/DSA paper were subsets of the iatr cohort and had similar characteristics (Table 1).
TABLE 1.
Characteristics of participants in the original cross-sectional cohort analyses
|
iatr paper update (N = 336) |
Cluster paper update (N = 237)a |
C4d and DSA paper update (N = 172) |
||||
|---|---|---|---|---|---|---|
| Number with data available | n (%) or mean (SD) | Number with data available | n (%) or mean (SD) | Number with data available | n (%) or mean (SD) | |
| Female gender | 336 | 177 (53) | 237 | 13 (54.6) | 172 | 84 (49) |
| Hispanic or Latino ethnicity | 335 | 4 (1.2) | 236 | 2 (0.8) | 172 | 0 |
| Race | ||||||
| Caucasian | 335 | 263 (78) | 236 | 188 (79.7) | 172 | 131 (76) |
| Black or African American | 335 | 46 (14) | 236 | 34 (14.4) | 172 | 25 (14) |
| Asian | 335 | 12 (3.6) | 236 | 6 (2.5) | 172 | 6 (3.5) |
| Native American/Aleutian Islander | 335 | 13 (3.9) | 236 | 7 (3.0) | 172 | 9 (5.2) |
| Multiracial | 335 | 1 (0.3) | 236 | 1 (0.4) | 172 | 1 (0.6) |
| Primary cause of kidney disease | ||||||
| Diabetes | 336 | 101 (30) | 237 | 72 (30) | 172 | 51 (30) |
| Glomerular disease | 336 | 94 (28) | 237 | 71 (30) | 172 | 48 (28) |
| Hypertensive nephrosclerosis | 336 | 33 (9.8) | 237 | 25 (11) | 172 | 23 (13) |
| Polycystic kidney disease | 336 | 24 (7.1) | 237 | 16 (7) | 172 | 9 (5.2) |
| Other | 336 | 67 (20) | 237 | 45 (19) | 172 | 32 (19) |
| Unknown | 336 | 17 (5.1) | 237 | 8 (3) | 172 | 9 (5.2) |
| Era | ||||||
| Before 1980 | 336 | 4 (1.2) | 237 | 3 (1.3) | 172 | 3 (1.7) |
| 1980–1989 | 336 | 22 (6.6) | 237 | 13 (5.5) | 172 | 9 (5.2) |
| 1990–1999 | 336 | 97 (29) | 237 | 68 (29) | 172 | 52 (30) |
| 2000–2005 | 336 | 213 (63) | 237 | 153 (65) | 172 | 108 (63) |
| At transplant | ||||||
| Diabetes | 335 | 138 (41) | 236 | 97 (41) | 171 | 73 (43) |
| Hypertension | 336 | 114 (34) | 237 | 91 (38) | 172 | 57 (33) |
| Retransplant | 336 | 52 (15) | 237 | 43 (18) | 172 | 27 (16) |
| Living donor transplant | 336 | 201 (60) | 237 | 149 (63) | 172 | 101 (59) |
| Donor age (y) | 295 | 37 (13) | 209 | 37 (13) | 151 | 36 (13) |
| Donor hypertension | 240 | 13 (5.4) | 162 | 7 (4.1) | 126 | 9 (7.1) |
| Number of HLA mismatches | 327 | 3.2 (1.7) | 231 | 3.1 (1.7) | 167 | 3.2 (1.6) |
| Class I mismatches | 327 | 2.1 (1.3) | 231 | 2.1 (1.2) | 167 | 2.1 (1.2) |
| Class II mismatches | 327 | 1.1 (0.7) | 231 | 1.0 (0.7) | 167 | 1.1 (0.7) |
| Last creatinine before 2006 (mg/dL) |
336 | 1.4 (0.3) | 237 | 1.4 (0.3) | 172 | 1.4 (0.3) |
| At biopsy | ||||||
| Creatinine (mg/dL) | 335 | 2.6 (1.5) | 237 | 2.6 (1.5) | 171 | 2.7 (1.6) |
| Recipient age (y) | 336 | 48 (14) | 237 | 47 (14) | 172 | 49 (14) |
| Time posttransplant (y) | 336 | 7.2 (5.9) | 237 | 7.0 (5.9) | 172 | 7.3 (6.1) |
| Cumulative cyclosporine exposure (y) | 307 | 4.0 (5.3) | 216 | 3.9 (5.0) | 160 | 4.4 (5.2) |
| Cumulative tacrolimus exposure (y) |
311 | 2.1 (2.8) | 220 | 2.0 (2.6) | 159 | 2.1 (3.0) |
| C4d + | 299 | 108 (36) | 208 | 83 (40) | 172 | 68 (40) |
| DSA + | 304 | 130 (43) | 220 | 99 (45) | 172 | 70 (41) |
DSA, donor specific antibody; iatr, inflammation in areas of atrophy; SD, standard deviation; y, years.
Data for the 7 small clusters are not shown (N = 25).
3.1 |. Inflammation in areas of atrophy (iatr)
Traditional Banff scoring ignored iatr. One of the first major findings of the DeKAF study was that iatr and tatr were associated with an increased postbiopsy risk of death-censored graft loss (iatr P < .0001 and tatr P = .03).9 This association remained when we corrected for serum creatinine level at biopsy and the traditional Banff scores of i (inflammation) and ct (tubular atrophy).
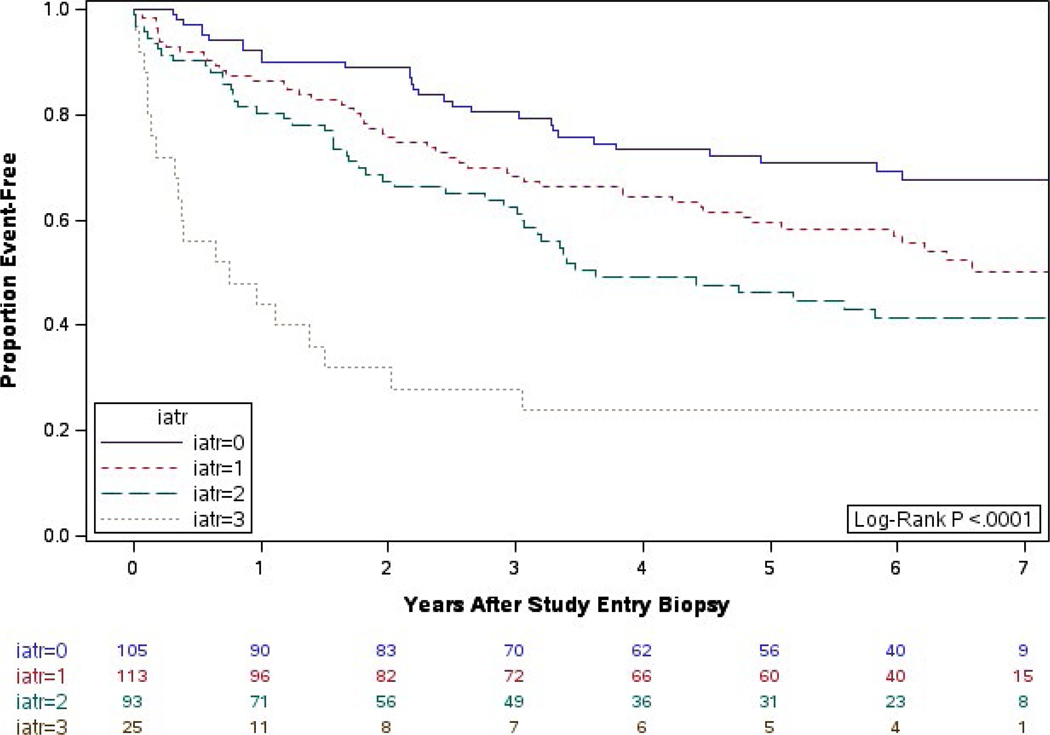
With 7 years of follow-up, the impact of iatr persists (Figure 1). Recipients with an iatr score of 0 had the best postbiopsy death-censored graft survival; death-censored graft survival declined as the degree of iatr increased (log-rank P < .0001). In our multivariable proportional hazards regression model that compared all iatr groups in pairwise fashion, severe iatr (score = 3) had worse graft survival than iatr = 0 (HR = 4.0 [95% CI = 1.6, 10.1], P = .018) (Table 2). Although the comparisons of iatr = 3 to iatr = 2 and iatr = 3 to iatr = 1 were significant univariately (P < .01), they were not significant after covariate adjustment (HR = 1.5 [0.7, 3.4], P = .728 vs HR = 2.3 [1.0, 5.3], P = .178). An iatr score of 2 had worse graft survival than a score of 0 (HR = 2.6 [1.4, 5.0], P = .019).
FIGURE 1.
Years from biopsy to graft failure by iatr scoring: extended follow-up
TABLE 2.
Univariable and multivariable results for the 3 analyses: inflammation, clusters, and C4d/DSA
| Univariate |
Multivariable |
|
|---|---|---|
| Comparison | HR [95% CI]; P-value; adjusted P* | HR [95% CI]; P-value; adjusted P* |
| iatr score (0–3)a (N = 336) | ||
| iatr = 3 vs iatr = 2 | 3.2 [1.9, 5.6]; P < .001; P*< .001 | 1.5 [0.7, 3.4]; P = .300; P* = .728 |
| iatr = 3 vs iatr = 1 | 4.4 [2.5, 7.6]; P < .001; P*< .001 | 2.3 [1.0, 5.3]; P = .043; P* = .178 |
| iatr = 3 vs iatr = 0 | 7.7 [4.2, 14.2]; P < .001; P*< .001 | 4.0 [1.6, 10.1]; P = .003; P* = .018 |
| iatr = 2 vs iatr = 1 | 1.3 [0.9, 2.0]; P = .138; P* = .449 | 1.5 [0.9, 2.5]; P = .091; P* = .328 |
| iatr = 2 vs iatr = 0 | 2.4 [1.5, 3.8]; P < .001; P* = .001 | 2.6 [1.4, 5.0]; P = .004; P* = .019 |
| iatr = 1 vs iatr = 0 | 1.8 [1.1, 2.8]; P = .015; P* = .073 | 1.7 [1.0, 2.9]; P = .044; P* = .183 |
| iatr and i scoresb (N = 336) | ||
| Some i vs i = 0, iatr>0 | 1.2 [0.9, 1.8]; P = .249; P* = .481 | 1.0 [0.6, 1.5]; P = .908; P* = .993 |
| Some i vs no inflammation | 2.4 [1.6, 3.8]; P < .001; P* < .001 | 1.8 [1.0, 3.0]; P = .030; P* = .076 |
| i = 0, iatr > 0 vs no inflammation | 2.0 [1.2, 3.1]; P = .004; P* = .012 | 1.8 [1.1, 3.0]; P = .022; P* = .057 |
| Cluster analysis (1–6)c (N = 237) | ||
| Clusters 6 vs 5 | 2.3 [1.0, 5.4]; P = .050; P* = .368 | 1.9 [0.8, 4.6]; P = .159; P* = .723 |
| Clusters 6 vs 4 | 1.4 [0.6, 3.3]; P = .497; P* = .984 | 1.2 [0.5, 3.0]; P = .706; P* = .999 |
| Clusters 6 vs 3 | 2.3 [1.0, 5.2]; P = .040; P* = .313 | 2.0 [0.8, 4.7]; P = .116; P* = .618 |
| Clusters 6 vs 2 | 3.5 [1.5, 8.0]; P = .004; P* = .042 | 3.4 [1.4, 8.0]; P = .006; P* = .065 |
| Clusters 6 vs 1 | 6.9 [3.1, 15.5]; P < .001; P* < .001 | 5.8 [2.5, 13.7]; P < .001; P*< .001 |
| Clusters 5 vs 4 | 0.6 [0.3, 1.2]; P = .157; P* = .717 | 0.6 [0.3, 1.4]; P = .251; P* = .861 |
| Clusters 5 vs 3 | 1.0 [0.5, 1.9]; P = .997; P* > .999 | 1.0 [0.5, 2.1]; P = .890; P* > .999 |
| Clusters 5 vs 2 | 1.5 [0.8, 2.9]; P = .245; P* = .855 | 1.8 [0.8, 4.0]; P = .165; P* = .734 |
| Clusters 5 vs 1 | 3.0 [1.5, 5.7]; P = .001; P* = .013 | 3.1 [1.5, 6.1]; P = .001; P* = .016 |
| Clusters 4 vs 3 | 1.7 [0.8, 3.5]; P = .140; P* = .680 | 1.7 [0.8, 3.4]; P = .165; P* = .735 |
| Clusters 4 vs 2 | 2.6 [1.2, 5.3]; P = .011; P* = .110 | 2.8 [1.3, 6.1]; P = .009; P*= .091 |
| Clusters 4 vs 1 | 5.1 [2.5, 10.3]; P < .001; P* < .001 | 4.9 [2.4, 9.9]; P < .001; P* < .001 |
| Clusters 3 vs 2 | 1.5 [0.8, 2.8]; P = .221; P* = .825 | 1.7 [0.8, 3.4]; P = .149; P* = .700 |
| Clusters 3 vs 1 | 3.0 [1.6, 5.4]; P < .001; P* = .004 | 2.9 [1.6, 5.4]; P < .001; P* = .007 |
| Clusters 2 vs 1 | 2.0 [1.1, 3.7]; P = .029; P* = .247 | 1.7 [0.8, 3.5]; P = .130; P* = .654 |
| C4d and DSAd (N = 172) | ||
| C4d+ / DSA+ vs C4d+ / DSA- | 1.6 [0.8, 3.2]; P = .186; P* = .549 | 0.8 [0.3, 1.9]; P = .598; P* = .953 |
| C4d+ / DSA+ vs C4d- / DSA+ | 1.5 [0.8, 2.9]; P = .220; P* = .610 | 0.8 [0.4, 2.0]; P = .708; P* = .982 |
| C4d+ / DSA+ vs C4d- / DSA- | 4.8 [2.5, 9.3]; P < .001; P*< .001 | 3.6 [1.5, 8.3]; P = .003; P* = .016 |
| C4d+ / DSA- vs C4d- / DSA+ | 0.9 [0.4, 2.1]; P = .871; P* = .998 | 1.1 [0.4, 2.8]; P = .876; P* = .999 |
| C4d+ / DSA- vs C4d- / DSA- | 3.0 [1.3, 6.8]; P = .007; P*=.035 | 4.5 [1.7, 11.7]; P = .002; P* = .010 |
| C4d- / DSA+ vs C4d- / DSA- | 3.2 [1.5, 6.7]; P = .002; P* = .009 | 4.2 [1.7, 10.2]; P = .001; P* = .008 |
Models are stratified by clinical center. Unadjusted and adjusted (Tukey-Kramer, P*) P values are provided.
CI, confidence interval; DSA, donor specific antibody; HR, hazard ratio; iatr, inflammation in areas of atrophy.
iatr score multivariable analyses were adjusted for creatinine at biopsy, treatment for acute rejection after biopsy (yes/no), and central i, t, ci, and ct scores.
iatr and i group multivariable analyses were adjusted for creatinine at biopsy, treatment for acute rejection after biopsy (yes/no), and central t, ci, and ct scores.
Cluster multivariable analyses were adjusted for creatinine at biopsy, time from transplant to biopsy and treatment for acute rejection after biopsy (yes/no).
C4d and DSA multivariable analyses were adjusted for age at biopsy, race (black or nonblack), prior transplant status, serum creatinine at biopsy, treatment for acute rejection after biopsy (yes/no), and central i, t, g, and ptc scores.
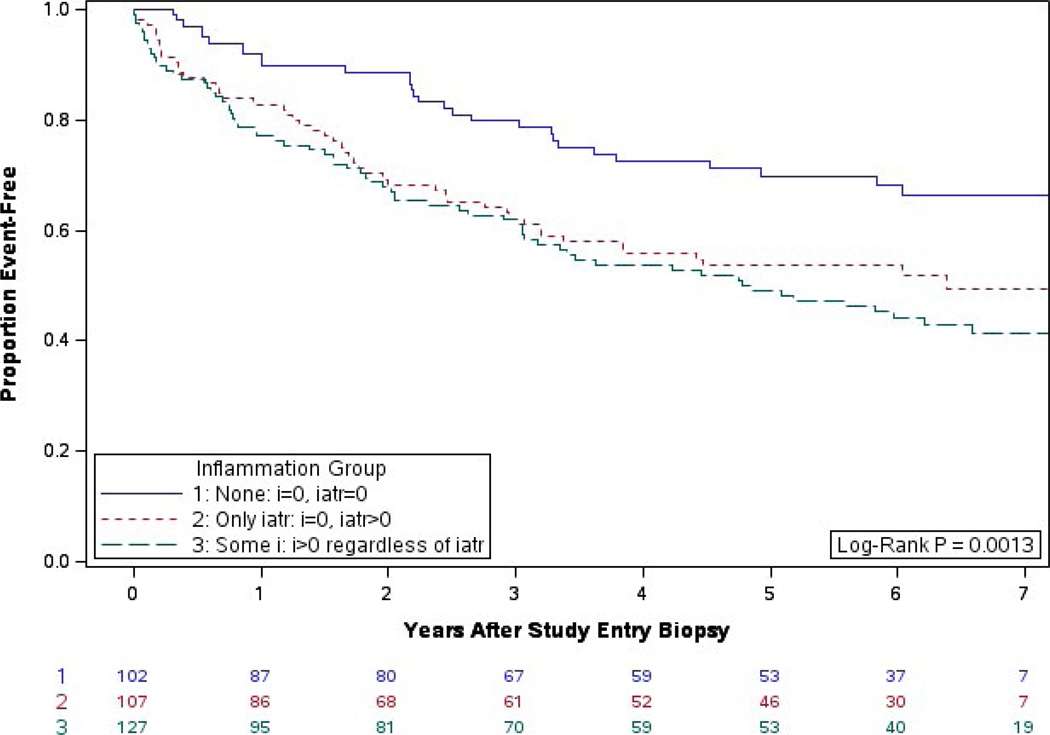
We also studied the relative impact of iatr and i on postbiopsy outcome. Biopsies with no inflammation (i = 0 and iatr = 0) were associated with the best graft survival; however, death-censored graft survival after biopsies showing iatr without i versus biopsies showing iatr with i did not differ (HR = 1.1 [0.7, 1.8], P = .75).9 With an additional 4 years of follow-up, there is still a significant difference in graft survival between the groups (log-rank P = .0013) (Figure 2). The difference between the group with no inflammation and the 2 groups with any inflammation remains after adjustment for covariates (HR = 1.8 [1.1, 3.0], P = .030 vs i = 0; HR = 1.8 [1.1, 3.0], P = .022 vs i > 0), however, those associations are no longer significant after controlling for the Type 1 error rate (P = .076 vs P = .057). There is still no difference between the presence of i group vs. the iatr-only group (HR = 0.97 [0.6, 1.5], P = .993).
FIGURE 2.
Years from biopsy to graft failure by iatr and i scoring: extended follow-up
3.2 |. Hierarchical cluster analysis
The population studied for the cluster analyses had a mean (SD) serum creatinine level as of January 2006 of 1.4 (0.3) mg/dL, which increased to 2.6 (1.5) mg/dL at the time of index biopsy for graft dysfunction (Table 1). The mean time from transplant to the index biopsy was 7.0 (5.9) years (median, 5.2).
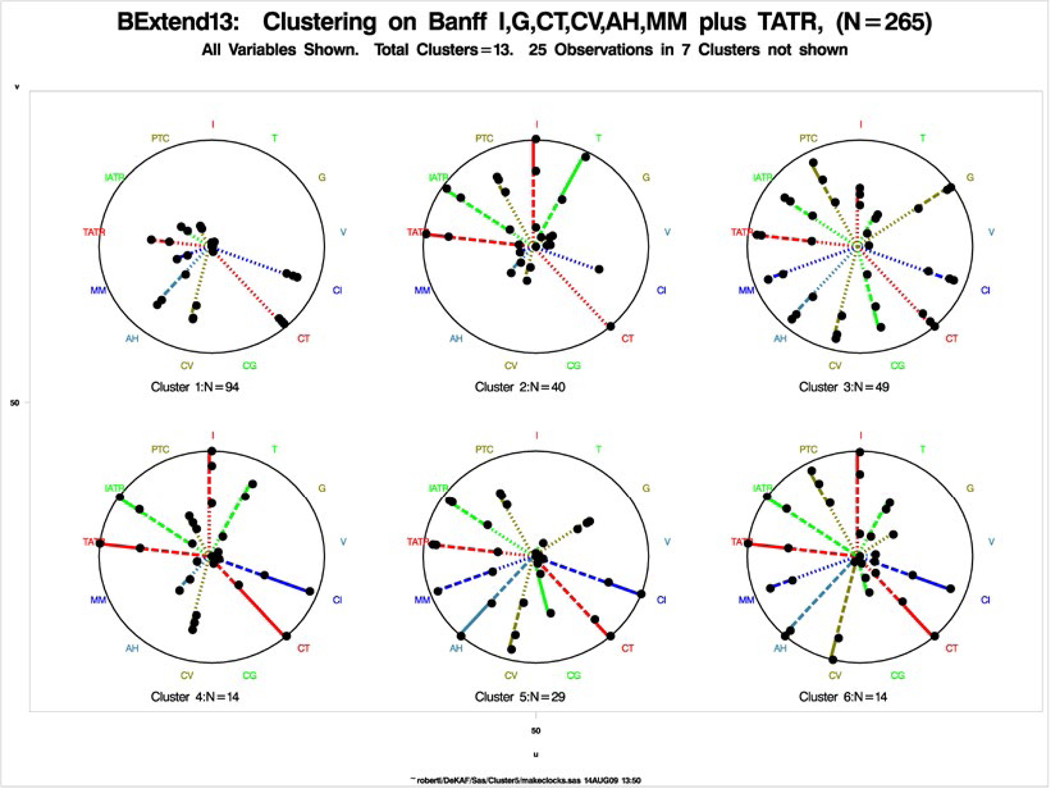
Per hierarchical cluster analysis based on central Banff scores plus tatr, we grouped biopsies into individual entities associated with different death-censored graft failure rates.10 In the initial analysis, we identified 6 major clusters. We depicted each of them as a wheel, with the individual Banff scores as spokes (Figure 3); the length of each spoke = the percent of biopsies within that cluster with a Banff score > 0. Mild lesions (eg, i = 1) are shown as a dotted line; moderate lesions (eg, i = 2) as a dashed line; and severe lesions (eg, i = 3) as a solid line. We observed clear differences between the clusters. For example, clusters 1 and 2 both showed mild fibrosis, but although cluster 1 had no inflammation, all the biopsies in cluster 2 also had inflammation (i) and tubulitis (t). Clusters 3-6 showed varying degrees of inflammation and tubulitis but also chronic changes, including varying degrees of tubular atrophy, intimal thickening, mesangial matrix increases, and arterial hyalinosis, with cluster 6 showing the most severe chronic changes.
FIGURE 3.
Original clusters based on Banff i, g, ct, cv, ah, mm, plus tatr (data shown for the 6 larger clusters N = 240). Only scores used in clustering are shown
The clusters were not significantly different with respect to gender, ethnicity, race, age (recipient or donor), primary cause of kidney disease, diabetes (recipient), hypertension (recipient or donor), prior kidney transplant, donor type (living or deceased), last stable creatinine prior to 2006, or cumulative cyclosporine or tacrolimus exposure prior to index biopsy (Table 3), but differed in the number of HLA Class I mismatches (P = .03) and total number of HLA mismatches (P = .01). In addition, at the time of index biopsy, we noted a significant difference between clusters in the time from transplant to biopsy (P < .001), in the mean creatinine level (P < .001), and in the prevalence of DSA (P < .001).
TABLE 3.
Characteristics of participants by cluster (N = 237)b
| Cluster 1 (N = 92) |
Cluster 2 (N = 40) |
Cluster 3 (N = 49) |
Cluster 4 (N = 14) |
Cluster 5 (N = 28) |
Cluster 6 (N = 14) |
||
|---|---|---|---|---|---|---|---|
| n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | P | |
| Female gender | 60 (65) | 19 (47) | 26 (53) | 7 (50) | 15 (54) | 5 (36) | .21 |
| Hispanic or Latinx ethnicity | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.6) | 0 (0.0) | .62 |
| Race, % | .36 | ||||||
| Caucasian | 74 (81) | 31 (78) | 39 (80) | 9 (64) | 22 (79) | 13 (93) | |
| Black or African American | 13 (14) | 6 (15) | 5 (10) | 5 (36) | 4 (14) | 1 (7.1) | |
| Asian | 2 (2.2) | 2 (5.0) | 0 (0.0) | 0 (0.0) | 2 (7.1) | 0 (0.0) | |
| Native American/Aleutian Islander | 2 (2.2) | 1 (2.5) | 4 (8.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Multiracial | 0 (0.0) | 0 (0.0) | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Primary cause of kidney disease, % | .13 | ||||||
| Diabetes | 35 (38) | 10 (25) | 16 (33) | 3 (21) | 4 (14) | 4 (29) | |
| Glomerular disease | 22 (24) | 13 (32) | 12 (24) | 5 (36) | 15 (54) | 4 (29) | |
| Hypertensive nephrosclerosis | 11 (12) | 6 (15) | 1 (2.0) | 3 (21) | 3 (11) | 1 (7.1) | |
| Polycystic kidney disease | 6 (6.5) | 5 (12) | 2 (4.1) | 0 (0.0) | 2 (7.1) | 1 (7.1) | |
| Other | 17 (18) | 4 (10) | 14 (29) | 3 (21) | 3 (11) | 4 (29) | |
| Unknown | 1 (1.1) | 2 (5.0) | 4 (8.2) | 0 (0.0) | 1 (3.6) | 0 (0.0) | |
| At transplant | |||||||
| Era | .01 | ||||||
| Before 1980 | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (7.1) | 0 (0.0) | |
| 1980-1989 | 5 (5.4) | 1 (2.5) | 1 (2.0) | 0 (0.0) | 4 (14) | 2 (14) | |
| 1990-1999 | 29 (31) | 6 (15) | 12 (24) | 4 (29) | 11 (39) | 6 (43) | |
| 2000-2005 | 57 (62) | 33 (82) | 36 (73) | 10 (71) | 11 (39) | 6 (43) | |
| Diabetes | 45 (49) | 13 (33) | 22 (45) | 4 (29) | 8 (29) | 5 (36) | .27 |
| Hypertension | 33 (36) | 14 (35) | 24 (49) | 7 (50) | 9 (32) | 4 (29) | .47 |
| Retransplant | 12 (13) | 7 (17) | 12 (24) | 3 (21) | 7 (25) | 2 (14) | .54 |
| Living donor transplant | 60 (65) | 27 (67) | 33 (67) | 10 (71) | 12 (43) | 7 (50) | .21 |
| Donor age (y) | 38 (12) | 33 (14) | 40 (12.3) | 40 (12) | 38 (15) | 32 (14) | .19 |
| Donor hypertension | 2 (2.8) | 0 (0.0) | 2 (5.0) | 0 (0.0) | 2 (12) | 1 (14) | .24 |
| Number of HLA mismatches | 2.8 (1.7) | 2.9 (1.8) | 3.8 (1.3) | 2.6 (1.9) | 2.8 (1.9) | 3.7 (1.3) | .01 |
| Class I mismatches | 1.9 (1.3) | 2.0 (1.3) | 2.6 (1.0) | 1.8 (1.1) | 2.0 (1.3) | 2.4 (0.9) | .03 |
| Class II mismatches | 0.9 (0.7) | 0.9 (0.7) | 1.2 (0.6) | 0.9 (0.9) | 0.9 (0.7) | 1.3 (0.8) | .07 |
| Last creatinine before 2006 (mg/dL) | 1.4 (0.3) | 1.3 (0.3) | 1.5 (0.3) | 1.5 (0.3) | 1.5 (0.3) | 1.5 (0.4) | .12 |
| At biopsy | |||||||
| Creatinine (mg/dL) | 2.2 (1.1) | 3.5 (2.5) | 2.3 (0.9) | 3.0 (1.2) | 2.4 (0.8) | 3.9 (1.9) | <.001 |
| Recipient age (y) | 48 (14) | 45 (14) | 48 (13) | 43 (17) | 50 (10) | 49 (17) | .60 |
| Time posttransplant (yrs) | 7.2 (5.5) | 4.4 (4.3) | 5.9 (4.4) | 4.9 (2.7) | 11 (8.8) | 10 (6.5) | <.001 |
| Cumulative cyclosporine exposure (y) |
11 (24) | 11 (27) | 8 (22) | 16 (32) | 16 (28) | 12 (24) | .80 |
| Cumulative tacrolimus exposure (y) | 9 (24) | 8 (24) | 8 (22) | 7 (24) | 1.9 (3.1) | 9 (25) | .80 |
| C4d + | 24 (29) | 17 (50) | 21 (48) | 5 (50) | 9 (38) | 7 (58) | .11 |
| DSA + | 17 (25) | 17 (55) | 24(69) | 7 (64) | 11 (50) | 8 (73) | <.001 |
DSA, donor-specific antibody; SD, standard deviation; y, years.
Data for the 7 small clusters are not shown (N = 25).
Local pathologist interpretations of biopsy results (primary and secondary diagnoses) for each cluster are summarized in Table 4. The interpretations were relatively similar across clusters with 2 exceptions: a high percentage of CAN (54%) and CNI nephrotoxicity (45%) in cluster 1 and a high percentage of acute rejection in cluster 2 (72%).
TABLE 4.
Local pathology primary or secondary biopsy diagnoses by cluster (N = 237)b
| Cluster (N = 92) |
Cluster 2 (N = 40) |
Cluster 3 (N = 49) |
Cluster 4 (N = 14) |
Cluster 5 (N = 28) |
Cluster 6 (N = 14) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | N | Percent | N | Percent | N | Percent | N | Percent | N | Percent | N | Percent |
| Acute rejection — cellular | 6 | 6.5 | 29 | 72.5 | 8 | 16.3 | 4 | 28.6 | 1 | 3.6 | 5 | 35.7 |
| Acute rejection — antibody-mediated | 3 | 3.3 | 5 | 12.5 | 8 | 16.3 | 1 | 7.1 | 1 | 3.6 | 1 | 7.1 |
| Chronic rejection — antibody-mediated | 1 | 1.1 | 0 | 0.0 | 5 | 10.2 | 0 | 0.0 | 0 | 0.0 | 1 | 7.1 |
| Acute tubular necrosis | 4 | 4.3 | 1 | 2.5 | 0 | 0.0 | 2 | 14.3 | 1 | 3.6 | 0 | 0.0 |
| Allograft nephropathy | 50 | 54.3 | 19 | 47.5 | 26 | 53.1 | 9 | 64.3 | 20 | 71.4 | 9 | 64.3 |
| Calcineurin inhibitor toxicity | 41 | 44.6 | 3 | 7.5 | 10 | 20.4 | 1 | 7.1 | 12 | 42.9 | 3 | 21.4 |
| Glomerulonephritis | 12 | 13.0 | 3 | 7.5 | 7 | 14.3 | 0 | 0.0 | 6 | 21.4 | 2 | 14.3 |
| Arterial nephrosclerosis | 13 | 14.1 | 3 | 7.5 | 11 | 22.4 | 1 | 7.1 | 8 | 28.6 | 3 | 21.4 |
| Polyoma (BK) virus infection | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Recurrent disease | 6 | 6.5 | 1 | 2.5 | 7 | 14.3 | 1 | 7.1 | 2 | 7.1 | 1 | 7.1 |
| Transplant glomerulopathy | 7 | 7.6 | 2 | 5.0 | 18 | 36.7 | 3 | 21.4 | 14 | 50.0 | 5 | 35.7 |
| CMV infection | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Pyelonephritis | 0 | 0.0 | 2 | 5.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Interstitial nephritis | 1 | 1.1 | 0 | 0.0 | 1 | 2.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PTLD | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Obstruction | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Subcapsular scar | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Cortical necrosis | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Microvascular inflammation | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 3.6 | 0 | 0.0 |
| Transplant arteriopathy | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Miscellaneous | 1 | 1.1 | 1 | 2.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Borderline changes | 9 | 9.8 | 1 | 2.5 | 4 | 8.2 | 3 | 21.4 | 0 | 0.0 | 0 | 0.0 |
| No abnormalities | 6 | 6.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Inadequate specimen for diagnosis | 2 | 2.2 | 0 | 0.0 | 1 | 2.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
CMV, cytomegalovirus; PTLD, postransplant lymphoproliferative disease.
Data for the 7 small clusters are not shown (N = 25).
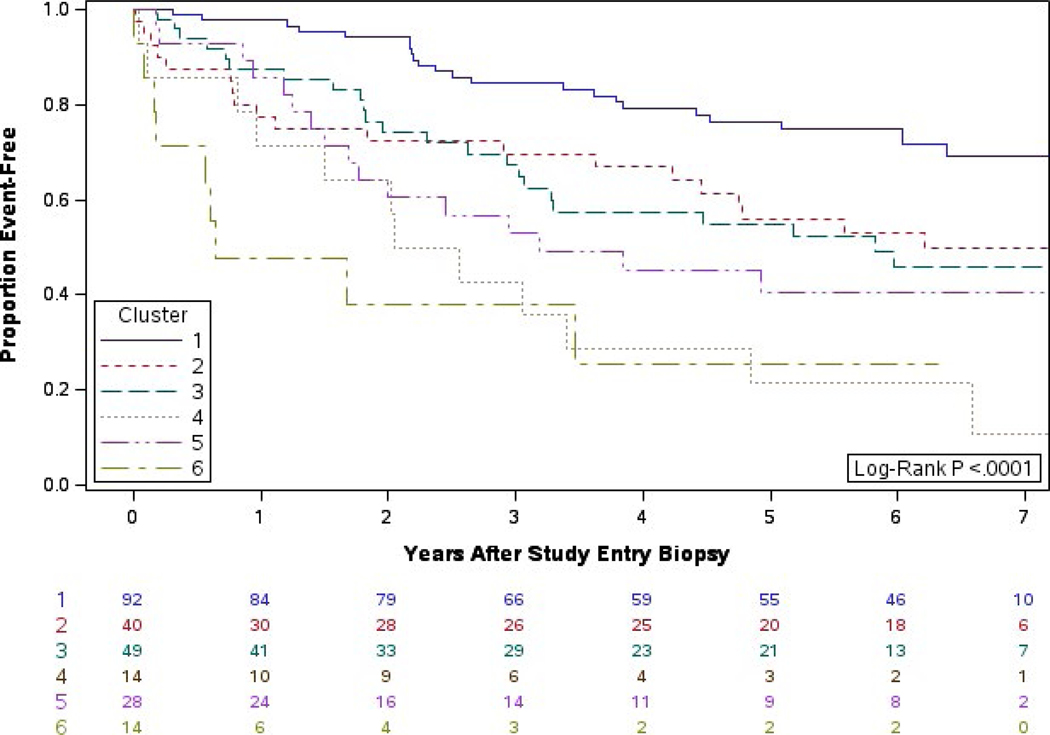
Our initial finding with a median of 18 months of follow-up was that the death-censored graft failure rate significantly differed by cluster (P = .0002). With 7 years of follow-up, there is increased differentiation between clusters in the death-censored graft failure rate (P < .0001, Figure 4). Using a proportional hazards model to perform pairwise comparisons, we found that cluster 1 had better graft survival than clusters 3, 4, 5, and 6: 6 vs 1: HR = 5.8 [2.5, 13.7], P < .001; 5 vs 1: HR = 3.1 [1.5, 6.1], P = .016; 4 vs 1: HR = 4.9 [2.4, 9.9], P < .001; 3 vs 1: HR = 2.9 [1.6, 5.4], P = .007 (Table 2).
FIGURE 4.
Years from biopsy to graft failure by cluster: extended follow-up
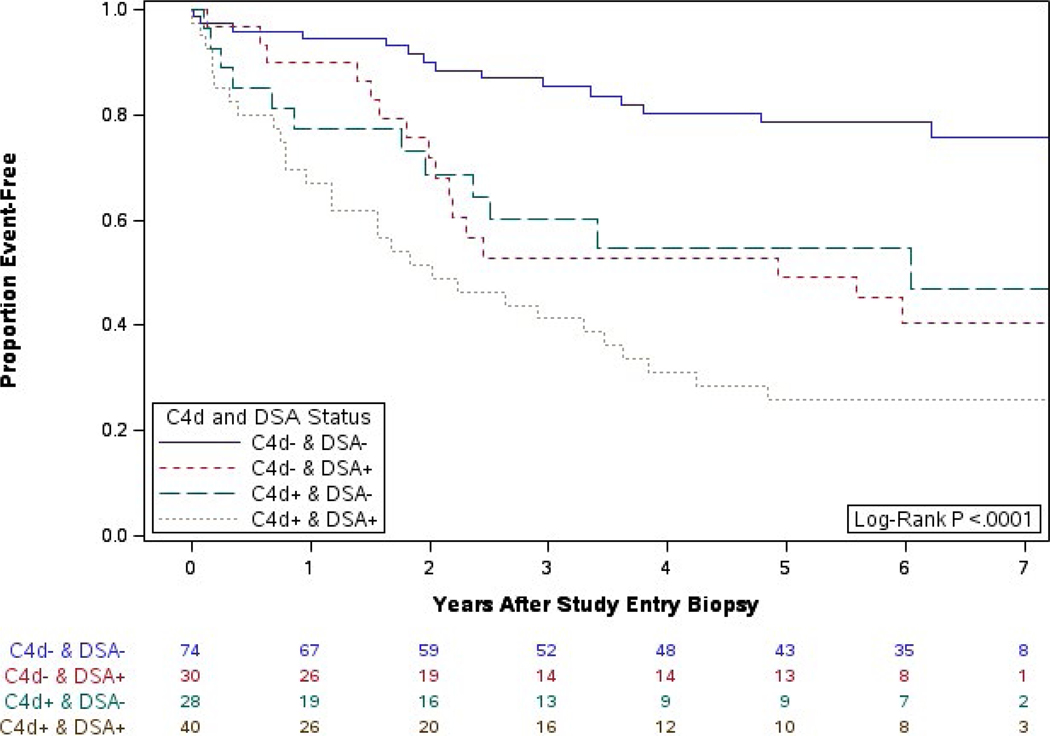
3.3 |. Impact of positive C4d staining and DSA
In contrast to the preceding analyses that looked at death-censored graft survival, our original C4d/DSA analysis examined the impact of C4d and DSA positivity on graft survival (including death and graft loss).11 Using proportional hazards regression, including ethnicity and serum creatinine at biopsy, we showed that C4d+ biopsy specimens irrespective of DSA status were associated with significantly worse graft survival than biopsy specimens that were C4d− and DSA−. Herein, to be consistent with the preceding analyses, we studied the impact of C4d and DSA positivity on death-censored graft failure.
With 7 years of follow-up, there is a significant difference between groups in death-censored graft survival (P < .0001, Figure 5). Participants whose biopsies were C4d- and DSA- had significantly better graft survival than the other three groups: vs C4d+ and DSA− HR = 4.5 [1.7, 11.7], P = .010; vs C4d− and DSA+ HR=4.2 [1.7, 10.2], P = .008; and vs C4d+ and DSA+ HR=3.6 [1.5, 8.3], P = .016 (Table 2).
FIGURE 5.
Years from biopsy to graft failure by C4d and DSA: extended follow-up
Importantly, in the initial analysis, there was a suggestion that outcome differed between C4d+/DSA− and C4d−/DSA+ groups. With long-term follow-up, it is clear that the postbiopsy death-censored graft failure rate for these 2 groups is similar (HR=1.1 [0.4, 2.8], P = .999) (Figure 5).
4 |. DISCUSSION
Since the beginning of the DeKAF study, a number of studies have used histopathology to gain a better understanding of the pathophysiology of late graft dysfunction and graft loss. Protocol biopsy studies have shown the natural history of graft histopathology and the evolution of histologic findings.13 Studies of biopsies done at the time of graft dysfunction have provided additional data on histopathology at the time of dysfunction and the impact on subsequent outcomes.
The DeKAF study focused on biopsies done at the time of new-onset graft dysfunction; its initial findings have already contributed to the understanding of late graft dysfunction and graft loss. With the addition of several years of follow-up, we have confirmed these initial findings. We found the best postbiopsy graft survival in cluster 1 (Figure 4), the cluster showing only interstitial fibrosis and tubular atrophy and no evidence of inflammation. Biopsies in that cluster had frequently been interpreted by local pathologists as showing CNI nephrotoxicity and/or CAN. Importantly, the DeKAF study was the first to show that the diagnoses of “chronic allograft nephropathy” and “CNI nephrotoxicity” are meaningless in terms of prognostic significance. One of the goals of the DeKAF study was to identify entities that could be subjected to clinical intervention trials. DeKAF indicated that the entity of tubular atrophy in the absence of inflammation, even when found in a biopsy performed for graft dysfunction, may not warrant intervention. We noted that acute inflammation (without significant chronic changes) is an entity for which there are ongoing clinical trials (cluster 2). For clusters 3-6, with different proportions of acute and chronic changes, we found differing outcomes. Analyses of data from the DeKAF prospective cohort, which followed a much larger cohort of recipients, may help further determine whether each of these clusters can be clearly defined to be used as criteria for entry into a clinical trial.
Moreover, our longer follow-up confirmed the finding that iatr is associated with an increased rate of death-censored graft failure. Mengel et al also showed that the total inflammation score (iatr plus inflammation in nonatrophic parenchyma [as routinely scored in Banff]) was associated with worse graft survival.14 These observations have been confirmed in 2 recent studies,15,16 and recently, inflammation in areas of atrophy/fibrosis has been incorporated into the latest Banff scoring, using the terminology “i-ifta”.17
At the initiation of the DeKAF study, the single-bead method for DSA determination was just becoming widely used. Now, after extensive use of that method, it has become clear that the presence of posttransplant DSA, particularly against Class II HLA antigens, is associated with an increased risk of graft dysfunction and graft loss. Not surprisingly, in both our initial and current analyses, we found that biopsies that were C4d+/DSA+ were associated with an increased death-censored graft failure rate. Of note, in our initial analysis, biopsies that were DSA+/C4d− had a better prognosis than those that were DSA−/C4d+. In contrast, with longer follow-up, biopsy specimens that were DSA+/C4d− and that were DSA−/C4d+ were associated with similar death-censored graft failure. That finding is consistent with recent studies reporting C4d− antibody-mediated rejection, as well as with recent studies confirming the histologic appearance of antibody-mediated rejection in the absence of DSA.18,19
There are limitations to our analyses. First, although the study design is a prospective cohort study, these analyses are post hoc in nature, which carries a potential risk of bias. Additionally, the usual care must be taken in comparing survival curves in plots as the standard error grows as the number of participants at risk declines over time. Therefore, Kaplan-Meier curves are provided with numbers of participants at risk. As seen in the figures, there are only a small number of patients remaining 7 years after the index biopsy. In summary, these longer-term data confirm a principal hypothesis of the DeKAF study. Namely, late graft dysfunction and graft loss are the consequence of active, ongoing injury. Even when elevated creatinine levels lead to a biopsy, grafts without evidence of active, ongoing injury have prolonged survival. New-onset late graft dysfunction, with inflammation at the time of for-cause biopsy, often accompanied by C4d positivity and circulating DSA, is associated with increased risk of graft loss.
ACKNOWLEDGMENTS
We thank Mary Knatterud for editorial review and Stephanie Taylor for preparation of the manuscript.
Funding information
Astellas Pharma US; Novartis Pharmaceuticals Corporation; Sanofi-Aventis; Pfizer; Bristol-Myers Squibb
This work was supported between 2005 and 2012 via a grant from the National Institutes of Health (5U01A1058013), and since 2013 by unrestricted grants from Astellas, Bristol-Myers Squibb, Novartis, Pfizer, and Sanofi-Aventis.
Abbreviations:
- CAN
chronic allograft nephropathy
- CI
confidence interval
- CNI
calcineurin inhibitor
- DeKAF
Deterioration of Kidney Allograft Function
- DSA
donor-specific antibody
- HR
hazard ratio
- iatr
inflammation in areas of atrophy
- IvIG
intravenous Immunoglobulin
- MFI
mean fluorescent intensity
- SD
standard deviation
- tatr
tubulitis in areas of atrophy
Footnotes
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation.
ENDNOTES
University of Alabama; University of Alberta; Hennepin County Medical Center; Mayo Clinic (Rochester, MN); University of Iowa; University of Manitoba; University of Minnesota.
Mayo Clinic (Rochester, MN).
University of California – Los Angeles.
University of Minnesota.
REFERENCES
- 1.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4(3):378–383. [DOI] [PubMed] [Google Scholar]
- 2.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 annual data report: kidney. Am J Transplant. 2015;15(suppl 2):1–34. [DOI] [PubMed] [Google Scholar]
- 3.Almond PS, Matas A, Gillingham K, et al. Risk factors for chronic rejection in renal allograft recipients. Transplantation. 1993;55(4):752–756; discussion 756-757. [DOI] [PubMed] [Google Scholar]
- 4.Cosio FG, Pelletier RP, Falkenhain ME, et al. Impact of acute rejection and early allograft function on renal allograft survival. Transplantation. 1997;63(11):1611–1615. [DOI] [PubMed] [Google Scholar]
- 5.Monaco AP, Burke JF Jr, Ferguson RM, et al. Current thinking on chronic renal allograft rejection: issues, concerns, and recommendations from a 1997 roundtable discussion. Am J Kidney Dis. 1999;33(1):150–160. [DOI] [PubMed] [Google Scholar]
- 6.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349(24):2326–2333. [DOI] [PubMed] [Google Scholar]
- 7.Nankivell BJ, P’Ng CH, O’Connell PJ, Chapman JR. Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology: comparison of cyclosporine and tacrolimus eras. Transplantation. 2016;100(8):1723–1731. [DOI] [PubMed] [Google Scholar]
- 8.Gourishankar S, Leduc R, Connett J, et al. Pathological and clinical characterization of the “troubled transplant”: data from the DeKAF study. Am J Transplant. 2010;10(2):324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannon RB, Matas AJ, Grande J, et al. Inflammation in areas of tubular atrophy in kidney allograft biopsies: a potent predictor of allograft failure. Am J Transplant. 2010;10(9):2066–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matas AJ, Leduc R, Rush D, et al. Histopathologic clusters differentiate subgroups within the nonspecific diagnoses of CAN or CR: preliminary data from the DeKAF study. Am J Transplant. 2010;10(2):315–323. [DOI] [PubMed] [Google Scholar]
- 11.Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90(1):68–74. [DOI] [PubMed] [Google Scholar]
- 12.Crary GS, Raissian Y, Gaston RC, et al. Optimal cutoff point for immunoperoxidase detection of C4d in the renal allograft: results from a multicenter study. Transplantation. 2010;90(10):1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stegall MD, Gaston RS, Cosio FG, Matas A. Through a glass darkly: seeking clarity in preventing late kidney transplant failure. J Am Soc Nephrol. 2015;26(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mengel M, Reeve J, Bunnag S, et al. Scoring total inflammation is superior to the current Banff inflammation score in predicting outcome and the degree of molecular disturbance in renal allografts. Am J Transplant. 2009;9(8):1859–1867. [DOI] [PubMed] [Google Scholar]
- 15.Nankivell BJ, Shingde M, Keung KL, et al. The causes, significance and consequences of inflammatory fibrosis in kidney transplantation: the Banff i-IFTA lesion. Am J Transplant. 2018;18(2):364–376. [DOI] [PubMed] [Google Scholar]
- 16.Lefaucheur C, Gosset C, Rabant M, et al. T cell-mediated rejection is a major determinant of inflammation in scarred areas in kidney allografts. Am J Transplant. 2018;18(2):377–390. [DOI] [PubMed] [Google Scholar]
- 17.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18(2):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272–283. [DOI] [PubMed] [Google Scholar]
- 19.Haas M. Evolving criteria for the diagnosis of antibody-mediated rejection in renal allografts. Curr Opin Nephrol Hypertens. 2018;27(3):137–143. [DOI] [PubMed] [Google Scholar]