Abstract
Background
Hyperoxia at resuscitation increases oxidative stress, and even brief exposure to high oxygen concentrations during stabilization may trigger organ injury with adverse long-term outcomes in premature infants. We studied the long-term effects of short-term perinatal oxygen exposure on cell cycle gene expression and lung growth in adult mice.
Methods
We randomized mice litters at birth to 21, 40, or 100%O2 for 30 min and recovered in room air for 4 or 12 weeks. Cell cycle gene expression, protein analysis, and lung morphometry were assessed at 4 and 12 weeks.
Results
The principal component analysis demonstrated a high degree of correlation for cell cycle gene expression among the three oxygen groups. Lung elastin was significantly lower in the 100%O2 groups at 4 weeks. On lung morphometry, radial alveolar count, alveolar number, and septal count were similar. However, the mean linear intercept (MLI) and septal length significantly correlated among the oxygen groups. The MLI was markedly higher in the 100%O2 groups at 4 and 12 weeks of age, and the septal length was significantly lower in the 100%O2 groups at 12 weeks.
Conclusion
Short-term exposure to high oxygen concentrations lead to subtle changes in lung development that may affect alveolarization. The changes are related explicitly to secondary crest formation that may result in alteration in lung elastin. Resuscitation with high oxygen concentrations may have a significant impact on lung development and long-term outcomes such as BPD in premature infants.
Keywords: Oxygen, Gene expression, Cell cycle, Lung, Resuscitation
Background
An abrupt transition of the fetus from a relatively hypoxic to a relatively hyperoxic environment results in physiologic oxidative stress in infants soon after birth [1]. Due to the lack of induction of antioxidant enzyme (AOE) systems [2], premature infants are particularly sensitive to the toxic effects of oxygen. Resuscitation with 100%O2 generates oxygen radicals [3], and subsequent reperfusion injury may further exacerbate free radical production and oxygen toxicity [4]. Furthermore, supplemental oxygen contributes to the development of bronchopulmonary dysplasia (BPD) [5], retinopathy of prematurity (ROP) [6], and brain injury [7] in premature infants. However, there is uncertainty as to whether initiating resuscitation post-birth with lower (FiO2 < 0.40) or higher (FiO2 ≥ 0.40) in the first ten minutes of birth has on mortality, morbidity, and other long-term outcomes in premature infants [8, 9]. Current neonatal resuscitation guidelines recommend initiation of resuscitation with low oxygen (FiO2: 0.21–0.30) and to titrate the oxygen concentration to achieve preductal oxygen saturation approximating the interquartile range measured in healthy term infants [10]. Due to concerns about oxidative injury, the guidelines do not recommend initiation of resuscitation with ≥ 65%O2 [10]. The optimal oxygen concentration required at resuscitation of premature infants is one of the contentious issues in newborn care.
We have demonstrated that ventilated premature lambs cannot appropriately increase antioxidant activity in response to hyperoxia, and that increased exposure to O2 aggravates oxidant lung injury in these lambs [11]. A brief period of high oxygen concentrations led to increased pulmonary arterial contractility at 24 h of newborn lambs [12]. Reactive oxygen species (ROS) from excess oxygen exposure influence the molecular and biological processes observed in cells during differentiation and aging [13]. Furthermore, these processes are affected by changes in gene expression and signal transduction that act as messengers for growth factors [14]. Because oxidative stress influence apoptosis and cell growth [15], hyperoxia may have long-term consequences on growth & development. Although the relationships among the stage of the cell cycle, redox state, and oxidant production are poorly understood, mitotic progression influences the redox state and vice versa [16, 17]. The mitotic index of the postnatal lung is significantly higher compared to the adult lung, and perinatal oxygen exposure may alter cell cycle gene expression, mitotic progression, and hence cell proliferation [18, 19].
Hyperoxia at resuscitation also increases systemic oxidative stress and oxidative lung injury [20]. Brief exposure to high oxygen concentrations during stabilization may trigger organ injury with adverse long-term outcomes in premature infants [21]. We studied the effects of oxygen exposure soon after birth on cell cycle gene expression and lung alveolarization in adult mice. We studied three different concentrations of oxygen (21%O2, 40%O2, and 100%O2) in an experimental model of resuscitation in newborn mice to assess long-term effects on the lung at four weeks and 12 weeks in adult mice. We hypothesize that a brief period of exposure to 100%O2 in the perinatal period may alter cell cycle gene expression with implications on lung alveolarization in adult mice.
Methods
Oxygen exposure
We performed an in vivo study of short-term exposure to O2 in newborn mice to mimic the exposure to hyperoxia during the resuscitation of premature infants after IACUC approval of the University at Buffalo (Project # PED24116N). Time-dated pregnant C57Bl/6 mice were acclimatized in the lab animal facility after purchasing from the vendor (Envigo RMS Inc, Indianapolis, IN) before delivery. Dams were observed on the day of expected delivery (q3h) with minimal disturbance. We had 16 dams delivering 80 pups (average of six pups/dam) with a mortality of 10% (8 pups) during the study. Oxygen exposures were performed as close to birth as possible and no later than 6 h of age. Litters were allocated by simple randomization using cards to one of the three groups (21%O2, 40%O2, or 100%O2). Four-week experiments were performed initially, followed by twelve-week mice experiments. Animal cages were placed in a Plexiglas chamber pre-filled with 100%O2 or 40%O2 and covered with a plastic wrap. Oxygen concentration was maintained in the chamber both before and during the experiment with ProOx 110 oxygen controller (BioSpherix, NY). ProOx-110 senses oxygen concentration inside the chamber and infuses either oxygen or nitrogen, respectively, to increase or decrease the oxygen concentration in the chamber. The chamber was monitored continuously during the 30 min of oxygen exposure to confirm the precise administration of oxygen concentrations into the cages. Humidity (50–60%) and the temperature was identical to all exposures. Mice in 21%O2 were subjected to an identical environment as hyperoxia-exposed mice.
All mice litters were recovered in room air (RA) after 30 min of oxygen exposure. Mice were sacrificed at 4 or 12 weeks of age by intraperitoneal injection of sodium pentobarbital. Gene expression and protein analysis were performed on frozen lung tissue in all the three O2 groups at 4 and 12 weeks of age (N = six in each group, each time point). Formalin studies were performed in a separate set of mice at both time points (N = six in each group, each time point).
RNA isolation
RNA was isolated from flash-frozen mouse lung using the RNeasy Mini kit (Qiagen, Valencia, CA) with on-column DNase digestion per manufacturer's protocol. RNA integrity was assessed using the Experion Automated Electrophoresis System (BioRad, Hercules, CA).
Whole lung gene expression profiling by RT2-qPCR
The cell cycle PCR array (Mouse Cell Cycle RT2 Profiler PCR Array; SA Biosciences, MD) profiles the expression of 84 specific genes that regulate the cell cycle. The PCR array performs gene expression analysis with real-time PCR sensitivity and the multi-gene profiling capability of a microarray. Using the RT2 first strand kit (SA Biosciences, MD), 300 ng of total RNA was reverse transcribed to cDNA, mixed with RT2-SYBR Green qPCR master mix. Aliquots of this mix were placed into each of the PCR array plates containing the predispensed gene-specific primer sets. PCR performed on a 96 well MyiQ thermocycler (BioRad, Hercules, CA) according to the manufacturer's protocol. The threshold cycle (Ct) values and the fold change in gene expression for pair-wise comparison were processed using the excel-based PCR Array Data Analysis software (SA Biosciences) applying the equation 2-∆∆C(t) by comparing to the corresponding RA group (21%O2-4 weeks; 21%O2-12 weeks). We used three housekeeping genes (Glyceraldehyde-3-phosphate dehydrogenase (Gapdh), Hypoxanthine guanine phosphoribosyl transferase (Hprt) & Actin, beta (Actb) based on stability between experimental conditions.
Cell cycle protein analysis
Cdkn1a (p21), a cell cycle inhibitor; Ki67, a marker of cell proliferation; tumor protein 63 (Trp63), a protein that governs tissue morphogenesis; cyclin B1 (CCNB1), a protein that is essential for the control of cell cycle at the G2/mitosis transition and elastin (ELN), a necessary protein for alveolar development were analyzed in frozen lung tissue. Tissues were pulverized on dry ice, suspended in PBS with protease inhibitors, spun at 16,000 g for 3 min, and the supernatant used for cytoplasmic protein ELISAs (p21, Elastin, Cyclin B1). The pellet was suspended in PBS and centrifuged × 2 (500 g for 15 min; 1000 g for 15 min), discarding the supernatant each time. The pellet was resuspended in buffer (20 mM HEPES;1%Triton-x-100), and nuclei lysed by passing the suspension through an 18 g needle 20 times; spun at 9000 g for 30 min. The resulting supernatant (nuclear protein) was used for the determination of Ki67 and Trp63 proteins by ELISA. Protein concentration for both fractions was determined by the BioRad DC Protein assay (Hercules, CA). ELISAs were performed according to the manufacturer's protocol. Mouse Trp63, MKi-67, Cdkn1A, and Cyclin B1 ELISAs obtained from MyBioSource (San Diego, CA) and Elastin ELISA from Elabscience (Wuhan, China).
Histopathology
The trachea was cannulated, and the lungs were instilled with 10% buffered formalin at 25 cm of H2O pressure. The lungs were embedded in paraffin, cut into 5 µm thick sections, and stained with hematoxylin–eosin and elastin (Verhoeff's elastin stain). Lung morphometry was assessed in twenty representative images per lung section and five sections per mouse from different regions of the lung (six animals per group; three groups each at 4 and 12 weeks). Lung morphometry measurements were performed at 200 × resolution in all groups (850 × 450 µm; 382,000 µm2). Alveolization was estimated by the radial alveolar count (RAC) method of Emery & Mithal [22], alveolar number, and alveolar surface area (excluding all conducting airways and blood vessels > 10 µm diameter). Mean linear intercept (MLI) was calculated from the H&E stained lung sections by dividing the total length of a line (in micrometers) drawn across the entire field by the total number of alveolar intercepts encountered along the length of the line. Septal count (secondary crests or septae) and septal length were assessed in elastin stained lung sections. The septal length was measured from the base of the primary septum to the tip of the secondary septum and expressed in µm. Lung morphometry assessments were performed by an experienced investigator blinded to the treatment groups by Aperio imaging software (Leica Biosystems, Buffalo Groove, IL).
KI67 immunohistochemistry
Antigen retrieval was performed on paraffin-embedded sections by heating in citrate buffer (pH-6.0) for 20 min. Slides were washed in PBS and incubated with 2% goat non-immune serum-2% BSA to block nonspecific binding. Lung slides were incubated with the primary antibody for Ki67 (rabbit anti-Ki67, LabVision, Fremont, CA) at 1:250 dilution for 30 min; the secondary antibody and DAB staining kit were used per manufacturer's protocol (Dako Envision-HRP-DAB; Carpinteria, CA). Nonspecific IgG and omission of primary antibody acted as controls for staining specificity. Lung sections were assessed by manual counting of the digitalized slide for Ki67 positive cells at a magnification of 400× (56,000 µm2; 20 images per slide; 5 sections/animal; 6 mice/group) by Aperio software. The intensity of brown staining varied from cell to cell. Any degree of brown nuclear staining was identified as a Ki67 positive cell. Cytoplasmic staining was not counted as a Ki67 + cell. Masking was observed to avoid bias in the quantification of Ki67 staining. Staining results were expressed as the number of Ki67 positive cells per HPF.
Statistical analysis
Sample size
The sample size was calculated based on the 'resource equation' method [23] as it was not possible to assess effect size or standard deviation from preliminary data for calculation. We aimed to find any level of differences among the groups. 'E", the degree of freedom of Analysis of Variance (ANOVA) (Total number of animals − Number of groups) was calculated, based on the number of animals (36) and number of groups (6) (36 − 6 = 30) (for gene expression analysis). E of < 10 indicates an increase in the number of animals per group is required, and E > 20 would suggest that adding more animals would not increase the chance of getting a significant result.
All data were expressed as mean ± standard deviation (SD) with n representing the number of animals studied (N = 6 in each group). Normal distribution of data was confirmed before analysis by student's t-test and ANOVA. Categorical data were analyzed by the Fisher Exact test. P values were calculated based on students’ test of the replicate 2-∆∆C(t) values for each gene in the control group and the treatment group. ANOVA was performed on 2-∆∆C(t) values to compare differences between the groups. Fisher's post-hoc test analyzed protein expression among the groups.
Principal component analysis (PCA)
Principal component analysis, a dimensionality reduction technique, was performed to explain the variance–covariance structure in gene expression data among the groups. We assessed the suitability and the adequacy of the dataset for PCA before analysis. The Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy was 0.73. Bartlett's test of sphericity was statistically significant (p < 0.0005), indicating that the dataset is suitable for data reduction techniques such as PCA or factor analysis. The assumption of normality was met by the rank transformation of data and homogeneity of variance confirmed by the Levene test (P = 0.747). A p-value of < 0.05 was considered significant.
Results
Cell cycle gene expression at four weeks
Cell cycle gene expression in the 40%O2 (Table 1) and 100%O2 (Table 2) is compared to the control group (21%O2). The fold change in gene expression in the control group is normalized to 1.0, and the fold change in the other two oxygen groups (40%O2 and 100%O2) is expressed as relative to the control group. Genes are considered overexpressed for a fold change of ≥ 2.0 fold; and under-expressed for fold changes of ≤ 0.5. Of the 84 cell cycle genes analyzed, 13 genes (16%) in the 100%O2 group were downregulated (FC ≤ 2; Table 2) compared to 4 genes (5%) in the 40%O2 group at four weeks (Table 1) (p < 0.01; Fisher's exact test). Ten genes (12%) in the 100%O2 group were overexpressed (FC ≥ 2.0, Table 2) compared to seven (8%) in the 40%O2 groups at four weeks (Table 1). Overall, significantly more genes were either over or under-expressed in the 100%O2 group than 40%O2 group at four weeks (23/84–100%O2 vs. 11/84–40%O2; p < 0.05, Fisher's exact test). Cdkn1a (p21), a cyclin-dependent kinase inhibitor, Ccna2, Mki67, a marker of cell proliferation, and Ccnb2 were significantly downregulated (p < 0.05) in the 100%O2 group at four weeks (Table 2) following short-term perinatal oxygen exposure.
Table 1.
Cell cycle gene expression by real-time PCR array in lung homogenate of mice at 4- and 12-weeks of age exposed to 30 min of 40%O2 within 6 h of birth
| Gene symbol | Gene description | Fold change in gene expression |
|---|---|---|
| 40%O2 Group—4 weeks (Control Group—21%O2 4 weeks) | ||
| Over-expressed genes | ||
| Cdk4 | Cyclin-dependent kinase 4 | 4.26 (0.01–16.5) |
| Dst | Dystonin | 4.34 (0.01–15.4) |
| Msh2 | MutS homolog 2 (E. coli) | 2.64 (0.01–9.35) |
| Psmg2 | Proteasome assembly chaperone 2 | 3.28 (0.01–10.3) |
| Rbl1 | Retinoblastoma-like protein 1 | 2.56 (0.01–7.06) |
| Shc1 | Src homology 2 transforming protein C1 | 2.21 (0.01–5.09) |
| Trp63 | Transformation related protein p63 | 2.37 (0.01–5.67) |
| Under-Expressed Genes | ||
| Ccnb1 | Cyclin B1 | 0.36 (0.01–1.04) |
| Ccnb2 | Cyclin B2 | 0.50 (0.19–0.82) |
| Gpr132 | G protein-coupled receptor 132 | 0.15 (0.01–0.76) |
| Mcm3 | Minichromosome maintenance complex-3 | 0.17 (0.01–0.70) |
| 40%O2 Group—12 weeks (Control Group—21%O2 12 weeks) | ||
| Over-expressed genes | ||
| Ccnb1 | Cyclin B1 | 5.9 (0.01–10.75) |
| Trp63 | Transformation related protein p63 | 4.16 (0.01–11.59) |
| Under-expressed genes | ||
| Abl1 | Non-receptor tyrosine protein kinase | 0.28 (0.01–0.67) |
Genes were considered over-expressed (Fold Change ≥ 2.0) or under-expressed (Fold Change ≤ 0.5) relative to the control group (21%O2). The fold change (FC) in gene expression in 21%O2 group = 1.0; values expressed as FC with 95% confidence intervals
Housekeeping genes: Gapdh (Glyceraldehyde-3-phosphate dehydrogenase), Hprt (Hypoxanthine guanine phosphoribosyl transferase) & Actb (Actin, beta); N = six mice / group, each time-point
Table 2.
Cell cycle gene expression by real-time PCR array in lung homogenate of mice at 4- and 12-weeks of age exposed to 30 min of 100%O2 within 6 h of birth
| Gene symbol | Gene description | Fold change in gene expression |
|---|---|---|
| 100%O2 Group—4 weeks (Control Group—21%O2 4 weeks) | ||
| Over-expressed genes | ||
| Cdk4 | Cyclin-dependent kinase 4 | 4.07 (0.01–15.8) |
| Dst | Dystonin | 3.45 (0.001–12.3) |
| Mre11a | Meiotic recombination 11 homolog A | 2.06 (0.001–4.38) |
| Mdm2 | mouse 3T3 cell double minute 2 | 2.01 (0.54–3.49) |
| Msh2 | MutS homolog 2 (E. coli) | 2.91 (0.01–10.32) |
| Npm2 | Nucleoplasmin 2 | 2.31 (0.02–4.62) |
| Psmg2 | Proteasome assembly chaperone 2 | 3.28 (0.01–10.3) |
| Rbl1 | Retinoblastoma-like protein 1 | 2.70 (0.01–7.56) |
| Shc1 | Src homology 2 transforming protein C1 | 2.52 (0.01–5.85) |
| Slfn1 | Schlafen 1 | 2.01 (0.51–3.45) |
| Under-expressed genes | ||
| Ak1 | Adenylate kinase 1 | 0.34 (0.01–0.68) |
| Abl1 | Non-receptor tyrosine protein kinase | 0.31 (0.01–0.98) |
| Ccna2 | Cyclin A2 | 0.35 (0.14–0.57)* |
| Ccnb1 | Cyclin B1 | 0.08 (0.001–0.31) |
| Ccnb2 | Cyclin B2 | 0.38 (0.13–0.64)* |
| Cdkn1a | Cyclin-dependent kinase inhibitor 1A | 0.50 (0.31–0.69)* |
| Gpr132 | G protein-coupled receptor 132 | 0.32 (0.001–1.61) |
| Mcm3 | Minichromosome maintenance complex-3 | 0.17 (0.001–0.71) |
| Mki67 | Antigen identified by monoclonal antibody Ki-67 | 0.39 (0.21–0.58)* |
| Ppm1d | Protein Phosphatase 1D | 0.28 (0.001–0.97) |
| Ran | RAs-related nuclear protein | 0.13 (0.01–0.62) |
| Rbl2 | Retinoblastoma-like protein-2 | 0.22 (0.01–0.81) |
| Sfn | Stratifin | 0.38 (0.001–0.94) |
| 100%O2 Group—12 Weeks (Control Group—21%O2 12 weeks) | ||
| Over-expressed genes | ||
| Atm | Ataxia Telangiectasia Mutated | 2.4 (0.01–7.38) |
| Ccnb1 | Cyclin B1 | 2.55 (0.001–6.75) |
| Ccnb2 | Cyclin B2 | 3.38 (0.001–11.3) |
| Ccnc | Cyclin C | 2.28 (0.01–7.15) |
| Trp63 | Transformation related protein p63 | 2.93 (0.01–8.79) |
| Under-expressed genes | ||
| Abl1 | Non-receptor tyrosine protein kinase | 0.33 (0.01–0.88) |
| Inha | Inhibin alpha | 0.52 (0.32–0.73)* |
| Skp2 | S-phase kinase-associated protein 2 (p45) | 0.62 (0.46–0.79)* |
Genes were considered over-expressed (Fold Change ≥ 2.0) or under-expressed (Fold Change ≤ 0.5) relative to the control group (21%O2). The fold change (FC) in gene expression in 21%O2 group = 1.0; values expressed as FC with 95% confidence intervals
Housekeeping genes: Gapdh (Glyceraldehyde-3-phosphate dehydrogenase), Hprt (Hypoxanthine guanine phosphoribosyl transferase) & Actb (Actin, beta); N = six mice / group, each time-point
*p < 0.01 vs control group
Cell cycle gene expression at 12 weeks
Gene expression data at 12 weeks are presented in Table 1 (40%O2) and Table 2 (100%O2). The majority of the cell cycle genes in the 40%O2 and 100%O2 groups recovered within ± twofold change of the 21%O2 groups at 12 weeks. Two cell cycle genes (Inha and Skp2) were significantly under-expressed in the 100%O2 group at 12 weeks (Table 2).
Principal component analysis of gene expression data
There were no significant differences in cell cycle gene expression (2-∆∆C(t) values) among the three groups at four weeks (p = 0.91) or 12 weeks (p = 0.74) by ANOVA.
PCA analysis at 4 weeks
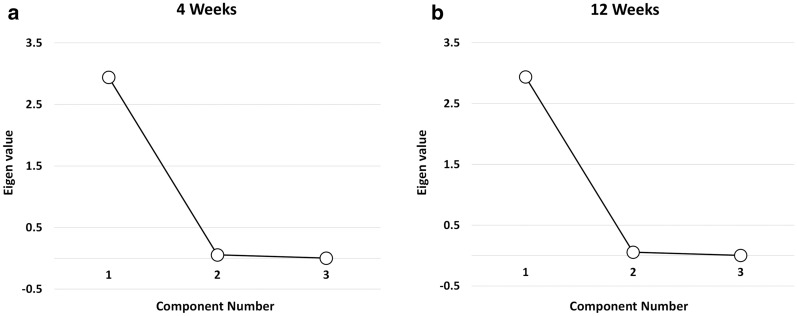
The proportion of variance accounted for by the PCA was 96.3, 99.1, and 98.7% for 21%O2, 40%O2, and 100%O2 groups respectively in 4-week-old mice. A scree plot (Fig. 1) displays the eigenvalues on the y-axis and the number of components on the x-axis. PCA revealed one component with an eigenvalue greater than one, explaining 98.03% of the total variance in gene expression (Fig. 1a). The leveling of the slope indicated that including components 2 and 3 would not offer additional benefits, and hence one component was retained for further analysis. The component matrix for the three groups at four weeks is consistent with strong loadings for 21%O2 (0.981), 40%O2 (0.995), and 100%O2 (0.994) (Table 3). The results indicate that the three oxygen groups themselves are highly correlated, suggesting the absence of variance in gene expression among the three oxygen groups.
Fig. 1.
Principal Component Analysis (PCA). The Scree plot demonstrates the Eigenvalues of the principal components in the PCA analysis. The PCA presents one component with an eigenvalue of > 1, which explains 98.03% of the total variation in cell cycle gene expression at 4 weeks (a). Similarly, one component with an eigenvalue of > 1 illustrates 97.92% of the total variation in cell cycle gene expression at 12 weeks (b)
Table 3.
Principal component analysis (PCA) correlation matrix
| Component 1 | |
|---|---|
| Correlation in gene expression among groups (4-week old mice) | |
| 21%O2 Group | 0.981 |
| 40%O2 Group | 0.995 |
| 100%O2 Group | 0.994 |
| Correlation in gene expression among groups (12-week old mice) | |
| 21%O2 Group | 0.981 |
| 40%O2 Group | 0.997 |
| 100%O2 Group | 0.991 |
Cell cycle gene expression in whole lung homogenate in 4- and 12-week-old mice following short-term (30 min) of perinatal oxygen exposure soon after birth. The principal component analysis revealed one component with Eigenvalue > 1. The PCA component matrix demonstrates a high correlation between each variable in 4- and 12-week-old mice
PCA analysis at 12 weeks
The proportion of variance accounted for by the PCA was 96.3%, 99.3%, and 98.2% for 21%O2, 40%O2, and 100%O2 groups respectively in 12-week-old mice. PCA revealed one component with an eigenvalue greater than one, explaining 97.92% of the total variance in gene expression (Fig. 1b). The Steep drop after component one, followed by leveling off the slope (component 2 and 3), indicate that we retain component one for matrix analysis. The component matrix for the three groups is consistent with strong loadings for 21%O2 (0.981), 40%O2 (0.997), and 100%O2 (0.991) in 12-week old mice (Table 3). The results indicate that the three oxygen groups are highly correlated, suggesting an absence of variance in gene expression among the three oxygen groups.
Cell cycle protein analysis
Cdkn1a (p21) and Trp6 protein were not significantly different among the groups at four weeks and 12 weeks (Table 4). Lung elastin was decreased considerably in the 100%O2 group compared to the 21%O2 group at four weeks (Table 4, p < 0.05; ANOVA). However, elastin levels in the lung were similar in the three oxygen groups at 12 weeks. Ki67 protein was significantly higher in the 40%O2 group than 21%O2 and the 100%O2 group at four weeks (Table 4, p < 0.05 vs. 21%O2 & 100%O2, ANOVA). However, Ki67 levels were not different among the groups at 12 weeks. Similarly, Cyclin B1 was significantly higher in the 40%O2 group than 21%O2 at four weeks (Table 4, p < 0.05 vs. 21%O2, ANOVA). However, cyclin B1 in the lung was not different among the groups at 12 weeks.
Table 4.
Protein expression of selected cell cycle proteins and elastin in lung homogenates measured by enzyme immunoassay (EIA) in mice at 4 and 12 weeks
| Protein | 4 Weeks | 12 Weeks | ||||
|---|---|---|---|---|---|---|
| 21% O2 | 40% O2 | 100% O2 | 21% O2 | 40% O2 | 100% O2 | |
| CCNB1 | 27.32 ± 3.54 | 34.60 ± 6.29* | 29.08 ± 3.26 | 28.29 ± 3.54 | 21.59 ± 4.73 | 25.04 ± 4.65 |
| MKI67 | 17.05 ± 2.98 | 25.16 ± 1.4*† | 19.24 ± 2.38 | 26.63 ± 5.67 | 22.35 ± 4.15 | 25.51 ± 7.39 |
| CDKN1A (p21) | 12.60 ± 0.31 | 12.58 ± 0.28 | 12.25 ± 0.28 | 14.33 ± 3.68 | 12.89 ± 0.30 | 12.71 ± 0.08 |
| Trp63 | 2.46 ± 2.71 | 3.55 ± 2.51 | 0.66 ± 0.15 | 1.67 ± 0.13 | 1.48 ± 1.01 | 1.37 ± 0.66 |
| ELN | 1.14 ± 0.37 | 0.96 ± 0.22 | 0.48 ± 0.24* | 0.23 ± 0.20 | 0.57 ± 0.40 | 0.62 ± 0.42 |
The mice were exposed to 30 min of oxygen exposure (21%O2, 40%O2, or 100%O2) within six hours after birth
Values were expressed as mean ± SD (N = 6 in each group, each time point)
CCNB1 cyclin B1, Ki67 marker of proliferation Ki-67, CDKN1A (p21) cyclin-dependent kinase inhibitor 1A, Trp63 tumor protein 63, ELN elastin, Cell cycle proteins and elastin expressed as pg/µg lung protein
*p < 0.05 vs. 21%O2 group
†p < 0.05 vs. 100% O2 group (Fisher's Post-Hoc test, ANOVA)
Ki67 immunostaining for cell proliferation
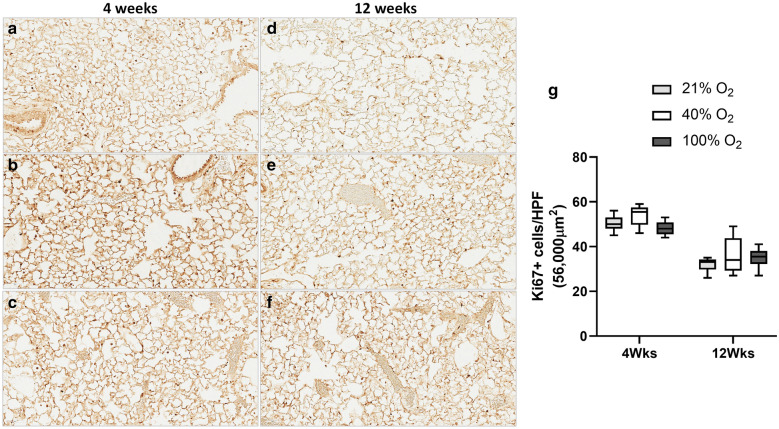
Nuclear staining for Ki67 was assessed by immunostaining at four weeks and 12 weeks of age (Fig. 2). Nuclear staining was quantified by counting Ki67 positive cells in the high power field. There was a significant interaction of the number of Ki67 positive cells over time (p < 0.001; Two-way ANOVA) but not for the oxygen groups. On multiple comparisons, there was no difference in the number of Ki67 positive cells among the three oxygen groups, suggesting that cell proliferation is not significantly different among the groups in mice at four weeks and 12 weeks (Fig. 2g).
Fig. 2.
Ki67 Immunostaining. Staining for Ki67, a marker of cellular proliferation was performed on lung sections at four weeks (a 21%O2, b 40%O2 & c 100%O2) and 12 weeks mice (d 21%O2, e 40%O2 & f 100%O2) following neonatal oxygen exposure (Scale bar: 60 µm). Ki67 positive cells for nuclear staining was calculated at 400 × resolution (56,000 µm2 area) in all the groups (g five sections/mice; 6 mice/group; 21%O2—grey bars; 40%O2—white bars; 100%O2—black bars). The number of Ki67 positive cells demonstrated a significant correlation over time but not with the oxygen groups (p < 0.001, Two-way ANOVA). On multiple comparisons, there was no significant difference among the oxygen groups
Lung morphometry
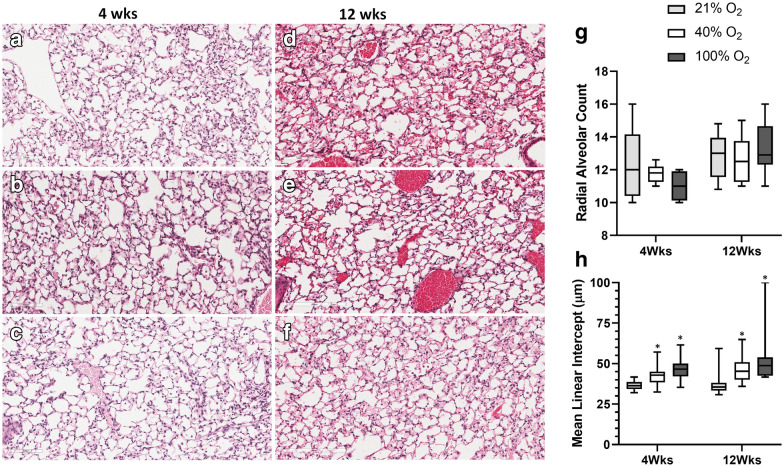
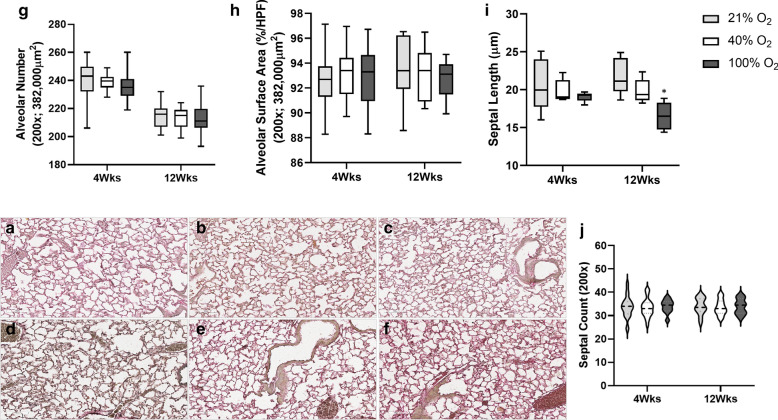
Histopathology, as assessed by H&E staining of lung sections, demonstrated no gross evidence of alveolar simplification among the groups at four weeks (Fig. 3a–c) and 12 weeks of age (Fig. 3d–f). The radial alveolar count was not significantly different among the groups at four weeks and 12 weeks in mice (Fig. 3g). However, MLI demonstrated a significant interaction concerning oxygen groups (p < 0.001; Two-way ANOVA). On multiple comparisons, exposure to 100%O2 soon after birth resulted in a significant increase in MLI at 4 and 12 weeks of age in adult mice (*p < 0.001 vs. 21%O2 & 40%O2 groups, Two-way ANOVA), suggesting subtle changes of impaired alveolarization following 30 min of oxygen exposure in the perinatal period. Morphometry of the lung was further detailed by assessing the alveolar number, alveolar surface area, septal count, and septal length on elastin stained sections of the three oxygen groups at four weeks (Fig. 4a–c) and 12 weeks (Fig. 4d–f). There was no difference in alveolar number (Fig. 4g), alveolar surface area (Fig. 4h), and septal count (Fig. 4j) among the three oxygen groups at both time points. However, septal length demonstrated a significant interaction among the three oxygen groups over time (p < 0.01, Two-way ANOVA). On multiple comparisons, exposure to 100%O2 soon after birth resulted in a significant reduction in length of the secondary septae at 12 weeks of age in adult mice (*p < 0.01 vs. 21%O2 & 40%O2 groups, Two-way ANOVA).
Fig. 3.
Lung histopathology. Lung sections were assessed by H&E staining in all the groups at four weeks (a 21%O2, b 40%O2 & c 100%O2) and 12 weeks of age (d 21%O2, e 40%O2 & f 100%O2) following neonatal oxygen exposure. Lungs did not demonstrate gross evidence of alveolar simplification (Scale bar: 100 µm). Radial alveolar count (RAC) (g) and mean linear intercept (MLI) (h) was estimated in all the groups (21%O2—grey bars; 40%O2—white bars; 100%O2—black bars; 400 × resolution). RAC was not significantly different in the groups at four weeks and 12 weeks in mice (g). However, MLI demonstrated a significant interaction concerning oxygen groups (p < 0.001; Two-way ANOVA). On multiple comparisons, exposure to 100%O2 soon after birth resulted in a significant increase in MLI at 4 and 12 weeks of age in mice (*p < 0.001 vs. 21%O2 & 40%O2 groups, Two-way ANOVA). (n = 6/group, each time-point)
Fig. 4.
Elastin Staining & Lung Morphology. Lungs were assessed by elastin staining in all groups at four weeks (a 21%O2, b 40%O2 & c 100%O2) and 12 weeks (d 21%O2, e 40%O2 & f 100%O2) of age following neonatal oxygen exposure (Scale bar: 100 µm). Alveolar number (g), alveolar surface area per high power field (HPF) (850 × 450 µm; 382,000 µm2) (h), septal length (i), and septal count (j) were assessed at 200 × resolution. There was no difference in alveolar number, alveolar surface area, and septal count among the three oxygen groups at both time points. Septal length demonstrated a significant interaction among the three oxygen groups over time (p < 0.01, Two-way ANOVA). On multiple comparisons, exposure to 100%O2 soon after birth resulted in a significant reduction in the length of the secondary septae at 12 weeks of age in adult mice (*p < 0.01 vs. 21%O2 & 40%O2 groups, Two-way ANOVA). (n = 6/group, each time-point)
Discussion
Exposure to a high concentration of oxygen in the neonatal period impairs lung growth and is a major contributing factor in the development of BPD. Oxygen concentrations that can be safely administered at resuscitation and subsequently in the postnatal management of premature infants at risk for BPD and ROP are uncertain at best. Studies on the effects of oxygen on gene expression are particularly relevant in newborns. Changes in gene expression at critical times of development can have long-lasting consequences with changes in lung structure and function [24]. Neonatal hyperoxia markedly inhibits lung epithelial cell proliferation [25] during the period of maximum alveolarization of the lung [26], implying alterations in gene expression, particularly relating to the regulation of cell cycle [27]. We studied the long-term effects of exposure to clinically relevant oxygen concentrations, including 100%O2 after birth on cell cycle gene expression and lung structure in adult mice.
Exposure to oxygen for 30 min in the perinatal period resulted in the downregulation of several cell cycle genes in 4-week-old mice, particularly in the 100%O2 group. An increased number of cell cycle genes were downregulated in the 100%O2 group compared to the 40%O2 group at four weeks, perhaps suggesting that oxygen effects on gene expression may be dose-dependent. However, gene expression among the groups was not different at 12 weeks of age. Among the 84 genes studied by array analysis, none of the genes in the 40%O2 group was statistically significant from room air controls either at four weeks or at 12 weeks. Microarray experiments allow simultaneous rapid assessment of hundreds of genes; however, several factors affect their reliability [28], requiring confirmation by RT-PCR or protein analysis. There were no significant differences noted in gene expression either by ANOVA or by PCA analysis. The scree plot retained only one principal component, and the component matrix demonstrated a high correlation of greater than 98% among the groups at both four weeks and 12 weeks. The high correlation among the groups suggests that the concentration of oxygen administered in the perinatal period had minimal effect on gene expression at four weeks and 12 weeks of age in adult mice. Species differences, biological variability, and array procedure may have affected the significant differences noted in the expression of several genes in the 100%O2 groups.
Ki67, a marker gene for cell proliferation, was significantly downregulated in the 100%O2 group; however, the Ki67 protein expression was not different from 21%O2 at 4 and 12 weeks. A significantly higher Ki67 protein in the lung was noted in the 40%O2 group despite the lack of differences in gene expression at four weeks. Ki67 expression in tissues is highly heterogeneous and is influenced by the stage of the cell cycle and the time spent in G0 [29]. Higher levels of Cyclin B1, a regulator of the cell cycle, suggest that elevation of Ki67 in lung tissue may be related to cell cycle changes [30]; however, the functional significance of Ki67 protein expression is often unclear [31]. Additionally, Ki67 immunostaining did not demonstrate differences in Ki67 + cells among the groups at both four weeks and 12 weeks. However, a significant difference in Ki67 staining over time may reflect active cell proliferation from lung development at four weeks. During the active stage of lung development in the postnatal lung, downregulation of p21 is essential for effective DNA synthesis, as cells expressing low levels of p21 progress through cell cycle upon release from S-phase arrest [32], facilitating lung recovery in developing mice. Higher expression of p21 was noted following exposure to 95%O2 for 24 or 48 h, suggesting overall inhibition of cell cycle progression [33]. The absence of any differences in p21 expression may indicate no significant differences in lung development. However, the study is limited by the lack of p21 measurement closer to hyperoxia exposure after birth.
Mice lung is composed of large sacculi by postnatal day 4 (P4), formed by branching morphogenesis; and alveolarization occurs from P4 to P21 with new septa formation from immature pre-existing septa [34, 35]. Lifting-up of new septae (also called 'secondary septae' or 'secondary crests') from pre-existing mature septa occurs from P14 into early adulthood and is virtually complete by P36 [34, 35]. Alveoli formation occurs at a faster pace from P4 to P14 and at a slower rate after that. The development of a single layer capillary network in the septa coincides with not only the completion of alveolarization but also microvascular maturation [34]. In our study, 4-week old mice are in the final phase of alveolarization corresponding to young adulthood, with alveolarization virtually complete in adult mice at 12 weeks.
A significant reduction in lung elastin in the 100%O2 group at four weeks indicates the enduring effects of even brief exposure to high oxygen concentration. Even though elastin measurements in lung homogenates were not different among the groups at 12 weeks, MLI was significantly higher in the 100%O2 groups at both 4 and 12 weeks of age. Septal length, a measure of secondary crest formation, was significantly different among the three oxygen groups, with a significant decrease in septal length in the 100%O2 group compared to 21%O2 and 40%O2 group adult mice. Not all measurements of lung morphometry demonstrate lung impairment, as differences in lung development may be challenging to determine from single measurements; however, septal thickness and MLI are sensitive objective tools of lung morphometry. Taken together, exposure to 30 min of 100%O2 administration may modify secondary crest maturation, alter lung elastin, and remodel lung development.
The postnatal alveolar formation is the most important and the least understood phase of lung development. Tightly regulated differentiation of alveolar fibroblasts towards a myogenic or a lipogenic phenotype is relevant to the septal formation [36]. This is driven to some extent by Platelet-Derived Growth Factor Receptor (PDGFRα). Postnatal PDGFRα is crucial in regulating elastogenesis [37]. The stability of the extracellular matrix involving collage and elastin is perturbed during the arrested alveolarization of the developing mouse lung exposed to hyperoxia [38]. We have shown that prolonged hyperoxia in the postnatal period results in permanent alveolar simplification in mice [39, 40]. Hyperoxia exposed cells undergo both apoptotic and nonapoptotic cell death. An increase in apoptosis from hyperoxia during a critical period of lung development may be an essential factor in impaired lung growth and remodeling [41]. Clinical studies have shown that resuscitation with high oxygen concentration (90%O2) results in higher oxidative stress, inflammation, and bronchopulmonary dysplasia than resuscitation with 30%O2 in premature infants [21].
Newborn mice at birth are equivalent to 26-week gestation human infant and 30 min of oxygen, in mice is similar to oxygen exposures premature infants experience at birth. As randomization was not based on littermates from different litters, bias might have been introduced due to possible litter effects. If 30 min of exposure would not affect gene expression, then probably anything less is less likely to affect newborn mice. In humans, the duration of pure O2 breathing needed to induce oxygen toxicity is not known. However, supraphysiologic levels of O2 prolong cellular dysfunction with increasing morbidity and mortality [42, 43]. Hyperoxic reoxygenation affects multiple signaling pathways in the lung that regulate cell growth, DNA repair, and survival [44]. An exposure period of 6–24 h is associated with clinical and histologic signs of lung injury in humans [45, 46]. The effects of prolonged hyperoxia on gene expression is well studied [47]; however, the effects of shorter duration (30 min to 6 h) needs further exploration. We analyzed gene expression in the whole lung instead of specific lung cells such as alveolar epithelial or airway epithelial cells, which is a limitation. The lack of gene expression, protein analysis, and oxidant injury markers closer to hyperoxia exposure could have been relevant to the study. The study is limited by the lack of exposure to oxygen at birth that usually occurs in infants requiring resuscitation. Early hyperoxia may induce changes in histone signatures in gene expression, altering vascular patterns in infants with BPD [48]. The timing, duration, and severity of hyperoxia relative to resuscitation need critical exploration. In a National Collaborative Perinatal Project, a slightly higher risk of cancer was noted in children exposed to > 3 min of oxygen at birth [49]. Even though 30 min of 100%O2 by itself does not produce changes in cell cycle gene expression, factors such as intrauterine or postnatal infection or respiratory depression at birth that may affect resuscitation and oxygen administration needs to be studied further. The development of lung injury during hyperoxia exposure is a complex process with the expression of several genes essential in the adaptive response to hyperoxia, including apoptosis, cytokine production, and extracellular matrix repair [33]. We focused on cell cycle gene expression and its impact on cell proliferation and lung development, one of the critical components of responses to hyperoxia.
Conclusions
The study demonstrates that short-term exposure to high oxygen concentrations leads to subtle changes in lung development that may affect alveolarization. These changes are related explicitly to secondary crest formation that may result in alteration in lung elastin. The effects of resuscitation with high oxygen concentrations may have a significant impact on lung development and long-term outcomes such as BPD in premature infants. Alveolar development resulting from the formation of the secondary crest and microvasculature maturation is being explored. The role of oxygen in alveolar development and its relationship to crest formation and lung elastin is not clear. The data generated from the study is not only a step in understanding lung development in the context of oxygen resuscitation but also its long-term impact, specifically in the lung. The information may be useful in term and premature newborns at resuscitation and beyond, wherein oxygen is routinely used in managing these infants.
Acknowledgements
We thank Mr. Imtiaz Mohammad, Department of Pathology (Elastin Staining), and Mrs. Sylvia Gugino (Digitalization of histopathology slides). Without whose help, the revision would not have been possible during the pandemic.
Abbreviations
- PCA
Principal Component Analysis
- BPD
Bronchopulmonary Dysplasia
- ROP
Retinopathy of Prematurity
Authors’ contributions
VK conceived and designed the study. HW was responsible for breeding, oxygen exposures & immunohistochemistry; LN performed gene expression and protein analysis; VK supervised the study and drafted the initial manuscript. All authors read and approved the final manuscript.
Funding
The study was funded by the American Academy of Pediatrics / Neonatal Resuscitation Program Grant to V. Kumar (No. 53831/2010), Department of Pediatrics, University at Buffalo, Buffalo, NY.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study approved by the Institutional Animal Care and Use Committee of the University at Buffalo, Buffalo, NY (IACUC#PED24116N).
Consent for publication
Not applicable (animal study).
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vasantha H. S. Kumar, Email: vkumar3@buffalo.edu
Huamei Wang, Email: huawang@acsu.buffalo.edu.
Lori Nielsen, Email: lnielsen@buffalo.edu.
References.
- 1.Buonocore G, Perrone S, Longini M, Vezzosi P, Marzocchi B, Paffetti P, et al. Oxidative stress in preterm neonates at birth and on the seventh day of life. Pediatr Res. 2002;52(1):46–49. doi: 10.1203/00006450-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Frank L, Sosenko IR. Prenatal development of lung antioxidant enzymes in four species. J Pediatr. 1987;110(1):106–110. doi: 10.1016/S0022-3476(87)80300-1. [DOI] [PubMed] [Google Scholar]
- 3.Kondo M, Itoh S, Isobe K, Kunikata T, Imai T, Onishi S. Chemiluminescence because of the production of reactive oxygen species in the lungs of newborn piglets during resuscitation periods after asphyxiation load. Pediatr Res. 2000;47(4 Pt 1):524–527. doi: 10.1203/00006450-200004000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res. 1997;41(5):599–606. doi: 10.1203/00006450-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003;8(1):39–49. doi: 10.1016/S1084-2756(02)00194-X. [DOI] [PubMed] [Google Scholar]
- 6.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38(7):397–432. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mickel HS, Vaishnav YN, Kempski O, von Lubitz D, Weiss JF, Feuerstein G. Breathing 100% oxygen after global brain ischemia in Mongolian Gerbils results in increased lipid peroxidation and increased mortality. Stroke. 1987;18(2):426–430. doi: 10.1161/01.STR.18.2.426. [DOI] [PubMed] [Google Scholar]
- 8.Lui K, Jones LJ, Foster JP, Davis PG, Ching SK, Oei JL, et al. Lower versus higher oxygen concentrations titrated to target oxygen saturations during resuscitation of preterm infants at birth. Cochrane Database Syst Rev. 2018;5:CD010239. doi: 10.1002/14651858.CD010239.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGuire W, Soll R. Commentary on ‘Lower versus Higher Oxygen Concentrations Titrated to Target OxygenSaturations during Resuscitation of PretermInfants at Birth’. Neonatology. 2019;115(3):278–282. doi: 10.1159/000495315. [DOI] [PubMed] [Google Scholar]
- 10.Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Perlman JM, et al. Part 13: neonatal resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S543–S560. doi: 10.1161/CIR.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 11.Patel A, Lakshminrusimha S, Ryan RM, Swartz DD, Wang H, Wynn KA, et al. Exposure to supplemental oxygen downregulates antioxidant enzymes and increases pulmonary arterial contractility in premature lambs. Neonatology. 2009;96(3):182–192. doi: 10.1159/000211667. [DOI] [PubMed] [Google Scholar]
- 12.Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, 3rd, et al. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res. 2006;59(1):137–141. doi: 10.1203/01.pdr.0000191136.69142.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohal RS, Allen RG. Oxidative stress as a causal factor in differentiation and aging: a unifying hypothesis. Exp Gerontol. 1990;25(6):499–522. doi: 10.1016/0531-5565(90)90017-V. [DOI] [PubMed] [Google Scholar]
- 14.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28(3):463–499. doi: 10.1016/S0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 15.Klein JA, Ackerman SL. Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest. 2003;111(6):785–793. doi: 10.1172/JCI200318182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messina JP, Lawrence DA. Cell cycle progression of glutathione-depleted human peripheral blood mononuclear cells is inhibited at S phase. J Immunol. 1989;143(6):1974–1981. [PubMed] [Google Scholar]
- 17.Smirnova IB. Thiols in mitosis and cleavage. Sov J Dev Biol. 1974;4(5):407–415. [PubMed] [Google Scholar]
- 18.Evans MJ, Bils RF. Identification of cells labeled with tritiated thymidine in the pulmonary alveolar walls of the mouse. Am Rev Respir Dis. 1969;100(3):372–378. doi: 10.1164/arrd.1969.100.3.372. [DOI] [PubMed] [Google Scholar]
- 19.Kauffman SL, Burri PH, Weibel ER. The postnatal growth of the rat lung. II. Autoradiography. Anat Rec. 1974;180(1):63–76. doi: 10.1002/ar.1091800108. [DOI] [PubMed] [Google Scholar]
- 20.Vento M, Asensi M, Sastre J, Lloret A, Garcia-Sala F, Vina J. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J Pediatr. 2003;142(3):240–246. doi: 10.1067/mpd.2003.91. [DOI] [PubMed] [Google Scholar]
- 21.Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 2009;124(3):e439–e449. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 22.Emery JL, Mithal A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child. 1960;35:544–547. doi: 10.1136/adc.35.184.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4(4):303–306. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright CJ, Dennery PA. Manipulation of gene expression by oxygen: a primer from bedside to bench. Pediatr Res. 2009;66(1):3–10. doi: 10.1203/PDR.0b013e3181a2c184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clement A, Edeas M, Chadelat K, Brody JS. Inhibition of lung epithelial cell proliferation by hyperoxia. Posttranscriptional regulation of proliferation-related genes. J Clin Invest. 1992;90(5):1812–1818. doi: 10.1172/JCI116056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275(1 Pt 1):L110–L117. doi: 10.1152/ajplung.1998.275.1.L110. [DOI] [PubMed] [Google Scholar]
- 27.Vuillaume M. Reduced oxygen species, mutation, induction and cancer initiation. Mutat Res. 1987;186(1):43–72. doi: 10.1016/0165-1110(87)90014-5. [DOI] [PubMed] [Google Scholar]
- 28.Jaksik R, Iwanaszko M, Rzeszowska-Wolny J, Kimmel M. Microarray experiments and factors which affect their reliability. Biol Direct. 2015;10:46. doi: 10.1186/s13062-015-0077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller I, Min M, Yang C, Tian C, Gookin S, Carter D, et al. Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep. 2018;24(5):1105–1112. doi: 10.1016/j.celrep.2018.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobecki M, Mrouj K, Colinge J, Gerbe F, Jay P, Krasinska L, et al. Cell-cycle regulation accounts for variability in Ki-67 expression levels. Cancer Res. 2017;77(10):2722–2734. doi: 10.1158/0008-5472.CAN-16-0707. [DOI] [PubMed] [Google Scholar]
- 31.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Gottifredi V, McKinney K, Poyurovsky MV, Prives C. Decreased p21 levels are required for efficient restart of DNA synthesis after S phase block. J Biol Chem. 2004;279(7):5802–5810. doi: 10.1074/jbc.M310373200. [DOI] [PubMed] [Google Scholar]
- 33.Perkowski S, Sun J, Singhal S, Santiago J, Leikauf GD, Albelda SM. Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am J Respir Cell Mol Biol. 2003;28(6):682–696. doi: 10.1165/rcmb.4692. [DOI] [PubMed] [Google Scholar]
- 34.Mund SI, Stampanoni M, Schittny JC. Developmental alveolarization of the mouse lung. Dev Dyn. 2008;237(8):2108–2116. doi: 10.1002/dvdy.21633. [DOI] [PubMed] [Google Scholar]
- 35.Pozarska A, Rodriguez-Castillo JA, Surate Solaligue DE, Ntokou A, Rath P, Mizikova I, et al. Stereological monitoring of mouse lung alveolarization from the early postnatal period to adulthood. Am J Physiol Lung Cell Mol Physiol. 2017;312(6):L882–L895. doi: 10.1152/ajplung.00492.2016. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Castillo JA, Perez DB, Ntokou A, Seeger W, Morty RE, Ahlbrecht K. Understanding alveolarization to induce lung regeneration. Respir Res. 2018;19(1):148. doi: 10.1186/s12931-018-0837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C, Lee MK, Gao F, Webster S, Di H, Duan J, et al. Secondary crest myofibroblast PDGFRalpha controls the elastogenesis pathway via a secondary tier of signaling networks during alveologenesis. Development. 2019;146:15. doi: 10.1242/dev.176354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizikova I, Ruiz-Camp J, Steenbock H, Madurga A, Vadasz I, Herold S, et al. Collagen and elastin cross-linking is altered during aberrant late lung development associated with hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2015;308(11):L1145–L1158. doi: 10.1152/ajplung.00039.2015. [DOI] [PubMed] [Google Scholar]
- 39.Kumar VH, Lakshminrusimha S, Kishkurno S, Paturi BS, Gugino SF, Nielsen L, et al. Neonatal hyperoxia increases airway reactivity and inflammation in adult mice. Pediatr Pulmonol. 2016;51(11):1131–1141. doi: 10.1002/ppul.23430. [DOI] [PubMed] [Google Scholar]
- 40.Kumar VHS, Wang H, Kishkurno S, Paturi BS, Nielsen L, Ryan RM. Long-Term Effects of Neonatal Hyperoxia in Adult Mice. Anat Rec (Hoboken). 2017. [DOI] [PubMed]
- 41.McGrath-Morrow SA, Stahl J. Apoptosis in neonatal murine lung exposed to hyperoxia. Am J Respir Cell Mol Biol. 2001;25(2):150–155. doi: 10.1165/ajrcmb.25.2.4362. [DOI] [PubMed] [Google Scholar]
- 42.Saugstad OD, Ramji S, Soll RF, Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology. 2008;94(3):176–182. doi: 10.1159/000143397. [DOI] [PubMed] [Google Scholar]
- 43.Yee M, White RJ, Awad HA, Bates WA, McGrath-Morrow SA, O'Reilly MA. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am J Pathol. 2011;178(6):2601–2610. doi: 10.1016/j.ajpath.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wollen EJ, Sejersted Y, Wright MS, Bik-Multanowski M, Madetko-Talowska A, Gunther CC, et al. Transcriptome profiling of the newborn mouse lung after hypoxia and reoxygenation: hyperoxic reoxygenation affects mTOR signaling pathway, DNA repair, and JNK-pathway regulation. Pediatr Res. 2013;74(5):536–544. doi: 10.1038/pr.2013.140. [DOI] [PubMed] [Google Scholar]
- 45.Sackner MA, Landa J, Hirsch J, Zapata A. Pulmonary effects of oxygen breathing. A 6-hour study in normal men. Ann Intern Med. 1975;82(1):40–43. doi: 10.7326/0003-4819-82-1-40. [DOI] [PubMed] [Google Scholar]
- 46.Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58(1):123–141. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, et al. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med. 2004;36(6):782–801. doi: 10.1016/j.freeradbiomed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Chao CM, van den Bruck R, Lork S, Merkle J, Krampen L, Weil PP, et al. Neonatal exposure to hyperoxia leads to persistent disturbances in pulmonary histone signatures associated with NOS3 and STAT3 in a mouse model. Clin Epigenetics. 2018;10:37. doi: 10.1186/s13148-018-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spector LG, Klebanoff MA, Feusner JH, Georgieff MK, Ross JA. Childhood cancer following neonatal oxygen supplementation. J Pediatrics. 2005;147(1):27–31. doi: 10.1016/j.jpeds.2005.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.