Abstract
Implementation and adoption of complex health information technology (HIT) is gaining momentum internationally. This is underpinned by the drive to improve the safety, quality, and efficiency of care. Although most of the benefits associated with HIT will only be realized through optimization of these systems, relatively few health care organizations currently have the expertise or experience needed to undertake this. It is extremely important to have systems working before embarking on HIT optimization, which, much like implementation, is an ongoing, difficult, and often expensive process. We discuss some key organization-level activities that are important in optimizing large-scale HIT systems. These include considerations relating to leadership, strategy, vision, and continuous cycles of improvement. Although these alone are not sufficient to fully optimize complex HIT, they provide a starting point for conceptualizing this important area.
Keywords: Health policy value, quality of health care, safety, leadership
INTRODUCTION
Health information technology (HIT) represents a fundamental component of health system improvement strategies internationally.1,2 Accordingly, implementation of these systems is picking up pace. Well over three-quarters of US hospitals have implemented at least a basic electronic health record system.3 Similar deployments are also being seen in many other countries.4
As health care systems and delivery organizations overcome the initial very substantial implementation hurdles, focus is shifting to how best use HIT to transform care processes, patient experiences, and outcomes.5–7 There is currently a great deal of frustration that, despite investing significant financial and human resources in HIT implementation, many organizations do not yet feel that they are achieving the hoped-for returns. What is often not appreciated is that deriving value is dependent on organizations putting in place carefully considered optimization strategies. These processes should be conceptualized as continuous organizational efforts to improve HIT systems and the ways they are used.8 This may involve refining advanced system functionalities, such as computerized decision support systems; developing data analytic functionalities to support quality improvement and/or research efforts; and developing sound approaches to deal with new emerging problems, some of which may have been introduced by the HIT systems themselves. We have summarized some exemplar overall optimization areas and specific strategies associated with these in Table 1, but this list is by no means exhaustive. These functionalities are central to the Stage 3 Final Recommendations of the Meaningful Use Incentive Program recently announced by the United States (US) Department of Health and Human Services.9
Table 1.
Examples of optimization strategies for HIT
| Exemplar overall optimization area | Advanced computerized provider order entry and clinical decision support (please also see Reference 23) | Data reuse and data linkage | Increased patient involvement and ownership | Integration of information and interfacing across settings |
|---|---|---|---|---|
| Examples of specific activities addressing the exemplar area | Drug-allergy, drug-drug interaction, and contraindication checking | Quality improvement | Patient access to records | Ability to exchange data across care settings (electronic links to primary and specialty care) |
| Laboratory test reminders | Research | Patient involvement in care activities | Electronic referral summaries | |
| Dosing support | Financial management | Patient education | Reconciliation activities | |
| Order entry of medication, laboratory tests, and diagnostic imaging linked to patient characteristics | Health care professional education and learning |
To help healthcare organizations achieve advanced HIT use, we build on our previous work detailing essential organizational considerations associated with implementing and adopting HIT.10,11 We draw on our experiences of working with benchmark sites in the US and United Kingdom (UK) and our ongoing research to share key findings, in the hope that this will helphealth care organizations in their continuing journey of refining HIT systems to maximize benefits. This work is intended as a framework for optimization deliberations, but it is important to keep in mind that our list is not intended to be comprehensive. There may be additional important points that will be necessary to fully optimize HIT, and these are likely to vary between systems and across organizational contexts.
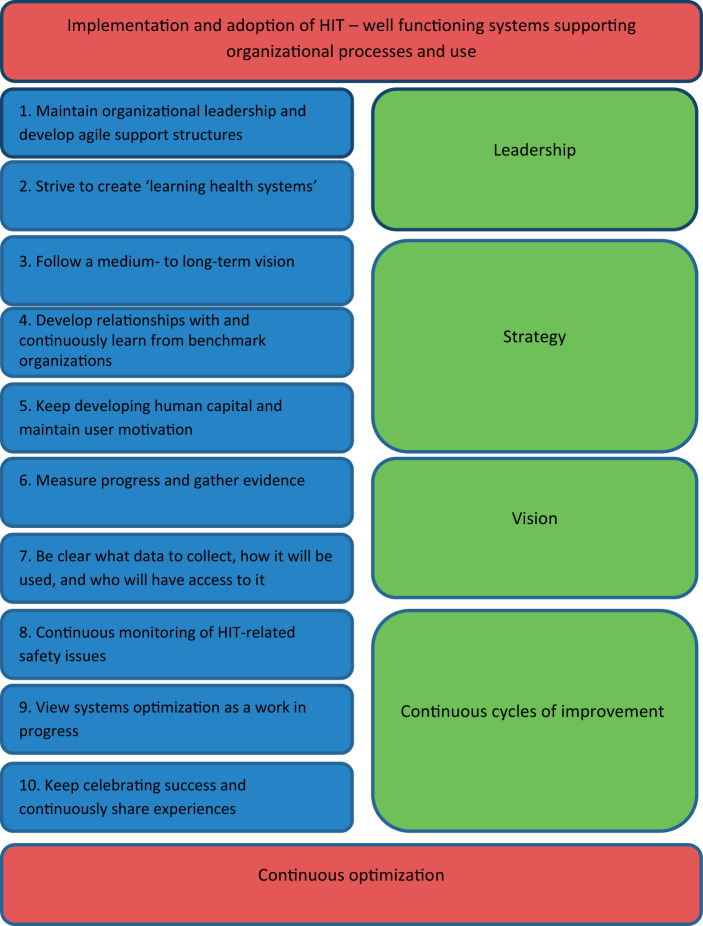
TEN KEY CONSIDERATIONS FOR THE SUCCESSFUL OPTIMIZATION OF LARGE-SCALE HEALTH INFORMATION TECHNOLOGY
Before substantive efforts are made on optimizing systems, it is vital that these are functioning as well as possible to support the organizational processes and work practices of users.12–15 This stabilization effort may include ironing out high-priority HIT-related safety threats and usability issues through either technical customization and/or changes in user/organizational practices. Once systems have achieved a certain level of stabilization, which in itself is typically a long and difficult process, the journey toward optimizing systems can begin.8 In this respect, it is important to note that quite often there are no clear boundaries between the various stages from implementation to optimization. We discuss the most essential elements of the optimization journey below.
1. Maintain organizational leadership and develop agile support structures
As with system implementation, ongoing high-level commitment is essential to ensure that sufficient resources are devoted to support optimization activities.16–20 Such leadership needs to continuously and consistently drive improvements by following the transformational vision, determining medium- and long-term priority areas, and providing financial support and incentives for related initiatives.1,21,22 At present, many organizations are trying to manage enormous numbers of requests for HIT changes, and if these are not actively addressed, value is not likely to be achieved, with the consequence that “sharp end” providers may become discouraged. The systems that vendors offer tend to be “bare-bones,” and the implicit assumption being made is that organizations will use the system tools offered to make care improvements. But achieving this requires organizations to continuously develop local human resource and governance structures. During this process, it is important to recognize that priorities may change and therefore a certain degree of flexibility is imperative. Useful practical guidance can be found in the US Office of the National Coordinator for Health Information Technology SAFER Self Assessment Guides, which are designed to help organizations optimize safety in high-priority areas.23
2. Strive to create “learning health systems”
The overall vision surrounding HIT optimization needs to be articulated within the framework of what the Institute of Medicine (now part of the National Academy of Sciences) has called the “learning health system.”24 HIT has great potential in this respect, since it allows the creation of a wealth of electronic data for analysis of trends, retrospective reviews/audits, identification of patterns, and analysis of potential contributing factors to adverse events.25 Such systems, therefore, if adequately optimized, should be able to facilitate collection and analysis of data that can then be used to inform future planning through iterative cycles of feedback to users, units, and organizations. Here it is important to consider that learning and sharing needs to occur at different levels. Ideally, individuals and organizations should make data available for analysis in exchange for being able to draw on data themselves (thereby resulting in reciprocal benefits).26 This implies that organizations need robust quality monitoring, tools, and organizational structures that enable relevant staff to get the data they need to improve care. This work should also involve embedding quality improvement and research from the start to drive quality and innovation.27 Intermountain Healthcare in Salt Lake City, Utah, for instance, routinely provides performance data to individual clinicians, which in turn motivates individual users to input high-quality data.28
3. Follow a medium- to long-term vision
Optimization of HIT takes time and needs to follow a medium- to long-term vision, as implementation-related activities are likely to consume a significant amount of initial resources.29–33 A degree of medium-term prioritization will likely be needed in order to agree on which optimization strategies should take precedence. For instance, integration of information and interfacing across settings is a strategic priority that transcends organizational boundaries and stakeholder groups (Table 1).34,35 This therefore presents an ideal starting point for considering medium-term goals associated with HIT optimization. Longer-term goals should be in line with the vision but also open to continuous revision, as priorities and circumstances are likely to change over time. Establishing firm organizational processes to periodically revise longer-term goals can be very helpful in maintaining focus.
4. Develop relationships with and continuously learn from benchmark organizations
Developing relationships with and continuously learning from benchmark sites that have already implemented and refined optimization approaches can prove very helpful. Although approaches need to be tailored to local needs and environments, there are likely to be important transferable lessons, particularly in relation to continuous system customization and data reuse. Important benchmark sites in the US include the Regenstrief Institute at the Indiana University School of Medicine, the Mayo Clinic in Rochester, Minnesota, Intermountain Healthcare, and the University of Vermont Medical Center Hospital.36,37
5. Keep developing human capital and maintaining user motivation
Project teams with necessary clinical, informatics, analytics, and organizational skills are essential for continuing system optimization, but once a system is implemented, many talented staff tend to leave to work on implementations in other institutions.11 As technologies become more advanced, there may also be a need to recruit staff with new skills and/or train existing staff in optimization strategies.38 Required skill sets include technical expertise to customize systems (eg, incorporating new computerized decision support alerts or interfaces and modeling their effects),39 organizational change capability to ensure that new functionalities are adequately used and desired data is collected,15 and development of analytics skills to ensure that data is effectively drawn on to improve quality.40 Some settings have made significant progress in this respect, at least in the short-term. More medium- and longer-term capacity development strategies are also needed in order to derive maximum value from HIT.41
Moreover, data to be collected through HIT needs to be of good quality. Otherwise there is a danger that analysis will produce misleading results.42 In order to develop and maintain high-quality data that can be used repeatedly, stakeholders need to be motivated to record the data and to use electronic health records and related HIT as intended.43 The more the data are used, the better they will get. The development of informal workarounds is a continuous threat to data quality and may jeopardize system optimization.14,15 Workarounds tend to be developed by users to get around concerns surrounding system usability, which highlights the underlying need for sustained engagement in relation to identifying, understanding, and responding to changing technical features.44 A periodic formal organizational review of shifting levels of engagement and potential associated unintended consequences should therefore be an essential part of organizational efforts.
6. Measure progress and gather evidence
Evaluation of optimization initiatives can help to create a baseline, track progress over time, and facilitate learning.45 This work ideally commences well before implementing any system and/or operational changes, and should be designed to obtain good-quality indicators for improvement surrounding optimization.46 In doing so, attention to both quantitative outcome measures and qualitative process measures is vital. Evidence also needs to reflect the complexity of the environment in which changes are taking place, paying attention to both technical and social dimensions, including improvements and risks.46 In relation to technical aspects, for instance, continued measurement and monitoring of technical functioning, including system reliability, response time, upgrades, and system-induced errors, can help in anticipating and mitigating risks.47,48 Some useful guidance in this respect can be found in a recent conceptual framework surrounding the monitoring and measurement of social and technical threats associated with HITs.48
Learning from experiences with using electronic data can help to improve data quality, and careful tracking of progress can help to ensure that this data has real-world applicability.49 A key to achieving this is establishing close links between academic and health care communities.
7. Be clear what data to collect, how it will be used, and who will have access to it
Agreement in relation to goals is important to ensure that the optimization efforts of stakeholders are aligned.50 Multi-stakeholder consensus around key organizational, professional, and patient priorities is therefore a crucial step in any optimization strategy.11,19 Specifying associated electronic data to address priority areas should follow.51 Here, it is important to consider which data is actually needed to achieve the desired goal, otherwise there is a danger that efforts will be spent on collecting information that is ultimately not used.52 For instance, the approach of Intermountain Healthcare’s data analytics team begins with the identification of a specific aim (eg, 5% reduction in hospital readmissions within 12 months), followed by an assessment of current and future states.41 After these important preliminary steps, relevant data items are identified and specified, which allows monitoring of progress toward this goal.
Another pitfall is that organizations frequently establish approaches that are too restrictive regarding who can access data after implementation of new systems, so that those responsible for quality and safety or researchers cannot access data in a timely fashion. Therefore, careful thought should be given to balancing the need to protect data with allowing access to support improvement efforts.
8. Continuous monitoring of HIT-related safety issues
HIT-related safety threats can occur at any stage of the implementation process, but given their critical importance, there is a continuing need to address these. This should include constant monitoring of risks and unintended consequences, as technical functionalities and ways of use are likely to change over time.13 Such activity needs to be characterized by a willingness to learn from user experiences and draw on formal reporting systems to respond to identified problems. Users must be encouraged to report adverse events, which can then be collected, analyzed, and interpreted. Systems and processes subsequently need to be put into place to prevent similar issues from occurring in the future.53–55
9. View systems optimization as a work in progress
The work surrounding HIT optimization activities is best conceptualized as an ongoing process of improvement.8,11 Although milestones are important, there is no actual endpoint. Quality improvement needs to adapt to technical changes (eg, emerging innovations and refinement of existing functionalities) and social dynamics (eg, national, organizational, and user factors),56,57 paying attention to both formal/planned and informal/emergent use.56,57 Emergent informal and potentially innovative optimization activities deserve special attention, as these often identify or address existing technical/social issues associated with new systems/practices. For example, workarounds employed by system users can be drawn on to improve system design and integration with work/organizational practices.58
10. Keep celebrating successes and continuously share experiences
In order to keep organizational actors motivated, successes need to be celebrated, and in order to promote learning across organizations, experiences and insights should be shared.15,21 This will likely entail establishing organizational structures that guide the collection of data associated with success (eg, the number of reduced readmissions) as well as mechanisms to record and disseminate lessons learned (eg, by establishing collaborations with academic centers and communications teams).
The larger the scale of such sharing, the better. Organizational learning can help to inform the efforts of establishments yet to embark on optimization activities.56 In this context, sharing of negative experiences is important, as it can potentially prevent unnecessary use of resources and iatrogenic harm.59 However, these should not be one-off activities; rather, they should become established as part of the core identity of healthcare organizations.52,53
CONCLUSIONS
We have outlined 10 key considerations for optimization that we hope will help to inform efforts to refine HIT systems as organizations are moving from implementation toward embedded use (Figure 1). This list of considerations is by no means exhaustive, and we hope that others will, in due course, build on it. The 10 areas we have identified can be conceptually divided into leadership, vision, strategy, and improving outcomes; covering technical, organizational, user, and patient dimensions. These need to be considered together and, if possible, aligned to effectively optimize HIT. It is important to keep in mind that before any of this optimization can begin, systems must be working appropriately and used as intended in individual settings.
Figure 1.
Ten key considerations for the successful optimization of large-scale health information technology
Many health care organizations have made major investments HIT. However, the extent to which these investments will result in the desired improvements is still uncertain, and is the subject of significant political debate in many countries, including the US. We believe that these benefits will be achieved, but it will take time, and that achieving them will be absolutely contingent on developing effective and robust optimization approaches. We strongly advocate a wider recognition that optimization efforts must be conceptualized as an ongoing journey and not as a destination that can be reached shortly after implementation. This journey will almost certainly be difficult, time consuming, and expensive, but the investments made will likely be worth it.
ACKNOWLEDGEMENTS
We are very grateful to all participants who kindly gave their time and to the extended project and program teams of work we have drawn on. We would also like to thank 2 anonymous expert reviewers for very helpful comments on an earlier draft of this manuscript.
Contributors and sources
A.S. and D.W.B. conceived this work. A.S. is currently leading a National Institute for Health Research–funded national evaluation of electronic prescribing and medicine administration systems. K.C. is employed as a researcher on this grant and led on the write-up and drafting of the initial version of the paper, with A.S. and D.W.B. commenting on various drafts.
Funding
This work has drawn on data funded by the National Health Service Connecting for Health Evaluation Programme (National Health Service Connecting for Health Evaluation Programme 001, National Health Service Connecting for Health Evaluation Programme 005, National Health Service Connecting for Health Evaluation Programme 009, National Health Service Connecting for Health Evaluation Programme 010), the National Institute for Health Research under its Programme Grants for Applied Research scheme (RP-PG-1209-10099), and The Commonwealth Fund. K.C. is supported by a Chief Scientist Office of the Scottish Government Postdoctoral Fellowship, and A.S. is supported by the Farr Institute. The views expressed are those of the authors and not necessarily those of the National Health Service, the Chief Scientist Office, the National Institute for Health Research, or the Department of Health.
Competing interest
The authors declare that they have no competing interests.
REFERENCES
- 1. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. NEJM. 2010;363:501–504. [DOI] [PubMed] [Google Scholar]
- 2. Sheikh A, Cornford T, Barber N, et al. Implementation and adoption of nationwide electronic health records in secondary care in England: final qualitative results from prospective national evaluation in “early adopter” hospitals. BMJ. 2011;343:d6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adoption of Electronic Health Record Systems among U.S. Non-Federal Acute Care Hospitals: 2008-2014. https://www.healthit.gov/sites/default/files/data-brief/2014HospitalAdoptionDataBrief.pdf Accessed October 14, 2015.
- 4. Global Market for Electronic Health Records (EHR) Expected to Reach $22.3 Billion by the End of 2015, According to Accenture. https://newsroom.accenture.com/subjects/research-surveys/global-market-for-ele ctronic-health-records-expected-to-reach-22-3-billion-by-the-end-of-2015-according-to-accenture.htm. Accessed October 14, 2015.
- 5. Cresswell KM, Sheikh A. Health information technology in hospitals: current issues and future trends. Future Hosp J. 2015;2:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kho AN, Cashy JP, Jackson KL, et al. Design and implementation of a privacy preserving electronic health record linkage tool in Chicago. J Am Med Inform Assoc. 2015; 10.1093/jamia/ocv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simpao AF, Ahumada LM, Desai BR, et al. Optimization of drug-drug interaction alert rules in a pediatric hospital's electronic health record system using a visual analytics dashboard. J Am Med Inform Assoc. 2015;22(2): 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cresswell K, Coleman J, Slee A, et al. A toolkit to support the implementation of electronic prescribing systems into UK hospitals: preliminary recommendations. J Royal Soc Med. 2014;107:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meaningful Use Stage 3 – 2015 Certification final rules released. http://www.practicefusion.com/blog/meaningful-use-stage-3/. Accessed October 14, 15.
- 10. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cresswell KM, Bates DW, Sheikh A. Ten key considerations for the successful implementation and adoption of large-scale health information technology. J Am Med Inform Assoc. 2013;20:e9–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berg M. Patient care information systems and health care work: a sociotechnical approach. Int J Med Inform. 1999;55:87–101. [DOI] [PubMed] [Google Scholar]
- 13. Koppel R, Metlay JP, Cohen A, et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005; 293:1197–1203. [DOI] [PubMed] [Google Scholar]
- 14. Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: the nature of patient care information system- related errors. J Am Med Inform Assoc. 2004;11:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sittig DF, Singh H. Electronic health records and national patient-safety goals. NEJM. 2012;367:1854–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ludwick DA, Doucette J. Adopting electronic medical records in primary care: lessons learned from health information systems implementation experience in seven countries. Int J Med Inform. 2009;78(1):22–31. [DOI] [PubMed] [Google Scholar]
- 17. Buntin MB, Burke MF, Hoaglin MC, et al. The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Aff. 2011;30:464–471. [DOI] [PubMed] [Google Scholar]
- 18. Hersh W. Health care information technology: progress and barriers. JAMA. 2004;292:2273–2274. [DOI] [PubMed] [Google Scholar]
- 19. Coiera E. Building a national health IT system from the middle out. J Am Med Inform Assoc. 2009;16:271–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cresswell K, Sheikh A. Organizational issues in the implementation and adoption of health information technology innovations: an interpretative review. Int J Med Inform. 2013;82:e73–e86. [DOI] [PubMed] [Google Scholar]
- 21. Gans D, Kralewski J, Hammons T, et al. Medical groups’ adoption of electronic health records and information systems. Health Aff. 2005;24: 1323–1333. [DOI] [PubMed] [Google Scholar]
- 22. Jha AK, Doolan D, Grandt D, et al. The use of health information technology in seven nations. Int J Med Inform. 2008;77:848–854. [DOI] [PubMed] [Google Scholar]
- 23. The Office of the National Coordinator for Health Information Technology. SAFER Guides for EHRs. https://www.healthit.gov/safer/safer-guides. Accessed December 13, 2015.
- 24. Kohn LT, Corrigan JM, Donaldson MS. eds. To err is human: building a Safer Health System. Vol. 6. Washington, D.C: National Academies Press; 2000. [PubMed] [Google Scholar]
- 25. Hersh WR. Adding value to the electronic health record through secondary use of data for quality assurance, research, and surveillance. Am J Manag Care. 2007;81:126–128. [PubMed] [Google Scholar]
- 26. Friedman CP, Wong AK, Blumenthal D. Achieving a nationwide learning health system. Sci Transl Med. 2010;2:57cm29. [DOI] [PubMed] [Google Scholar]
- 27. Ferlie EB, Shortell SM. Improving the quality of health care in the United Kingdom and the United States: a framework for change. Milbank Quart. 2001;79:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. When Health Care Gets a Healthy Dose of Data. http://sloanreview.mit.edu/case-study/when-healthcare-gets-a-healthy-dose-of-data/. Accessed Octo ber 14, 2015.
- 29. Wanless D. Securing our Future Health: Taking a Long-term View. London: HM Treasury; 2002:16. [Google Scholar]
- 30. Cresswell K, Sheikh A. Electronic health record technology. JAMA. 2012;307:2255–2255. [DOI] [PubMed] [Google Scholar]
- 31. DesRoches CM, Campbell EG, Vogeli C, et al. Electronic health records’ limited successes suggest more targeted uses. Health Aff. 2010;29: 639–646. [DOI] [PubMed] [Google Scholar]
- 32. Hillestad R, Bigelow J, Bower A, et al. Can electronic medical record systems transform health care? Potential health benefits, savings, and costs. Health Aff. 2005;24:1103–1117. [DOI] [PubMed] [Google Scholar]
- 33. John K, Holger R, James BA. Our Iceberg Is Melting: Changing and Succeeding Under Any Conditions. The Academy of Management Perspectives. 2009;23(3):101–103. [Google Scholar]
- 34. Crawford MJ, Rutter D, Manley C, et al. Systematic review of involving patients in the planning and development of health care. BMJ. 2002;325:1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goodwin N, Smith J, Davies A, et al. Integrated Care for Patients and Populations: Improving Outcomes by Working Together. London: King’s Fund; 2012. [Google Scholar]
- 36. Panesar S, Carson-Stevens A, Salvilla S, Sheikh A. Patient Safety and Healthcare Improvement at a Glance. Chichester, UK: John Wiley & Sons; 2014. [Google Scholar]
- 37. Baker GR, MacIntosh-Murray A, Porcellato C, et al. Intermountain Healthcare. High Performing Healthcare Systems: Delivering Quality by Design. Toronto: Longwoods Publishing; 2008: 151–178. [Google Scholar]
- 38. Rippen HE, Pan EC, Russell C, et al. Organizational framework for health information technology. Int J Med Inform. 2013;82:e1–e13. [DOI] [PubMed] [Google Scholar]
- 39. Tiwari R, Tsapepas DS, Powell JT, et al. Enhancements in healthcare information technology systems: customizing vendor-supplied clinical decision support for a high-risk patient population. J Am Med Inform Assoc. 2013;20:377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grossman RL, Siegel KP. Organizational models for big data and analytics. J Org Design. 2014;3:20–25. [Google Scholar]
- 41. Building an Analytics Team. http://files.meetup.com/1608444/030514_CS_BuildingAnalyticsTeam-Intermountain_WWA_Final.pdf Accessed October 14, 2015.
- 42. Rose LT, Fischer KW. Garbage in, garbage out: having useful data is everything. Measurement. 2011;9:222–226. [Google Scholar]
- 43. Ammenwerth E, Gräber S, Herrmann G, et al. Evaluation of health information systems—problems and challenges. Int J Med Inform. 2003;71(2):125–135. [DOI] [PubMed] [Google Scholar]
- 44. Halbesleben JR, Wakefield DS, Wakefield BJ. Work-arounds in health care settings: literature review and research agenda. Health Care Manage Rev. 2008;33:2–12. [DOI] [PubMed] [Google Scholar]
- 45. Catwell L, Sheikh A. Evaluating eHealth interventions: the need for continuous systemic evaluation. PLoS Med. 2009;6:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cresswell KM, Sheikh A. Undertaking sociotechnical evaluations of health information technologies. Inform Prim Care. 2014;21:78–83. [DOI] [PubMed] [Google Scholar]
- 47. Meeks DW, Smith MW, Taylor L, et al. An analysis of electronic health record-related patient safety concerns. J Am Med Inform Assoc. 2014;21: 1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singh H, Sittig DF. Measuring and improving patient safety through health information technology: the Health IT Safety Framework. BMJ Qual Saf. 2015:bmjqs-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Botsis T, Hartvigsen G, Chen F, et al. Secondary use of EHR: data quality issues and informatics opportunities. AMIA Summits on Translational Science Proceedings. 2010;1. [PMC free article] [PubMed] [Google Scholar]
- 50. Bryson JM. Strategic Planning for Public and Nonprofit Organizations: A Guide to Strengthening and Sustaining Organizational Achievement, Vol. 1 San Francisco, CA: John Wiley & Sons; 2011. [Google Scholar]
- 51. Christensen C. The Innovator's Dilemma: When New Technologies Cause Great Firms to Fail. Boston, MA: Harvard Business Review Press; 2013. [Google Scholar]
- 52. Coulter A, Locock L, Ziebland S, et al. Collecting data on patient experience is not enough: they must be used to improve care. BMJ. 2014;348:g2225. [DOI] [PubMed] [Google Scholar]
- 53. Barach P, Small SD. Reporting and preventing medical mishaps: lessons from non-medical near miss reporting systems . BMJ. 2000;320:759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hutchinson A, Young TA, Cooper KL, et al. Trends in healthcare incident reporting and relationship to safety and quality data in acute hospitals: results from the National Reporting and Learning System. Qual Saf Health Care. 2009;18:5–10. [DOI] [PubMed] [Google Scholar]
- 55. Department of Health, Social Services and Public Safety. Risk Register Guidance. https://www.dhsspsni.gov.uk/articles/risk-register-guidance-0. Accessed December 15, 2015.
- 56. Phaal R, Farrukh CJ, Probert DR. Technology roadmapping—a planning framework for evolution and revolution. Technol Forecast Soc Change. 2004;71:5–26. [Google Scholar]
- 57. Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Safadi H, Faraj S. The Role of workarounds during an OpenSource Electronic Medical Record System Implementation. ICIS. 2010:47. [Google Scholar]
- 59. Levitt B, March JG. Organizational learning. Ann Rev Sociol. 1988;319–340. [Google Scholar]