Abstract
Objective: This paper outlines the implementation of a comprehensive clinical pharmacogenomics (PGx) service within a pediatric teaching hospital and the integration of clinical decision support in the electronic health record (EHR).
Materials and Methods: An approach to clinical decision support for medication ordering and dispensing driven by documented PGx variant status in an EHR is described. A web-based platform was created to automatically generate a clinical report from either raw assay results or specified diplotypes, able to parse and combine haplotypes into an interpretation for each individual and compared to the reference lab call for accuracy.
Results: Clinical decision support rules built within an EHR provided guidance to providers for 31 patients (100%) who had actionable PGx variants and were written for interacting medications. A breakdown of the PGx alerts by practitioner service, and alert response for the initial cohort of patients tested is described. In 90% (355/394) of the cases, thiopurine methyltranferase genotyping was ordered pre-emptively.
Discussion: This paper outlines one approach to implementing a clinical PGx service in a pediatric teaching hospital that cares for a heterogeneous patient population. There is a focus on incorporation of PGx clinical decision support rules and a program to standardize report text within the electronic health record with subsequent exploration of clinician behavior in response to the alerts.
Conclusion: The incorporation of PGx data at the time of prescribing and dispensing, if done correctly, has the potential to impact the incidence of adverse drug events, a significant cause of morbidity and mortality.
Keywords: pharmacogenomics, clinical, genetics, child, electronic medical record
OBJECTIVE
This paper outlines the development and implementation of a comprehensive clinical pharmacogenomics service within a pediatric tertiary care urban teaching hospital and the integration of related results with clinical decision support in the electronic health record (EHR).
BACKGROUND AND SIGNIFICANCE
The clinical application of pharmacogenomic (PGx) principles has consistently been cited as a major opportunity for improving patient care. In multiple well-conducted research studies, the results of PGx testing have been shown to guide therapy choice and dosing modifications to expedite and improve treatment efficacy and reduce the incidence of adverse drug reactions (harm not related to medication error).1,2
There are over 100 US Food and Drug Administration drug labels containing PGx marker information and several medications that require the use of pharmacogenetic testing prior to drug initiation, including ivacaftor, trastuzumab, maraviroc, dasatinib, and eliglustat.3 Established peer-reviewed guidelines from the Clinical Pharmacogenomics Implementation Consortium (CPIC) and Royal Dutch Association for the Advancement of Pharmacy’s Pharmacogenetics Working Group guide application of genotype results in the clinical realm.3–5 Nonetheless, application has been limited and PGx has yet to be fully implemented into routine clinical practice.6 In 2011 the Pharmacogenomics Research Network established the Translational Pharmacogenetics Program to understand real-world barriers to PGx adoption within six diverse healthcare systems (see Supplementary materials).6
A clinical PGx service has been launched and incorporated into routine patient care at Boston Children’s Hospital (BCH). Whereas previously published PGx translational studies were performed under research protocols with constraints on result return and selection bias, the BCH Clinical Pharmacogenomic Service (CPS) serves all BCH patients, independent of a research protocol.2 To date, the primary focus has been on patients with significant adverse drug reactions or extended periods of nonresponse to medications. However all patients are eligible for consult or referral at the discretion of their provider, regardless of medical service, medication ordered or diagnosis.
In many cases for PGx genes of interest, the enzyme name is the same as the gene responsible for encoding. Some of the more common terms that will be discussed in this paper include thiopurine methyltransferase (TPMT), the cytochrome P450 enzymes such as CYP2C19 and CYP2C9, the vitamin K epoxide reductase complex, subunit 1 (VKORC1) and the major histocompatatibility complexes (HLAs). At the time of the study, BCH offered clinical single gene sequencing for TPMT genotyping for thiopurine dosing and CYP2C9/VKORC1 genotyping for warfarin dosing with full clinical decision support in the EHR. Additionally a more comprehensive 225 gene single nucleotide polymorphism-based PGx panel is available for patients with more complicated histories of multiple adverse drug reactions and/or nonresponse to various therapies (AffyMetrix DMET Plus with three additional CYP2D6 probes) as well as an HLA panel (HLA-A*31:01, HLA-B*15:02, HLA-B*57:01, and HLA-B*58:01) performed by Medical College of Wisconsin. Clinical decision support is currently available for TPMT, CYP2C9, VKORC1, and CYP2C19 and is linked to over 30 medications based on the CPIC guidelines.7–9 The HLA variant build is currently underway and is supporting ordering and dispensing of carbamazepine, phenytoin/fosphenytoin, abacavir, and allopurinol.
The CPS has focused on the following: (i) acquiring accurate genotype information in an acceptable time frame allowing for clinical application; (ii) completing an EHR build that allows for easily accessible displays of genotype results leveraging the gracious help and extensive knowledge of the previous work done by the team at St Jude Children’s Research Hospital; (iii) choosing drug-gene pairs with conclusive support in the literature; (iv) providing discipline-based education to providers and clinicians in both meeting-based and computer-based learning formats; (v) encouraging the various services in the hospital to assess, analyze, and determine when PGx testing makes the most sense for their patients and subsequently building algorithms to support those practices; (vi) leveraging and expanding upon the previous work of the PGx community, unique clinical reporting infrastructures and customized EHR alerts and data fields were developed; and (vii) interacting with payers, building the mechanisms for clinical service reimbursement through collaborative drug therapy management practice.
A key requirement for clinical use of PGx data is development of an appropriate clinical decision support (CDS) system within the EHR. CDS may be implemented in either an active or passive manner. Passive systems make information available should the practitioner seek it, but generally provide little guidance as to how such information may be applied to a particular clinical scenario. In this case, it is not only the responsibility of the clinician to know how and when to access the information, but also then to deduce if action should be taken. Additionally, documentation may be text heavy and difficult to interpret or navigate at the point of care. Active CDS, in contrast, involves the automatic, interruptive displaying of dynamic alerts, messages, or reminders based on specified rules or logic. The display typically requires some action on the part of the user, such as a manual override or closing of a text box. The general benefits of active versus passive CDS have been documented and include a more natural integration into computerized prescriber order entry workflow and increased physician adherence to recommendations and clinical practice guidelines.10,11 These advantages are particularly valuable within the realm of PGx, where it has been shown that many practitioners lack confidence in applying genomic results and would find an active system providing guidance and additional resources useful in practice.12,13 Active alerts at the point of care requiring acknowledgement and providing both clinical insight and resources for further guidance have the potential to enrich the prescribing process, improving the safety and effectiveness of therapies. In the absence of an active alert system, it is possible that genetic results would sit dormant as scanned PDF documents and may be forgotten or lost within the EHR over the course of a patient’s lifetime.
Even with the most robust of systems the question remains as to which genes to include within the CDS framework. This can be challenging as PGx evidence is rapidly evolving and literature is being generated daily with varying levels of clinical strength. To address this, the CPIC was established by the National Institutes of Health's Pharmacogenomics Research Network and the Pharmacogenomics Knowledge Base to publish guidelines providing insight in identifying which drug–gene pairs have sufficient evidence to implement clinically actionable recommendations into practice.14 These publications are peer reviewed, regularly updated, and primarily focus on a particular drug or gene. Articles include grading levels of evidence linking genotypes to phenotypes, strategies for determining phenotype based on genotype, and prescribing algorithms by genotype and phenotype. These guidelines help institutions to determine which drugs and genes may be eligible for CDS within the EHR, and what recommendations to include within the alert.
CPIC guidelines are useful in directing CDS with existing PGx results, however they do not address the question of when to pre- emptively complete PGx testing.13 This decision is in some cases determined by drug labeling recommendations but more is often left up to institutional policies, which can vary even among different departments. Published data suggests that pre-emptive testing may provide clinical benefit and is the most effective way to utilize pharmacogenetic data in practice with results readily available at the time of prescribing.2 In a study published by St Jude Children’s Research Hospital, 78% of patients tested were found to have at least one genotype eligible to be entered into the EHR as clinically actionable. With this and other mounting evidence, the case for pre-emptive standardized screening for clinical variants holds promise for the future of PGx in practice. Additionally as PGx testing moves rapidly into the next generation sequencing realm as part of the natural progression of personalized medicine, the necessity for clear policies around security and privacy of a large amount of genomic data together with return of secondary findings is paramount.
MATERIALS AND METHODS
In January 2012, BCH implemented a new comprehensive clinical service for PGx testing to improve patient care. Importantly, clinical PGx is now considered to be part of standard care for defined indications at BCH such as prior to the initiation of 6-mercaptopurine in patients with inflammatory bowel disease. Initially the infrastructure was designed to support genetic testing for TPMT and thiopurines (azathioprine, thioguanine, and mercaptopurine). The clinical service was then expanded to support reflexive and pre-emptive genetic testing using the Affymetrix DMET Plus array (225 genes) supplemented with CYP2D6 copy number assay, and now are implementing an HLA panel to support the safe prescribing and dispensing of four target medications (carbamazepine, phenytoin/fosphenytoin, abacavir, and allopurinol). All tests are performed in Clinical Laboratory Improvement Amendments certified laboratories. All results are recorded in the EHR and a subset of approved relevant variant statuses are placed in the patient’s problem list to trigger CDS alerts.
This study was determined to be exempt by the institutional IRB at BCH.
Prior to implementing the service, BCH established a Clinical Pharmacogenomics Oversight Committee to provide formal governance of clinical pharmacogenomics at BCH and provide necessary oversight to the CPS. The committee is comprised of experts in genetics, clinical pharmacy, pharmacology, research, bioinformatics, clinical informatics, and various specialties. The Clinical Pharmacogenomics Oversight Committee is a subcommittee of the Pharmacy and Therapeutics Committee, which reports to the Medical Executive Committee. The CPS Oversight Committee guides the decisions around implementation of pre-emptive testing alerts, the display of information and approval of the final content of alert messages.
When adequate evidence is available through publication of CPIC guidelines or evaluation of primary literature using criteria outlined by CPIC, decisions to migrate gene–drug pairs into the EHR and the corresponding use of clinical decision support rules and reports are made by the CPS Oversight Committee. To ensure consistent evaluation and patient safety, we developed a scoring system to rate gene–drug pairs based on 16 different criteria, such as benefit to patient, cost, and actionability and may be found in the Supplementary Materials. By using the scoring system, the Oversight Committee is able to prioritize EHR build that competes for Informatics Service Department resources with other hospital initiatives.
The clinical alerts used at BCH are modeled after the approach designed by St Jude Children’s Hospital and implemented on the Cerner EHR platform.1 However, the use cases at BCH in a clinical setting are substantially different and alerts were therefore adapted accordingly.
In order to support the laboratory process of reporting results to the EHR in a usable manner, the team designed an automated system to interpret individual nucleotide base calls and determine patient diplotypes which are then added to a clinical report that is input directly into the EHR. A web-based platform (written in Python using the Django Framework and powered by a MySQL database) was created to automatically generate a clinical report from either raw assay results or specified diplotypes. The platform is able to parse and translate TaqMan, Sanger sequencing and Affymetrix DMET Plus software outputs into star allele nomenclature by extracting reference and alternate nucleotides and corresponding rsID numbers to compute star allele haplotypes. rsID numbers represent unique single nucleotide polymorphisms and are important for accurate interpretation. The star allele nomenclature is unique to PGx and requires careful implementation in the EHR, as the asterisk symbol is often used as a wild card in proprietary systems. The haplotypes are then combined into a diplotype call for each individual and compared to the reference lab call. If any discrepancy is found, an error is created and a report cannot be generated without further manual review.
The reporting framework is based on pre-defined templates that can be combined computationally to generate a final clinical report; thus, a small number of templates can result in a large number of unique reports. Templates include report header, patient information (laboratory accession number, medical record number, patient name, specimen type), test ordered, genotype reported, interpretation, gene-specific information, general information, test methods, regulatory information, and references. Within each template, dynamic variables are used as placeholders that are filled in with the appropriate information in real-time when generating the report. Variables include genes, haplotypes, diplotypes, phenotypes, and some special case results where additional information is required. The language in all the report templates was carefully crafted to ensure proper grammar including pluralization. For the TPMT gene test, three primary report templates were created – wild type (*1/*1), heterozygous variant and homozygous variant – that can handle any of the 29 current diplotype combinations (note that diplotypes *1/*3A and *1/*8 have some unique text and require additional report modification). In the preliminary work done for the Affymetrix DMET Plus output for CYP2C9/VKORC1 warfarin reports, 8 report templates were created to return results for any of the 18 possible genotype combinations (note that the report templates were more complex as compared to TPMT due to variants called in 2 genes). In some cases, there is not enough information available to uniquely identify a haplotype. The software is able to recognize these situations and return the more common haplotype (or diplotype) with an appropriate rationale based on recommendations from the clinical PGx oversight committee. For each patient, a brief provider-friendly summary report is also created that details all of the genotypes reported in an easy to read, color-coded table (ie, yellow for heterozygous variants, red for homozygous variants, green for gain of function variants), and an interpretation highlighting any recommendations.
The CPS has designed daily reports that are automatically emailed to the specially trained PGx pharmacists to alert them when results are posted in the EHR. The alerts have been built to compare identified genetic variant problems in the patient problem list when affected medications are ordered. The choice to use the problem list presented the most flexibility in light of the varied way genetic data is currently entered into the EHR. The pharmacist is responsible for updating the patient problem list with any clinically relevant variants (see Supplementary Materials) within 24 h. Additional reports run weekly alerting the pharmacist to any changes to problem lists – either a change or deletion of a CPS entered (verified) problem, and entry of non-coded problems that look similar in the Systematized Nomenclature of Medicine (SNOMED) terms but are not coded in the CDS rules. This allows the CPS to correct any changes that would adversely affect the triggering of CDS alerts. As can be seen below, the SNOMED codes are often not granular enough to describe the genotype adequately. This challenge is currently overcome with workarounds and requires advocacy with the National Library of Medicine to adopt SNOMED codes that are adequate for clinical PGx use.
Once the problem has been listed in the problem list of the patient’s chart an alert will fire if a prescriber orders a related medication, recommending action to incorporate the genotype information into their prescribing decision. The subsequent actions range from 90% dosing modification as seen with a homozygous variant status *3B/*3C for TPMT and a thiopurine drug to recommending increased monitoring for effect in the case of a CYP2C19 *1/*17 genotype and sertraline.15,16 The language used in the alerts to both prescribers and the pharmacists has been carefully crafted. As pharmacogenetics is only one part of a much larger picture, we refrained from using words such as “should” or “must.” Multiple variables are considered when choosing a drug and dose, and some carry more weight than others. For instance, in the case of warfarin, clinical factors such as diet play a much larger role in INR disparities than does the CYP2C9 variant status.17 By design, the BCH alerts do not dictate exact dosing adjustment recommendations. Other institutions have opted to include the percent increase or decrease in dosage, or even a firm number, in the alert text when applicable. BCH alerts instead refers the recipient of the alert to either consult the CPS or refer to their departmental algorithms as the clinical situations can be significantly different and require dosing alterations on the opposite ends of the spectrum. For example, consider TPMT dose reduction recommendations for two patients, one with Crohn’s disease and one status post a renal transplant, both of whom returned a variant status of *1/*2 (CPIC recommendation decrease dose of the thiopurine by 30–70%).3 In this case, the prescriber for the patient with Crohn’s disease is likely to use the conservative end of the dosing recommendations and decrease the dose of 6-mercaptopurine by 70%, minimizing the chance of leukopenia and initiating a slower taper up to a tolerated dose that also controls the symptoms. Meanwhile the transplant prescriber faced with the possibility of a patient losing a graft, may choose to dose reduce azathioprine only by 30% to insure maximum therapeutic benefit, while monitoring closely for adverse effects. The alerts are only activated on medications that have been determined by the Oversight Committee to be significantly affected by the genotype status in order to reduce alert fatigue. The BCH formulary medications and possible combination drugs that could be prescribed for outpatient use that are currently coded for a CDS alert can be found in Supplementary Materials.
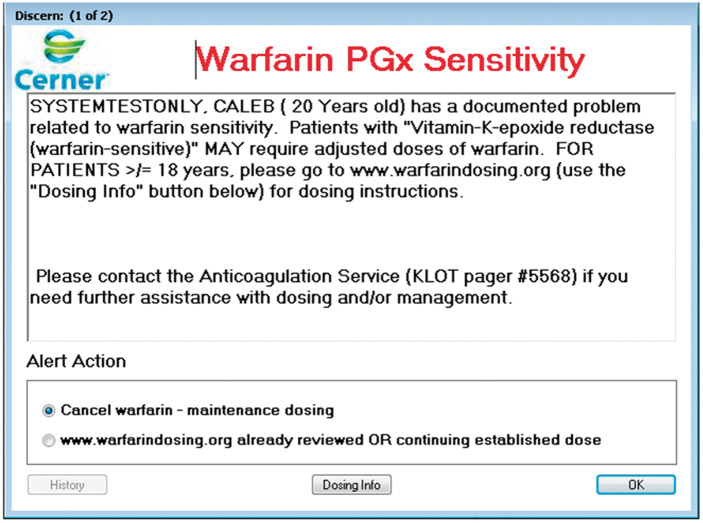
The action a clinician performs when presented with an alert (also referred to as alert behavior) is reviewed on a quarterly basis. An automated report is generated listing the name of the rule, the rule event ID code, the name of the patient, the primary name of the drug (eg, generic name) that triggered the alert, the date and time the alert fired, the clinician name, the clinician position (House Officer, Pharmacist, etc.), the order ID code, the name of the medication as typed into the order (includes drug formulation), the order details including dose, route and frequency, the override default action (did the practitioner override the alert or not), the override reason chosen, and override free text reasons if listed. The override reasons are chosen by the prescriber from a pre-defined list that are essentially the same with some slight modifications based on drug and level of actionability associated with the variant status. For example, in the case of the HLA-B*57:01 and abacavir, the choices are “Cancel abacavir” or “Alert acknowledged AND/OR continuing established therapy.” For the example of a patient with either a CYP2C9 variant and/or a VKORC1 variant combined with warfarin, the override choices are “Cancel warfarin” or “Anticoagulation Service involved AND/OR continuing established dose” for patients under 18 years of age. For patients over the age of 18 years, the choices change to “Cancel warfarin” or www.warfarindosing.org already reviewed OR continuing established dose with an active hyperlink to the website (Figure 1). In the case of TPMT and the thiopurines, the choices are “Cancel order,” “Acknowledge and Override,” and “Modify.” The modify option was added to allow for dose modification without creating an override and the subsequent documentation of a reason (including free text reasons). The Oversight Committee is ultimately responsible for deciding the language used in each alert and solicits input from the clinician stakeholders. The actions are reviewed for consistency of response to ensure that the alerts are not consistently overridden with no action and thus are only contributing to alert fatigue. The doses of the medications are pulled out of the clinical display line and charted against all other dose changes, drug levels, and other lab values of interest to monitor outcome.
Figure 1.
Alert display to a prescriber ordering warfarin for a patient > 18 years old with a relevant VKORC1 variant status.
RESULTS
During the study period that is reflective of the first 2 years of operation (August 2012 to August 2014), 394 patients had TPMT single gene sequencing and 15 patients with Affymetrix DMET Plus testing performed and resulted to the EHR. During the study timeframe, no isolated CYP2C9/VKORC1 assays were performed. HLA testing was not added until 2015. For the TPMT patients we achieved an average turnaround time of 7.9 days and reported a clinically actionable variant incidence of 9.6%, consistent with the literature.15 The Affymetrix DMET Plus assay results are also listed below, however no alerts fired during the study timeframe. The patient demographics are as outlined in Table 1.
Table 1.
TPMT and AffyMetrix DMET Plus assay sequencing demographics
| TPMT | |||||
|---|---|---|---|---|---|
| Patient characteristics | N = 400 patients, 394 results (6 patients had cancelled tests) | ||||
| Gender | Male = 197 | Female = 203 | |||
| Race | Caucasian = 264 | African American = 18 | Asian = 4 | American Indian = 1 | Declined to answer or Other = 113 |
| Ethnicity | White = 177 | Hispanic or Latino = 17 | Declined to answer or Other = 206 | ||
| Age | Average = 13.6 years | Median = 14.6 years | Range = 0.6–25.7 years | ||
| AffyMetrix DMET Plus | |||||
| Patient characteristics | N = 15 patients | ||||
| Gender | Male = 8 | Female = 7 | |||
| Race | Caucasian = 15 | African American = 0 | Asian = 0 | American Indian = 0 | Declined to answer or Other = 0 |
| Ethnicity | American = 7 | Hispanic or Latino = 0 | Declined to answer or Other = 8 | ||
| Age | Average = 23 years | Median = 17 years | Range = 11–58 years | ||
The analysis of the TPMT genotypes is as follows: *1/*1 (n = 356), *1/*3A (n = 26), *1/*3C (n = 7), *1/*2 (n = 2), a rare *1/*8 (n = 1), *3A/*3A (n = 1), and *8/*33 (n = 1), which follow the expected population allele frequencies (Table 2). Initially the infrastructure was designed to support genetic testing for TPMT and thiopurines (azathioprine, thioguanine, and 6-mercaptopurine) using a TaqMan assay. Support for Sanger sequencing was subsequently added (Mutation Surveyor format).
Table 2.
TPMT results and population frequency
| Reported Genotype | Expected Enzyme Activity | BCH Population (%) | General Population (%) |
|---|---|---|---|
| *1/*1 | High/Normal | 90.4 | 89 |
| *1/*3A | Intermediate | 6.6 | 11 |
| *1/*2 | 0.5 | ||
| *1/*3C | 1.8 | ||
| *1/*8 | 0.25 | ||
| *3A/*3A | Low/Deficient | 0.25 | 0.3 |
| *8/*33 | Likely Low/Deficient4 | 0.25 |
When the patient result was a non-wild type genotype, a problem of “TPMT enzyme deficiency” was added to the patient problem list in EHR. For patients with TPMT testing ordered from August 1, 2012 to August 1, 2014, a total of 160 alerts fired for 31 unique patients and 131 unique orders (Table 3). Seven patients who had TPMT deficiency recorded in the problem list subsequently never had an order or prescription written for a thiopurine, thus only 31 of the 38 patients qualified for the alert to fire. The primary practitioners who received the alert were physicians 36 (53%) followed by pharmacists 15 (22%), nursing with physician co-signature 12 (18%), and nurse practitioners 5 (7%). The majority (89%) of orders were written as outpatient prescriptions. Currently the institutional pharmacists do not review prescriptions and therefore only the prescriber would receive the alert and not the pharmacist. Although the average number of alerts per practitioner was 2.3, most received three or less (Table 3).
Table 3.
Alert data and provider actions (8 January 2012 to 8 January 2014)
| Number of Patients with TPMT deficiency added to the problem list | Number of TPMT alerts that fired | Number of patients that the TPMT alert fired | Average number of TPMT alerts firing per patient (for prescribers and pharmacists) | Number of unique practitioners for whom the TPMT alert fired | Average number of TPMT alerts firing per practitioner | Percentage of prescribers who cancelled the order in response to the TPMT alert (%) | Percentage of prescribers who initiated a modified dose after receiving the alert for the initial prescription (does not include subsequent alerts on refills) | Ratio of inpatient orders to outpatient prescriptions |
|---|---|---|---|---|---|---|---|---|
| 38 | 160 | 31 | 5 (range 1–21) | 69 | 2.3 (range 1–18) | 23 | 71% (5/7) | 15:116 |
The Gastroenterology service uses pre-emptive testing as standard of care prior to ordering a thiopurine (97% of gastroenterology patients were tested) and thus were responsible for 317 of the 355 pre-emptive tests ordered. The other pre-emptive tests were ordered by various services including hematology, pulmonary, oncology, renal, and rheumatology. Among these groups, renal and oncology had the highest percentage of reactive testing following an unexplained adverse reaction at 100 and 60%, respectively (see Table 4). In these cases, TPMT testing was ordered in the setting of neutropenia while the patient was actively receiving a thiopurine drug.
Table 4.
Characterization of timing of PGx testing by service (8 January 2012 to 8 January 2014)
| Service | Pre- emptive | Reactive | Simul taneous | Unknown | Cancelled | Total |
|---|---|---|---|---|---|---|
| Allergy | 1 | 1 | ||||
| Ambulatory Transfer Treatment | 1 | 1 | ||||
| Gen Peds | 1 | 1 | ||||
| General Pediatrics | 1 | 1 | ||||
| General Surgery | 2 | 2 | ||||
| GI | 317 | 11 | 4 | 332 | ||
| Hematology | 10 | 1 | 8 | 19 | ||
| Hepatology | 1 | 1 | ||||
| ICP | 2 | 2 | ||||
| Immunology | 2 | 1 | 3 | |||
| Neurology | 1 | 1 | ||||
| Oncology | 2 | 3 | 5 | |||
| Ophthalmology | 1 | 1 | ||||
| Outpatient Lab | 7 | 1 | 8 | |||
| Pulmonary | 4 | 1 | 2 | 7 | ||
| Renal | 3 | 3 | ||||
| Rheumatology | 5 | 2 | 3 | 2 | 12 | |
| Total | 355 | 10 | 26 | 3 | 6 | 400 |
The timing of the TPMT genotyping test order in relation to a prescription for 6-mercaptopurine was analyzed. In 90% (355/394) of the cases, the test was ordered pre-emptively and the drug was not ordered prior to result return in the medical record. In 26 cases, prescriptions for the thiopurine drug were ordered either in conjunction with the ordering of the TPMT test or during the interim period between the ordering of the test and result return. Reactive genotyping was done in 10 cases in light of toxicity manifesting as neutropenia. In 3 cases, the reason for testing was unable to be determined from the EHR and 6 tests were canceled after ordering without a reason specified. Pre-emptive testing alerts that would instruct the prescriber to order testing where feasible prior to starting the medication have been submitted to our informatics department and are under review. As there usually is no need to repeat testing for an individual patient, the ideal behavior for these alerts would look back across all encounters and direct the prescriber to the result when there was a previous test available. There is concern that this will slow system performance to unacceptable levels and requires significant testing.
All patients undergoing PGx screening with the AffyMetrix DMET plus panel were referred for testing due to significant or multiple adverse drug reactions or a history of nonresponse. Actionable variants in the genotypes approved for return to the medical record with CDS were found in 100% of patients and recorded in the problem list of the medical record. For the study timeframe, the CDS alerts were active for TPMT, CYP2C9, VKORC1, and CYP2C19. The CDS rules for CYP2C9 and CYP2C19 were activated in January 2014 and July 2014, respectively. Unfortunately none of the 15 patients were ordered for medications that would be affected by the CYP2C9, VKORC1, or CYP2C19 variants in the timeframe of the study, therefore there is no resultant alert data.
DISCUSSION
The above results demonstrate the utility of TPMT genotypes in clinical practice. In 6 months, the CPS went from planning to production for thiopurine/TPMT testing hospital-wide. Future reports will contain clinical utility data for CYP2C9, CYP2C19, and VKORC1 as well as the HLA markers as they are implemented.
Bringing the knowledge to the bedside necessitates interruptive point of care alerts, ie, the right information to the right provider at the right time. However that must be tempered with the real phenomena of alert fatigue. In order to balance this in our design, the alerts have been built only to fire if there is an action that is recommended to be taken by the provider, such as a dose alteration, a monitoring parameter to follow, or an alternative drug selection. Therefore the wild-type variant statuses do not trigger alerts to the provider. It could be argued that with this model, the provider does not know if a drug has been screened against available genetic data. While that is true, it is far less common for that data to be available in the general population at this point in time. Additionally if a provider attempts to order a duplicate PGx test, the system will fire an alert, displaying the date when the order was last placed and resulted. In the future it is likely the provider will need to know that no genetic data was available for screening as it will come to be expected, similar to allergy/adverse effect screening today. Our automated reporting saves genetic counselor time by calculating diplotypes and assembling report content, while also ensuring consistent and reproducible reporting. Documentation of code versioning allows for historical transparency of changes over time to the context of the report. We expect these methods are generalizable enough that they can be transferred to other EHR systems.
The field is continually evolving and it will become more commonplace for patients to have pre-emptive genetic test results. Therefore it is incumbent upon health care entities to facilitate a process for assigning patient specific pharmacogenetic markers. It is important for hospitals, primary care and specialty practices, and pharmacies to consider other data sources, in addition to their standard lab result and screening fields. For example, we have successfully prototyped linking our report generation to 23andMe’s application program interface (API) and could create a report directly if and when that data is deemed clinically actionable. We recommend that any future tools have the capability to read in data from any Clinical Laboratory Improvement Amendments lab. For this to be acceptable, labs will need to agree on standards for genomic data formatting and transmission.
CONCLUSION
Clinical implementation of PGx data into the medication use cycle poses a significant challenge for healthcare entities. As the field progresses and the knowledge base expands, healthcare providers will be expected to consider PGx implications at the individual patient level. This paper describes the successful implementation and evolution of a PGx service, focusing on EHR integration for both inpatient orders and outpatient prescriptions. This paper also outlines key focus areas to consider when implementing clinical PGx in a healthcare setting. The incorporation of PGx data at the time of prescribing and dispensing, if done correctly, has the potential to impact the incidence of adverse drug events, a significant cause of morbidity and mortality. Avoidance of these events will improve lives and save healthcare dollars.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to acknowledge the staff at St Jude’s Research Hospital, particularly Kris Crews, James Hoffman, and Mary Relling. Additionally we could not have made the improvements to our reporting and overall service without the help of the staff at the Medical College of Wisconsin, particularly Rachel Lorier and Ulrich Broeckel. Thank you to all of the CPS Oversight Committee members and the PGx pharmacists at BCH. A very special thanks to Brenda Dodson who makes the magic behind the scenes happen!
AUTHOR CONTRIBUTIONS
S.M. drafted the initial manuscript. S.M. and L.C. analyzed the data. S.M., L.C., J.H., C.C., C.B., W.W. contributed significant portions of the manuscript. V.F. and K.M. reviewed and revised the manuscript. All authors approved the final manuscript as written.
CONFLICT OF INTEREST/DISCLOSURE
The authors have no potential conflicts of interest or financial relationships relevant to this article to disclose. There was no external funding received.
SUPPLEMENTARY MATERIAL
Supplementary material is available online at http://jamia.oxfordjournals.org/.
REFERENCES
- 1. Bell GC, Crews KR, Wilkinson MR, et al. Development and use of active clinical decision support for preemptive pharmacogenomics. J Am Med Inform Assoc. 2014;21(e1):e93–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffman JM, Haidar CE, Wilkinson MR, et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014;166C(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 2011;89(3):464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: from bench to byte–an update of guidelines. Clin Pharmacol Ther. 2011;89(5):662–673. [DOI] [PubMed] [Google Scholar]
- 5. Swen JJ, Wilting I, de Goede AL, et al. Pharmacogenetics: from bench to byte. Clin Pharmacol Ther. 2008;83(5):781–787. [DOI] [PubMed] [Google Scholar]
- 6. Shuldiner AR, Relling MV, Peterson JF, et al. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: overcoming challenges of real-world implementation. Clin Pharmacol Ther. 2013;94(2): 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin Pharmacol Ther. 2015;98(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caudle KE, Rettie AE, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation C. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Ther. 2014;96(5):542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson JA, Gong L, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011;90(4):625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cannon DS, Allen SN. A comparison of the effects of computer and manual reminders on compliance with a mental health clinical practice guideline. J Am Med Inform Assoc. 2000;7(2):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–1238. [DOI] [PubMed] [Google Scholar]
- 12. Devine EB, Lee CJ, Overby CL, et al. Usability evaluation of pharmacogenomics clinical decision support aids and clinical knowledge resources in a computerized provider order entry system: a mixed methods approach. Int J Med Inform. 2014;83(7):473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Overby CL, Erwin AL, Abul-Husn NS, et al. Physician Attitudes toward Adopting Genome-Guided Prescribing through Clinical Decision Support. J Pers Med. 2014;4(1):35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 2012;92(4): 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones TS, Relling M. Thiopurines. In Pharmacogenomic Testing in Current Clinical Practice. 1st edn. Wu AHB, Yeo KTJ, eds. Humana press; 2011: 91–114. [Google Scholar]
- 16. Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tatarunas V, Lesauskaite V, Veikutiene A, et al. The effect of CYP2C9, VKORC1 and CYP4F2 polymorphism and of clinical factors on warfarin dosage during initiation and long-term treatment after heart valve surgery. J Thromb Thrombolysis. 2014;37(2):177–185. [DOI] [PubMed] [Google Scholar]
- 18. Ford LT, Berg JD. “Thiopurine S-Methyltransferase (TPMT) Assessment Prior to Starting Thiopurine Drug Treatment; a pharmacogenomic test whose time has come. J Clin Pathol. 2010;63(4):288–295. [DOI] [PubMed] [Google Scholar]
- 19. Kornbluth A, Sachar DB, Practice Parameters Committee of the American College of Gastroenterology. Ulcerative Colitis Practice Guidelines in Adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501–523. [DOI] [PubMed] [Google Scholar]
- 20. Sanderson J, Ansari A, Marinaki T, et al. Thiopurine methyltransferase: should it be measured before commencing thiopurine drug therapy? Ann Clin Biochem. 2004;41(Pt 4):294–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.