Abstract
Background:
Monitoring for acute allograft rejection improves outcomes after cardiac transplantation. Endomyocardial biopsy is the gold standard test defining rejection, but carries risk and has limitations. Cardiac magnetic resonance T2 mapping may be able to predict rejection in adults, but has not been studied in children. Our aim was to evaluate T2 mapping in identifying pediatric cardiac transplant patients with acute rejection.
Methods:
Eleven pediatric transplant patients presenting 18 times were prospectively enrolled for non-contrast cardiac magnetic resonance at 1.5T followed by endomyocardial biopsy. Imaging included volumetry, flow and T2 mapping. Regions of interest were manually selected on the T2 maps using the middle-third technique in the left ventricular septal and lateral wall in a short axis and four-chamber slice. Mean and maximum T2 values were compared with Student’s t-tests analysis.
Results:
Five cases of acute rejection were identified in three patients, including 2 cases of grade 2R on biopsy, and 3 cases of negative biopsy treated for clinical symptoms attributed to rejection (new arrhythmia, decreased exercise capacity). A monotonic trend between increasing T2 values and higher biopsy grades was observed: grade OR T2 53.4±3ms, grade 1R T2 54.5ms±3ms, grade 2R T2 61.3±1ms. The 5 rejection cases had significantly higher mean T2 values compared to cases without rejection (58.3±4ms vs 53±2ms, p=0.001).
Conclusions:
Cardiac magnetic resonance with quantitative T2 mapping may offer a non-invasive method for screening pediatric cardiac transplant patients for acute allograft rejection. More data is needed to understand the relationship between T2 and rejection in children.
Keywords: pediatric, cardiac magnetic resonance, T2 mapping, heart transplant, allograft rejection
Introduction:
Pediatric orthotopic heart transplantation is standard of care for end-stage heart failure from congenital heart disease or cardiomyopathy.1 Acute allograft rejection remains the third leading cause of post-transplant mortality.2 The Pediatric Heart Transplant Society database demonstrates that although there has been a decline in the rates of early rejection, the incidence of rejection with hemodynamic compromise or associated mortality has remained unchanged.3 Similarly, despite a decline in rates of late rejection, affected patients continue to be at significant risk for coronary vasculopathy, need for re-transplantation, and mortality.4
Early detection of allograft rejection can alter clinical course and is therefore essential in the care of a heart transplant patient. Periodic endomyocardial biopsy is the current gold standard test for rejection surveillance. However, its utility and diagnostic accuracy have been debated.5,6 Endomyocardial biopsy is a safe procedu but has risk of serious adverse events including cardiac perforation, tricuspid valve injury, and arrhythmias.7,8 Also, endomyocardial biopsy with its blinded sampling solely of the right ventricle carries a chance of false negative results.9 The right ventricle portion sampled is that which is easily reached, and therefore the same endomyocardial portions tend to be sampled. Over repeated catheterizations and years since transplantation, this leads to fibrosis which can additionally compromise diagnostic yield.8 This is especially of concern in pediatric patients who require a lifetime of rejection surveillance.
There is a need for a non-invasive and more accurate method of detecting acute allograft rejection, however echocardiography10,11 and serum biomarkers12 have shown limited correlation. Cardiovascular magnetic resonance offers a diagnostic advantage in its ability to characterize the entire myocardium for evidence of scar or edema using T1 and T2-weighted techniques.9 In addition, quantitative T2 mapping can uniquely assign a number to the degree of myocardial edema. The T2-relaxation time is known to be prolonged in states of increased myocardial edema,13,14 such as in acute allograft rejection.15–17 This has been extensively studied in adult heart transplant patients,9,15,18,19 but to date there is limited data on its application in the pediatric population.
In addition, use of pre-contrast quantitative T1 mapping has been validated as a marker of myocardial fibrosis in children and adults with cardiomyopathy, and can predict heart failure onset and mortality when prospectively applied.20 T1 mapping also correlates with degree of histologic fibrosis in pediatric heart transplant recipients, which may provide prognostic insight into the degree of adverse myocardial remodeling and overall long-term graft health.21
The ability to non-invasively detect and monitor rejection within healthy myocardium of a transplanted heart may be influential in guiding clinical management and counseling. Our aim was to evaluate T2 mapping in identifying episodes of acute allograft rejection in a cohort of pediatric heart transplant patients. Our secondary aim was to compare this to native T1 data in these patients.
Materials and Methods:
With IRB approval and informed consent/assent, 18 encounters of 11 outpatient pediatric heart transplant patients were prospectively and consecutively enrolled from January 2016 to June 2018 at Children’s National Hospital. This cohort underwent a comprehensive, non-contrast cardiac magnetic resonance scan on a 1.5T MR scanner (Aera, Siemens Healthcare, Erlangen, Germany), co-located with an interventional cardiac catheterization suite. The patients were sedated under general anesthesia as was clinically indicated for the cardiac magnetic resonance and MR-guided right heart catheterization and endomyocardial biopsy sampling, standard of care at our institution.
At the completion of cardiac magnetic resonance imaging, patients were transferred through a shared door into the adjoining cardiac catheterization suite, where at least four endomyocardial biopsy samples were obtained from the right ventricular septum. The samples were stored in sterile saline and immediately sent for review in pathology, where tissue processing was performed according to routine clinical standards. Histopathology of endomyocardial biopsy samples was performed according to the International Society for Heart Lung Transplant classification of acute cellular rejection from grade OR to 3R.22 Samples were additionally sent to an outside laboratory and graded by International Society for Heart Lung Transplant nomenclature of acute humoral rejection from pAMR 0 to pAMR 3.23
Patient charts were reviewed for history of prior rejection, defined as any of the following since transplantation: acute cellular rejection with prior endomyocardial biopsy of grade 2R or higher, prior endomyocardial biopsy with acute humoral rejection, or prior treatment for clinical rejection.
Cardiac magnetic resonance imaging:
Cardiac magnetic resonance included cine volumetric analysis using motion-corrected real-time cine imaging to obtain left and right ventricular ejection fractions (standard of care at our institution) and cardiac index.24 Parametric maps of T2 and T1 were generated in one short axis and one four-chamber slice. Table 1 lists the sequence parameters for the T1 and T2 map acquisitions, including two types of MOLLI acquisitions based on heart rate (greater than and less than 90 beats per minute).
Table 1.
Sequence parameters for T2 mapping and T1 mapping using MOLLI technique
| Sequence parameter | T2 | T1 MOLLI HR <90 | T1 MOLLI HR >90 |
|---|---|---|---|
| FOV (mm) | 360 × 270 | 360 × 270 | 360 × 270 |
| Matrix | 256 × 144 | 256 × 144 | 192 ×120 |
| Resolution (mm) | 1.4 × 1.4 | 1.4 × 1.9 | 1.9 × 2.3 |
| Slice thickness (mm) | 8 | 8 | 8 |
| TE (msec) | 1.18 | 1.12 | 1.01 |
| TR (msec) | 2.8 | 2.7 | 2.44 |
| Flip angle (°) | 18 | 35 | 35 |
| Acquisition window (msec) | 830 | 167 | 126 |
| Parallel imaging acceleration | off | 2 | 2 |
| Partial Fourier | off | 7/8 | 7/8 |
CMR sequence parameters for T2 mapping and T1 mapping using MOLLI technique, the latter was adjusted for patient’s baseline heart rate to improve resolution for HR < 90 and HR > 90. HR heart rate FOV field of view, TE echo time, TR repetition time
The MOLLI acquisition sampled the inversion recovery using a 5s (3s) 3s scheme for native T1 contrast during a breath hold. The different T1-weighted images were aligned prior to map creation using a motion-correction algorithm used in several other large studies to minimize through-plane motion.25–27 The T2 map was obtained during a breath hold through use of T2-weighted SSFP images acquired with T2 prep times of 4ms, 25ms, and 55ms.28,29
Parametric map analysis:
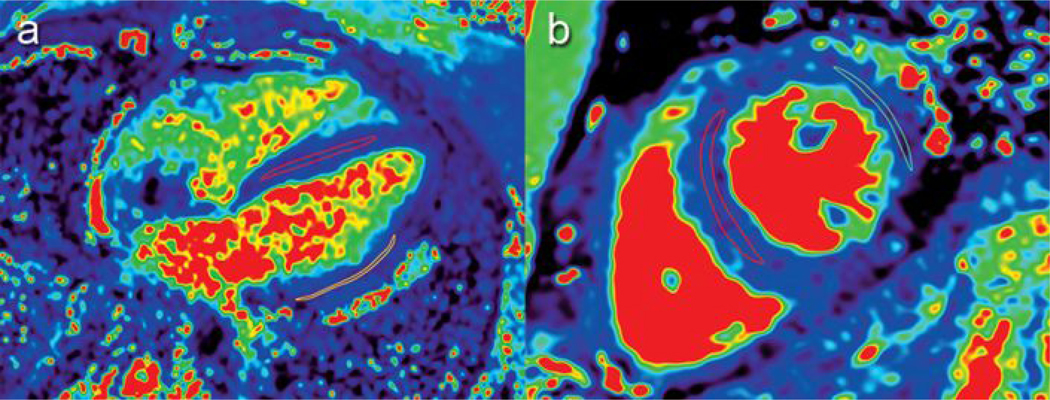
Following cardiac magnetic resonance, parametric maps were de-identified and transferred for analysis (OsiriX, Bernex, Switzerland). Two regions of interest were manually traced by one blinded reviewer onto T1 and T2 parametric maps in the septal and left ventricle lateral wall of a mid-ventricular short axis and four-chamber slice. The “middle-third” technique was used to generate an average, regional pixel value from that parametric map with care to avoid artifacts and blood pool at the endocardial border, consistent with earlier work in our lab.30 A second, more experienced blinded reviewer examined the first reviewer’s regions of interest tracings and made any necessary adjustments, which were usually minimal. Figure 1 demonstrates a native T2 map with the region of interest from a patient in the typical short axis and four-chamber positions. The T1 and T2 mean values were noted for the septal and lateral positions for each short axis and four-chamber slice. Each T1 and T2 mean value in the septal and lateral positions in the short axis and four-chamber slices were averaged to generate an overall mean T1 and T2 value for each study, similar to work from other groups 15,19. T1 reference values are locally maintained per our institution’s lab standard. T2 reference values are derived from prior published work.31–33
Figure 1.
T2 parametric map in the septal and left ventricular lateral wall of a four-chamber (a) and mid-ventricular short axis (b) slice, with representative regions of interest drawn demonstrating the standard “middle-third” technique
Statistical analysis:
All statistical analyses were performed in Stata 14 (StataCorp, USA). For analysis, acute allograft rejection was defined by decision to treat by the clinical team, i.e. an endomyocardial biopsy with acute cellular rejection grade 2R or greater, an endomyocardial biopsy with acute humoral rejection, or presence of clinical symptoms. In order to identify potential differences between cases with and without acute allograft rejection, the two groups were compared using Student’s t-tests. Characteristics analyzed included mean T1 and T2 values, maximum T2 value, and maximum T1 value in the region of maximum T2 value. Exact logistic regression analyses were performed to estimate the odds of acute rejection as a function of each individual covariate. Due to small sample size, multivariable logistic regression analyses or a receiver operator characteristic curve were not performed. For all analyses, a p-value less than alpha of 0.05 was considered statistically significant.
Results:
Study cohort:
The 11 heart transplant patients ranged from 4–17 years old at the time of the study, with nearly equal gender distribution of 6 males with 5 females (Table 2). Cardiac allograft age ranged from 0.6–17.4 years at the time of the study. In 6 patients with operative data available, average total ischemic time during the transplant surgery was 271 ± 93 minutes, bypass time 197 ± 35 minutes, and cross clamp time 64 ± 9 minutes. Two were transplanted for critical congenital heart disease; nine patients were transplanted for primary or secondary cardiomyopathies: restrictive cardiomyopathy (n=1), dilated cardiomyopathy (n=3), hypertrophic cardiomyopathy (n=1), non-compaction cardiomyopathy (n=1), anthracycline-induced cardiomyopathy (n=1), myocarditis (n=1), and pacing-induced dilated cardiomyopathy (n=1).
Table 2.
Demographic table of heart transplant subjects
| Patient | Number of studies performed | Case Number | Initial cardiac diagnosis | Patient age at study (years) | Cardiac allograft age (years) |
|---|---|---|---|---|---|
| 1 | 1 | 1 | Myocarditis | 17 | 6.4 |
| 2 | 2 | 2 and 6 | RCM | 4–5 | 3.1–3.9 |
| 3 | 1 | 4 | Non-compaction cardiomyopathy | 4 | 2.4 |
| 4 | 2 | 3 and 14 | Anthracycline induced DCM | 15–17 | 0.6–2.7 |
| 5 | 3 | 5, 10, and 11 | Familial DCM | 13 | 1.0–1.4 |
| 6 | 1 | 8 | Familial HCM | 5 | 4.5 |
| 7 | 1 | 9 | HLHS | 17 | 17.4 |
| 8 | 1 | 12 | PA/IVS with RVDCC | 15 | 13.9 |
| 9 | 3 | 13, 17, and 18 | DCM | 10–11 | 8.5–9.8 |
| 10 | 2 | 15 and 16 | DCM | 16–17 | 14–14.7 |
| 11 | 1 | 7 | PM induced DCM | 16 | 15.5 |
RCM restrictive cardiomyopathy, DCM dilated cardiomyopathy, HCM hypertrophic cardiomyopathy, HLHS hypoplastic left heart syndrome, PA/IVS pulmonary artery with intact ventricular septum, RVDCC right ventricular dependent coronary circulation, PM pacemaker
All patients were on a stable immunosuppressive regimen, with recent therapeutic tacrolimus or sirolimus levels prior to surveillance studies. Five patients had a prior history of biopsy-proven rejection (ranging from 0.25 – 13.9 years prior to cardiac magnetic resonance-catheterization for this study), with associated ventricular diastolic but not systolic dysfunction evident on cardiac catheterization hemodynamics done at the time of positive endomyocardial biopsy results. There was normalization of rejection-mediated ventricular diastolic function and clinical status in all patients prior to cardiac magnetic resonance-catheterization for this study. There was no patient history of significant non-adherence to immunosuppressive medications.
Clinical course and endomyocardial biopsy results:
All endomyocardial biopsy samples from the 18 encounters were considered adequate and underwent pathology review. There were 3 cases with grade 1R, 2 cases of grade 2R, and none with grade 3R. All but one sample underwent immunofluorescence for acute humoral rejection, and all returned negative or pAMR 0.
Five cases of acute allograft rejection occurred in three patients requiring treatment. Two episodes of rejection involved an asymptomatic patient with endomyocardial biopsy confirming rejection. The remaining three episodes of rejection had clinical symptoms and normal endomyocardial biopsy results. Coronary angiography performed in these three cases on the study date showed no evidence of coronary vasculopathy. Of these three, two were in one patient with two consecutive studies six months apart (cases 15 and 16) with clinical concerns of acute graft dysfunction with baseline tachycardia and reduced maximal oxygen uptake on exercise stress test. The third case of clinical rejection was a patient (case 7) with new ventricular tachycardia. After treatment of clinical rejection with intensified immunosuppression, both patients had resolution of these clinical symptoms.
Cardiac magnetic resonance results:
Table 3 displays the averaged findings of ventricular systolic function on cardiac magnetic resonance, subdivided by cases of rejection. There were no patient limitations to image acquisition and all cardiac magnetic resonance exams were analyzed. There is no statistically significant difference in cardiac magnetic resonance parameters of cardiac systolic function in cases with none compared to cases with histologic or clinical rejection. To note, two cases of clinical rejection did have biventricular systolic dysfunction on cardiac magnetic resonance (discussed later). However, the other rejection cases have normal cardiac systolic function and so there is no statistically significant difference in cardiac magnetic resonance cardiac systolic function between cases with and without rejection as a whole. There is also no significant difference indexed right ventricular end-diastolic volume in cases with none compared to cases with histologic or clinical rejection.
Table 3.
CMR results for cases with and without acute allograft rejection
| CMR Ventricular Function | No Rejection (n = 13) | Rejection (n = 5) | p-value |
|---|---|---|---|
| LV end-diastolic volume, mL/m2 (SD) | 67.0 (13.4) | 62.8 (7.3) | NS, p = 0.53 |
| LV end-systolic volume, mL/m2 (SD) | 29.4 (8.2) | 29.8 (9.8) | NS, p = 0.92 |
| Stroke volume, mL/m2 (SD) | 37.6 (7.0) | 33.2 (6.1) | NS, p = 0.24 |
| Ejection fraction, % (SD) | 56.5 (5.8) | 53.2 (11.5) | NS, p = 0.43 |
| Cardiac index (QAo), L/min/m2 (SD) | 3.2 (0.5) | 2.9 ( 0.6) | NS, p = 0.3 |
| Myocardial mass index, gm/m2 (SD) | 53.2 (9.2) | 49.2 (12.4) | NS, p = 0.47 |
CMR ventricular function results comparing all-cause rejection to no rejection. There are no differences in all CMR parameters between the two groups. CMR cardiac magnetic resonance, LV left ventricle, QAo flow in the ascending aorta, NS not significant
T2 mapping results:
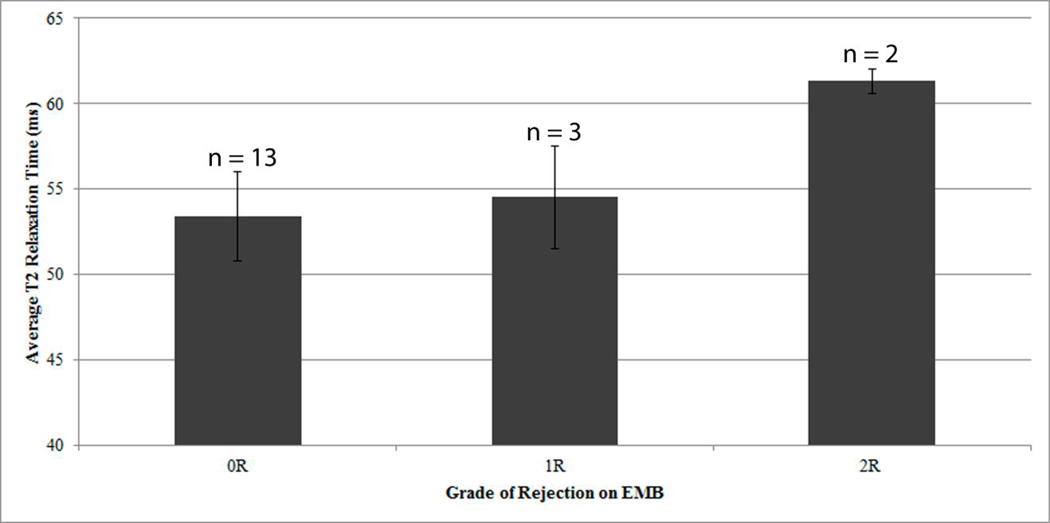
All T2 parametric maps were able to be analyzed. Figure 2 displays the mean T2 value for the three endomyocardial biopsy categories: no rejection, grade 1R, and grade 2R. To note, the two cases of clinical rejection are included in the grade 0R group. There is a notable monotonic trend between increasing T2 values and higher endomyocardial biopsy grades: grade 0R averaged T2 53.4 ± 3ms, grade 1R averaged T2 54.5 ± 3ms, and grade 2R averaged T2 61.3 ± 1ms.
Figure 2.
Average T2 values for Grade 0R, Grade 1R, and Grade 2R biopsy categories. There is a monotonic trend with increasing T2 values and higher grades on EMB (Grade 0R T2 53.4 ± 3ms; Grade 1R T2 54.5 ± 3ms; Grade 2R averaged mean T2 61.3 ± 1ms)
The mean T2 values were evaluated between cases with and without acute allograft rejection, including the 3 clinical rejection cases and the 2 with grade 2R acute cellular rejection (Table 4). There is a statistically significant difference in the mean T2 value, with an average of 58 ± 4ms in the rejection group versus 53 ± 2ms in the non-rejection group (p=0.001).
Table 4.
T2 and T1 mapping results for cases with all-cause rejection compared to no rejection
| CMR Parameter | No rejection (n = 13) | Rejection (n = 5) | p-value |
|---|---|---|---|
| Mean T2, mean (SD) | 53 (2.1) | 58.3 (3.6) | p = 0.001 |
| Maximum T2, mean (SD) | 56.5 (2.5) | 62.3 (3.4) | p = 0.001 |
| No rejection (n = 10) | Rejection (n = 5) | ||
| Mean T1, mean (SD) | 1023.8 (34) | 1055.9 (70.5) | NS, p = 0.25 |
| Maximum T1 at T2, mean (SD) | 1023.7 (47) | 1069.6 (62) | NS, p = 0.13 |
There is a statistically significant difference in the mean and maximum T2 values in cases of all-cause rejection compared to no rejection. There is no difference in the mean T1 or maximum T1 value at the region of the maximum T2 value between the two groups. CMR cardiac magnetic resonance, SD standard deviation NS not significant
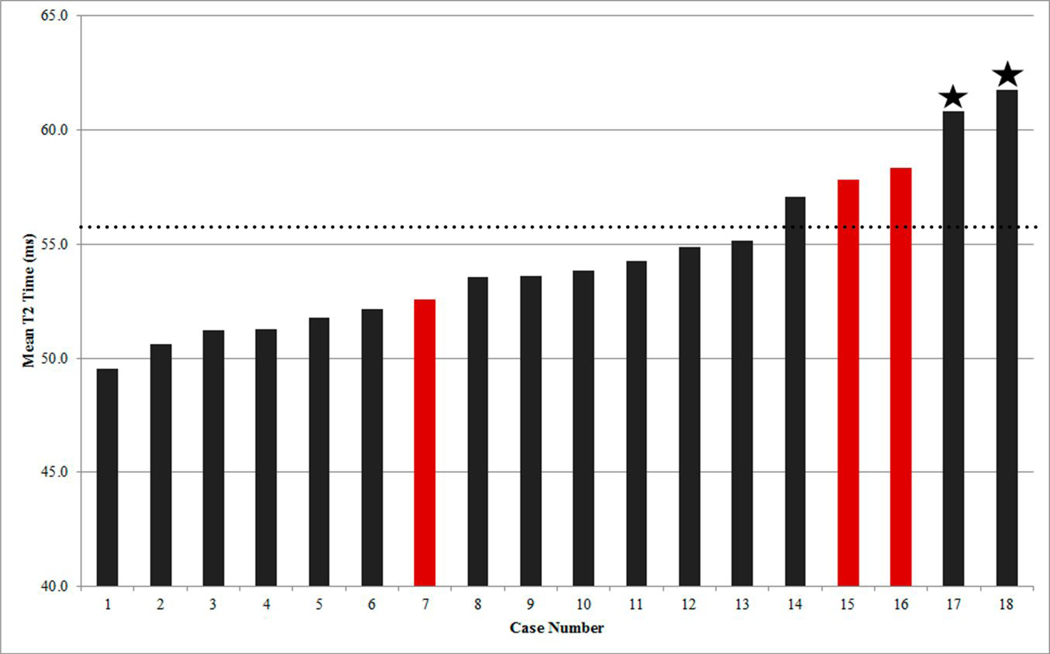
Figure 3 presents the 18 cases in order of ascending mean T2 time, with the cases of acute rejection highlighted. The two asterisked cases of acute cellular rejection both had mean T2 values greater than 60ms. Notably, one patient serially studied accounted for both cases of acute cellular rejection (grade 2R). With treatment for rejection with intensified immunosuppression, the rejection improved (grade 1R) on a repeat encounter 16 months later. Cardiac magnetic resonance at that study case demonstrated a T2 time that had shortened from greater than 60ms to 55ms.
Figure 3.
The 18 studies in sequential order of ascending mean T2 value. The two cases of Grade 2R are asterisked; both with a mean T2 value greater than 60ms. The three clinical rejection cases are highlighted in red; two of the three had higher mean T2 values of 57.8–58.3ms. A dotted line has been drawn at a T2 time of 56 ms; reported to be a cut off to capture treatment warranted rejection episodes based on a receiver operating curve in adult transplant patients15.
Of the three clinical rejection cases with normal endomyocardial biopsy (cases 7, 15, and 16), cases 15 and 16 had higher mean T2 values of 57.8–58.3ms in both the left ventricular septal and lateral walls. Both cases also had biventricular systolic dysfunction on cardiac magnetic resonance (right ventricle ejection fraction 43–45%, left ventricle ejection fraction 42%), raising the possibility of false negative endomyocardial biopsy results. To note, the T2 time of the right ventricular septal or lateral wall was not studied and so, it is uncertain whether the T2 time in the right ventricular apical septum where endomyocardial biopsy is typically obtained was suggestive of rejection or not.
In order to account for regional variability that the calculated mean T2 for each study may average out, the maximum mean T2 value for each study among the four slice positions was also evaluated (Table 4). The maximum T2 time in cases with all-cause rejection is significantly different than in non-rejection cases (62.3 ±3 ms vs 56.5 ± 3ms, p=0.001).
Following exact logistic regression analysis, both mean and maximum T2 values are significantly associated with an episode of acute allograft rejection. The odds of acute rejection are 1.85 and 1.83 times greater for each 1-unit increase in mean and maximum T2 values respectively (p=0.004 and 0.002, respectively).
T1 mapping results:
Fifteen of the 18 cases also had T1 mapping performed (Table 4). All T1 parametric maps were able to be analyzed. There is no significant difference in the mean T1 in cases with and without rejection, 1056 ± 70.5ms vs 1024 ± 34ms (p=0.247). The T1 value in the region of maximum T2 value in the two groups is also not significantly different, 1069.6 ± 62ms vs 1023.7 ± 47ms (p=0.13). Using exact logistic regression, the mean T1 values and T1 values at the area of maximum T2 are not significantly associated with acute rejection (p=0.248 and 0.136, respectively).
Studies in cases with a history of prior rejection (n=8) had a significantly higher mean T1 than cases with no prior rejection (n=7), 1063±42ms compared to 1002±35ms (p=0.009). In contrast, there is no significant difference in mean T2 value between cases with a history of prior rejection (56.2 ± 4.3ms) vs cases with no prior rejection (53.8 ± 1.7ms), p=0.196. Notably, two of the five patients with a history of prior biopsy-proven rejection were in acute rejection at the time of the study and did have prolonged T2 times. However, the other three patients with a history of biopsy-proven rejection were not in rejection at the time of the study and had normal T2 times. This suggests that the prolonged T2 time in those two patients is reflective of the acute rejection episode at the time of the study rather than related to the history of prior biopsy-proven rejection episodes.
Finally, there is no statistically significant correlation between T1 or T2 values and graft age (T1 correlation coefficient −0.18, p valve 0.5; T2 correlation coefficient 0.34, p 0.17).
Discussion:
This study is the first to feature the application of cardiac magnetic resonance based quantitative T2 mapping in describing acute cellular rejection in routine surveillance of pediatric heart transplant recipients. Additionally, this study is the first to present data comparing cardiac magnetic resonance T2 mapping and endomyocardial biopsy done sequentially on the same study day. In our cohort, there is a clear monotonic trend with prolongation of the T2-relaxation time with higher endomyocardial biopsy grades. Furthermore, the T2 values in cases of histologic or clinical rejection were statistically significantly higher than the T2 values in non-rejection cases. Notably, the cardiac systolic function by cardiac magnetic resonance and echocardiography in cases of biopsy-proven rejection was within normal range, suggesting that T2 mapping can identify rejection before late findings, such as significant cardiac systolic dysfunction develops.
Three cases of clinical rejection with normal endomyocardial biopsy are included in this study, and two had prolongation of the mean and maximum T2 times compared to the rest of the cohort. Interestingly, those two cases of clinical rejection also had biventricular systolic depression on cardiac magnetic resonance in the absence of coronary arteriopathy on angiography, which also raised concern for acute cellular rejection. This suggested that the negative endomyocardial biopsy results for those two cases may have been falsely negative. These findings indicate that quantitative T2 myocardial imaging may add value to the endomyocardial biopsy in the detection of acute allograft rejection.
T2 time in pediatric heart transplant patients appear to rise similarly with acute rejection as in adult patients. In a recent prospective study of adult heart transplant patients, there was a significant rise in T2 time in cases of all-cause rejection. In addition, the prolonged T2 values returned to baseline after the episode of acute allograft rejection resolved with intensified immunosuppression. Based on a generated receiver operating characteristic curve, a cut off T2 of 56ms was proposed to maximize sensitivity and specificity in capturing true rejection cases that warrant treatment.15 This is consistent with findings in our pediatric cohort, in which a threshold T2 mean of 56ms would have detected both cases of biopsy-proven rejection as well as the two cases of clinical rejection with suspected false-negative biopsies. One case with clinical rejection would have been missed and one non-rejecting case would have been falsely positive.
To date, there is little research on the use of cardiac magnetic resonance T2 quantitative imaging in pediatric patients despite its well established presence in adult heart transplant literature. An initial pediatric study did not demonstrate a difference in the T2 in significant allograft rejection. However, it was limited in that the ratio of myocardial to skeletal muscle T2 signal intensities was studied in a single short axis slice.34 This does not comprehensively account for the myocardial tissue relaxation properties over a period of time. In contrast, the quantitative T2-mapping technique used in our study provides a more extensive and objective assessment of the degree of myocardial edema.28 Thus, our ability to detect allograft rejection was augmented and more successful.
In our cohort, native T1 values were not significantly different in cases with clinical or histologic acute allograft rejection, which differs from recent adult literature where native T1 was found to be increased in rejection cases.20 Classically, an increase in native T1 value has been associated with myocardial fibrosis, though it is nonspecific to disease state.35 In the cardiac transplant population, T1 values may be reflective of nonspecific graft fibrosis, as native T1 values have been shown to be higher in transplant recipients compared to healthy controls.5 We identified a statistically significant difference in mean T1 value in cases with a history of prior rejection. We hypothesize that these prior episodes of rejection result in increased myocardial fibrosis, which manifests as increased native T1 values. T1 times in the right ventricular septal wall were not studied to differentiate between repeated endomyocardial biopsy induced fibrosis.
In contrast to the Butler et al paper9, there were no significant differences in the indexed right ventricular end diastolic volume between the group with and without rejection, making it unlikely that this parameter would add value to the T2 relaxation time in predicting clinical rejection in our cohort, however our sample size is small, and our data are in growing children and right ventricular size is known to be a function of age, body surface area and gender and thus may not be the ideal parameter to use. More data are needed to fully understand the value of right ventricular size in predicting transplant rejection in children.
Limitations of this work include typical limitations of cardiac magnetic resonance techniques in children with smaller hearts and faster heart rates, which require more spatial and temporal resolution and introduces the possibility of inconsistent results at high heart rates, although we attempted to account for this with use of a smaller matrix size for faster heart rates. There has been a recent interest in T1 values to predict rejection; however our sample size was too small to perform a statistical test of the interaction between T1 and T2. As cardiac magnetic resonance imaging with contrast was not clinically indicated for our patient population, we did not want to introduce contrast for research purposes alone and so data such as the presence late gadolinium enhancement or extracellular volume quantification are not available for analysis. The small sample size and relatively low rate of high-grade rejection also limits results.
In addition, serially studying a patient with rejection has the potential for introduction of bias. However, the number of rejection episodes amongst the cohort and the fact that individuals had recurrent rejection is typical for a pediatric heart transplant practice and thus unavoidable. Because our study aimed to investigate the utility of T2 values as a possible biomarker for rejection, we considered each patient encounter as an independent study case regardless of the patient’s prior history of rejection. There is a large body of evidence, both animal and human studies, demonstrating the association of graft rejection with an increase of T2 time from baseline, suggesting that T2 reproducibly increases in response to acute graft rejection9,15,19,36–38. In addition, in sequential studies the prolonged T2 time returns to baseline after the episode of rejection resolves. In our cohort, one patient had two episodes of histologic rejection (grade 2R) studied during which the T2 was prolonged to greater than 60ms. With intensified immunosuppression, the rejection improved (grade 1R) on a repeat encounter. The T2 at that subsequent encounter shortened to 55ms. Thus, the prolonged T2 time in our cases of rejection is likely more a reflection of graft rejection rather than intrinsic myocardial characteristics of the patients.
Finally, this pilot study obtained standard, limited imaging planes of the heart for T2 maps and was able to detect 4 of the 5 cases of rejection in limited views. It remains to be seen if more comprehensive imaging of the entire heart with T2 mapping could detect clinically significant rejection.
A larger multi-institutional trial is needed to obtain a larger sample size to better study the sensitivity and specificity of T2 mapping in detecting acute allograft rejection. A multi-institutional trial with standardized cardiac magnetic resonance pulse sequences will also enhance our understanding of the range in T2 time in normal control subjects as well as the variations in T2 time of heart transplant patients with and without rejection. In addition, there is a need to longitudinally study a large pediatric sample size to assess whether a prolonged T2 time reproducibly returns to baseline once a rejection episode resolves with intensified immunosuppression. Furthermore, collecting longitudinal data in a larger sample size will enable exploration of other potential cardiac magnetic resonance markers of graft rejection such as the rate of rise of T2 time above baseline in a patient as opposed to the absolute T2 time in itself. In conclusion, this study is the first to demonstrate the novel use of cardiac magnetic resonance with quantitative T2 mapping as a non-invasive method in the surveillance of pediatric cardiac transplant patients for acute allograft rejection. A larger multi-institutional investigation is needed for further validation, but this early data suggests that T2 mapping is a promising imaging biomarker for monitoring myocardial edema related to acute allograft rejection. Secondarily, we found higher native T1 values in patients with a history of allograft rejection, indicating that T1 mapping may be a noninvasive imaging biomarker for myocardial fibrosis and perhaps overall graft health.
Acknowledgments
Financial support: This work was supported by the National Heart, Lung, and Blood Institute (contract number NHLBI-CSB-(HL)-2014–013-JML).
Footnotes
Conflicts of Interest: None.
Compliance with Ethical Standards:
Ethical approval and informed consent:
The authors assert that all procedures contributing to this work comply with the ethical standards with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional review committee at Children’s National Hospital. Informed consent: Informed consent/assent was obtained from all individual participants included in the study.
References:
- 1.Thrush PT, Hoffman TM. Pediatric heart transplantation-indications and outcomes in the current era. J Thorac Dis. 2014;6(8):1080–1096. doi: 10.3978/j.issn.2072-1439.2014.06.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dipchand AI, Rossano JW, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Eighteenth Official Pediatric Heart Transplantation Report - 2015; Focus Theme: Early Graft Failure. J Hear Lung Transplant. 2015;34(10):1233–1243. [DOI] [PubMed] [Google Scholar]
- 3.Gossett JG, Canter CE, Zheng J, et al. Decline in rejection in the first year after pediatric cardiac transplantation: A multi-institutional study. J Hear Lung Transplant. 2010;29(6):625–632. doi: 10.1016/j.healun.2009.12.009 [DOI] [PubMed] [Google Scholar]
- 4.Ameduri RK, Zheng J, Schechtman KB, et al. Has late rejection decreased in pediatric heart transplantation in the current era? A multi-institutional study. J Hear Lung Transplant. 2012;31(9):980–986. doi: 10.1016/j.healun.2012.05.016 [DOI] [PubMed] [Google Scholar]
- 5.Vermes E, Pantaleon C, Ucheux J, Aupart M, Cazeneuve N, Brunereau L. Cardiac Magnetic Resonance in heart transplant patients: Diagnostic value of quantitative tissue markers (T2 mapping and ECV) for acute cardiac rejection diagnosis. JCardiovascMagn Reson. 2016;18:1–12. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L72182792%5Cnhttp://rug.on.worldcat.org/atoztitles/link/?sid=EMBASE&issn=10976647&id=doi:&atitle=Cardiac+Magnetic+Resonance+in+heart+transplant+patients%3A+Diagnostic+value+of+quanti. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel J, Kittleson M, Rafiei M, et al. The natural history of biopsy negative rejection after heart transplantation. J Hear Lung Transplant. 2012;1):S235. doi: 10.1155/2013/236720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Entrez A, Version T. Complications of endomyocardial biopsy in children. 2005; 10588231(7):1–2. [Google Scholar]
- 8.Daly KP, Marshall AC, Vincent JA, et al. Endomyocardial biopsy and selective coronary angiography are low-risk procedures in pediatric heart transplant recipients: Results of a multicenter experience. J Hear Lung Transplant. 2012;31(4):398–409. doi: 10.1016/j.healun.2011.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler CR, Savu A, Bakal JA, et al. Correlation of cardiovascular magnetic resonance imaging findings and endomyocardial biopsy results in patients undergoing screening for heart transplant rejection. J Hear Lung Transplant Off Publ Int Soc Hear Transplant. 2015;34(5):643–650. doi: 10.1016/j.healun.2014.12.020 [DOI] [PubMed] [Google Scholar]
- 10.Thorn EM, de Filippi CR. Echocardiography in the Cardiac Transplant Recipient. Heart Fail Clin. 2007;3(1):51–67. doi: 10.1016/j.hfc.2007.02.008 [DOI] [PubMed] [Google Scholar]
- 11.Kindel SJ, Hsu HH, Hussain T, Johnson JN, McMahon CJ, Kutty S. Multimodality Noninvasive Imaging in the Monitoring of Pediatric Heart Transplantation. J Am Soc Echocardiogr. 2017;30(9):859–870. doi: 10.1016/j.echo.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Labarrere CA, Jaeger BR. Biomarkers of heart transplant rejection: The good, the bad, and the ugly! Transl Res. 2012;159(4):238–251. doi: 10.1016/j.trsl.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 13.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57(5):891–897. doi: 10.1002/mrm.21215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornicelli MD, Rigsby CK, Rychlik K, Pahl E, Robinson JD. Diagnostic performance of cardiovascular magnetic resonance native T1 and T2 mapping in pediatric patients with acute myocarditis. 2019;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Usman AA, Taimen K, Wasielewski M, et al. Cardiac magnetic resonance T2 mapping in the monitoring and follow-up of acute cardiac transplant rejection: A pilot study. Circ Cardiovasc Imaging. 2012;5(6):782–790. doi: 10.1161/CIRCIMAGING.111.971101 [DOI] [PubMed] [Google Scholar]
- 16.Vermes E, Pantaleaon C, Pucheux J, Mirza A, Delhommais A, Sirinelli A. Diagnostic Value of Quantitative Tissue Markers (T2 Mapping and ECV) for Acute Cardiac Rejection Diagnosis: A Preliminary Experience. J Hear Lung Transplant. 2016;35(4):S193. [Google Scholar]
- 17.Butler CR, Thompson R, Haykowsky M, Toma M, Paterson I. Cardiovascular magnetic resonance in the diagnosis of acute heart transplant rejection: a review. J Cardiovasc Magn Reson. 2009;11(1):7. doi: 10.1186/1532-429X-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnemains L, Villemin T, Escanye J, et al. Diagnostic and prognostic value of MRI T2 quantification in heart transplant patients. 2005:69–76. doi: 10.1111/tri.12222 [DOI] [PubMed] [Google Scholar]
- 19.Markl M D PH. Multiparametric Cardiac Magnetic Resonance Imaging Can Detect Acute Cardiac Allograft Rejection After Heart Transplantation. 2019:1–10. doi: 10.1016/jjcmg.2019.01.026 [DOI] [Google Scholar]
- 20.Imran M, Wang L, McCrohon J, et al. Native T1 Mapping in the Diagnosis of Cardiac Allograft Rejection. JACC Cardiovasc Imaging. 2019:2864. doi: 10.1016/j.jcmg.2018.10.027 [DOI] [PubMed] [Google Scholar]
- 21.Ide S, Riesenkampff E, Chiasson DA, et al. Histological validation of cardiovascular magnetic resonance T1 mapping markers of myocardial fibrosis in paediatric heart transplant recipients. J Cardiovasc Magn Reson. 2017:1–11. doi: 10.1186/s12968-017-0326-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Lung Rejection. J Hear Lung Transplant. 2007;26(12):1229–1242. doi: 10.1016/j.healun.2007.10.017 [DOI] [PubMed] [Google Scholar]
- 23.Berry GJ, Burke MM, Andersen C, et al. The 2013 international society for heart and lung transplantation working formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Hear Lung Transplant. 2013;32(12):1147–1162. doi: 10.1016/j.healun.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 24.Cross R, Olivieri L, O’Brien K, Kellman P, Xue H, Hansen M. Improved workflow for quantification of left ventricular volumes and mass using free-breathing motion corrected cine imaging. J Cardiovasc Magn Reson. 2016;18(1):1–12. doi: 10.1186/s12968-016-0231-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellman P, Hansen MS. T1-mapping in the heart: Accuracy and precision. J Cardiovasc Magn Reson. 2014;16(1): 1–20. doi: 10.1186/1532-429X-16-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messroghli DR, Arai AE, Neubauer S. Cardiovascular Magnetic Resonance ( SCMR ) and CMR Working Group of the Journal of Cardiovascular Magnetic Resonance. 2013. doi: 10.1186/1532-429X-15-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1 , T2 , T2 * and extracellular volume : A consensus statement by the Society for Cardiovascular Magnetic Resonance ( SCMR ) endorsed by the European Association for Cardiovascular. 2017:1–25. doi: 10.1186/s12968-017-0389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammer-Hansen S, Ugander M, Hsu L-Y, et al. Distinction of salvaged and infarcted myocardium within the ischaemic area-at-risk with T2 mapping. Eur Hear J-Cardiovasc Imaging. 2014;15(9):1048–1053. doi: 10.1093/ehjci/jeu073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giri S, Chung Y-C, Merchant A, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11(1):56. doi: 10.1186/1532-429X-11-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olivieri LJ, Kellman P, McCarter RJ, Cross RR, Hansen MS, Spurney CF. Native T1 values identify myocardial changes and stratify disease severity in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson. 2017;18(1):72. doi: 10.1186/s12968-016-0292-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagio T, Huang C, Abidov A, et al. T2 mapping of the heart with a double-inversion radial fast spin-echo method with indirect echo compensation. J Cardiovasc Magn Reson. 2015;17:24. doi: 10.1186/s12968-015-0108-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparrow P, Amirabadi A, Sussman MS, Paul N, Merchant N. Quantitative assessment of myocardial T2 relaxation times in cardiac amyloidosis. J Magn Reson Imaging. 2009;30(5):942–946. doi: 10.1002/jmri.21918 [DOI] [PubMed] [Google Scholar]
- 33.Blume U, Lockie T, Stehning C, et al. Interleaved T(1) and T(2) relaxation time mapping for cardiac applications. J Magn Reson Imaging. 2009;29(2):480–487. doi: 10.1002/jmri.21652 [DOI] [PubMed] [Google Scholar]
- 34.Greenway SC, Dallaire F, Kantor PF, et al. Magnetic resonance imaging of the transplanted pediatric heart as a potential predictor of rejection. World J Transplant. 2016;6(4):751. doi: 10.5500/wjt.v6.i4.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2 and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imagin. J Cardiovasc Magn Reson. 2017;19(1): 1–3. doi: 10.1186/s12968-017-0389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aheme T, Tscholakoff D, Finkbeiner W, et al. Magnetic resonance imaging of cardiac transplants: the evaluation of rejection of cardiac allografts with and without immunosuppression. Circulation. 1986;74(1):145–156. http://www.ncbi.nlm.nih.gov/pubmed/3518982. [DOI] [PubMed] [Google Scholar]
- 37.Aherne TYETDGGHCEP. Diagnosis of acute and chronic cardiac rejection by magnetic resonance imaging: a non-invasive in-vivo study. J Cardiovacular Surg. 1988;29(5):587–590. [PubMed] [Google Scholar]
- 38.Tscholakoff D Magnetic resonance tomography of the heart. Experimental study of a non-invasive characterization of myocardial tissue. Rofo. 1987;146(1):82–88. [DOI] [PubMed] [Google Scholar]