Abstract
BACKGROUND:
Disrupted proactive cognitive control, a form of early selection and active goal maintenance, is hypothesized to underlie the broad cognitive deficits observed in patients with schizophrenia (SPs). Current research suggests that the disrupted activation within and connectivity between regions of the cognitive control network contribute to disrupted proactive cognitive control; however, no study has examined these mechanisms using an AX Continuous Performance Test task in schizophrenia.
METHODS:
Twenty-six SPs (17 male subjects; mean age 34.46 ± 8.77 years) and 28 healthy control participants (HCs; 16 male subjects; mean age 31.43 ± 7.23 years) underwent an electroencephalogram while performing the AX Continuous Performance Test. To examine the extent of activation and level of connectivity within the cognitive control network, power, intertrial phase clustering, and intersite phase clustering metrics were calculated and analyzed.
RESULTS:
SPs exhibited expected general decrements in behavioral performance relative to HCs and a more selective deficit in conditions requiring proactive cognitive control. Additionally, SPs exhibited deficits in midline theta power and connectivity during proactive cognitive control trials. Specifically, HCs exhibited significantly greater theta power for B cues relative to A cues, whereas SPs exhibited no significant differences between A- and B-cue theta power. Additionally, differential theta connectivity patterns were observed in SPs and HCs. Behavioral measures of proactive cognitive control predicted functional outcomes in SPs.
CONCLUSIONS:
This study suggests that low-frequency midline theta activity is selectively disrupted during proactive cognitive control in SPs. The disrupted midline theta activity may reflect a failure of SPs to proactively recruit cognitive control processes.
Keywords: AX-CPT, Electroencephalography, Functional outcomes, Proactive cognitive control, Schizophrenia, Theta
Impaired cognitive function is linked to decreased objective quality of life (1) and daily functioning in schizophrenia (2,3). Disrupted proactive cognitive control is hypothesized to underlie the broad cognitive deficits observed in patients with schizophrenia (SPs). Therefore, understanding the mechanisms contributing to this proactive cognitive control impairment is an important step toward developing targeted treatments for the cognitive dysfunction observed in schizophrenia.
Cognitive control is defined as a set of processes required to pursue a goal, which includes context representation and maintenance, especially when distraction and/or strong competing responses must be overcome (4,5). Proactive cognitive control is a form of early selection in which goal-relevant information is actively maintained, requiring sustained activity of the lateral prefrontal cortex. The functioning of the dorsolateral prefrontal cortex (DLPFC) is thought to be a key component of proactive cognitive control (6,7), which is involved in the early, sustained maintenance of context information. This ability and corresponding DLPFC activation is consistently impaired in schizophrenia as evidenced by a reduction in blood oxygen level–dependent signal (4,8) and gamma activity (9), in addition to disrupted connectivity between the DLPFC and broader cognitive control network (CCN) (6), during cognitive control. It is unclear, however, whether the deficits in DLPFC functioning reflect a local failure (e.g., DLPFC fails to respond to input) or, rather, a failure of a preceding signal that would indicate the need for proactive cognitive control.
Electrophysiological studies have suggested that midline theta power reflects the operations of a conflict-and-control mechanism (10), and that theta connectivity facilitates both proactive and reactive cognitive control processes (11). The cerebral generators of midline theta power are currently actively investigated and may reflect activity from several brain regions (12–14); however, increasing evidence highlights the midcingulate cortex (MCC) as a key generator, including electroencephalography (EEG) source estimation (15,16), EEG-informed functional magnetic resonance imaging (17–20), magnetoencephalography (21), and invasive recordings in humans and monkeys (13,22–26). MCC is consistently implicated in the demand for control, either proactively or reactively, with varied theoretical accounts of its computational function (5,27,28). Given the roles of MCC and midline theta, the current study aimed to examine whether a deficit in proactive cognitive control was associated with disrupted midline theta activation.
In addition to theta activity, prefrontal cortex–related gamma activity is disrupted in SPs during working memory and cognitive control (29), particularly in the DLPFC. This failure to maintain oscillatory activity may form the basis for deficits in top-down support to task-relevant circuits across the brain (30), an effect that is also present in persons experiencing a first episode of schizophrenia (31). Notably, in healthy control participants (HCs), higher frequencies (gamma) establish synchronization in local cortical networks (32,33), whereas lower frequencies (such as theta) establish synchronization over longer distances (34) and modulate power in the gamma spectrum (35). Thus, the disruption in gamma oscillations in SPs, particularly in the lateral frontal region, may be related to alterations in low-frequency activity, such as midline theta (36). There is emerging evidence that not only is theta disrupted during cognitive function in SPs (37,38), but the coupling between theta and gamma is also impaired (39), suggesting that SPs exhibit deficits in theta activity as well as the coordination of theta and gamma signaling.
While recent research highlights that theta-band dysfunction represents a key deficit of schizophrenia (40), to date no study has comprehensively examined theta activity during proactive cognitive control using the AX Continuous Performance Test (AX-CPT) in SPs. The central hypothesis of the current study is that deficits in proactive cognitive control result from aberrant theta and gamma power and phase clustering within and between regions within the CCN. These deficits, in turn, affect everyday functioning.
METHODS AND MATERIALS
Participants and Assessment Measures
SPs were recruited from the University of New Mexico Psychiatric Center. All participants provided informed consent according to institutional guidelines at the University of New Mexico. Refer to Supplemental Methods for additional information on inclusion and exclusion criteria and clinical assessments.
Multisensory AX-CPT Task
The Presentation software package (Neurobehavioral Systems, Berkeley, CA) was used for stimulus presentation and recording of behavioral data. Prior to EEG assessment, participants received instructions and completed practice until their performance indicated an understanding of task (see Supplementary Methods for details). Participants monitored a continuous series of visual cues (the letters A, R, V, P, S, and E; duration, 500 ms) and auditory probes (the letters X, Q, F, I, M, and U; duration, 500 ms) and were instructed to respond “yes” when the letter X follows the letter A (Figure 1). The total number of trials collected was as follows: 280 AX trials (70% of the total trials) and 40 of each of the remaining trial types (AY, BX, BY; each 10% of the total trials). A visual cue was used with an auditory probe, as previous studies indicate that this combination results in maximum cross-modal cueing effects (41). Non-A (hereafter referred to as “B cues”) and non-X (hereafter referred to as “Y probes”) letters were selected to be visually or aurally distinct from their respective counterparts. The interstimulus interval was 3220 ms jittered by 460 ms. The intertrial interval was 4520 ms, again jittered by 460 ms. Figure 1 provides further details of the task. The following contrasts were examined: B versus A cues, AX versus AY probes, and AX versus BX probes. See Supplemental Methods for further details.
Figure 1.
Diagrammatic representation of the multisensory AX Continuous Performance Test and behavioral results. (A) The required correct response (CR) was “yes” (Y) when the letter X followed the letter A (target sequence AX; denoted with an asterisk; 70% of trials) (left panel). The three remaining conditions—AY in right panel (A) and BX and BY in panel (B) —all required a “no” (N) response to both cue and probe and each occurred on 10% of trials. Letter sequences were presented in a pseudorandom order but always maintained the same probability structure within each of the eight runs. The total number of trials collected was as follows: 280 AX trials and 40 of each of the remaining trial types. Panel (B) depicts an exemplar B cue and BX probe trial. The interstimulus interval was 3220 ms jittered by 460 ms. The intertrial interval was 4520 ms, again jittered by 460 ms. Delays were used to decrease temporal expectations and to maximize the proactive demands of the task. Box-and-whisker plots depict (C) reaction time (RT) and (D) accuracy (AC) for all task conditions for healthy control participants (HC) and patients with schizophrenia (SP). The center line of each box plot represents the median RT, with whiskers representing the first value more extreme than 1.5 times the interquartile range.
Behavioral Analyses
AX Continuous Performance Test.
Accuracy (percent correct) and median reaction time (RT) for correct trial data were computed for cues (A and B) and three of the probe trial types (AX, AY, and BX). A series of 2 × 2 (group [SPs vs. HCs] × condition [e.g., A cues vs. B cues]) mixed-measures analyses of variance examined RT and accuracy differences across the following planned contrasts: B versus A cues, AX versus AY probes, and AX versus BX probes.
RT and Functional Outcomes.
The relationship between the three functional outcome measures [University of California–San Diego Performance-Based Skills Assessment–Brief (UPSA-B) (42), Schizophrenia Quality of Life Questionnaire 18 (S-QoL 18) (43), and Specific Levels of Functioning Scale–Informant Report (SLOF-I) (44)] was examined, and correlations indicated that the UPSA-B and S-QoL 18 were relatively independent (r = −.04). Therefore, three separate multiple regressions were conducted using two RT metrics of proactive cognitive control (B–A cue RT and BX–AX RT) as the independent variables and functional outcome measures as the dependent measure accounting for medication (olanzapine equivalent), extrapyramidal symptoms (Abnormal Involuntary Movement Scale, Barnes Akathisia Rating Scale, and Simpson–Angus Scale), and smoking history (Fagerstrom Test for Nicotine Dependence).
Electrophysiological Data Processing
Data were collected on a Biosemi EEG system (Amsterdam, Netherlands) utilizing a 128-electrode EEG cap and a sampling rate of 512 Hz. EEG data were collected in a shielded room with active electrodes, with no observed spectral peaks at 60 Hz. Similar to previous publications (45), the data underwent standard preprocessing steps using MATLAB (MathWorks; The MathWorks, Inc., Natick, MA) and EEGLAB, an opensource toolbox available for EEG signal processing (46). See Supplemental Materials for details. Data were epoched relative to stimulus onset (−2000 to +2000 ms), and artifacts were identified and removed using a combination of automated and manual selection processes. As some time–frequency metrics are heavily biased by the number of trials (47), we randomly sampled from conditions that contained greater that 40 trials to ensure equivalent trial count across trials and across groups. The EEG time series were used to calculate time–frequency measures by multiplying the fast Fourier transformed power spectrum of the single trial data with the fast Fourier transformed power spectrum of a set of complex Morlet wavelets. Wavelets were defined as a Gaussian windowed complex sine wave: where t is time, f is frequency (which increased from 1 to 80 Hz in 50 logarithmically spaced steps), and s defines the width (or “cycles”) of each frequency band. Scaled cycles were used whereby s = 4 to 7 cycles logarithmically spaced and . Power was extracted from these large windows and converted to decibel (dB) scale (10 × log10[power(t)/power(baseline)]), based on the average precue activity from −300 to −200 ms. As wavelets smear data temporally, we wanted to avoid near-zero time points, as the convolved data (particularly in low frequencies) may contain postevent activities when time approaches zero.
Intertrial phase clustering (ITPC) was calculated to determine the extent of phase coherence within electrodes of interest. Intersite phase clustering (ISPC) was calculated to quantify the extent to which phase angle differences between electrode sites are consistent across trials; these data were again extracted from the large windows (48). Prior to computing ISPC, a surface Laplacian was applied to all EEG epochs using previously published methods (49). See Supplementary Methods for further details.
Electrophysiological Data Analyses
An a priori approach was used to identify time windows, electrodes, and frequencies of interest to examine power and ITPC. As midline activation is heavily implicated in recruiting cognitive control (10), FCz theta (4–7 Hz) and gamma (30–80 Hz) were examined. Additionally, we aimed to identify whether early, sustained gamma activity (indicative of proactive cognitive control) was disrupted in SPs in the lateral prefrontal cortex, and therefore we examined left lateral frontal electrode (F5) and the right lateral frontal electrode (F6) (45). Time–frequency plots averaged across cue and probe conditions, and groups were used to identify the time window of theta activity. For gamma power, an approach similar to that of previous studies was taken (31), whereby a long gamma window was selected (500–1000 ms). As visual and auditory responses differ in their temporal activations [typically visual responses occur ~100 ms later than auditory responses (50)], two separate windows were examined for theta windows: visual cues (250–450 ms) and auditory probes (125–325 ms).
A series of 2 × 2 (group [SPs vs. HCs] × condition [e.g., B vs. A cues]) mixed-measures analyses of variance examined differences in power and ITPC within theta and gamma bands across the following contrasts: B versus A cues, AX versus AY probes, and AX versus BX probes. To examine reliable effects outside our a priori regions of interest, additional analyses were conducted using within-subject t tests on same the comparisons as above, correcting for multiple comparisons via weighted cluster-based thresholding (51). See details in the Supplemental Methods.
ISPC between the frontal midline FCz electrode and either F5 or F6 within the theta and gamma-frequency bands were extracted to examine connectivity between the frontal midline electrode and lateral electrodes during each of the conditions of interest. For each comparison, a 2 × 2 × 2 [group (SPs vs. HCs) × condition (e.g., B vs. A cues) × electrode (e.g., FCz–F5 vs. FCz–F6)] mixed-measures analysis of variance was conducted.
A series of multiple regressions was conducted to examine whether the neural disruption in proactive cognitive control (e.g., B vs. A; BX vs. AX) predicted functional outcomes (UPSA-B total score, S-QoL 18 total score, and SLOF-I total score), accounting for medication effects and/or smoking history and extrapyramidal symptoms.
RESULTS
Demographics and Clinical Data
Thirty-seven clinically stable SPs (outpatients) and 37 age- and sex-matched HCs were enrolled in the current study. A total of 26 SPs (17 male subjects; mean age 34.46 ± 8.77 years) and 28 HCs (16 male subjects; mean age 31.43 ± 7.23 years) were included in the behavioral and EEG analyses. See Supplemental Methods for further details. There were no significant differences in age (p = .17) or sex (p = .73) between the two groups. Significant group differences were observed in education (t59 = 2.36, p = .04) and estimated premorbid intelligence (t59 = 2.27, p = .03), with SPs exhibiting lower education and estimated intelligence levels than HCs, as is commonly observed, which is consistent with the observed disease course (52,53). See Table 1 for remainder of clinical demographics.
Table 1.
Demographics and Clinical Measures
| SP Group (n = 26) | HC Group (n = 28) | p Value | Cohen’s d | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 34.46 (8.77) | 31.43 (7.23) | .174 | 0.38 |
| Sex, male, % | 57 | 65 | .733 | |
| Education level, yearsa | 13.62 (2.77) | 15.18 (2.00) | .022 | 0.65 |
| WTAR, t score | 51.87 (8.94) | 56.81 (6.80) | .028 | 0.63 |
| Clinical Measures | ||||
| Age of symptom onset, years | 21.50 (6.65) | |||
| Illness duration, years | 12.96 (9.06) | |||
| Olanzapine equivalent, mg/d | 14.76 (12.02) | |||
| CGI | 3.42 (0.81) | |||
| AIMS | 0.62 (0.94) | |||
| BAS | 0.31 (0.74) | |||
| SAS | 1.50 (2.69) | |||
| PANSS positive | 12.38 (4.47) | |||
| PANSS negative | 13.92 (5.45) | |||
| PANSS total | 24.38 (6.03) | |||
| FTND | 1.36 (2.60) | |||
| SLOF-I totalb | 30.49 (5.52) | |||
| UPSA-B total | 69.68 (13.69) | 77.21 (9.67) | .025 | 0.64 |
| S-QoL 18 total | 56.07 (13.45) | 67.58 (9.18) | < .001 | 1.01 |
Values are mean (SD) unless otherwise indicated.
AIMS, Abnormal Involuntary Movement Scale; BAS, Barnes Akathisia Scale; CGI, Clinical Global Impressions Scale; FTND, Fagerstrom Test for Nicotine Dependence; HC, healthy control participant; PANSS, Positive and Negative Syndrome Scale; S-QoL 18, Schizophrenia Quality of Life Questionnaire 18; SAS, Simpson–Angus Scale; SLOF-I, Specific Levels of Functioning Scale–Informant Report; SP, person with schizophrenia; UPSA-B, University of California–San Diego Performance-Based Skills Assessment–Brief; WTAR, Wechsler Test of Adult Reading.
Education level was determined based on number of years in school.
Only a subset of SPs (n = 21) had data for the SLOF-I measures.
Behavioral Data
AX Continuous Performance Test.
RT and accuracy data are presented in Figure 1 and Table 2. Examination of accuracy indicated no significant differences (all p values > .10). For RT to cues, significant main effects of group (SPs > HCs; F1,52 = 18.05, p < .001) and cue condition (B > A; F1,52 = 30.27, p < .001) were observed. For RT to AX and AY probes, significant main effects of group (F1,52 = 5.44, p = .02) and condition (F1,52 = 168.00, p < .001) were observed. SPs exhibited slower RTs relative to those of HCs, and RT for AY probes was significantly slower than that for AX probes. Examination of AX and BX probes indicated a significant interaction between group and condition (F1,52 = 8.65, p = .01). Follow-up tests indicated that HCs exhibited no significant difference (p = .17) in RT for AX relative to that for BX trials, whereas SPs exhibited significantly (t25 = 2.60, p = .02) slower RT on BX relative to that for AX trials. In sum, while SPs had globally slower RTs than HCs did, the operational definition of proactive control (BX vs. AX RT) was specifically delayed in SPs compared with HCs, suggesting a deficit in proactive cognitive control.
Table 2.
Behavioral Data
| SP Group (n = 26) | HC Group (n = 28) | |
|---|---|---|
| Accuracy, % (SD) | ||
| A cues | 96 (5) | 98 (3) |
| B cues | 97 (5) | 98 (3) |
| AX targets | 94 (9) | 92 (12) |
| AY targets | 94 (6) | 92 (6) |
| BX targets | 90 (13) | 98 (8) |
| BY targets | 97 (5) | 99 (2) |
| Median RT, ms (SD) | ||
| A cues | 609.43 (173.47) | 434.00 (130.08) |
| B cues | 651.35 (137.38) | 486.68 (157.55) |
| AX targets | 566.46 (183.39) | 487.46 (110.16) |
| AY targets | 771.81 (135.89) | 694.06 (106.91) |
| BX targets | 656.64 (277.16) | 447.96 (179.20) |
| BY targets | 692.54 (202.91) | 518.07 (162.15) |
HCs, healthy control participants; RT, reaction time; SPs, patients with schizophrenia.
RT and Functional Outcomes.
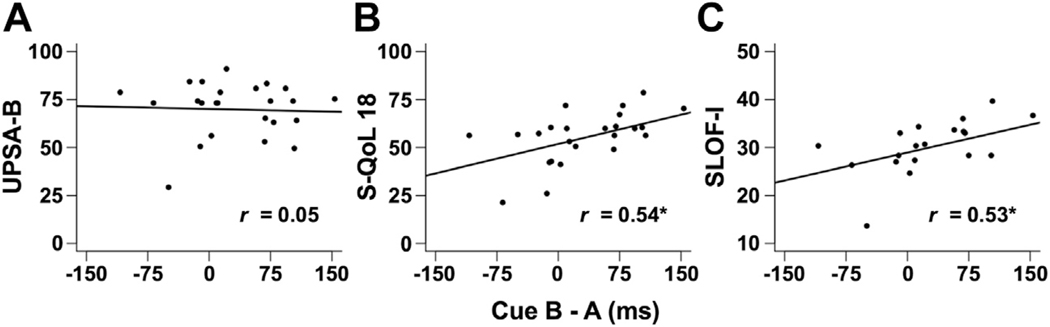
In the SP group, three separate regression analyses were run with each of the functional outcome measures serving as the dependent measure, accounting for medication effects and/or smoking history and extrapyramidal symptoms. Results indicated significant relationships between the difference in cue RT (B–A RT) and both the subjective quality of life total (S-QoL 18; β = .11, t19 = 2.54, p = .02) and informant report of functioning (SLOF-I; β = .06, t15 = 3.38, p = .01; Figure 2).
Figure 2.
AX Continuous Performance Test: Relationships between functional measures and behavioral data in patients with schizophrenia. Scatter plots depicting relationship between (A) University of California–Performance-Based Skills Assessment–Brief (UPSA-B), (B) Schizophrenia Quality of Life Questionnaire 18 (S-QoL 18), and (C) the Specific Levels of Functioning Scale–Informant Report (SLOF-I) and the difference in reaction time between B and A cues on the AX Continuous Performance Test in milliseconds. *Pearson’s correlation coefficients (r) are significant at p < .05.
Electrophysiological Data Analyses
Frontal Midline.
Examination of the theta-band power to cue trials indicated a significant interaction (F1,52 = 4.23, p = .04, ηp2 = .07) between cue conditions and group and a main effect of cue condition (F1,52 = 5.4, p = .02, ηp2 = .10). Follow-up tests indicated that HCs exhibited a significantly greater theta power for B cues (t27 = 3.05, p = .01; mean ± SD, 1.15 ± 1.43 dB, Cohen’s d = 0.56) relative to A cues (0.46 ± 0.98 dB), whereas SPs exhibited no significant differences between A and B-cue theta power (p = .85, Cohen’s d = 0.03) (Figure 3). Comparison of AX and AY probe trials indicated a significant main effect of group (F1,52 = 4.85, p = .03, ηp2 = .09) and condition (F1,52 = 32.97, p < .001, ηp2 = .39), with SPs exhibiting lower theta power (0.59 ± 1.27 dB) relative to HC (1.29 ± 1.07 dB; Cohen’s d = 0.60) and AY conditions exhibiting greater theta power (1.41 ± 1.51 dB) relative to that of AX (0.50 ± 1.17 dB, Cohen’s d = 0.67) across groups. Comparison of AX and BX probe trials indicated a significant main effect of group (F1,52 = 5.52, p = .02; ηp2 = .10), with SPs exhibiting lower theta power (0.59 ± 1.27 dB) than HCs (1.29 ± 1.07 dB, Cohen’s d = 0.60). In addition to the observed theta effects in the region-of-interest analysis, examination of power differences between probe conditions of interest across time and frequency bands, correcting for multiple comparisons, indicated that both groups exhibited significantly decreased beta power (defined as 12.5–30 Hz) for AY relative to AX probes (Figure 4). BX relative to AX showed no significant differences across groups (Figure 4). No significant results were observed in the gamma-frequency band.
Figure 3.
AX Continuous Performance Test: Cue-related time–frequency results at the FCz electrode. Plots depict (A) power across time averaged across cue conditions, with boxes indicating a priori regions of interest used in analyses (top panel) and box plots depicting theta power within regions of interest (bottom panel). (B) Results of examination across all frequencies (top panel) and within theta band (4–7 Hz) (bottom panel). Healthy control participants (HC) demonstrate greater delta and theta power following B cues, whereas patients with schizophrenia (SP) demonstrate minimal activation. Plots of t values (top far right panel) depict the differences between groups. Bold line indicates significant group differences in B cues after controlling for multiple comparisons.
Figure 4.
AX Continuous Performance Test: Probe-related time–frequency results at the FCz electrode. (A) Time–frequency plots in which t values represent the magnitude of the differences between AY and AX conditions (top row) and BX and AX conditions (bottom row). A significant main effect was observed for AY relative to AX in which healthy control participants (HC) and patients with schizophrenia (SP) demonstrate significantly greater theta power in response to AY relative to AX (HC differences are significant after corrections for multiple comparisons, as indicated by the bold outline). Additionally, a significant main effect was observed whereby SPs also demonstrated reduced theta power across conditions. No significant main effect of BX relative to AX was observed. (B) Box plots of theta power across probe conditions.
Examination of frontal midline theta ITPC yielded similar results with the exception that there was no significant interaction between cue conditions and group assignment. See Supplemental Results for details.
Lateral Prefrontal.
Examination of ISPC (phase synchrony) between the midline electrode and lateral frontal electrodes across cue conditions indicated a significant three-way interaction of cue condition by electrode by group (F1,52 = 4.06, p = .04). Follow-up analyses within F5 demonstrated a significant group by cue condition effect (F1,52 = 4.42, p = .04) in which HCs demonstrated a trend-level greater medial and lateral phase synchrony (ISPC) in response to B (0.18 ± 0.06) relative to that of A cues (0.16 ± 0.04; t27 = 1.74, p = .10), whereas SPs demonstrated no differences (t25 = 1.36, p = .19), with a relative greater synchrony (ISPC) for A cues (0.21 ± 0.10) than for B cues (0.19 ± 0.13). Follow-up within F6 indicated no significant effects. There were no significant differences in lateral prefrontal cortex theta power and ITPC and no significant relationships with functional outcomes.
Exploratory Analysis.
Conceptualization of frontal theta as a mechanism of proactive cognitive control suggests that greater midline theta activity during B cues and greater phase coherence between the medial and lateral prefrontal cortices would be larger in individuals with faster BX RTs (i.e., reflections of proactive cognitive control). To formally examine this relationship, a multiple-regression analysis was conducted to predict BX RT via the influence of B–A theta power and B–A FCz–F5 ISPC, with group added as a contrast-coded variable. The difference in B and A cue theta power predicted subsequent BX RT across participants (β = 74.92, t49 = 2.88, p = .006), with no moderating influence of group. The extent of phase coherence (ISPC) did not predict subsequent RT.
DISCUSSION
The current study investigated theta and gamma power and phase clustering within the medial and lateral frontal lobe in SPs. Results provide evidence that midline theta power is significantly disrupted in SPs. Specifically, an interaction was observed whereby SPs failed to demonstrate increased midline theta activity following a 100% predictive cue (B cue), suggesting a failure to recruit proactive cognitive control. Less theta activation during this predictive-cue condition predicted subsequently slower BX RT, suggesting that this failure is related to poor performance on measures of proactive control. Additionally, there was an indication that HCs exhibited coherence between the medial and left lateral frontal lobes for proactive cues, whereas the SPs did not. Notably, the slower the RT for B relative to that for A indicates the extent to which an individual is recruiting proactive control early, predicting better functional outcomes. In addition to the selective impairment in proactive cognitive control, we observed general decrements in theta power and performance, suggesting that reactive cognitive control is also disrupted, but this finding may reflect more generalized cognitive disturbances in SPs.
While much work emphasizes that early, sustained DLPFC function is critically disrupted in SPs (6), the current results suggest that disrupted midline theta may reflect a failure to initiate cognitive control (10) in SPs. While midline theta has historically been examined within highly reactive conditions, such as in response to error detection and correction (45,54) or conflict (55,56), there is increasing evidence of increased midline theta during proactive conditions (57–59), with greater activation reflecting greater proactive control demands, such as an increased need for updating goals (60). To determine whether theta disruption predicted performance, we examined the relationship between midline theta (B–A cue) and subsequent BX probe RT and found that increased theta activation to B relative to A cues predicted better performance (faster BX RT) on behavioral responses that require proactive control (e.g., prepare early). Thus, the reduction in midline theta during B cues suggests that SPs likely fail to update goals, leading to a decrement in subsequent performance.
The results of the current study also suggest that the failure in theta power during proactive conditions corresponds to a failure to exhibit coherence between the medial and left lateral prefrontal cortex in SPs. The brain relies on high-frequency gamma oscillations for local processes and low-frequency bands to coordinate activity across widespread regions (61). Theta serves as the primary communicator across the widespread CCN regions (62). Source localization suggests that midline theta reflects activation of the MCC and supplemental motor area (16,55,63,64), which are consistently implicated in the demand for control (65,66). The MCC is thought to function as a hub for theta phase-synchronized information transfer (67). Further evidence suggests theta coherence is critical to cognitive control, with specialized networks involved in proactive cognitive control (11). Of note, sometimes the signals of need for control occur in a slightly lower spectral range (~3–4 Hz), which we observed when we examined the broader time–frequency plots. This lower frequency response is phenomenologically similar to classic frontal theta-band effects (68), suggesting that although these phenomena are technically delta band, they index a similar neural computation. Given this role of low-frequency power in cognitive control, a failure to initially signal the need for control likely leads to decrements in coherence and communication among regions of the CCN in SPs, consistent with our findings.
Aberrant rhythmic activity is consistently observed in SPs (69,70), along with associated cognitive deficits (29,71). While a wealth of studies has emphasized the role of gamma disruption in SPs, it is increasingly appreciated that theta power (37,38) and theta–gamma coupling are altered as well (39,72). As theta activity modulates gamma (35), our results suggest that altered theta may serve as a more robust signal to detect disrupted activity. Notably, this may partially account for why the current study did not observe gamma deficits, as observing gamma via scalp EEG often requires averaging across many electrodes, as has been done in previous studies (31). Furthermore, gamma is heavily implicated in maintaining working-memory representations (73,74). The task used in our study had low-working-memory load, suggesting that the task may have required less robust gamma engagement. Subtle gamma effects may have been present but undetected in the current study, but robust effects were observed in theta, highlighting the utility of examination of theta in future studies and as a possible outcome measure in future studies.
While the disruption in gamma has been strongly linked to inhibitory/excitatory cortical dynamics, it is less clear whether disrupted theta and low-frequency oscillations occurred because of these same mechanisms. As inhibitory interactions contribute to oscillatory processes (75), some suggest the inhibitory/excitatory disruption in SPs accounts for both (70). Alternatively, long-range glutamatergic projections may underlie low-frequency oscillations, consistent with N-methyl-D-aspartate receptor hypofunction in SPs (76). Future research is necessary to identify what neural mechanisms contribute to theta power. Additionally, we included a broader analysis to determine whether other frequency bands exhibited significant effects to ensure that significant effects were not missed by the region-of-interest approach. Results indicated that both groups exhibited significantly decreased beta power for AY relative to AX probes. The beta effect was not significant for the cue conditions (proactive control conditions) and was observed in the reactive probe conditions across groups (e.g., no group differences). This finding highlights that both groups utilized beta in response to reactive probe conditions.
The functional outcomes examined measured three different aspects of functioning, including a performance-based measure (UPSA-B) (42), a self-report of health-related quality of life (S-QoL 18) (43), and an outside (informant) report of everyday functioning (44). As mentioned, cognitive dysfunction is a core deficit in schizophrenia and is related to objective quality of life (1) and functional outcomes (2,3). Numerous approaches to quantifying cognitive control exist (8,77–79), and the current study highlights that the behavioral measure of proactive control (e.g., B cue–A cue RT) was the best predictor of functional outcomes. While SPs showed a general reduction in their RTs across cues, the extent to which they demonstrated slowed RT for B cues relative to A (suggesting utilization of proactive cognitive control) was significantly related to quality of life and outside report of function.
There are several considerations that should be taken into account regarding these results. Specifically, it is possible that medication also affected time–frequency metrics and thus may have contributed to the observed dysfunction in the current study; however, the observed theta deficit was specific to proactive control conditions and not broadly absent. Future studies with unmedicated participants, who are earlier in the disease course, are necessary to replicate these findings. We also used a hypothesis-driven approach, which limited breadth of spatial and temporal aspects of cognitive control to be examined. Relatedly, we did not attempt to localize the observed effects, limiting the ability to infer where the disruption occurs within the brain. As source localization is challenging and poses several known issues in EEG (80), future studies using magnetoencephalography are necessary to identify the underlying sources of the disrupted oscillatory function we observed.
In conclusion, the current study is the first examination of midline frontal theta during proactive cognitive control processes in SPs. Results indicate that SPs fail to exhibit midline theta activation during a proactive condition, which results in slower RT to a probe that required them to be proactively prepared. In addition, faster RT during the proactive cue condition was related to worse functional outcome, suggesting that this failure to recruit proactive control is linked to impairment within real-world settings. Given these results, midline theta may serve as a fruitful treatment target, particularly using techniques such as transcranial direct-current stimulation and transcranial magnetic stimulation (81–83).
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Institutes of Health (Grant No. 1R01MH101512-03 to ARM). This is the first publication from this data set.
We thank Kim Paulson for his assistance with data collection.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2018.04.021.
REFERENCES
- 1.Tolman AW, Kurtz MM (2012): Neurocognitive predictors of objective and subjective quality of life in individuals with schizophrenia: A meta-analytic investigation. Schizophr Bull 38:304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fett A-KJ, Viechtbauer W, Penn DL, van Os J, Krabbendam L (2011): The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neurosci Biobehav Rev 35:573–588. [DOI] [PubMed] [Google Scholar]
- 3.Nuechterlein KH, Subotnik KL, Green MF, Ventura J, Asarnow RF, Gitlin MJ, et al. (2011): Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr Bull 37:S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesh TA, Niendam TA, Minzenberg MJ, Carter CS (2010): Cognitive control deficits in schizophrenia: Mechanisms and meaning. Neuropsychopharmacology 36:316–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shenhav A, Botvinick MM, Cohen JD (2013): The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 79:217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barch DM, Ceaser A (2012): Cognition in schizophrenia: Core psychological and neural mechanisms. Trends Cogn Sci 16:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barch DM, Sheffield JM (2014): Cognitive impairments in psychotic disorders: Common mechanisms and measurement. World Psychiatry 13:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesh TA, Westphal AJ, Niendam TA, Yoon JH, Minzenberg MJ, Ragland JD, et al. (2013): Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. Neuroimage Clin 2:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C-MA, Stanford AD, Mao X, Abi-Dargham A, Shungu DC, Lisanby SH, et al. (2014): GABA level, gamma oscillation, and working memory performance in schizophrenia. Neuroimage Clin 4:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavanagh JF, Frank MJ (2014): Frontal theta as a mechanism for cognitive control. Trends Cogn Sci 18:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper PS, Wong ASW, Fulham WR, Thienel R, Mansfield E, Michie PT, Karayanidis F (2015): Theta frontoparietal connectivity associated with proactive and reactive cognitive control processes. Neuroimage 108:354–363. [DOI] [PubMed] [Google Scholar]
- 12.Bonini F, Burle B, Liégeois-Chauvel C, Régis J, Chauvel P, Vidal F (2014): Action monitoring and medial frontal cortex: Leading role of supplementary motor area. Science 343:888–891. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J (2008): Medial frontal cortex and response conflict: Evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res 1238:127–142. [DOI] [PubMed] [Google Scholar]
- 14.Emeric EE, Leslie MW, Pouget P, Schall JD (2010): Performance monitoring local field potentials in the medial frontal cortex of primates: Supplementary eye field. J Neurophysiol 104:1523–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Noordt SJR, Segalowitz SJ (2012): Performance monitoring and the medial prefrontal cortex: A review of individual differences and context effects as a window on self-regulation. Front Hum Neurosci 6:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh MM, Anderson JR (2011): Learning from delayed feedback: Neural responses in temporal credit assignment. Cogn Affect Behav Neurosci 11:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker MPI, Nitsch AM, Miltner WHR, Straube T (2014): A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. J Neurosci 34:3005–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debener S, Ullsperger M, Siegel M, Fiehler K, Cramon DY, von, Engel AK (2005): Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci 25:11730–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards BG, Calhoun VD, Kiehl KA (2012): Joint ICA of ERP and fMRI during error-monitoring. Neuroimage 59:1896–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser TU, Iannaccone R, Stämpfli P, Drechsler R, Brandeis D, Walitza S, Brem S (2014): The feedback-related negativity (FRN) revisited: New insights into the localization, meaning and network organization. Neuroimage 84:159–168. [DOI] [PubMed] [Google Scholar]
- 21.Doñamayor N, Marco-Pallarés J, Heldmann M, Schoenfeld MA, Münte TF (2011): Temporal dynamics of reward processing revealed by magnetoencephalography. Hum Brain Mapp 32:2228–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsujimoto T, Shimazu H, Isomura Y (2006): Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. J Neurophysiol 95:2987–3000. [DOI] [PubMed] [Google Scholar]
- 23.Tsujimoto T, Shimazu H, Isomura Y, Sasaki K (2010): Theta oscillations in primate prefrontal and anterior cingulate cortices in forewarned reaction time tasks. J Neurophysiol 103:827–843. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E (2005): Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci 25:604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Womelsdorf T, Johnston K, Vinck M, Everling S (2010): Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc Natl Acad Sci U S A 107:5248–5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Womelsdorf T, Vinck M, Leung LS, Everling S (2010): Selective theta-synchronization of choice-relevant information subserves goal-directed behavior. Front Hum Neurosci 4:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holroyd CB, Coles MGH (2002): The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 109:679–709. [DOI] [PubMed] [Google Scholar]
- 28.Brown JW, Braver TS (2005): Learned predictions of error likelihood in the anterior cingulate cortex. Science 307:1118–1121. [DOI] [PubMed] [Google Scholar]
- 29.Senkowski D, Gallinat J (2015): Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol Psychiatry 77:1010–1019. [DOI] [PubMed] [Google Scholar]
- 30.Cho R, Konecky R, Carter C (2006): Impairments in frontal cortical γ synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A 103:19878–19883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS (2010): Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology 35:2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray CM, König P, Engel AK, Singer W (1989): Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338:334–337. [DOI] [PubMed] [Google Scholar]
- 33.Womelsdorf T, Schoffelen J-M, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P (2007): Modulation of neuronal interactions through neuronal synchronization. Science 316:1609–1612. [DOI] [PubMed] [Google Scholar]
- 34.von Stein A, Chiang C, König P (2000): Top-down processing mediated by interareal synchronization. Proc Natl Acad Sci U S A 97:14748–14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. (2006): High gamma power is phase-locked to theta oscillations in human neocortex. Science 313:1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran LV, Hong LE (2011): High vs low frequency neural oscillations in schizophrenia. Schizophr Bull 37:659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griesmayr B, Berger B, Stelzig-Schoeler R, Aichhorn W, Bergmann J, Sauseng P (2014): EEG theta phase coupling during executive control of visual working memory investigated in individuals with schizophrenia and in healthy controls. Cogn Affect Behav Neurosci 14:1340–1355. [DOI] [PubMed] [Google Scholar]
- 38.Schmiedt C, Brand A, Hildebrandt H, Basar-Eroglu C (2005): Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cogn Brain Res 25: 936–947. [DOI] [PubMed] [Google Scholar]
- 39.Barr MS, Rajji TK, Zomorrodi R, Radhu N, George TP, Blumberger DM, Daskalakis ZJ (2017): Impaired theta-gamma coupling during working memory performance in schizophrenia. Schizophr Res 189:104–110. [DOI] [PubMed] [Google Scholar]
- 40.Boudewyn MA, Carter CS (2018): Electrophysiological correlates of adaptive control and attentional engagement in patients with first episode schizophrenia and healthy young adults. Psychophysiology 55:e12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z, Mayer AR (2012): An event-related fMRI study of exogenous orienting across vision and audition. Hum Brain Mapp 35:964–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV (2001): UCSD Performance-Based Skills Assessment: Development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull 27:235–245. [DOI] [PubMed] [Google Scholar]
- 43.Auquier P, Simeoni M, Sapin C, Reine G, Aghababian V, Cramer J, Lancon C (2003): Development and validation of a patient-based health-related quality of life questionnaire in schizophrenia: The S-QoL. Schizophr Res 63:137–149. [DOI] [PubMed] [Google Scholar]
- 44.Schneider LC, Struening EL (1983): SLOF: A behavioral rating scale for assessing the mentally ill. Soc Work Res Abstr 19:9–21. [DOI] [PubMed] [Google Scholar]
- 45.Cavanagh JF, Cohen MX, Allen JJB (2009): Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci 29:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delorme A, Makeig S (2004): EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21. [DOI] [PubMed] [Google Scholar]
- 47.Cohen MX (2014): Analyzing Neural Time Series Data: Theory and Practice. Cambridge, Massachusetts: The MIT Press. [Google Scholar]
- 48.Mathalon DH, Sohal VS (2015): Neural oscillations and synchrony in brain dysfunction and neuropsychiatric disorders: It’s about time. JAMA Psychiatry 72:840–844. [DOI] [PubMed] [Google Scholar]
- 49.Perrin F, Pernier J, Bertrand O, Echallier J (1989): Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol 72:184–187. [DOI] [PubMed] [Google Scholar]
- 50.Luck SJ (2014): An Introduction to the Event-Related Potential Technique. Cambridge, Massachusetts: The MIT Press. [Google Scholar]
- 51.Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujino H, Sumiyoshi C, Yasuda Y, Yamamori H, Fujimoto M, Fukunaga M, et al. (2017): Estimated cognitive decline in patients with schizophrenia: A multicenter study. Clin Neurophysiol 71:294–300. [DOI] [PubMed] [Google Scholar]
- 53.Resnick SM (1992): Matching for education in studies of schizophrenia. Arch Gen Psychiatry 49:246. [DOI] [PubMed] [Google Scholar]
- 54.Trujillo LT, Allen JJB (2007): Theta EEG dynamics of the error-related negativity. Clin Neurophysiol 118:645–668. [DOI] [PubMed] [Google Scholar]
- 55.Hanslmayr S, Pastötter B, Bäuml K-H, Gruber S, Wimber M, Klimesch W (2007): The electrophysiological dynamics of interference during the Stroop task. J Cogn Neurosci 20:215–225. [DOI] [PubMed] [Google Scholar]
- 56.Nigbur R, Ivanova G, Stürmer B (2011): Theta power as a marker for cognitive interference. Clin Neurophysiol 122:2185–2194. [DOI] [PubMed] [Google Scholar]
- 57.Cooper PS, Darriba Á, Karayanidis F, Barceló F (2016): Contextually sensitive power changes across multiple frequency bands underpin cognitive control. Neuroimage 132:499–511. [DOI] [PubMed] [Google Scholar]
- 58.van Driel J, Swart JC, Egner T, Ridderinkhof KR, Cohen MX (2015): (No) time for control: Frontal theta dynamics reveal the cost of temporally guided conflict anticipation. Cogn Affect Behav Neurosci 15:787–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Noordt SJR, Desjardins JA, Gogo CET, Tekok-Kilic A, Segalowitz SJ (2017): Cognitive control in the eye of the beholder: Electrocortical theta and alpha modulation during response preparation in a cued saccade task. Neuroimage 145:82–95. [DOI] [PubMed] [Google Scholar]
- 60.Cooper PS, Wong ASW, McKewen M, Michie PT, Karayanidis F (2017): Frontoparietal theta oscillations during proactive control are associated with goal-updating and reduced behavioral variability. Biol Psychol 129:253–264. [DOI] [PubMed] [Google Scholar]
- 61.Siegel M, Donner TH, Engel AK (2012): Spectral fingerprints of largescale neuronal interactions. Nat Rev Neurosci 13:121–134. [DOI] [PubMed] [Google Scholar]
- 62.Cohen MX (2014): A neural microcircuit for cognitive conflict detection and signaling. Trends Neurosci 37:480–490. [DOI] [PubMed] [Google Scholar]
- 63.Cohen MX, Elger CE, Ranganath C (2007): Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage 35:968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeung N, Botvinick MM, Cohen JD (2004): The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol Rev 111:931–959. [DOI] [PubMed] [Google Scholar]
- 65.Ide JS, Shenoy P, Yu AJ, Li CR (2013): Bayesian prediction and evaluation in the anterior cingulate cortex. J Neurosci 33:2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, et al. (2012): Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. Neuroimage 59:2860–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen MX (2011): Error-related medial frontal theta activity predicts cingulate-related structural connectivity. Neuroimage 55:1373–1383. [DOI] [PubMed] [Google Scholar]
- 68.Cavanagh JF, Zambrano-Vazquez L, Allen JJB (2012): Theta lingua franca: A common mid-frontal substrate for action monitoring processes. Psychophysiology 49:220–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uhlhaas PJ, Singer W (2010): Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11:100–113. [DOI] [PubMed] [Google Scholar]
- 70.Uhlhaas PJ, Singer W (2015): Oscillations and neuronal dynamics in schizophrenia: The search for basic symptoms and translational opportunities. Biol Psychiatry 77:1001–1009. [DOI] [PubMed] [Google Scholar]
- 71.Lewis DA, Curley AA, Glausier JR, Volk DW (2012): Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirihara K, Rissling AJ, Swerdlow NR, Braff DL, Light GA (2012): Hierarchical organization of gamma and theta oscillatory dynamics in schizophrenia. Biol Psychiatry 71:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Basar-Eroglu C, Brand A, Hildebrandt H, Karolina Kedzior K, Mathes B, Schmiedt C (2007): Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol 64:39–45. [DOI] [PubMed] [Google Scholar]
- 74.Roux F, Uhlhaas PJ (2014): Working memory and neural oscillations: Alpha–gamma versus theta–gamma codes for distinct WM information? Trends Cogn Sci 18:16–25. [DOI] [PubMed] [Google Scholar]
- 75.Buzsaki G (2006): Rhythms of the Brain. Oxford, United Kingdom: Oxford University Press. [Google Scholar]
- 76.Olney JW, Newcomer JW, Farber NB (1999): NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 33:523–533. [DOI] [PubMed] [Google Scholar]
- 77.MacDonald AW, Pogue-Geile MF, Johnson MK, Carter CS (2003): A specific deficit in context processing in the unaffected siblings of patients with schizophrenia. Arch Gen Psychiatry 60:57–65. [DOI] [PubMed] [Google Scholar]
- 78.Paxton JL, Barch DM, Racine CA, Braver TS (2008): Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb Cortex 18:1010–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paxton JL, Barch DM, Storandt M, Braver TS (2006): Effects of environmental support and strategy training on older adults’ use of context. Psychol Aging 21:499–509. [DOI] [PubMed] [Google Scholar]
- 80.Wendel K, Väisänen O, Malmivuo J, Gencer NG, Vanrumste B, Durka P, et al. (2009): EEG/MEG source imaging: Methods, challenges, and open issues. Comput Intell Neurosci 2009:656092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reinhart RMG (2017): Disruption and rescue of interareal theta phase coupling and adaptive behavior. Proc Natl Acad Sci U S A 114:11542–11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reinhart RMG, Zhu J, Park S, Woodman GF (2015): Medial–frontal stimulation enhances learning in schizophrenia by restoring prediction error signaling. J Neurosci 35:12232–12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reinhart RMG, Zhu J, Park S, Woodman GF (2015): Synchronizing theta oscillations with direct-current stimulation strengthens adaptive control in the human brain. Proc Natl Acad Sci U S A 112:9448–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.