Abstract
Glucocorticoid excess is an under-recognised cause of cardiovascular adverse effects. The sources can be either endogenous (Cushing’s syndrome) or exogenous (Anabolic steroid abuse). Cardiovascular complications due to excess glucocorticoid includes hypertension, left ventricular hypertrophy, myocardial infarction, and heart failure. Although anabolic steroid-induced cardiomyopathy is a well-recognised phenomenon, endogenous corticosteroid-induced cardiomyopathy and heart failure are rarely reported sequelae of glucocorticoid excess in the body. We report a glucocorticoid-induced dilated cardiomyopathy in a 26-year-old African–American man with cushingoid features and symptomatic heart failure.
Keywords: heart failure, adrenal disorders, drugs: endocrine system
Background
Hypercortisolism or Cushing’s syndrome is the systemic manifestation of excess exposure to cortisol or its derivative compounds. Endogenous sources of cortisol include either directly from the adrenal gland, or indirectly from pituitary or ectopic tumours. If hypercortisolism is due to excess Adrenocorticotropic hormone (ACTH) production from pituitary, it is called Cushing’s disease. Exogenous sources of cortisol include anabolic androgen steroid (AAS), glucocorticoids and mineralocorticoids.1 Cushing’s syndrome is a very common disorder affecting 10–15 per million people with multisystem involvement, including osteoporosis, diabetes mellitus, hypertension, dyslipidaemia, depression and alterations of coagulation factors.2 Among the cardiovascular complications of Cushing’s syndrome, hypertension is the most common one.3 Other severe outcomes include left ventricular (LV) hypertrophy, myocardial ischaemia and, very rarely, dilated cardiomyopathy.4
Prolonged and excessive exposure to cortisol has a direct effect on cardiac myocytes, which initiate cardiac remodelling via activation of the renin–angiotensin–aldosterone system (RAAS) pathway and heart failure pathophysiology. Long-term exposure to cortisol significantly correlates to asymmetric LV wall thickening.5 Chronically, this can lead to cardiac fibrosis and irreversible heart failure.4 6 AAS abuse is similarly associated with hypertension, ventricular remodelling, cardiomyopathy and myocardial infarction. AAS binds to mineralocorticoid receptors on cardiac and skeletal myocytes, causing hypertrophy via upregulation of the RAAS pathway, a pathway similar to cortisol.7
We report a case of symptomatic heart failure in a 26-year-old African–American male college student with Cushing’s syndrome. Our case highlights the importance of keeping in mind a broad differential diagnosis, a multidisciplinary approach, and thorough literature review and investigation of even the more common causes to reach a diagnosis.
Case presentation
A 26-year-old obese African–American male college student presented with 3 weeks of shortness of breath, lower extremity oedema and extreme fatigue. He denied fever, chills, any recent viral illness, recent travel or sick contacts. He was initially treated for suspected community-acquired pneumonia with doxycycline for 5 days. However, due to persistent symptoms, chest X-ray on follow-up visit showed signs concerning congestive heart failure (CHF). His medical history included a pituitary adenoma complicated by central hypothyroidism and Cushing’s disease, resected 2 months before. He was on maintenance hydrocortisone 20 mg/day. He had no history of smoking, alcohol, insect bites, animal exposure or allergies.
On admission, he appeared non-toxic; was afebrile, normotensive, tachycardic and tachypneic; and his O2 saturation was 97% on room air. Physical examinations significant for typical cushingoid features were appreciated, including acne on the forehead, moon facies, buffalo hump, abdominal obesity, prominent striae on the upper extremities and abdomen. Cardiovascular examination revealed normal heart rate and rhythm, no murmurs, gallops or rubs, nor jugular venous distension. Bibasilar crackles were appreciated. Peripheral pulses were strong and equal bilaterally. Extremities showed bilateral lower extremity oedema with upper extremity striae and melanin deposition in the fingernails.
Investigations
Laboratory findings showed the following: white cell count 9.5×109/L, haemoglobin 0.132 g/L, sodium 139 mEq/L, creatinine (Cr) 1.45 mg/dL (baseline Cr=1.0 mg/dL), International Normalized Ratio (INR) 2.6, troponin <0.01 ng/mL, lactate 2.2 mmol/L and Brain Natriuretic Peptide (BNP) 2298 pg/mL; otherwise, the basic metabolic panel was within normal limits. The toxicology screen was negative; procalcitonin was negative; and the viral respiratory pathogen panel was negative. HIV, antinuclear antibody and autoimmune panel were negative. Thyroid Stimulating Hormone (TSH) and T4 was within normal limits.
ECG revealed sinus tachycardia with normal rhythm, with no prolongation in the QT interval.
Chest radiography revealed cardiomegaly and pulmonary vascular congestion, suggestive of CHF. Nodular density in the left mid-lung was noted suspected of focal pulmonary nodule. Diagnosis of new-onset heart failure was made and workup was undertaken to investigate the cause of it.
CT angiography revealed pulmonary vascular congestion, oedema and focal consolidation in the left upper lobe with possible small central cavitation and bilateral pleural effusion (see figure 1).
Figure 1.
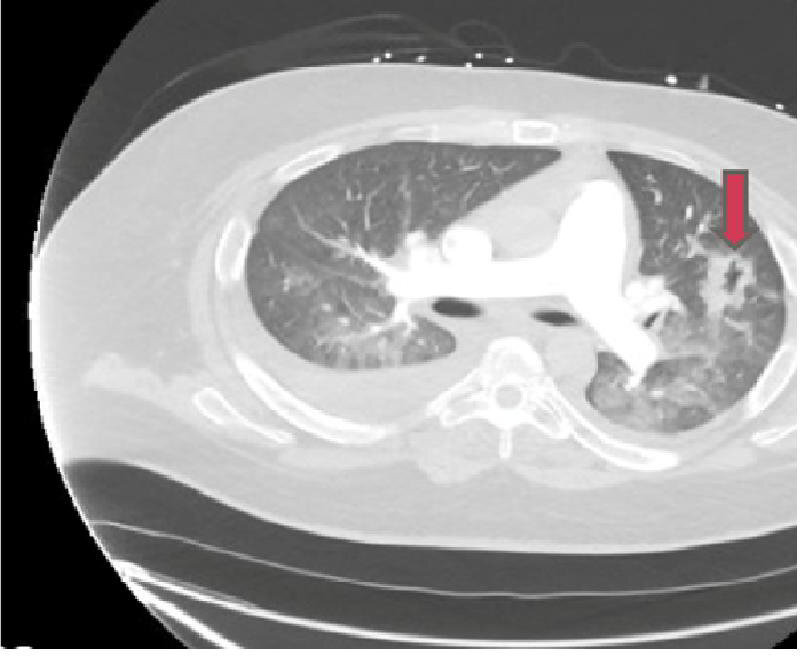
CT angiography of the chest showing pulmonary vascular congestion and focal consolidation in the left upper lobe. The red arrow points to the cavitating lesion in the left lung field, which on biopsy was a non-specific inflammation and fibrosis.
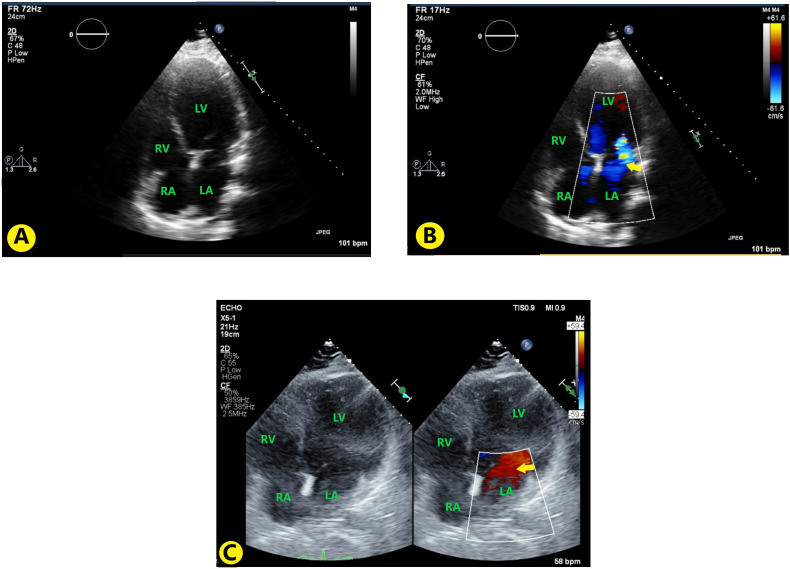
Hypertrophic cardiomyopathy was suspected due to young age and new-onset heart failure. Transthoracic echocardiography showed no ventricular hypertrophy. However, it showed new-onset severe LV dysfunction with global hypokinesis. Left ventricular ejection fraction (LVEF) of <10% with grade III restrictive diastolic dysfunction was also seen. Besides these, dilation of the left atrium, right atrium, moderate mitral regurgitation and moderate tricuspid regurgitation (figure 2A, B). Transoesophageal echocardiography was negative for any vegetation, thrombus or patent foramen ovale.
Figure 2.
(A) TTE images with apical four-chamber view without colour; (B) TTE with apical four-chamber view with colour Doppler showing mitral regurgitation (yellow arrow); (C) Follow-up TTE 3 months after discharge, showing decrease in mitral regurgitation (yellow arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; TTE, transthoracic echocardiography.
Right heart catheterisation revealed right atrial pressure of 3 mm Hg, right ventricular pressure of 26/2 mm Hg, pulmonary arterial pressure of 29/11 mm Hg, pulmonary capillary wedge pressure of 10 mm Hg and cardiac output of 4.9 L/min. Left ventriculography showed severe global LV dysfunction. Ischaemic cardiomyopathy was ruled out as there was no coronary artery disease on left heart catheterisation. Aortogram revealed no aortic insufficiency.
Cardiac MRI revealed an ejection fraction of 18% (normal: 56%–78%), end diastolic diameter of 69 mm (normal: 36–56 mm), stroke volume of 52 mL, end diastolic volume 298 mL (normal: 52–195 mL), end systolic volume of 245 mL (normal: 13–72 mL) and severely diminished LV systolic function with moderate LV dilatation. There was no delayed myocardial enhancement or evidence of myocardial fibrosis or scarring (see figure 3).
Figure 3.
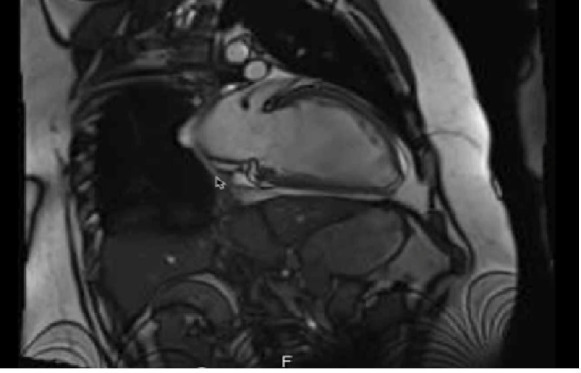
Cardiac MRI shows moderate left ventricular dilatation. There was no delayed myocardial enhancement or evidence of myocardial fibrosis or scarring.
Differential diagnosis
After ruling out ischaemic cardiomyopathy, a wide differential diagnosis was considered for possible aetiology. Viral myocarditis was ruled out by negative viral respiratory and enteric pathogen panel. Takatsubo cardiomyopathy was ruled out by the absence of typical features on echocardiography, especially apical ballooning of ventricles. Inherited cardiomyopathy/channelopathies were excluded by the absence of family history, lack of ECG changes and new onset of symptoms. Negative history of alcohol consumption negated ethanol-induced cardiomyopathy. The negative autoimmune panel ruled out lupus or autoimmune myocarditis. Absence of hilar adenopathy excluded sarcoidosis. No myocardium abnormalities on cardiac MRI, combined with absence of wild-type transthyretin gene and normal protein electrophoresis, excluded amyloidosis. Toxicology screen was negative; there was no history of exposure to heavy metals or recent travel. Thyroid panel was within normal limits. No evidence of vitamin and mineral deficiencies or toxicities was found. No cardiac biopsy was performed as cardiac MRI showed normal myocardium uptake. The pronounced cushingoid features, along with prolonged use of hydrocortisone, suggested glucocorticoid-induced cardiomyopathy.
Treatment
Experts from cardiology and endocrinology were consulted for cardiomyopathy and Cushing’s disease management, respectively. Goal-directed medical therapy for heart failure was instituted for management of heart failure. The patient’s symptoms improved dramatically after aggressive diuresis with loop and thiazide diuretic combination. Valsartan–sacubitril, ivabradine and metoprolol succinate were also initiated after the patient’s shortness of breath started to improve. He was evaluated for loop recorder and life vest due to risk of arrhythmia and severe systolic heart failure, respectively. However, the patient refused due to concerns of impairment in quality of life.
Endocrinology started to taper the patient’s steroids as the patient had cushingoid features. His symptoms improved after gradual decrease in steroid therapy and continued diuresis.
He was discharged to follow-up with pulmonary who advised for bronchoscopy and biopsy of the cavitary lesion. He had no signs of heart failure exacerbation on follow-up.
Outcome and follow-up
The patient returned to college within a week of discharge from the hospital. He followed up with pulmonary medicine in 2 months and underwent bronchoscopic biopsy of the pulmonary lesion, which showed non-specific inflammation and fibrosis, likely secondary to cavity formed by old pneumonia infection. On his follow-up with the cardiologist on the third month, he had no signs of heart failure and his diuretics were reduced as tolerated. His echo at the time showed slight improvement in Ejection Fraction (EF) from <10% to 20%–30% (figure 2C). He had also been following up with a dietitian and lost 50 lb after enrolling in a weight loss programme. He had a 6-month follow-up with an endocrinologist where, besides being asymptomatic, he was found to have normalisation of his cortisol levels at the reduced dose of 10 mg hydrocortisone/day.
Discussion
Cardiovascular complications in patients with Cushing’s syndrome are associated with a four times higher mortality rate compared with a normal population for both age and gender.4 LV hypertrophy and cardiomegaly is appreciated in 50%–70% of patients with Cushing’s syndrome; however, CHF is a rare finding. Approximately 20 cases of acute heart failure secondary to Cushing’s disease have been reported since 1994.4
There have been 15 case reports of dilated cardiomyopathy associated with Cushing’s syndrome, 7 attributed to pituitary macroadenoma, 6 to cortical adenoma, 1 to adrenocortical carcinoma, and in 1 case no obvious cause was found.5 8–10 All of these cases showed significant improvement in LVEF after cortisol-level normalisation, except one case due to other cardiovascular abnormalities, including ischaemia and valvular defect.5 We followed similar guidelines for our patient and observed dramatic improvement after a reduction in hydrocortisone dose besides goal-directed medical therapy for heart failure.
Frustaci et al reported that the major morphological changes caused by Cushing’s syndrome include cardiomyocyte hypertrophy, myofibrillolysis and myocardial fibrosis.2 Pathophysiology of Cushing’s cardiomyopathy based on endomyocardial biopsies has been studied. Cardiomyocyte hypertrophy is attributed to glucocorticoid-mediated angiotensin II release and increased responsiveness of cardiac cells to angiotensin II. High levels of glucocorticoid account for 28 times increase of atrogin 1 and ubiquitin (via activation of FOXO transcription factor) causing myofibrillolysis.2 Prolonged/excessive exposure to cortisol has a direct effect on cardiac myocytes, which initiates cardiac remodelling via activation of the RAAS pathway and heart failure physiology; chronically, this can lead to cardiac fibrosis and irreversible heart failure.4 6 Cushing’s cardiomyopathy is, however, reversible and the symptoms significantly improve after resection of the tumour and normalisation of cortisol levels.3
Treatment for patients with Cushing’s disease and non-ischaemic heart failure includes goal-directed cardioprotective heart failure therapy with agents that directly or indirectly inhibit the RAAS pathway (beta-blockers, potassium-sparing diuretics, Angiotensin Converting Enzyme inhibitors, Angiotensin Receptor Blockers, Angiotensin Receptor-Neprilysin Inhibitor) as well as cardiac monitoring in a heart failure clinic.9 Intuitively, optimising supplemental glucocorticoid dosage in a patient with insufficient endogenous glucocorticoid production and cushingoid features is paramount to long-term morbidity and mortality.10
Patient’s perspective.
My story started with always being a big kid, being lazy, eating a lot and not being very active. I was always horrible at PE classes, always ridiculed, but I was good at studies. So, I focused all my time studying and getting into the college of my choice. I spent hours studying, during which time I gained most of my weight. I was never into sports, mostly because I felt out of breath from the get-go. I always thought it was my weight. I never honestly tried to lose the weight either because it took very little for me to put on weight and almost impossible to lose any, especially with running out of breath so fast. My family wasn’t too concerned as most of us were fat to begin with and thought it is just hereditary, and for the longest time, I was okay with it. However, no one ran out of breath like I did, and I was somewhat worried about it. I always thought that once I reach a certain weight, I would just get weight loss surgery and get it over with. I researched what would qualify me for bariatric surgery and just waited until then. It was not until after I got into college and had a girlfriend who cared enough to make me think about my performance. I was able to talk to someone about my weight. She was also a little on the healthy side of the weight, but with her, I was able to differentiate what is related to being overweight and that my shortness of breath, weakness, constipation and weird skin discoloured patches may be more than just my weight. I finally went to a primary care physician where it was unveiled that I had low thyroid levels. I started taking thyroxine, but it helped only so much with my energy levels. I kept seeing the same physician group until they finally decide to test me for other hormones. I was surprised to find that my body was making excess steroids. I always had the impression that steroids are supposed to make one more macho, and that is why athletes take it to make more muscle mass. However, apparently, within me it was having the opposite effect. My doctor did some scans of the belly and head and found that I had a tumour which was producing this hormone! I was shocked to know that someone as young as me was having a tumour and did not know! I was not sure if it was my excess use of technology and radiation exposure? How long I had the tumour I did not know. Was my weight entirely due to a tumour? Or was I to play a part of it?
What had I done to deserve this tumour? My young life was wasted sitting home being overweight and unable to do things because of it. Was it always treatable from the start and no one cared enough to address it? Many regretful questions loomed in my mind. However, fortunately, the tumour was operable, and I saw a ray of hope that maybe I will finally have normal weight. My doctor referred me to a neurosurgeon. Within a week I had my tumour in pituitary removed. I thought after surgery I would feel like a new man, but that was not the case. I continued to feel weak; doctors told me that it was because I just had surgery, and it may take some time before I regain my strength. I was told that now I need to take some replacement meds since I lost my pituitary. I was okay with most of them but that steroid they gave me made feel worse. I knew my problems were because of steroids and I asked why I had to continue taking more steroids, but they said that we are giving those in moderation and that the body needs it.
Weeks went on with the off-and-on weakness, but then about a month later, I felt really short of breath.
I went to my primary care physician, where he gave me doxycycline, thinking that it may be pneumonia. I gotta admit I did not get an X-ray right away despite saying I would get one. I was in college and I had already missed a lot of homework due to time off for surgery. I needed to make up for that. However, the antibiotics did not do a thing, so I went in again. Seeing the X-ray my doctor flipped. He told me I had heart failure! Heart failure? That is an old man’s problem; I asked if he was sure. He told me that the symptoms fit and that I had to get myself checked into the hospital.
So yet another round of experimenting on me began. Here I was thinking I would never see a hospital ever again after my tumour was removed. They started to give me water pills to get rid of all the swelling. Finally, after years, I felt like I could breathe from parts of my lung I did not know existed! I was happy but also worried what was to come. I had to go through so many scans, so many blood draws and even had a study where they saw my arteries to see if there was any blockage. I was surprised when they said I did not despite all that fast food. The scariest part was getting the MRI; I had a taste of what getting buried alive may feel like. I hope no one must go through that. I had enough by then! I was happy that the meds they gave me were working. So, when they asked if I was okay getting a biopsy of my heart, I declined. I had already undergone way too much for a 26-year-old. I asked if it would change therapy and they said they cannot be sure unless the results are seen. I was not willing to miss out on anymore college days. I got my meds that worked for me; I do not need anything else if I can do well on these. They alternatively offered that I wear a monitor to look for funny heart rhythms and some jacket that will revive me if my heart stops. I did not like that idea either. I finally wanted to live my life, the best I can. I was tired of being in the hospital with people the age of my grandparents. I am glad the doctors did not take offence to that, understood my situation and continued to help me. They reduced the steroid dose I was getting, and I felt amazing! I have been following up with them and I have been doing well. I lost 50 lb after joining a weight loss programme! It would have been impossible for me to even think about losing a pound before I had the surgery. Now I can jog without running out of breath after five steps. My life has changed! Thank you everyone!
Learning points.
Steroid-induced cardiomyopathy is an under-recognised cause of heart failure in all age groups.
Cortisol levels should be checked in patients with heart failure if they are showing signs of concurrent Cushing’s syndrome.
Normalisation of cortisol levels early in the course leads to reversal and improvement of symptoms in heart failure.
Prolonged exposure to steroid leads to irreversible cardiac remodelling and dilated cardiomyopathy leading to heart failure.
Footnotes
Contributors: TS and SRZ were involved in the conception and design of the work. TS, AH and HS were involved in manuscript writing. TS and SRZ were involved in reviewing and revising the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Delivanis DA. Chapter 15 - Advances in the Diagnosis and Medical Management of Cushing’s Syndrome. Advances in Treatment and Management in Surgical Endocrinology 2020:151–74. [Google Scholar]
- 2.Frustaci A, Letizia C, Verardo R, et al. Cushing syndrome cardiomyopathy: clinicopathologic impact of cortisol normalization. Circ Cardiovasc Imaging 2016;9:e004569. 10.1161/CIRCIMAGING.116.004569 [DOI] [PubMed] [Google Scholar]
- 3.Bİ A, Gerede DM, Canpolat AG, et al. Cushing’s Disease Presented by Reversible Dilated Cardiomyopathy. Case Rep Cardiol 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibusawa N, Yamada M, Hashida T, et al. Dilated cardiomyopathy as a presenting feature of Cushing's syndrome. Intern Med 2013;52:1067–71. 10.2169/internalmedicine.52.9051 [DOI] [PubMed] [Google Scholar]
- 5.Marchand L, Segrestin B, Lapoirie M, et al. Dilated cardiomyopathy revealing Cushing disease: a case report and literature review. Medicine 2015;94:e2011. 10.1097/MD.0000000000002011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yong TY, Li JYZ. Reversible dilated cardiomyopathy in a patient with Cushing's syndrome. Congest Heart Fail 2010;16:77–9. 10.1111/j.1751-7133.2009.00123.x [DOI] [PubMed] [Google Scholar]
- 7.Youssef MYZ, Alqallaf A, Abdella N. Anabolic androgenic steroid-induced cardiomyopathy, stroke and peripheral vascular disease. BMJ Case Rep 2011;2011:bcr1220103650. 10.1136/bcr.12.2010.3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill A, Dean N, Cushing’s A-AR. Dilated cardiomyopathy and stroke: case report and literature review. Can J Gen Intern Med 2016;11. [Google Scholar]
- 9.Abdulla MC. Adrenocortical carcinoma presenting as reversible dilated cardiomyopathy. Heart Views 2018;19:71. 10.4103/HEARTVIEWS.HEARTVIEWS_125_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valente do Couto H, Sampaio K, Peres B, et al. MON-404 Reversible Dilated Cardiomyopathy in a Severe Case of Cushing’s Disease. J Endocr Soc 2019;3:MON–404. 10.1210/js.2019-MON-404 [DOI] [Google Scholar]