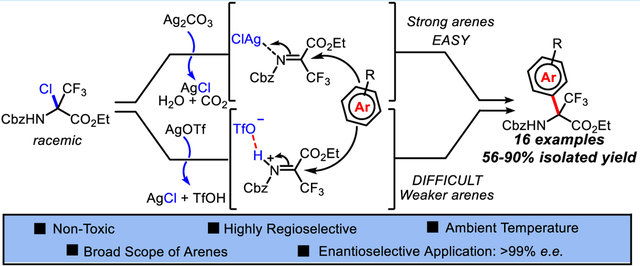
Abstract
A versatile synthetic protocol of aza-Friedel-Crafts alkylation has been developed for the synthesis of quaternary α-amino esters. This operationally simple alkylation proceeds under ambient conditions with high efficiency, regioselectivity, and an exceptionally broad scope of arene nucleophiles. A key feature of this alkylation is the role associated with the silver(I) salt counteranions liberated during the reaction. Taking advantage of a phase-transfer counteranion/Brønsted acid pair mechanism, we also report a catalytic enantioselective example of the reaction.
Graphical Abstract
α,α-Disubstituted α-amino acids found in carbonaceous chondritic meteorites1 have been suggested to be at the origin of symmetry breaking (up to 20% ee) on earth, eventually leading to the homochirality (single enantiomeric form) of terrestrial proteinogenic α-amino acids.2 Although several aspects of abiogenesis remain unclear, the role of α,α-disubstituted amino acids in transferring chirality to other proteinogenic α-amino acids renders these building blocks unique in nature. α,α-Disubstituted “quaternary” α-amino acids are also essential motifs of small molecules and nonribosomal peptides having a variety of biological activities (e.g., enzyme inhibitors, ion-channel blockers, or antibiotics).3 Given that these building blocks are highly constrained compared to monosubstituted α-amino acids (Thorpe-Ingold effect), they impart valuable conformational rigidity when embedded in synthetic molecules.4 Notably, α,α-disubstituted amino acids have a remarkable influence on peptide secondary structures, often producing well-defined helical conformations characteristic of many classes of biomolecules.5 Even so, the asymmetric synthesis of this class of noncanonical amino acids remains mostly untapped and complicated.6 On the other hand, fluorinated amino acids have recently attracted considerable attention owing to their unique medicinal and physicochemical properties (i.e., high stability to metabolic degradation, increased lipophilicity, and hydrogen bond acceptor ability).7 Therefore, establishing a scalable and practical (optically active) synthesis of α,α-disubstituted α-trifluoromethyl amino esters is desirable.
In stark contrast to the activation of α-iminoglycinate A into iminium B, which is one of the most studied and versatile enantioselective strategies for synthesizing monosubstituted nonproteinogenic α-amino esters,8 few diastereo-9 and enantioselective10 methods are currently available for the synthesis of acyclic α,α-disubstituted α-trifluoromethyl amino esters 4. Indeed, the important electrophilicity of α-imino ester 3 results in a weak Lewis basicity of the imine nitrogen, seemingly contributing to a lack of reactivity toward Lewis and Brønsted acid catalysts (Scheme 1). Of particular interest, the synthesis of α,α-trifluoromethyl-aryl amino esters by Friedel-Crafts reactions remains challenging by both diastereo-11 and enantioselective strategies.12 Seemingly, the relatively harsh acidic conditions required for condensing amines with α-trifluoromethyl ketoesters often limit the choice of N-protecting groups, leading to a two-step condensation/dehydration with water scavengers via hemiaminal 1.13 A milder alternative was also devised from the direct aza-Wittig reaction with phosphazenes to prepare the most moisture-sensitive α-imino ester 3.14 The lack of convenient synthesis and reactivity of α-trifluoromethyl α-imino esters 3 (weak Lewis basicity) combined with a high moisture sensitivity (hydration, 3 → 1) are factors that largely hindered the use of this approach. To address this methodological gap, we initially envisioned avoiding the isolation of 3 by studying the direct reactivity of chloroaminal 2. Given that glycinyl chloroaminals were successfully exploited as effective surrogates of α-iminoglycinates A by halide abstraction and anion binding catalysis for carbon-carbon bond formation at the α-center,15 we intuitively hypothesized that if a similar maneuver could be achieved from a tetrasubstituted chloroaminal 2, several classes of α,α-disubstituted α-trifluoromethyl amino esters 4 could become synthetically accessible. Herein, we report a highly practical and general Friedel-Crafts alkylation via a silver(I)-mediated halide abstraction combined with hydrogen bonding that enables the addition of a broad range of arene nucleophiles to the α-trifluoromethyl α-iminopyruvate 3.16
Scheme 1.
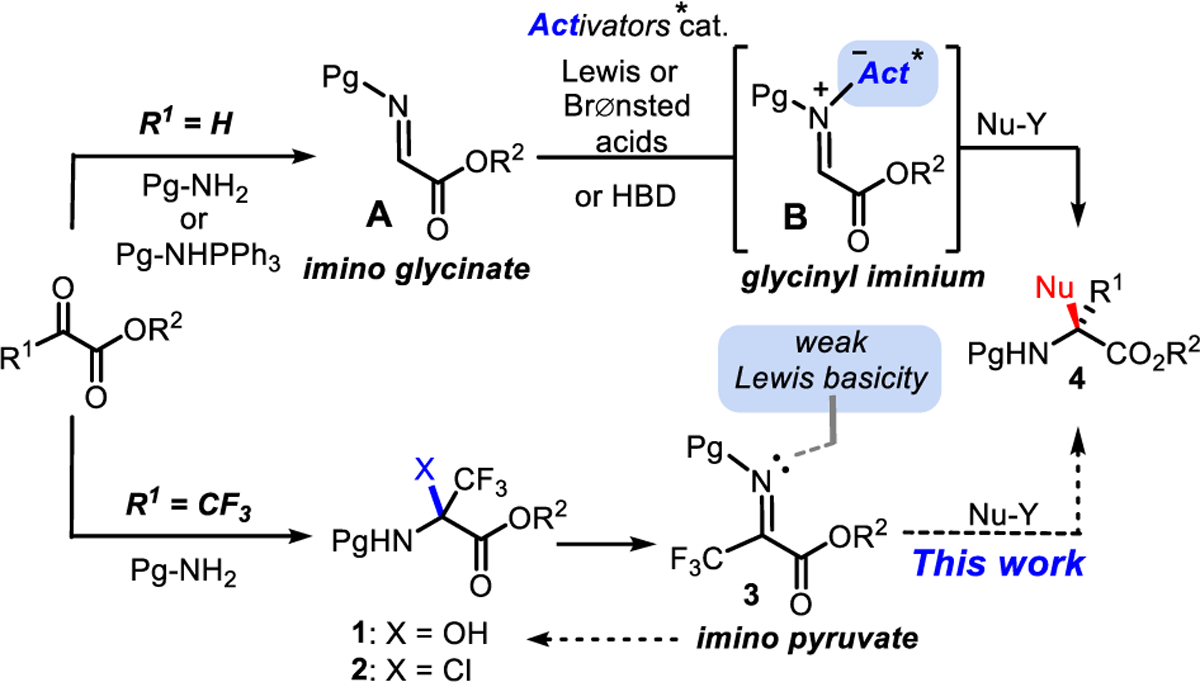
Challenging Activation of α-Trifluoromethyl Imino Pyruvate versus the Typical Imino Glycinate Reactivity
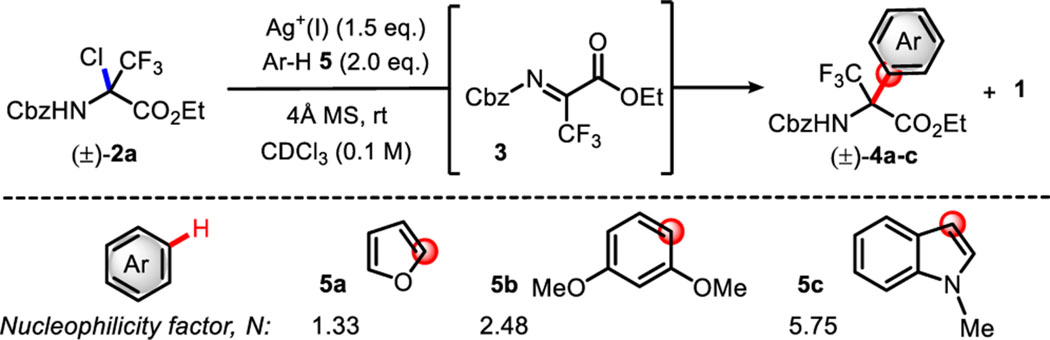
The starting material, α-trifluoromethyl chloroaminals (±)-2a and (±)-2b bearing common N-carbamoyl protecting groups (Cbz or Fmoc), can be synthesized in >90% yield and kept intact for >8 weeks under low-moisture conditions. In collaboration with scientists from Eli Lilly, a silver(I)-mediated Friedel-Crafts alkylation of chloroaminal (±)-2a was extensively assessed through the screening of solvents, nucleophiles, and other reaction parameters on the Automated Synthesis laboratory (ASL) platform.17,18 As a result of this screen, a couple of silver(I) salts emerged as efficient stoichiometric reagents capable of generating Friedel-Crafts products cleanly via a putative halide abstraction mechanism.19 The initial results obtained via the robot synthesizer were further optimized manually to study both reaction intermediates (e.g., 3) and any potential byproducts formed during the reaction by 1H and 19F NMR (Table 1). Indeed, using Ag2CO3 as a promoter, the halide abstraction took place rather slowly, leading to ~60% conversion of imine 3 after 4 h (entries 1 and 2). The reaction carried out without a desiccant (entry 1) produced imine 3, which rapidly transformed into hemiaminal 1 (~1:1 ratio after 4 h), leading to a >95% yield of 1 after 24 h. The same reaction in the presence of molecular sieves delivered imine 3 in a quantitative manner as the sole reaction product (entry 2). Initial Friedel-Crafts conditions were tested with rather weak π-nucleophile arenes (entries 3 and 4) such as furan 5a (N ~ 1.3) and 1,3-dimethoxybenzene 5b (N ~ 2.5).20 In both cases, the desired arylation did not take place as suggested by the large amount of imine 3 being formed over time (>95% NMR yield). These results suggest that arenes 5a and 5b are not nucleophilic enough to engage in the Friedel-Crafts alkylation with imine 3. Therefore, a stronger nucleophile, N-methylindole 5c (N ~ 5.8), was tested under the same reaction conditions. While small amounts of imine 3 were observed, the desired product 4c formed rapidly over the course of the reaction (entry 5, ≤98% NMR yield after 24 h). To circumvent the lack of reactivity of weak arene nucleophiles and expand the initial success to a broader scope of arenes, other common silver salts were evaluated.18 While reactions with AgOAc or the more ionizing AgBF4 and AgSbF6 do not deliver the desired Friedel-Crafts products, several silver salts such as AgNO3, AgOTs, and AgOTf enabled the reaction to occur with 5b as the nucleophile. Optimum reactivity was observed with AgOTf, leading to the formation of arylated product 4b in a 36% yield (entry 6). Reaction conditions were further optimized by evaluating several solvents and concentrations.18 Reactions in diethyl ether showed a cleaner profile, leading to the formation of 4b in 54% and 71% yields at concentrations of 0.1 and 0.3 M, respectively (entries 7 and 8, respectively). The presence of molecular sieves in addition to AgOTf did not affect the reaction outcome, leading to the full conversion of (±)-2a after only 1 h, but significant amounts of hemiaminal intermediate 1 persisted [entries 6–8 (vide infra)]. The fact that imine 3 was not observed in these reactions suggested that AgOTf or TfOH, a byproduct of halide abstraction, might account for activating imine 3 in the Friedel-Crafts alkylation.
Table 1.
 | |||||
|---|---|---|---|---|---|
| conversion ratio (2:3:1:4)b | product yieldb,c (%) |
||||
| entry | arene | silver source | 1 h | 4 h | 1 day |
| 1d | - | Ag2CO3 | 66:34:0:0 | 25:40:35:0 | >95 (1) |
| 2 | - | Ag2CO3 | 61:36:3:0 | 39:59:2:0 | 98 (3) |
| 3 | 5a | Ag2CO3 | 53:45:2:0 | 17:78:5:0 | >95 (3) |
| 4 | 5b | Ag2CO3 | 70:2:2:0 | 26:70:1:0 | >98 (3)e |
| 5 | 5c | Ag2CO3 | 54:9:5:32 | 36:9:5:50 | 98 (4c) |
| 6 | 5b | AgOTf | 42:0:17:20 | 0:0:16:15 | 36 (4b) |
| 7f | 5b | AgOTf | 0:0:32:42 | 0:0:52:33 | 54 (4b) |
| 8f,g | 5b | AgOTf | 0:0:40:39 | 0:0:24:53 | 71 (4b) |
Reactions were carried out under argon with 2 (0.10 mmol) with arenes 5a-c(2.0 equiv), silver reagent [1.5 equiv in Ag(I)], and 30 mg of 4 Å molecular sieves in CDCl3 (2.0 mL).
NMR ratios and yields determined for the crude reaction mixture by 19F NMR with C6F6 as the internal standard.
NMR yields determined for the crude reaction mixture by 1H NMR with mesitylene as the internal standard.
Reaction carried out without 4 Å molecular sieves.
The reaction was also carried out at higher temperatures (up to 60 °C), and the formation of 4b was not observed.
Reaction carried out in anhydrous Et2O.
Reaction carried out at 0.3 M.
Two silver-mediated methods therefore emerged to cover a broad scope of arene nucleophiles encompassing (a) electron-rich substrates using Ag2CO3 and (b) electron-poor arenes by switching the reagent to AgOTf (Scheme 2). For reactions mediated by Ag2CO3 (0.75 equiv), reaction times varied from 18 to 72 h to deliver the quaternary α-trifluoromethyl amino esters 4c-j in a range of yields of 56–89% with high regioselectivity in most cases. Using the same method, Fmoc-chloroaminal (±)-2b can also be functionalized in good yield, as shown by the synthesis of 4f in 89% yield. Interestingly, reactions with 1,3-dimethoxybenzene (N ~ 2.5) did not proceed after 3 days under these conditions, or at a higher temperature, thus delineating the limit of reactivity of Ag2CO3-mediated Friedel-Crafts alkylation. Even so, the reactivity of 3 with weaker π-nucleophiles (N ≤ 2.5) requires the use of AgOTf (2.0 equiv) to promote the Friedel-Crafts reactions. Under the optimum reaction conditions described in Scheme 2, reaction times could be decreased (<3 h) and α-trifluoromethyl amino esters 4a, 4b, and 4k-p were obtained in a remarkable range of yields of 71–90% with high regioselectivity.
Scheme 2. Substrate Scope for the Synthesis of α,α-Disubstituted Amino Esters 4a-p by Friedel-Crafts Reactionsa–b–c.
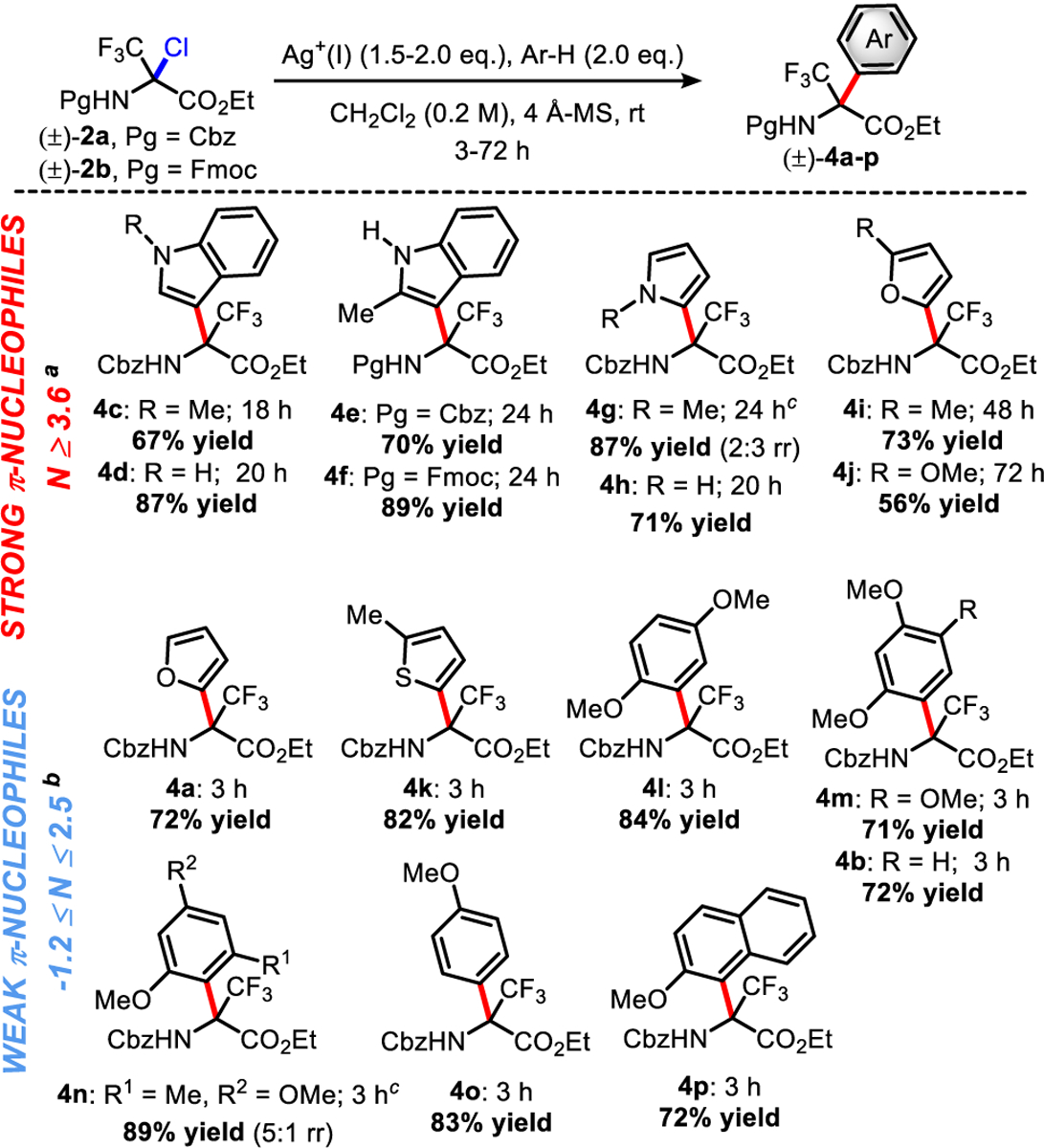
aStandard reactions carried out on a 0.2 mmol scale for 2 (1.0 equiv) with arenes 5a-o (2.0 equiv) in CH2Cl2 (0.2 M) with Ag2CO3 (0.75 equiv). Isolated yields reported. bStandard reactions carried out on a 0.2 mmol scale for 2 (1.0 equiv) with arenes 5a-o (2.0 equiv) in CH2Cl2 (0.2 M) with AgOTf (2.0 equiv). Isolated yields reported. cMajor regioisomers drawn for the sake of simplicity. Regioisomers separated by chromatography (see the Supporting Information).
Given the innate sensitivity of the 19F nucleus, and the large chemical shift dispersion δF(CF3) observed among the starting material 2 (−76.14 ppm), products 4b and 4c (−71.20 and −71.70 ppm, respectively), imine 3 (−70.05 ppm), and hemiaminal 1 (−80.63 ppm), reactions can be easily and quantitatively monitored by 19F NMR spectra calibrated on C6F6 (see Scheme 3A).21 To test our reactivity hypothesis, imine 3 was synthesized, isolated (highly hydroscopic → 1), and further reacted with the moderately reactive arene 5b (N ~ 2.5) and stoichiometric amounts of either AgOTf, Ag2CO3, or catalytic TfOH (Scheme 3B). Analysis of reaction progress by 19F NMR revealed no measurable formation of 4b in the presence of silver salts, while using 30 mol % TfOH afforded product 4b in 37% yield. The innate electrophilicity of imine 3 was further evaluated with arenes 5b, 5h, and 5i without any external additive. Arenes 5b and 5i were shown to be unreactive, while pyrrole 5h (N ~ 4.6) reacted adequately with 3 (20% yield). The electrophilicity factor of imine 3 can therefore be roughly estimated to be E = −5.0 − [(3.6 + 4.6)/2] = −9.1,20b which is in line with some of the most electrophilic imines reported to date in the empirical Ofial-Mayr reactivity scale.22 Taken together, these results suggest that the TfOH byproduct formed during halide abstraction on 2 plays a pivotal role in catalyzing the Friedel-Crafts alkylations.11a With this piece of mechanistic information, a catalytic enantioselective activation of imine 3 was evaluated with (R)-TRIP as the Brønsted acid catalyst and indole 5d as the arene nucleophile.23 While the uncatalyzed reaction yielded (±)-4d in 20% as a single C3 regioisomer, the TRIP-catalyzed reaction delivered the same regioisomer product (+)-4d (79:21 er) along with the C2 regioisomer in a 86:14 ratio. The C2/C3 regioisomers could not be separated by either silica gel chromatography or reverse-phase HPLC, but the comparable er and rr suggest that (+)-4d might be obtained with high enantiomeric purity.18
Scheme 3. (A) Reaction Profile Monitored by 19F NMRa and (B) Mechanistic Information from Control Experimentsb.

aCrude reactions analyzed by 1H and 19F NMR with C6F6 as the internal chemical shift reference set at −161.64 ppm in CDCl3. bReagents and conditions for imine 3 (1.0 equiv) with arenes 5b, 5d, 5h, and 5i (2.0 equiv) at rt: (a) AgOTf or Ag2CO3 (Ag+, 1.5 equiv) in CDCl3, no reaction observed; (b) TfOH (30 mol %) in CDCl3; (c) 4 Å molecular sieves (50 wt %) in CDCl3; (d) 4 Å molecular sieves (50 wt %) with (R)-TRIP (10 mol %) in CDCl3 for 24 h, C3/C2 rr of 86:14, (+)-4d er determined by chiral NP-HPLC analysis.
Given the role played by TfOH and (R)-TRIP as Brønsted acid catalysts in the key C-C bond-forming step from imine 3,24 we became interested in evaluating such a catalyst in combination with Ag2CO3 for halide abstraction. In principle, the cooperative action of an achiral transition metal with a chiral Brønsted acid could translate into a chiral counteranion catalysis approach (Scheme 4).25 In this reaction, (R)-TRIP should be easily deprotonated by Ag2CO3, leading to a transient silver phosphate catalyst which could further achieve the halide abstraction and a phase transfer resulting in a chiral iminium-phosphate pair 6. An iminium/imine equilibrium 6/6′ might be operating due to the low Brønsted basicity of imine 6′, likely leading to a H-bonding catalysis for the facial enantiodiscrimination of imine 6′. The addition of indole 5d was selected as a model reaction. We tested this approach with the enantiopure (R)-TRIP phosphoric acid catalyst (10 mol %) and observed that lower loadings of Ag2CO3 (0.6 equiv) and molecular sieves (50 wt %) reduced the proportion of the undesired C2 regioisomer.18 Under the optimized conditions, (+)-4d was obtained in 85% yield with a 90:10 er and a 91:09 rr. Crystallization of an EtOAc/EtOH solution of 4d by slow evaporation delivered (+)-4d in the mother liquor as a single regioisomer with an er of >99.5:0.5. The residual solid was analyzed and shown to be rich in the C2 regioisomer (80:20 rr), similar to the measured er of 78:22. Taken together, these results suggest that the enantiodiscrimination induced by the TRIP catalyst is remarkable at rt (>99% ee), yet further optimizations will be necessary to avoid the formation of regioisomers. The Friedel-Crafts alkylation with indole 5d was successfully scaled up to 1.0 mmol of (±)-2a with 5 mol % TRIP catalyst loading to afford product (+)-4d in 70% yield and >99% ee, which is more efficient than the original reaction of Bolm at −78 °C.11 In comparison to this study, the absolute configuration of α-amino ester (+)-4d should be (R) as depicted.
Scheme 4. Application to an Enantioselective Catalytic Aza-Friedel-Crafts Transformationa–c.
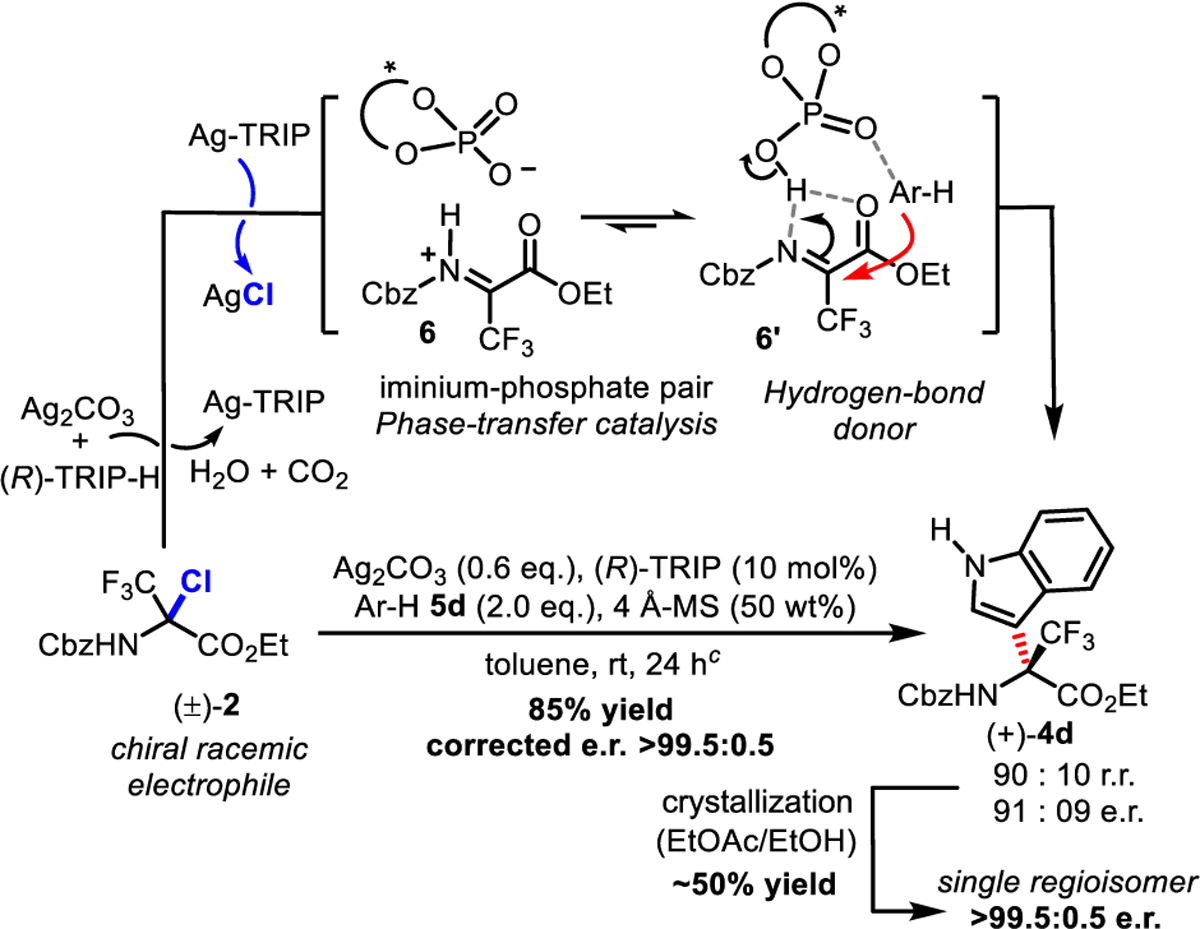
aer determined by NP-HPLC using an enantiodiscriminating Chiralcel OD-H stationary phase, and rr determined by 19F NMR. bThe estimated er should be corrected on the basis of the measured rr given that the minor C3 regioisomer co-elutes with the minor enantiomer (−)-4d in NP-HPLC (see the text for explanations). cReactions carried out at −20 and −78 °C afforded product (+)-4d with a similar er.
In summary, a versatile aza-Friedel-Crafts alkylation has been developed for the synthesis of quaternary α-trifluoromethyl-aryl amino esters. The combined halide abstraction/alkylation process is operationally simple under ambient conditions, highly efficient, regioselective, and amenable to a remarkable broad scope of arenes, spanning 6 orders of magnitude in nucleophilicity (indole, 5.5 ≥ N ≥ −1.2, anisole). The key feature of this reaction is the role associated with the silver(I) salt counteranions. In the course of silver-mediated halide abstraction, the silver counteranion is liberated as a conjugated Brønsted acid, likely resulting in an H-bond activation of the trifluoromethyl imine intermediate. This putative mechanism was exploited by replacing the achiral silver counteranion with a chiral phosphate in a catalytic enantioselective phase-transfer counteranion/Brønsted acid pair system to achieve an initial example of halide abstraction and aza-Friedel-Crafts alkylation with high stereoinduction (>99% ee). Further development of this asymmetric aza-Friedel-Crafts methodology for the synthesis of novel quaternary α-amino esters is of ongoing interest to our group. Ultimately, we anticipate that the present study will offer a useful new approach for the asymmetric synthesis of several classes of quaternary α-trifluoromethyl α-amino esters.
Supplementary Material
ACKNOWLEDGMENTS
The authors are very grateful for the financial support from the National Institutes of Health (NIH) (National Institute of General Medical Sciences Grant R15GM116025 to S.P.R. and S.S.S. and Grant R21GM132754 to G.Z.). The authors thank Dr. M. Zaghouani from Florida Atlantic University for obtaining some preliminary data for this project and Dr. G. Theodore from Theogen Corp. for manuscript proofreading and editing. The authors also thank Dr. C. D. Beadle for the training and mentoring of S.S.S, on the Automated Synthesis laboratory platform at Eli Lilly. Finally, the authors thank Dr. K. B. Basso at the Mass Spectrometry Research and Education Center from the Department of Chemistry at the University of Florida for the high-resolution mass spectrometry analysis supported by the NIH (S10 OD021758-01A1).
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.orglett.0c01895
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c01895.
Tables of selected results from the reaction optimization screen at Eli Lilly, complete experimental procedures and characterization data, including 1H, 13C, and 19F NMR spectra, and HPLC chromatograms for er determination (PDF)
The authors declare no competing financial interest.
Contributor Information
Guangkuan Zhao, Department of Chemistry and Biochemistry, Florida Atlantic University, Boca Raton, Florida 33431, United States.
Shyam S. Samanta, Department of Chemistry and Biochemistry, Florida Atlantic University, Boca Raton, Florida 33431, United States
Jessica Michieletto, Department of Chemistry and Biochemistry, Florida Atlantic University, Boca Raton, Florida 33431, United States.
Stéphane P. Roche, Department of Chemistry and Biochemistry and Center for Molecular Biology and Biotechnology, Florida Atlantic University, Boca Raton, Florida 33431, United States;.
REFERENCES
- (1).(a) Hein JE; Blackmond DG On the Origin of Single Chirality of Amino Acids and Sugars in Biogenesis. Acc. Chem. Res 2012, 45, 2045. [DOI] [PubMed] [Google Scholar]; (b) Elsila JE; Aponte JC; Blackmond DG; Burton AS; Dworkin JP; Glavin DP Meteoritic Amino Acids: Diversity in Compositions Reflects Parent Body Histories. ACS Cent. Sci 2016, 2, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Levine M; Kenesky CS; Mazori D; Breslow R Enantioselective Synthesis and Enantiomeric Amplification of Amino Acids under Prebiotic Conditions. Org. Lett 2008, 10, 2433. [DOI] [PubMed] [Google Scholar]; (b) Breslow R; Cheng Z-L On the origin of terrestrial homochirality for nucleosides and amino acids. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).For selected examples of α,α-disubstituted amino acids in bioactive molecules, see:; (a) Stilz HU; Jablonka B; Just M; Knolle J; Paulus EF; Zoller G Discovery of an Orally Active Non-Peptide Fibrinogen Receptor Antagonist. J. Med. Chem 1996, 39, 2118. [DOI] [PubMed] [Google Scholar]; (b) Hirayama R; Yamamoto M; Tsukida T; Matsuo K; Obata Y; Sakamoto F; Ikeda S Synthesis and biological evaluation of orally active matrix metalloproteinase inhibitors. Bioorg. Med. Chem 1997, 5, 765. [DOI] [PubMed] [Google Scholar]; (c) Ilies M; Di Costanzo L; Dowling DP; Thorn KJ; Christianson DW Binding of α,α-Disubstituted Amino Acids to Arginase Suggests New Avenues for Inhibitor Design. J. Med. Chem 2011, 54, 5432. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Van Zandt MC; Whitehouse DL; Golebiowski A; Ji MK; Zhang M; Beckett RP; Jagdmann GE; Ryder TR; Sheeler R; Andreoli M; Conway B; Mahboubi K; D’Angelo G; Mitschler A; Cousido-Siah A; Ruiz FX; Howard EI; Podjarny AD; Schroeter H Discovery of (R)-2-Amino-6-borono-2-(2-(piperidin-1-yl)ethyl)hexanoic Acid and Congeners As Highly Potent Inhibitors of Human Arginases I and II for Treatment of Myocardial Reperfusion Injury. J. Med. Chem 2013, 56, 2568. [DOI] [PubMed] [Google Scholar]
- (4).(a) Toniolo C; Bonora GM; Bavoso A; Benedetti E; Di Blasio B; Pavone V; Pedone C Preferred conformations of peptides containing α,α-disubstituted α-amino acids. Biopolymers 1983, 22, 205. [Google Scholar]; (b) Toniolo C; Crisma M; Formaggio F; Peggion C Control of peptide conformation by the Thorpe-Ingold effect (Cα-tetrasubstitution). Biopolymers 2001, 60, 396. [DOI] [PubMed] [Google Scholar]; (c) Demizu Y; Tanaka M; Doi M; Kurihara M; Okuda H; Suemune H Conformations of peptides containing a chiral cyclic α,α-disubstituted α-amino acid within the sequence of Aib residues. J. Pept. Sci 2010, 16, 621. [DOI] [PubMed] [Google Scholar]; (d) Maisch D; Wadhwani P; Afonin S; Böttcher C; Koksch B; Ulrich AS Chemical Labeling Strategy with (R)- and (S)-Trifluoromethylalanine for Solid State 19F NMR Analysis of Peptaibols in Membranes. J. Am. Chem. Soc 2009, 131, 15596. [DOI] [PubMed] [Google Scholar]; For a review discussing conformational analysis, see:; (e) Tanaka M Design and Synthesis of Chiral α,α-Disubstituted Amino Acids and Conformational Study of Their Oligopeptides. Chem. Pharm. Bull 2007, 55, 349. [DOI] [PubMed] [Google Scholar]
- (5).(a) Byrne L; Solà J; Boddaert T; Marcelli T; Adams RW; Morris GA; Clayden J Foldamer-Mediated Remote Stereocontrol: > 1,60 Asymmetric Induction. Angew. Chem., Int. Ed 2014, 53, 151. [DOI] [PubMed] [Google Scholar]; (b) Karnes MA; Schettler SL; Werner HM; Kurz AF; Horne WS; Lengyel GA Thermodynamic and Structural Impact of α,α-Dialkylated Residue Incorporation in a β-Hairpin Peptide. Org. Lett 2016, 18, 3902. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tomsett M; Maffucci I; Le Bailly BAF; Byrne L; Bijvoets SM; Lizio MG; Raftery J; Butts CP; Webb SJ; Contini A; Clayden J A tendril perversion in a helical oligomer: trapping and characterizing a mobile screw-sense reversal. Chem. Sci 2017, 8, 3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).For the most recent reviews, see:; (a) Vogt H; Bräse S Recent approaches towards the asymmetric synthesis of α,α-disubstituted α-amino acids. Recent approaches towards the asymmetric synthesis of α,α-disubstituted α-amino acids. Org. Biomol. Chem 2007, 5, 406. [DOI] [PubMed] [Google Scholar]; (b) Metz AE; Kozlowski MC Recent Advances in Asymmetric Catalytic Methods for the Formation of Acyclic α,α-Disubstituted α-Amino Acids. J. Org. Chem 2015, 80, 1. [DOI] [PubMed] [Google Scholar]; (c) Cativiela C; Ordóñez M; Viveros-Ceballos JL Stereoselective synthesis of acyclic α,α-disubstituted α-amino acids derivatives from amino acids templates. Tetrahedron 2020, 76, 130875. [Google Scholar]
- (7).(a) Salwiczek M; Nyakatura EK; Gerling UIM; Ye S; Koksch B Fluorinated amino acids: compatibility with native protein structures and effects on protein-protein interactions. Chem. Soc. Rev 2012, 41, 2135. [DOI] [PubMed] [Google Scholar]; (b) Marsh ENG Fluorinated Proteins: From Design and Synthesis to Structure and Stability. Acc. Chem. Res 2014, 47, 2878. [DOI] [PubMed] [Google Scholar]; (c) Berger AA; Völler J-S; Budisa N; Koksch B Deciphering the Fluorine Code—The Many Hats Fluorine Wears in a Protein Environment. Acc. Chem. Res 2017, 50, 2093 and references cited therein. [DOI] [PubMed] [Google Scholar]
- (8).For a review, see:; Eftekhari-Sis B; Zirak M α-Imino Esters in Organic Synthesis: Recent Advances. Chem. Rev 2017, 117, 8326. [DOI] [PubMed] [Google Scholar]
- (9).(a) Chaume G; Van Severen M-C; Marinkovic S; Brigaud T Straightforward synthesis of (S)- and (R)-α-trifluoromethyl proline from chiral oxazolidines derived from ethyl trifluoropyruvate. Org. Lett 2006, 8, 6123. [DOI] [PubMed] [Google Scholar]; (b) Min Q-Q; He C-Y; Zhou H; Zhang X Highly diastereoselective synthesis of quaternary α-trifluoromethyl α-amino acids from chiral imines of trifluoropyruvate. Chem. Commun 2010, 46, 8029. [DOI] [PubMed] [Google Scholar]; (c) Yang J; Min Q-Q; He Y; Zhang X Highly diastereoselective synthesis of quaternary α-trifluoromethyl α-amino acids by addition of benzylzinc reagents to chiral imines of trifluoropyruvate. Tetrahedron Lett. 2011, 52, 4675. [Google Scholar]; (d) Zhang F; Liu Z-J; Liu J-T Asymmetric aza-Henry reaction of chiral fluoroalkyl α,β-unsaturated N-tert-butanesulfinyl ketoimines: an efficient approach to enantiopure fluoroalkylated α,β-diamines and α,β-diamino acids. Org. Biomol. Chem 2011, 9, 3625. [DOI] [PubMed] [Google Scholar]; (e) Liu P; Liu Z-J; Wu F Highly regio- and diastereoselective addition of organolithium reagents to chiral fluoroalkyl α,β-unsaturated N-tert-butanesulfinyl ketimines: A general and efficient access to α-tertiary fluoroalkyl allylic amines and α-fluoroalkyl α-amino acids. Adv. Synth. Catal 2015, 357, 818. [Google Scholar]
- (10).(a) Huang G; Yang J; Zhang X Highly enantioselective zinc/BINOL-catalyzed alkynylation of α-ketoimine ester: a new entry to optically active quaternary α-CF3 α-amino acids. Chem. Commun 2011, 47, 5587. [DOI] [PubMed] [Google Scholar]; (b) Morisaki K; Sawa M; Nomaguchi J-Y; Morimoto H; Takeuchi Y; Mashima K; Ohshima T Rh-Catalyzed Direct Enantioselective Alkynylation of α-Ketiminoesters. Chem. - Eur. J 2013, 19, 8417. [DOI] [PubMed] [Google Scholar]; (c) Morisaki K; Sawa M; Yonesaki R; Morimoto H; Mashima K; Ohshima T Mechanistic Studies and Expansion of the Substrate Scope of Direct Enantioselective Alkynylation of α-Ketiminoesters Catalyzed by Adaptable (Phebox)-Rhodium(III) Complexes. J. Am. Chem. Soc 2016, 138, 6194. [DOI] [PubMed] [Google Scholar]; (d) Winter M; Faust K; Himmelsbach M; Waser M Synthesis of α-CF3-proline derivatives by means of a formal (3 + 2)-cyclization between trifluoropyruvate imines and Michael acceptors. Org. Biomol. Chem 2019, 17, 5731. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Winter M; Kim H; Waser M Pd-Catalyzed Allylation of Imines to Access α-CF3-Substituted α-Amino Acid Derivatives. Eur. J. Org. Chem 2019, 2019, 7122. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Bhakta U; Kattamuri PV; Siitonen JH; Alemany LB; Kurti L Enantioselective Catalytic Allylation of Acyclic Ketiminoesters: Synthesis of α-Fully-Substituted Amino Esters. Org. Lett 2019, 21, 9208. [DOI] [PubMed] [Google Scholar]
- (11).(a) Abid M; Teixeira L; Török B Triflic Acid-Catalyzed Highly Stereoselective Friedel-Crafts Aminoalkylation of Indoles and Pyrroles. Org. Lett 2008, 10, 933. [DOI] [PubMed] [Google Scholar]; (b) Török B; Sood A; Bag S; Kulkarni A; Borkin D; Lawler E; Dasgupta S; Landge S; Abid M; Zhou W; Foster M; LeVine H; Török M tionships of Organofluorine Inhibitors of β-Amyloid Self-Assembly. ChemMed-Chem 2012, 7, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Husmann R; Sugiono E; Mersmann S; Raabe G; Rueping M; Bolm C Enantioselective organocatalytic synthesis of quaternary α-amino acids bearing a CF3 moiety. Org. Lett 2011, 13, 1044. [DOI] [PubMed] [Google Scholar]
- (13).(a) Saaby S; Nakama K; Lie MA; Hazell RG; Jorgensen KA The first catalytic highly enantioselective alkylation of ketimines - a novel approach to optically active quaternary α-amino acids. Chem. - Eur. J 2003, 9, 6145. [DOI] [PubMed] [Google Scholar]; (b) Skarpos H; Vorob’eva DV; Osipov SN; Odinets IL; Breuer E; Roeschenthaler G-V Methyltrifluoropyruvate imines possessing N-oxalyl and N-phosphonoformyl groups-precursors to a variety of α-CF3-α-amino acid derivatives. Org. Biomol. Chem 2006, 4, 3669. [DOI] [PubMed] [Google Scholar]
- (14).(a) Bravo P; Crucianelli M; Vergani B; Zanda M Sulfinimines of trifluoropyruvate: novel intermediates for chiral non racemic α-trifluoromethyl α-amino acids. Tetrahedron Lett. 1998, 39, 7771. [Google Scholar]; (b) Fustero S; Miró J; Sánchez-Roselló M; del Pozo C Tandem Gold Self-Relay Catalysis for the Synthesis of 2,3-Dihydropyridin-4(1 H)-ones: Combination of σ and π Lewis Acid Properties of Gold Salts. Chem. - Eur. J 2014, 20, 14126. [DOI] [PubMed] [Google Scholar]; (c) Yang J; Wang Z; He Z; Li G; Hong L; Sun W; Wang R Organocatalytic Enantioselective Synthesis of Tetrasubstituted α-Amino Allenoates by Dearomative γ-Addition of 2,3-Disubstituted Indoles to β,γ-Alkynyl-α-imino Esters. Angew. Chem., Int. Ed 2020, 59, 642. [DOI] [PubMed] [Google Scholar]
- (15).(a) Wasa M; Liu RY; Roche SP; Jacobsen EN Asymmetric Mannich Synthesis of α-Amino Esters by Anion-Binding Catalysis. J. Am. Chem. Soc 2014, 136, 12872. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Samanta SS; Roche SP In situ-generated glycinyl chloroaminals for a one-pot synthesis of non-proteinogenic α-amino esters. J. Org. Chem 2017, 82, 8514. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bendelsmith AJ; Kim SC; Wasa M; Roche SP; Jacobsen EN Enantioselective Synthesis of α-Allyl Amino Esters via Hydrogen-Bond-Donor Catalysis. J. Am. Chem. Soc 2019, 141, 11414. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Samanta SS; Roche SP Synthesis and reactivity of α-haloglycine esters: Hyperconjugation in action. Eur. J. Org. Chem 2019, 2019, 6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Zhao G; Samanta SS; Michieletto J; Roche SP Synthesis of Quaternary t-Amino Esters: A Remarkably Broad Substrate Scope in Aza-Friedel-Crafts Alkylation. ChemRxiv 2020, DOI: 10.26434/chemrxiv.12111309.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).(a) Hands-Off Chemistry. Chem. Eng. News 2012, 90, 12. [Google Scholar]; (b) Godfrey AG; Masquelin T; Hemmerle H A remote-controlled adaptive medchem lab: an innovative approach to enable drug discovery in the 21st Century. Drug Discovery Today 2013, 18, 795. [DOI] [PubMed] [Google Scholar]
- (18). See the Supporting Information for complete experimental details.
- (19).For selected reviews of silver(I)-mediated and catalyzed reactions, see:; (a) Weibel J-M; Blanc A; Pale P Ag-Mediated Reactions: Coupling and Heterocyclization Reactions. Chem. Rev 2008, 108, 3149. [DOI] [PubMed] [Google Scholar]; (b) Yanagisawa A; Yamamoto H Catalytic Asymmetric Carbon-Carbon Bond Forming Reactions Using Chiral Silver Complexes. Yuki Gosei Kagaku Kyokaishi 2005, 63, 888. [Google Scholar]
- (20).For a detailed explanation of the empirical π-nucleophilicity scale, see:; (a) Mayr H; Bug T; Gotta MF; Hering N; Irrgang B; Janker B; Kempf B; Loos R; Ofial AR; Remennikov G; Schimmel H Reference Scales for the Characterization of Cationic Electrophiles and Neutral Nucleophiles. J. Am. Chem. Soc 2001, 123, 9500. [DOI] [PubMed] [Google Scholar]; (b) Mayr H; Kempf B; Ofial AR π-Nucleophilicity in Carbon-Carbon Bond-Forming Reactions. Acc. Chem. Res 2003, 36, 66. [DOI] [PubMed] [Google Scholar]; (c) Lakhdar S; Westermaier M; Terrier F; Goumont R; Boubaker T; Ofial AR; Mayr H Nucleophilic Reactivities of Indoles. J. Org. Chem 2006, 71, 9088. [DOI] [PubMed] [Google Scholar]; (d) Nigst TA; Westermaier M; Ofial AR; Mayr H Eur. J. Org. Chem 2008, 2008, 2369. [Google Scholar]
- (21).Rosenau CP; Jelier BJ; Gossert AD; Togni A Exposing the Origins of Irreproducibility in Fluorine NMR Spectroscopy. Angew. Chem., Int. Ed 2018, 57, 9528. [DOI] [PubMed] [Google Scholar]
- (22).Appel R; Chelli S; Tokuyasu T; Troshin K; Mayr H Electrophilicities of Benzaldehyde-Derived Iminium Ions: Quantification of the Electrophilic Activation of Aldehydes by Iminium Formation. J. Am. Chem. Soc 2013, 135, 6579. [DOI] [PubMed] [Google Scholar]
- (23).(a) Yonesaki R; Kondo Y; Akkad W; Sawa M; Morisaki K; Morimoto H; Ohshima T 3-Mono-Substituted BINOL Phosphoric Acids as Effective Organocatalysts in Direct Enantioselective Friedel-Crafts-Type Alkylation of N-Unprotected α-Ketiminoester. Chem. - Eur. J 2018, 24, 15211–15214. [DOI] [PubMed] [Google Scholar]; (b) Hatano M; Okamoto H; Kawakami T; Toh K; Nakatsuji H; Sakakura A; Ishihara K Enantioselective aza-Friedel-Crafts reaction of furan with α-ketimino esters induced by a conjugated double hydrogen bond network of chiral bis(phosphoric acid) catalysts. Chem. Sci 2018, 9, 6361–6367. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Miyagawa M; Yoshida M; Kiyota Y; Akiyama T Enantioselective Friedel-Crafts Alkylation Reaction of Heteroarenes with N-Unprotected Trifluoromethyl Ketimines by Means of Chiral Phosphoric Acid. Chem. - Eur. J 2019, 25, 5677–5681. [DOI] [PubMed] [Google Scholar]
- (24).For reviews, see:; (a) Terada M Chiral phosphoric acids as versatile catalysts for enantioselective transformations. Synthesis 2010, 2010, 1929. [Google Scholar]; (b) Parmar D; Sugiono E; Raja S; Rueping M Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev 2014, 114, 9047. [DOI] [PubMed] [Google Scholar]
- (25).(a) Mayer S; List B Angew. Chem., Int. Ed 2006, 45, 4193. [DOI] [PubMed] [Google Scholar]; (b) Hamilton GL; Kang EJ; Mba M; Toste FD Science 2007, 317, 496. [DOI] [PubMed] [Google Scholar]; (c) Hamilton GL; Kanai T; Toste FD J. Am. Chem. Soc 2008, 130, 14984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


