Abstract
Objective:
To compare proteomics and biological function of human dentin matrix molecules (hDMMs) and bovine dentin matrix molecules (bDMMs).
Design:
Dentin powder from human or bovine teeth (n=4) was demineralized in 10% (v/v) ethylenediaminetetraacetic acid for 7 days. The extracts were dialyzed, lyophilized and proteins were characterized using liquid chromatography-tandem mass spectrometry and shotgun proteomic analysis. To study biological function, mouse-derived undifferentiated dental pulp cells (OD21) were treated with 0.01, 0.1 or 1μg/mL of hDMMs or bDMMs and proliferation was measured after 24 hours and 48 hours using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cell migration was assessed after 24 hours using a Boyden chamber. Alizarin Red S staining was used to evaluate mineral formation.
Results:
There were 307 proteins identified, of which 93 proteins were common to both species. Gene Ontology functional analysis demonstrated similar pattern of biological process in both species which consisted mainly of tissue development and biomineralization. hDMMs and bDMMs both enhanced cell proliferation. After 24 hours, all concentrations of bDMMs promoted cell proliferation (p≤0.05), while hDMMs did not affect proliferation. After 48 hours, groups with 1μg/mL of bDMMs and 0.01μg/mL of hDMMs had increased cell proliferation compared to control (p≤0.0001). All concentrations of hDMMs and bDMMs enhanced cell migration and mineralization (p≤0.0001).
Conclusion:
bDMMs has similar biological functions as hDMMs. Moreover, bDMMs stimulated cell proliferation, migration and differentiation similar to hDMMs.
Keywords: dentin, growth factors, proteomics, dentin matrix components, cell differentiation
1. Introduction
The structure and composition of dentin matrix play an important role in the regeneration and function of the dental pulp. Harmful stimuli to dentinal tissue, such as caries and some dental materials stimulate progenitor cells in the pulp to proliferate, migrate and differentiate into odontoblast-like cells to produce reparative dentin (Cooper, Chicca, Holder, & Milward, 2017; Simon et al., 2011). Also, it has long been suggested that these noxious stimuli induce the release of bioactive molecules previously immobilized within the dentin extracellular matrix (ECM), thus resulting in their diffusion into the dental pulp. Once released, these bioactive molecules can stimulate regenerative processes in the dentin-pulp complex, playing an important role in tertiary dentin formation by inducing the underlying odontoblasts to secrete new dentin and activating differentiation of odontoblast-like cells, or both (Cooper et al., 2010; Smith et al., 1995; Smith, Murray, Sloan, Matthews, & Zhao, 2001; Smith et al., 1994).
The human dentin ECM is composed of collagenous and non-collagenous organic components, such as dentin sialoproteins, phophoproteins and proteoglycans (Goldberg & Smith, 2004; Jagr et al., 2014; Stankoska et al., 2016). Type I collagen fibrils represent the major collagenous component in the tissues, and serve as a scaffold for dentin mineralization, although trace amounts of collagen type VI have also been reported (Stankoska et al., 2016). Non-collagenous matrix molecules include a wide range of bioactive proteins and proteoglycans (Goldberg & Smith, 2004; Jágr, Eckhardt, Pataridis, & Miksik, 2012; Park et al., 2009; Smith et al., 2012), including dentin sialophosphoprotein (DSPP), dentin matrix protein-1 (DMP-1), bone sialoprotein, osteocalcin, all which are known to regulate the dentin mineralization process (Smith et al., 2012); as well as decorin and biglycan, which are proteoglycans from the Small Leucine-rich Repeat Proteoglycans (SLRP) family, known for their structural and mechanical roles (Bertassoni & Swain, 2014), among other things. Dentin non-collagenous matrix also contains growth factors, such as transforming growth factor beta (TGFβ), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF), which can stimulate vascular formation, growth and mineralization of the dentin-pulp complex (Goldberg & Smith, 2004; Smith et al., 2012). It has been reported, both in vitro and in vivo, that these dentin matrix molecules (DMMs) extracted from dentin can stimulate dental pulp cell growth, mineral matrix deposition and tertiary dentin formation (Athirasala et al., 2018; Okamoto, Takahashi, Komichi, Cooper, & Hayashi, 2018; Petridis et al., 2018; Salehi, Cooper, Smith, & Ferracane, 2016; Smith et al., 1995). Therefore, they have been extensively proposed and used for their potential as regenerative materials, or as part of the composition of bioactive scaffolds for dental pulp regeneration.
While the applicability of DMMs for dental pulp regeneration has been well documented, methods of processing and scaling up the isolation of DMMs for their use as tooth-derived bioactive materials have been limited. One important aspect to be considered in the use of these bioactive molecules for the development of dentin-derived biomaterials for pulp regeneration in the clinic, is the fact that not only are human teeth difficult to procure in large quantities, but also there are important ethical concerns with using allogeneic human teeth for regeneration of dental pulp in patients. For these reasons, an easily sourced, well-characterized and established source of animal teeth for large scale isolation and processing of DMM for dental pulp regeneration may be useful for the future development of dental materials for regenerative therapies. Bovine teeth are a suitable source of DMMs for dental pulp regeneration given the size, availability and well-regulated sourcing of bovine tissues for biomedical use by the Food and Drug Administration (FDA) and the United States Department of Agriculture (USDA). However, studies characterizing the biological equivalence of human and bovine DMMs with respect to their composition and odontogenic potential are not available, but are required before recommending the use of bovine teeth for isolation of bioactive DMMs.
To address these goals, here we aimed to (i) investigate and compare the proteomes of human and bovine dentin matrix molecules (hDMMs and bDMMs, respectively), and (ii) to identify potential similarities regarding their key biological functions, such as their comparative effect on dental pulp cell proliferation, migration and odontogenic differentiation. We hypothesized that bDMMs have similar composition and biological function as those derived from human teeth and can be used as an alternative to hDMMs for pulp regeneration.
2. Materials and methods
2.1. Isolation of dentin matrix molecules
Bovine incisors were extracted from 30-month-old slaughtered heifers from an establishment inspected by the (USDA #M9233/P9223). Human third molars, used as a control, were extracted at the Oregon Health & Science University dental clinics from donors with informed consent and after institutional ethics approval. The teeth were kept in a 0.5% chloramine T solution (Sigma-Aldrich, St. Louis, MO, US) at 4°C for no more than one week before processing.
For processing, as previously described by Tomson et al. (2007), teeth were subjected to thorough irrigation with saline, deionized water and a toothbrush. Soft tissue was removed using a scalpel, while enamel and cementum were removed using a diamond bur under water cooling. Endodontic files were used to remove the pulp tissue. Subsequently, the teeth were stored at 4°C in a phosphate-buffered saline solution (1x) until further use. The remaining pieces of dentin were crushed into a fine powder using a 6700 Spex SamplePrep freezer/mill (Glen Creston Ltd, Stanmore, UK) under liquid nitrogen and operated via a magnetically driven impactor. Then the powdered dentin was sieved through a 60 μm mesh sieve (≤ 0.251 mm2). Next, 5 g of pulverized dentin powder was weighted and immersed in 20 mL of an extraction solution of 10% (w/v) ethylenediaminetetraacetic acid (EDTA) in Tris buffer, pH 7.2, containing Halt protease inhibitor cocktail (1 μl/mL) (ThermoFisher Scientific, Waltham, MA, US). During the following seven days the extraction solution was changed daily, the supernatants were collected and kept frozen at −80°C. Subsequently, the supernatants were thawed, dialyzed at 4°C for 5 days and lyophilized for 48 hours. The powder was kept in −80°C until further use.
2.2. Quantification of extracted matrix molecules
The amount of matrix obtained from human and bovine dentin were evaluated by using bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford, IL, USA). Human and bovine teeth sizes are different, so to avoid a potential bias on the final amount of protein concentration, the total pulverized powder from the each tooth was weighted before DMM extraction. Next, for the BCA assay, same amounts of bovine and human DMM extracts were dissolved in deionized water, mixed with BCA working reagent and incubated for 30 minutes. The absorbance was then measured at 562 nm using a microplate reader (BioTek, Vermont, USA) and the protein concentrations were the calculated based on the albumin standard curve, as recommended by the manufacturer’s protocol. To obtain an estimate of dentin matrix molecules yielded by each tooth, the protein concentration data was normalized by the original weight of the tooth and volume of the DMM extracts.
2.3. Proteomics
Human and bovine lyophilized extracts were digested via enhanced filter-aided sample preparation (eFASP) (Erde, Loo, & Loo, 2014) and characterized using liquid chromatrography/mass spectrometry (LC/MS) and an Orbitrap Fusion Tribrid mass spectrometer (ThermoFisher Scientific). DMMs were dissolved in deionized water and the protein content was determined by a BCA assay (Pierce Biotechnology). As described above, four samples from each group were used for protein digestion. Briefly, 60 μg of protein from each sample were dissolved in 4% sodium dodecyl sulphate, 0.2% deoxycholic acid and 100mM triethylammonium bicarbonate, samples heated at 90°C for 15 minutes, followed by enhanced filter-aided sample preparation (eFASP) digestion and peptide assay. Next, 3 μg of digested peptides from four biological replicates from each group were pooled to create a total of 12 μg peptides for bDMM and hDMM samples, respectively. The pooled samples were dried, dissolved in 12 μL of 5% formic acid,3% acetonitrile, and 4 μL/sample (4 μg) injected into a Thermo Orbitrap Fusion with Dionex Ultimate 3000 Nano LC system equipped with Thermo Pepmap 100 C18 column (ThermoFisher Scientific) and analyzed using a single 4-hour liquid chromatography-mass spectrometry (LC-MS/MS) method.
Peptides were identified from the tandem mass spectrometry data using MaxQuant (version 1.6.5.0) (Cox & Mann, 2008). Protein relative abundances were estimated from the parent-ion mass analysis (MS1) feature intensities using the intensity-based absolute quantification (iBAQ) option. The searches used UniProt (www.uniprot.org) canonical reference proteomes for bovine (22007 sequences) and human (21053 sequences) downloaded November 2018 using utilities at https://github.com/pwilmart/fasta_utilities. Default MaxQuant parameters were used: 20 parts per million (ppm) first search, 4.5 ppm second search, tryptic cleavage with maximum of two missed cleavages, variable oxidized Methionine and protein N-term acetylation, static alkylated Cysteine, minimum peptide length of 7, peptide-spectrum match false discovery rate (FDR) of 1%, and minimum one peptide per protein. MaxQuant is distributed with common laboratory contaminant sequences and creates reversed sequence decoys internally (revert method). Bovine and human samples were analyzed in separate MaxQuant analyses. Gene ontology annotation for functional analysis were performed using the Panther database (Mi et al., 2005).
2.4. Cell culture
An undifferentiated murine dental pulp cell (OD-21) line (Hanks et al., 1998) was maintained in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Grand Island, NY,USA) containing 4.5 g/L glucose, 10% fetal bovine serum (FBS; Hyclone, Utah, USA), and 1 % (v/v) penicillin/streptomycin (Corning, NY, USA) at 37°C in a humidified atmosphere containing 5% CO2.
2.5. Proliferation Assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Assay was used to assess the metabolic activity of the OD21 cells. Cells were seeded in 96-well plates at a density of 4 × 103 cells/well in complete medium as described above. After cells had completely adhered to the bottom of the plate, cell culture medium was changed to medium with 5% fetal bovine serum containing 0.01, 0.1, or 1 μg/mL of hDMMs or bDMMs. OD21 cells were then cultured for 24 and 48 hours, followed by addition of 0.5 mg/mL of MTT (Millipore, Burlington, MA, USA) working solution and incubated for 4 hours. Dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) was used to dissolve the formazan salt and absorbance was measured at 570 nm. A standard curve of optical density for known cell numbers was established by making serial dilutions of cells in two 96-well plates. For both cell plates, cells were incubated for 24 hours, then for one plate, cells were trypsinized and counted, while the other plate was incubated with MTT solution and had absorbance measured using a microplate reader (BioTek). The data for cell number and optical density were correlated and plotted as a standard curve.
2.6. Migration Assay
Cell migration illustrates the chemotactic effect of dentin matrix molecules on dental pulp cells (Lauffenburger & Horwitz, 1996) and was measured via a Boyden chamber assay. Briefly, OD21 cells at a density of 2 × 104 cells were seeded in the upper chamber of a 24-well plate with 8.0 μm pore size membrane (Corning Life Sciences, Tewksbury, MA, US) in serum-free medium. The lower chamber contained 0.01, 0.1, or 1 μg/mL of human or bDMMin serum free medium. Negative control samples were comprised of serum free medium without DMMs. After 24 hours of incubation, cells were fixed in in 10% neutral buffered formalin (Sigma-Aldrich) and stained with 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) (Molecular Probes, Life Technologies). The images were analyzed using ImageJ (National Institutes of Health, NIH free software, Bethesda, Maryland, US) and migration was calculated as a percentage of cells that migrated to the bottom of the membrane.
2.7. Mineralization Assay
Cells were seeded in 24-well plates at a density of 5 × 104 cells/well in cell culture medium, and after reaching confluency, they were treated with odontogenic medium containing 50 μg/ml of ascorbic acid (Fisher Scientific, Hampton, New Hampshire, US) and 10 mM β-glycerophosphate (Millipore) supplemented with different concentrations of human or bovine dentin matrix molecules (0.01, 0.1, or 1 μg/mL) for 5 days. Two separate controls were used, (i) cells cultured in odontogenic medium without DMMs and (ii) cells kept in basal cell culture medium. For positive controls, cells were cultured in odontogenic medium supplemented with 10 mM dexamethasone (Sigma-Aldrich). At the end of 5 days, cells were fixed in 10% (v/v) neutral buffered formalin and stained with 2% (w/v) Alizarin red S (Electron Microscopy Sciences, Hatfield, PA, USA). Acetic acid extraction method was used for quantification of alizarin red stain, according to the protocol described by (Gregory, Gunn, Peister, & Prockop, 2004). In short, 10% (v/v) acetic acid (Sigma-Aldrich) was added to each well and incubated for 30 minutes, then the samples were neutralized with 10% (v/v) ammonium hydroxide (Sigma-Aldrich). Absorbance was measured at 405 nm with a microplate reader (BioTek).
2.8. Statistical Analysis
All experiments were performed in quadruplicate and values are expressed as mean ± standard deviation. Statistical analysis was performed using Graphpad Prism software. The differences between three or more groups were analyzed by One/Two-way Analysis of variance (ANOVA) followed by Tukey’s post hoc test (α = 0.05), and differences between two groups were analyzed by independent samples t-test (α = 0.05).
3. Results
3.1. Relative concentration of dentin matrix molecules in human and bovine dentin
The normalized data comparing DMMs concentration from human and bovine dentin showed that after 7 days of extraction, bovine dentin extracts yielded an average of 21.61 mg of total protein per tooth, while the human extracts yielded only 10.79 mg (n=3, Student’s t-test, *p≤ 0.05) (Fig. 1).
Fig. 1.
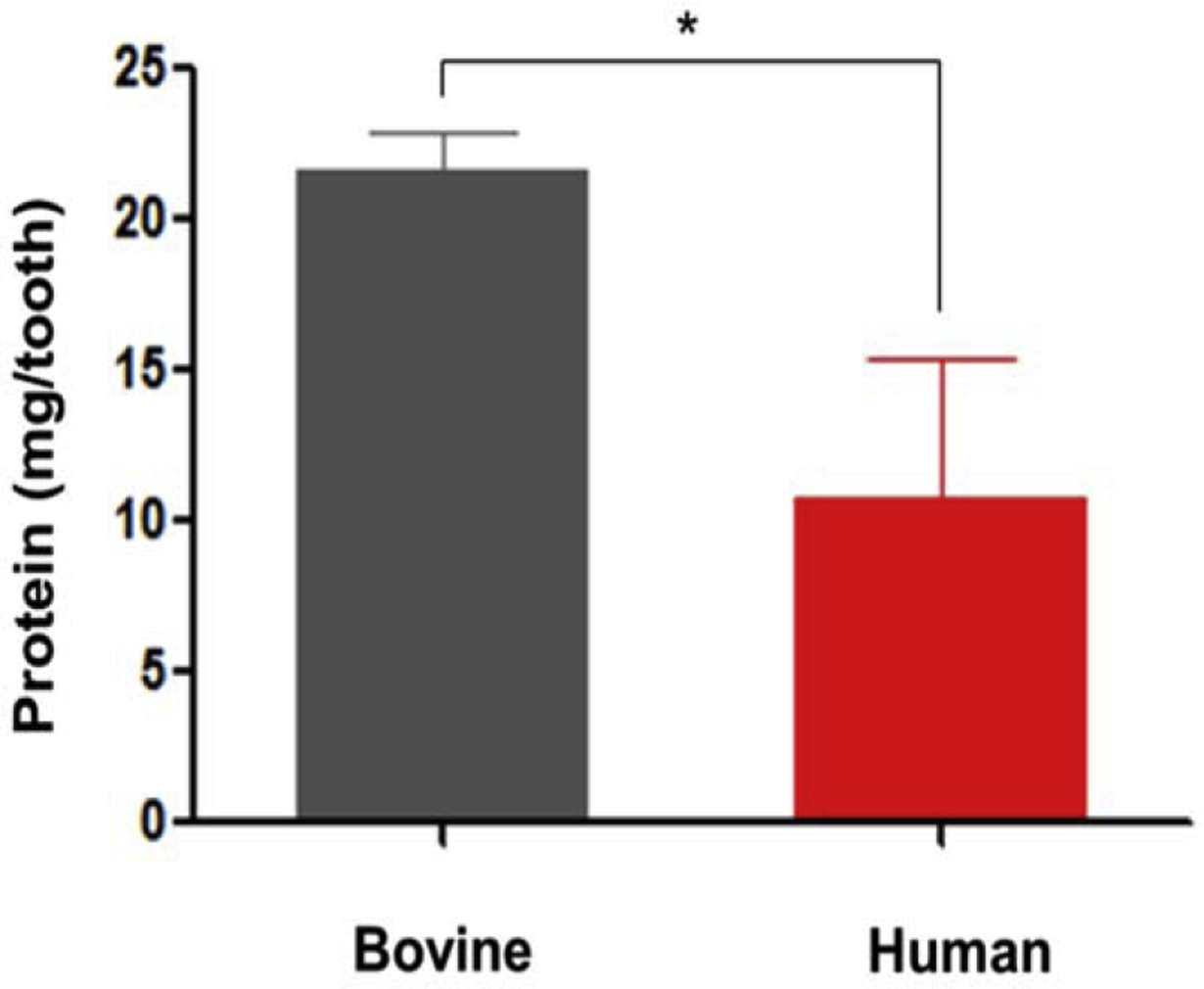
Comparison of protein content in bovine and human dentin matrix extracts. Bovine teeth yield 2 times more protein than human teeth (n=3), bars and error bars are mean ± standard deviation, (*p≤ 0.05) (t-test, α=0.05).
3.2. Proteomic profiles of human and bovine dentin matrix molecules
Proteomic profiles obtained from pooled dentin sample (n=4) for each group. A total of 307 proteins were identified through liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis. Of these identified proteins, 93 proteins were present in both human and bovine dentin extracellular matrix (Fig. 2, and S1–S3 Table).
Fig. 2.
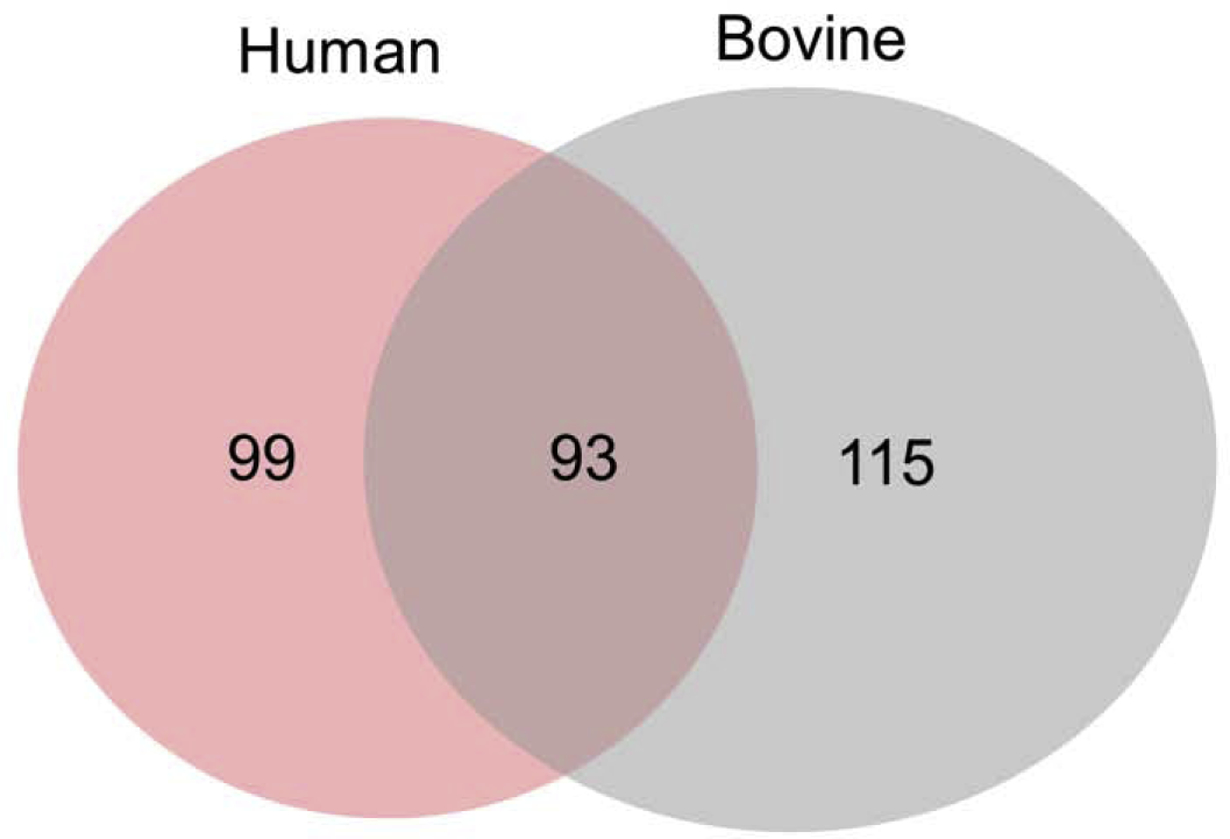
Venn diagram showing the unique and overlapped proteins of human and bovine dentin extracellular matrix. Pooled dentin sample from 4 teeth (n=4) for each group.
Of the 93 proteins found in human and bovine samples, collagenous proteins are the major component in both species (Fig. S1). From the large set of non-collagenous proteins present in both tissues, we selected matrix molecules that are known to regulate dentin formation and mineral matrix deposition. Comparison of the selected molecules found in both species is shown in Fig. 3A, B, and C. Matrix Gla protein (MGP) was the most abundant non-phosphorylated protein detected in both species whereas, biglycan and decorin were the major components of the Small leucine-rich Repeat Proteoglycans family Moreover, TGFβ1 was the most abundant growth factor found in both species with higher levels detected in human dentin, while higher levels of IGF binding protein (IGFBP5) were found in bovine dentin.
Fig. 3.
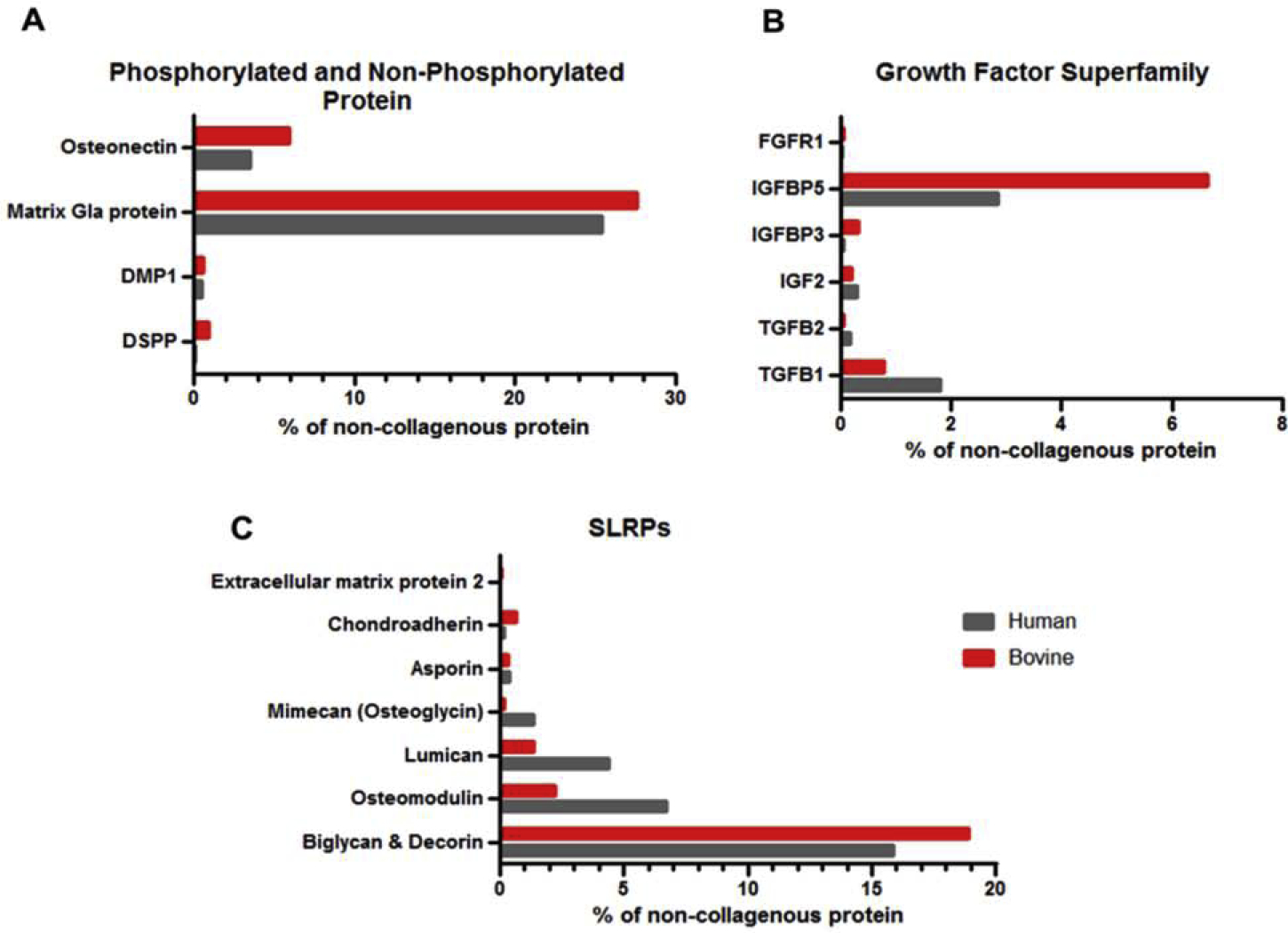
Comparison of DMMs present both in human and bovine dentin that were related to dental tissue regeneration. A: Phosphorylated and non-phosphorylated proteins, B: Growth factor superfamily, C: Small Leucine-Rich Proteoglycans (SLRPs), (DMP1: Dentin matrix acidic phosphoprotein 1, DSPP: Dentin sialophosphoprotein, TGFB1: Transforming growth factor beta-1, TGFB2: Transforming growth factor beta-2 , IGF2: Insulin-like growth factor 2, IGFBP3: Insulin-like growth factor-binding protein 3, IGFBP5: Insulin-like growth factor-binding protein 5, FGFR1: Fibroblast growth factor receptor 1). The x-axes are the fractions of the iBAQ intensities of the individual proteins out of the iBAQ intensity total for that protein class.
Although, the amount of individual proteins found in each species were different, the functional enrichment analysis of dentin samples using Gene Ontology annotation database (Mi et al., 2005) showed that dentin extracellular matrix from both species have similar distribution of biological processes for regeneration and matrix mineralization (Fig. 4), with the major difference being an increase in the percentage of proteins involved in extracellular matrix organization in human versus bovine teeth. Of note, molecules involved in wound healing and the transforming growth factor β receptor signaling had relatively lower concentrations in each respective proteome. Proteins involved in proliferation, adhesion and migration were also clearly identified.
Fig. 4.
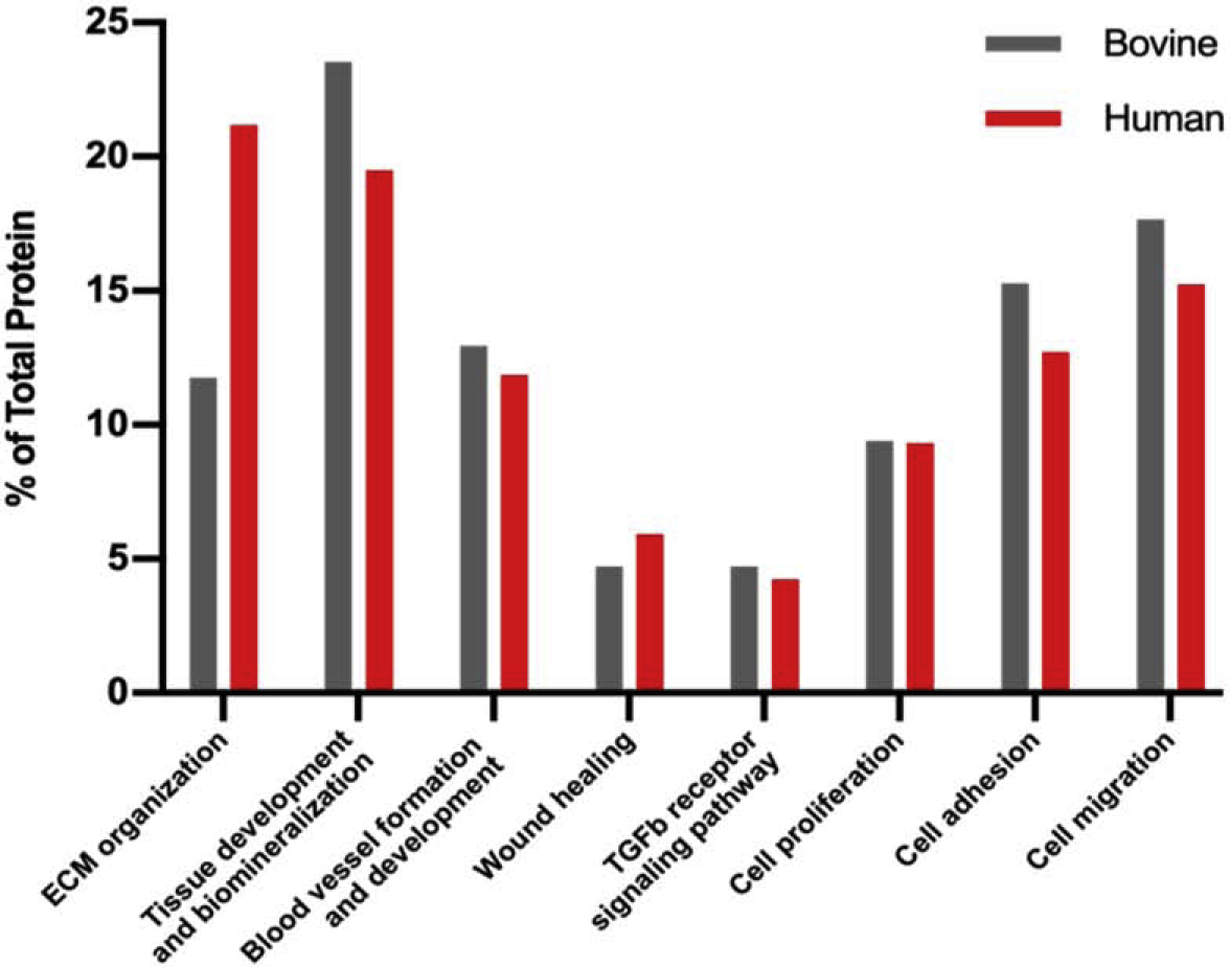
Comparison of biological process of protein found in human and bovine dentin ECM.
3.3. Proliferation Assay
When cells were cultured in hDMMs for at least 24 hours, no effects on cell proliferation were observed. In contrast, bDMMat concentrations of 0.01, 0.1, or 1 μg/mL significantly increased the number of the cells in comparison to controls (p≤ 0.05, p≤ 0.0001, p≤ 0.05, respectively). After 48 hours, hDMM (0.01 μg/mL) and bDMMs (1 μg/mL) elicited greater cell proliferation effects when compared to control (no DMMs treatment) (p≤ 0.0001) (Fig. 5).
Fig. 5.
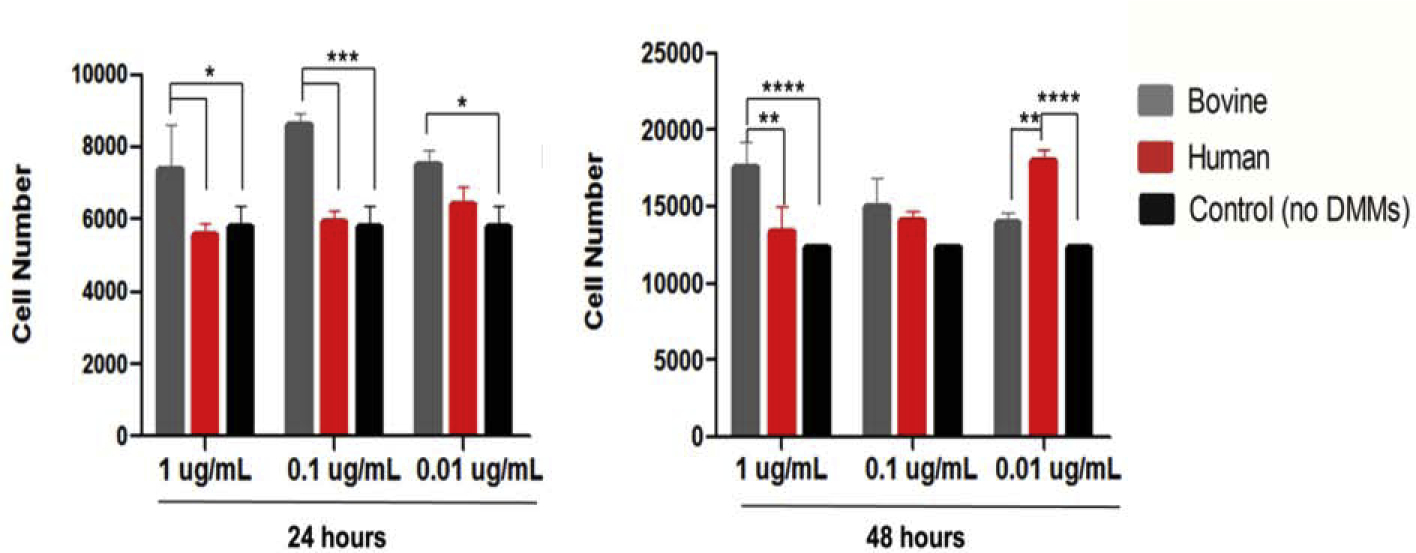
The effects of various concentrations of human and bovine DMMs on proliferation of OD-21 cells evaluated after 24 hours (A) and 48 hours (B), bars and error bars are mean ± standard deviation, (*p≤ 0.05, **p≤ 0.01, ***p≤ 0.001, ****p≤ 0.0001) (ANOVA, α=0.05).
3.4. Migration Assay
All concentrations of both bovine and human dentin matrix molecules elicited significantly greater migration of OD21 cells (p≤ 0.0001) compared to controls (no DMMs treatment). No differences were observed between each concentration of DMMs or the source (human or bovine) of the proteins (Fig. 6).
Fig. 6.
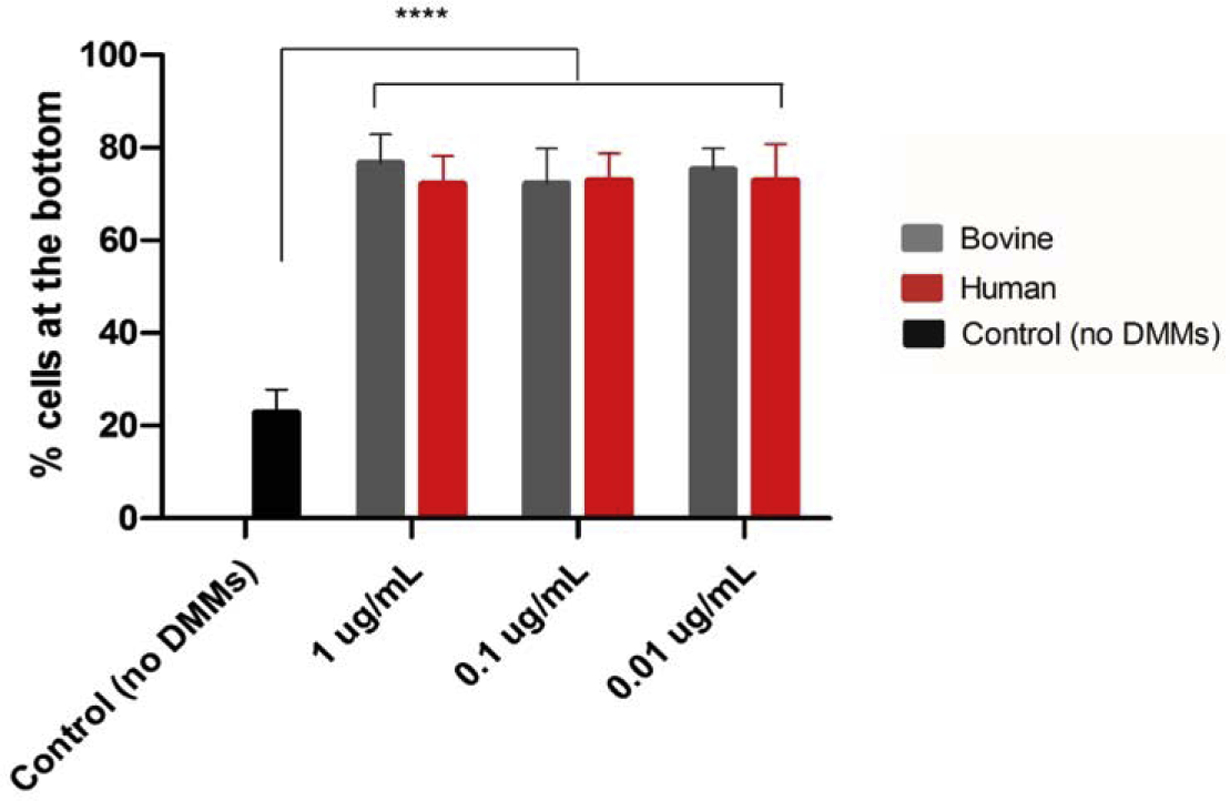
The effects of various concentrations of human and bovine DMMs on migration of OD-21 cells evaluated after 24 hours DMMs exposure, bars and error bars are mean ± standard deviation, (****p≤ 0.0001) (ANOVA, α=0.05)
3.5. Mineralization Assay
The Alizarin red S stain assay showed that both human and bovine dentin matrix molecules promoted cell mineralization (Fig. 7 A, B) and that all tested concentrations of hDMMs and bDMMs significantly induced mineral formation in OD21 cells, compared with cells that were incubated in odontogenic medium only (p≤ 0.0001). Cells that were exposed to dexamethasone, which served as a positive control, showed significantly higher mineral deposition (p<0.0001). No effects of the concentration of DMMs were observed on the amount of mineral deposition in OD21 cells.
Fig. 7.
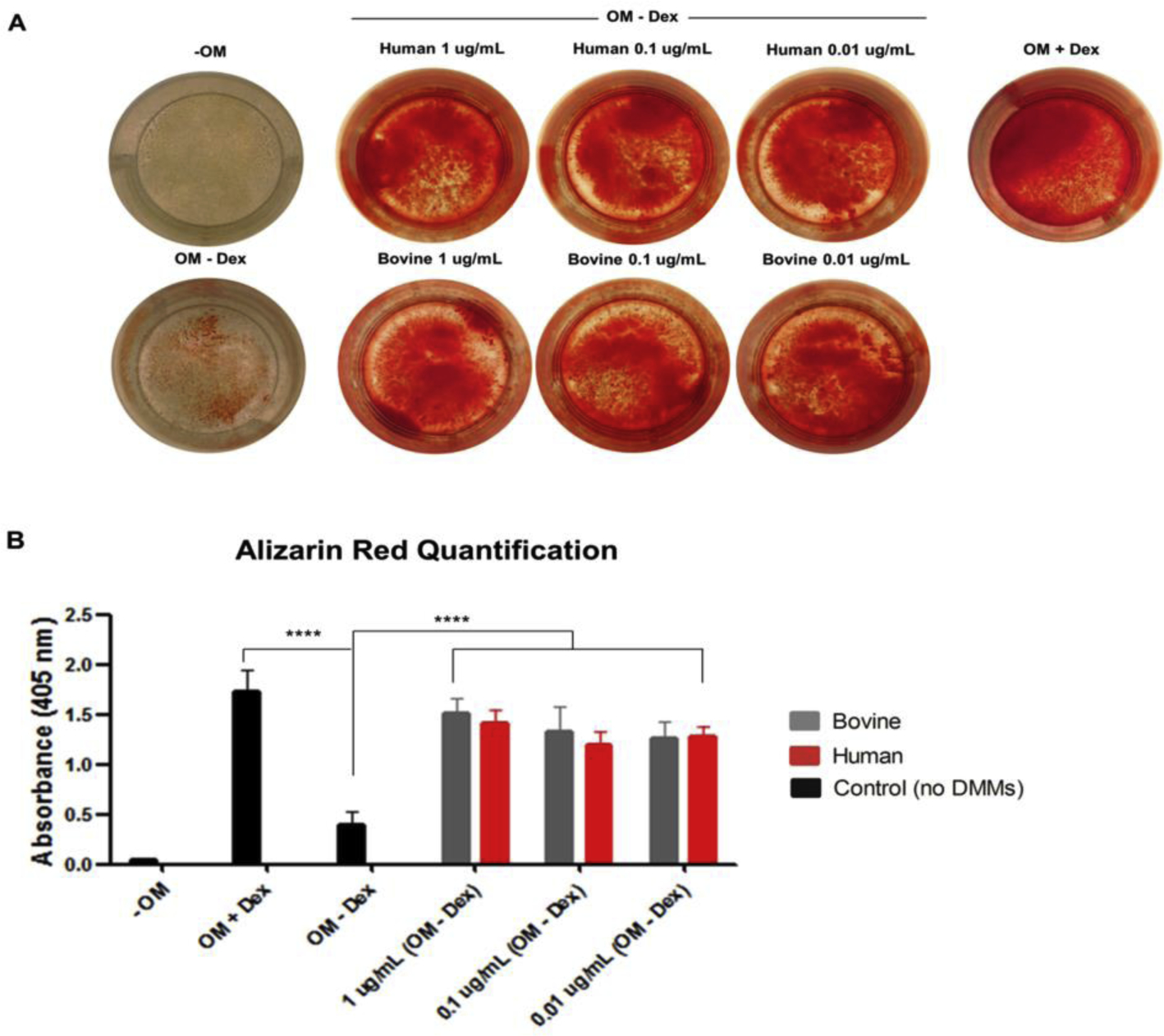
The effects of various concentrations of human and bovine DMMs on mineral matrix formation of OD-21 cells. (A: Alizarin red S staining, B: quantification of Alizarin red S stain, OM: Odontogenic medium, Dex: dexamethasone, bars and error bars are mean ± standard deviation, ****p≤ 0.0001) (ANOVA, α=0.05).
4. Discussion
Dentin matrix molecules have been shown to play an important role in the formation, healing, and regeneration of the dentin-pulp complex, and are considered a promising component of dental biomaterials which are intended to induce pulp regeneration (Athirasala et al., 2018; Petridis et al., 2018; Salehi et al., 2016; Smith et al., 2001; Smith et al., 1994). However, scaling-up the manufacture of DMM-based biomaterials remains impractical due to inherent limitations of using human teeth as a source of materials.
Therefore, we propose to use bDMMas a substitute for hDMMs. We hypothesized that bovine teeth, which are inherently larger and easier to procure, would yield more dentin matrix molecules than human teeth and would have similar protein composition and biological functions. We found that the total weight of proteins that bovine teeth yielded was 2 times higher than human teeth (Fig. 1). Moreover, out of 307 proteins identified in the proteomics analysis, 93 were present in both species, showing similar distribution of biological processes, regarding regeneration and matrix mineralization (Fig. 2). Moreover, bDMMs stimulated greater cell proliferation than those form human sources, while the three concentrations of dentin matrix molecules that were studied induced cell migration and differentiation equally for both species. We then accepted the hypothesis that bDMMs have similar composition and biological function as those derived from human teeth. Our data suggest that bDMMs may be a suitable substitute for hDMMs in material development for novel biomaterials.
Current proteomic tools allow large-scale, high-throughput analyses for the detection, identification, and functional investigation of proteome (Chandramouli & Qian, 2009). In our study, the proteomes of human and bovine dentin extracellular matrix were analyzed using shotgun liquid chromatography with tandem mass spectrometry (LC-MS/MS) proteomics. Comparing the proteomes of specialized tissues between different species is challenging. In proteomes with wider dynamic ranges, lower abundance proteins can suffer from sample-to-sample variability and detection. To reduce this source of ambiguity, the biological replicates were pooled to average out individual biological variability, providing more representative dentin proteomes for each species. Our study identified 192 proteins in human dentin extracellular matrix, in contrast to Park et al. (2009) that have reported 223 proteins and Jágr et al. (2012) that detected 289 proteins in human dentin extracellular matrix. Although, the number of identified proteins varied among the studies, consistently collagenous protein remained the major identified component of human dentin ECM. Interestingly, bovine dentin extracellular matrix displayed a higher number of total identified proteins than human dentin (208 and 192 respectively) but for both species, type I collagen, which is comprised of collagen alpha-1(COL1α1) and alpha-2 chains (COL1α2), was the major component of the extracts.
For a deeper comparison, from the 93 proteins common to human and bovine extracts, we selected the set of non-collagenous proteins and proteoglycans for deeper comparison, because these molecules are known to play a significant role in dental tissue regeneration. The selected proteins were categorized into 4 groups: 1) phosphorylated proteins, 2) non-phosphorylated proteins, 3) Small Leucine-rich Repeat Proteoglycans and 4) growth factor superfamilies. The phosphorylated proteins found in both species, consisted primarily of dentin sialophosphoprotein and dentin matrix protein-1. Both dentin sialophosphoprotein and dentin matrix protein-1 are secreted by odontoblasts and commonly found in dentin (Goldberg & Smith, 2004; Simon et al., 2009; Smith et al., 2012). These proteins are members of Small Integrin-Binding Ligand N-linked Glycoproteins (SIBLINGs), which contain negatively charged domains that act as nucleators for crystallization of hydroxyapatite (George & Veis, 2008). We found differences in other Small Integrin-Binding Ligand N-linked Glycoproteins (SIBLINGs) components between the species, i.e., osteopontin was only detected in human samples whereas bone sialoprotein was found only in the bovine samples. The differences in the amount and composition of protein identified in dentin matrix from each species may also be because odontoblasts usually express different matrix molecules at various stages of their life cycle (Simon et al., 2009). Among non-phosphorylated proteins, the most abundant in both species is Matrix Gla protein (MGP). MGP has high calcium binding affinity and is a potent inhibitor of mineralization (Roy & Nishimoto, 2002). Mgp deficient mice show extensive interstitial mineralization of arteries and premature cartilage mineralization, while transgenic mice expressing Col1a1-Mgp specifically in osteoblasts, odontoblasts and cementoblasts present with hypomineralization of dentin and alveolar bone (Kaipatur, Murshed, & McKee, 2008). Osteonectin is another non-collagenous protein that we found in both species. This protein is involved in collagen assembly and mineralization due to its capacity to bind to calcium and collagen (Rosset & Bradshaw, 2016). The Small Leucine-rich Repeat Proteoglycans had high levels of biglycan and decorin identified in both human and bovine samples. These proteoglycans contain tandem repeats of leucine rich motifs and play regulatory roles in collagen fibril growth, organization and extracellular matrix assembly (Chen & Birk, 2013), as well as tissue mechanics (Bertassoni & Swain, 2014). Biglycan and decorin share a similar structure and are classified as a class I SLRP based on their homology (Schaefer & Iozzo, 2008), being considered key molecules due to their critical role during dentinogenesis. They are involved in collagen fibril formation, matrix assembly, and control of dentin mineralization (Sloan, 2015). In addition, they specifically bind to transforming growth factor β1 (TGFβ1) which is the major isoform of the TGFβ superfamily found in dentin matrix from both species (Baker et al., 2009), and serve as a reservoir of bioactive molecules within dentin extracellular matrix (Sloan, 2015).
The growth factor superfamily was the last DMM component studied. Some of these growth factors are sequestrated and bound within the dentin extracellular matrix, playing an important role during dental tissue repair. Even though transforming growth factor β and insulin-like growth factor (IGF) were the most abundant growth factors common to both species, human dentin matrix molecules presented higher levels of transforming growth factor β, whereas bovine dentin matrix molecule extracts had more insulin-like growth factor (Fig 3). Both growth factors have been shown to stimulate odontoblast differentiation and dentin formation (Alkharobi, Al-Khafaji, Beattie, Devine, & El-Gendy, 2018; Dobie, Smith, Sloan, & Smith, 2002).
Regardless of the differences among identified proteins and their compositions between the two species, gene oncology functional analysis demonstrated similar patterns of biological function, regarding healing process, tissue regeneration, and formation of mineral tissue (Fig 4). Likewise, our study identified similar key bioactive molecules in both bovine and human dentin extracellular matrix, suggesting the suitability of using bDMMs as a substitute for hDMMs.
Previous studies showed that dentin matrix molecules contained bioactive molecules that regulate dental pulp regeneration and dentin formation and can potentially be used as a biomedical material for dental pulp regeneration (Athirasala et al., 2018; Smith et al., 2012; Smith et al., 1994). DMMs extracted from rabbit dentin promote tertiary dentin formation in ferret teeth (Smith et al., 2001; Smith et al., 1994) and odontoblast like cell differentiation in dog dental pulp (Smith et al., 1990; Tziafas et al., 1995). DMMs extracted from human dentin proved to induce proliferation, migration and odontogenic differentiation of dental pulp cells. Moreover, enhanced mineralization was observed in human umbilical cord mesenchymal stromal cells and dental pulp stromal cells (DPSCs) (Petridis et al., 2018). Despite many investigations on the composition and biological function of human DMMs (Jágr et al.; 2012, Okamoto et al., 2018; Salehi et al., 2016), the information on bovine DMMs, which can be more easily sourced and are more abundant, has never been studied. Based on the proteomic analysis that has demonstrated similarities in composition between the two species, we investigated the effects of bovine dentin matrix molecules on mouse dental pulp cell proliferation, migration and mineral matrix formation.
No effects on cell proliferation were observed after OD21 cells were exposed to human dentin matrix molecules for 24 hours (Fig. 5). However, after 48 hours incubation, hDMMs at a concentration of 0.01 ug/mL promoted cell proliferation (Fig. 5). Conversely, all concentrations of bDMMs enhanced cell proliferation after 24 hours. However, after 48 hours of treatment, only the 1 μg/mL bDMM group showed increased cell numbers. The differences in the dose dependent effect of dentin matrix molecules between species may be because human and bovine dentin have the different ratio of inter-tubular and peri-tubular dentin and also the differences in tubular size and density, which could alter the composition of bioactive molecules required for cell stimulation (Goldberg, Kulkarni, Young, & Boskey, 2011; Schilke, Lisson, Bauss, & Geurtsen, 2000; Sui et al., 2018). Collectively, these results suggest that (i) both human and bovine dentin matrix molecules can enhance cell proliferation, (ii) human DMMs induce cell proliferation after 48 hours and only at the lowest concentration (0.01 μg/mL), and (iii) the effects of bDMMs on cell proliferation might occur at an earlier time point than occurs for hDMMs. In contrast to our study, Salehi et al. (2016) reported that human dentin matrix molecules only stimulated OD21 cell proliferation when higher concentrations (10 μg/mL) were used. This may be explained by the fact that their proliferation experiment involved cells cultured in serum-free medium, and the fetal bovine serum is expected to enhance the growth of cultured cells (Even, Sandusky, & Barnard, 2006). Therefore, in the absence of serum in the medium, higher concentrations of human dentin matrix molecules are required for cell growth. Higher levels of these molecules have also been suggested to inhibit proliferation of human mesenchymal stromal cells (hMSCs). Petridis et al. (2018) observed a decrease in proliferation after human mesenchymal stromal cells were exposed to 5, 1 and 0.1 mg/mL of hDMMs in an inverse dose-dependent manner. Thus, it is possible that even lower concentrations of hDMMs might induce further enhanced proliferation of cells in our experiment had they been tested. As for bDMMs, all concentrations significantly induced cell proliferation at the first 24 hours. After 48 hours, all concentrations of bDMMs still showed to increase cell numbers, however, significant difference was found only at the highest concentration (1 μg/mL), suggesting that a much higher amount of bDMMs may be required in order to induce cell proliferation in the late time point, while for hDMMs lower concentration may be used to stimulate cell proliferation. We are uncertain of the specific mechanisms leading up to these outcomes, and this forms the basis for future investigations.
Cell migration plays an essential role in tissue repair and regeneration (Lauffenburger & Horwitz, 1996). We used a trans-well migration method to compare the chemotactic effects of human and bovine dentin matrix molecules. Our results showed that all concentrations of both human and bovine DMMs were able to induce significant migration of the cells and in a similar manner. Previous studies using human dentin matrix molecules reported that conditioning of human dentin discs with ethylenediaminetetraacetic acid promoted migration of dental pulp stem cells towards the discs (Galler et al., 2016). Of relevance to these findings, Tomson, Lumley, Smith, and Cooper (2017), found that human dentin matrix molecules solubilized by mineral trioxide aggregate and calcium hydroxide induced the migration of rat dental pulp cells, confirming the chemotactic effect of hDMMs on dental pulp cells. Interestingly, we observed the same positive effects of bovine dentin matrix molecules on cell migration, suggesting that bDMMs may have similar chemotactic effects as hDMMs. Again, it would be interesting to see if lower concentrations of dentin matrix molecules of either species would show an intermediate effect between the lowest concentration and the control, as one would expect eventually to see a typical dose response.
Lastly, we investigated the effects of dentin matrix molecules on dental pulp cell (OD21) differentiation and mineral secretion. Cells were exposed to different concentrations of human or bovine DMMs in the presence of odontogenic medium containing ascorbic acid and β-glycerophosphate. Higher alizarin red staining was observed in cells that were treated with dentin matrix molecules from both species, when compared to controls (odontogenic medium without DMMs). However, different from other studies (Salehi et al., 2016), here we found no dose dependent effect of DMMs on calcium deposition. Interestingly, no difference in mineral formation was observed between dentin matrix molecules from human and bovine group in our study, which confirmed the similar odontogenic effects of bovine and human DMMs on dental pulp cells.
Dentin matrix molecules was proven to successfully stimulate healing and regeneration in dentin-pulp complex. Tertiary dentin was reported to be induced by DMMs. In vivo implantation of DMMs on the floor of the cavity without exposing the pulp (transdentinal delivery) showed to stimulate the underlying odontoblasts to form reactionary dentin (Smith etal., 2001; Smith et al., 1994). Moreover, when applied as direct pulp capping material, dentin matrix molecules showed to stimulate dentin bridge formation by inducing the differentiation of dental pulp stem cells into odontoblasts-like cells and form reparative dentin (Okamoto et al., 2018). Based on the current literature, DMMs were proposed to be used as a base, lining or pulp capping dental material. Recently, we have developed three-dimensional printable alginate hydrogel containing dentin matrix molecules, which showed cytocompatibility and odontogenic capacity and can be a used as scaffolds for regenerative dentistry in the future (Athirasala et al., 2018).
Due to the limitation of acquiring human teeth as a source for dental materials, bovine teeth were considered to be more practical source for scaling-up the manufacture of DMM-based biomaterials. Dentin matrix molecules from both species showed similar protein composition and biological function, favoring the use of bDMMs. However, biocompatibility between species and immunological reaction when using bovine dentin matrix molecules on patients still need further investigation.
5. Conclusion
In summary, the concentration of dentin matrix molecules extracted from bovine dentin was greater than that of human dentin, while the general composition of proteins and bioactive molecules and their correlation to biological processes appeared to be highly comparable. Each concentration of dentin matrix molecules from both human and bovine sources significantly enhanced migration and mineralization of OD21 cells with the similar results, while some concentrations of bovine and human dentin matrix molecules can promote cell proliferation. Therefore, we suggest the possible use of bDMMs as a substitute for hDMMs for pulp regeneration therapy, with the abundance of ease of scalability and sourcing.
Supplementary Material
Highlights:
Bovine dentin contains active molecules with similar biological function to human
Dentin matrix from both species stimulated regenerative processes of dental pulp
Bovine dentin could be a human dentin substitute for dental pulp regeneration
Acknowledgements
This project was supported by funding from the National Institute of Dental and Craniofacial Research (R01DE026170 and 3R01DE026170-03S1 to LEB), the OHSU-Mahidol University cooperation agreement (to SH), the Oregon Clinical & Translational Research Institute (OCTRI) - Biomedical Innovation Program (BIP), the Innovation in Oral Care Awards sponsored by GlaxoSmithKline (GSK), International Association for Dental Research (IADR), the Michigan-Pittsburgh-Wyss Resource Center - Regenerative Medicine Resource Center (MPW-RM), the OHSU Fellowship for Diversity and Inclusion in Research (OHSU-OFDIR to CMF). Mass spectrometric analysis was performed by the OHSU Proteomics Shared Resource with partial support from NIH core grants P30EY010572 and P30CA069533, and S10OD012246. Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0060/2557) to S Horsophonphong and R Surarit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
There are no conflicts to declare.
References
- Alkharobi HE, Al-Khafaji H, Beattie J, Devine DA, & El-Gendy R (2018). Insulin-like growth factor axis expression in dental pulp cells derived from carious teeth. Frontiers in bioengineering and biotechnology, 6, 36 10.3389/fbioe.2018.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athirasala A, Tahayeri A, Thrivikraman G, França CM, Monteiro N, Tran V, … Bertassoni LE (2018). A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication, 10(2), 024101 10.1088/1758-5090/aa9b4e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SM, Sugars RV, Wendel M, Smith AJ, Waddington RJ, Cooper PR, & Sloan AJ (2009). TGF-beta/extracellular matrix interactions in dentin matrix: a role in regulating sequestration and protection of bioactivity. Calcified Tissue International, 85(1), 66–74. 10.1007/s00223-009-9248-4 [DOI] [PubMed] [Google Scholar]
- Bertassoni LE, & Swain MV (2014). The contribution of proteoglycans to the mechanical behavior of mineralized tissues. Journal of the mechanical behavior of biomedical materials, 38, 91–104. 10.1016/j.jmbbm.2014.06.008 [DOI] [PubMed] [Google Scholar]
- Chandramouli K, & Qian P-Y (2009). Proteomics: challenges, techniques and possibilities to overcome biological sample complexity. Human genomics and proteomics, 2009, 239204 10.4061/2009/239204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, & Birk DE (2013). The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. The FEBS journal, 280(10), 2120–2137. 10.1111/febs.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PR, Chicca IJ, Holder MJ, & Milward MR (2017). Inflammation and regeneration in the dentin-pulp complex: net gain or net loss? Journal of endodontics, 43(9S), S87–S94. 10.1016/j.joen.2017.06.011 [DOI] [PubMed] [Google Scholar]
- Cooper PR, Takahashi Y, Graham LW, Simon S, Imazato S, & Smith AJ (2010). Inflammation-regeneration interplay in the dentine-pulp complex. Journal of Dentistry, 38(9), 687–697. 10.1016/j.jdent.2010.05.016 [DOI] [PubMed] [Google Scholar]
- Cox J, & Mann M (2008). MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nature biotechnology, 26(12), 1367–1372. 10.1038/nbt.1511 [DOI] [PubMed] [Google Scholar]
- Dobie K, Smith G, Sloan AJ, & Smith AJ (2002). Effects of alginate hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connective tissue research, 43(2–3), 387–390. [DOI] [PubMed] [Google Scholar]
- Erde J, Loo RR, & Loo JA (2014). Enhanced FASP (eFASP) to increase proteome coverage and sample recovery for quantitative proteomic experiments. Journal of proteome research, 13(4), 1885–1895. 10.1021/pr4010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even MS, Sandusky CB, & Barnard ND (2006). Serum-free hybridoma culture: ethical, scientific and safety considerations. Trends in biotechnology, 24(3), 105–108. 10.1016/j.tibtech.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Galler KM, Widbiller M, Buchalla W, Eidt A, Hiller KA, Hoffer PC, & Schmalz G (2016). EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. International endodontic journal, 49(6), 581–590. 10.1111/iej.12492 [DOI] [PubMed] [Google Scholar]
- George A, & Veis A (2008). Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chemical reviews, 108(11), 4670–4693. 10.1021/cr0782729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M, Kulkarni AB, Young M, & Boskey A (2011). Dentin: structure, composition and mineralization. Frontiers in bioscience (Elite edition), 3, 711–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M, & Smith AJ (2004). Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Critical reviews in oral biology and medicine, 15(1), 13–27. 10.1177/154411130401500103 [DOI] [PubMed] [Google Scholar]
- Gregory CA, Gunn WG, Peister A, & Prockop DJ (2004). An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Analytical biochemistry, 329(1), 77–84. 10.1016/j.ab.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Hanks CT, Sun ZL, Fang DN, Edwards CA, Wataha JC, Ritchie HH, & Butler WT (1998). Cloned 3T6 cell line from CD-1 mouse fetal molar dental papillae. Connective tissue research, 37(3–4), 233–249. 10.3109/03008209809002442 [DOI] [PubMed] [Google Scholar]
- Jágr M, Eckhardt A, Pataridis S, Broukal Z, Dušková J, & Mikšík I (2014). Proteomics of human teeth and saliva. Physiological reearchs, 63 (Suppl 1), S141–S154. [DOI] [PubMed] [Google Scholar]
- Jágr M, Eckhardt A, Pataridis S, & Mikšík I (2012). Comprehensive proteomic analysis of human dentin. European journal of oral sciences, 120(4), 259–268. 10.1111/j.1600-0722.2012.00977.x [DOI] [PubMed] [Google Scholar]
- Kaipatur NR, Murshed M, & McKee MD (2008). Matrix Gla protein inhibition of tooth mineralization. Journal of dental research, 87(9), 839–844. 10.1177/154405910808700907 [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, & Horwitz AF (1996). Cell migration: a physically integrated molecular process. Cell, 84(3), 359–369. 10.1016/s0092-8674(00)81280-5 [DOI] [PubMed] [Google Scholar]
- Mi H, Lazareva-Ulitsky B, Loo R, Kejariwal A, Vandergriff J, Rabkin S, … Thomas PD (2005). The PANTHER database of protein families, subfamilies, functions and pathways. Nucleic acids research, 33(Database issue), D284–288. 10.1093/nar/gki078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Takahashi Y, Komichi S, Cooper PR, & Hayashi M (2018). Dentinogenic effects of extracted dentin matrix components digested with matrix metalloproteinases. Scientific reports, 8, 10690 10.1038/s41598-018-29112-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ES, Cho HS, Kwon TG, Jang SN, Lee SH, An CH, Cho JY (2009). Proteomics analysis of human dentin reveals distinct protein expression profiles. Journal of proteome research, 8(3), 1338–1346. 10.1021/pr801065s [DOI] [PubMed] [Google Scholar]
- Petridis X, Beems BP, Tomson PL, Scheven B, Giepmans BNG, Kuipers J, … Harmsen MC (2018). Effect of Dentin Matrix Components on the Mineralization of Human Mesenchymal Stromal Cells. Tissue engineering part A. 10.1089/ten.TEA.2018.0192 [DOI] [PubMed]
- Rosset EM, & Bradshaw AD (2016). SPARC/osteonectin in mineralized tissue. Matrix biology, 52–54, 78–87. 10.1016/j.matbio.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy ME, & Nishimoto SK (2002). Matrix Gla protein binding to hydroxyapatite is dependent on the ionic environment: calcium enhances binding affinity but phosphate and magnesium decrease affinity. Bone, 31(2), 296–302. 10.1016/s8756-3282(02)00821-9 [DOI] [PubMed] [Google Scholar]
- Salehi S, Cooper P, Smith A, & Ferracane J (2016). Dentin matrix components extracted with phosphoric acid enhance cell proliferation and mineralization. Dental materials, 32(3), 334–342. 10.1016/j.dental.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Schaefer L, & Iozzo RV (2008). Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. The Journal of biological chemistry, 283(31), 21305–21309. 10.1074/jbc.R800020200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilke R, Lisson JA, Bauss O, & Geurtsen W (2000). Comparison of the number and diameter of dentinal tubules in human and bovine dentine by scanning electron microscopic investigation. Archives of oral biology, 45(5), 355–361. 10.1016/s0003-9969(00)00006-6 [DOI] [PubMed] [Google Scholar]
- Simon SR, Berdal A, Cooper PR, Lumley PJ, Tomson PL, & Smith AJ (2011). Dentin-pulp complex regeneration: from lab to clinic. Advances in dental research, 23(3), 340–345. 10.1177/0022034511405327 [DOI] [PubMed] [Google Scholar]
- Simon S, Smith AJ, Lumley PJ, Berdal A, Smith G, Finney S, & Cooper PR (2009). Molecular characterization of young and mature odontoblasts. Bone, 45(4), 693–703. 10.1016/j.bone.2009.06.018 [DOI] [PubMed] [Google Scholar]
- Sloan AJ (2015). Chapter 29 - Biology of the Dentin-Pulp Complex In Vishwakarma A, Sharpe P, Shi S & Ramalingam M (Eds.), Stem cell biology and tissue engineering in dental sciences (pp. 371–378). Boston: Academic Press. [Google Scholar]
- Smith AJ, Cassidy N, Perry H, Bègue-Kirn C, Ruch JV, & Lesot H (1995). Reactionary dentinogenesis. The International journal of developmental biology, 39(1), 273–280. [PubMed] [Google Scholar]
- Smith AJ, Murray PE, Sloan AJ, Matthews JB, & Zhao S (2001). Trans-dentinal stimulation of tertiary dentinogenesis. Advances in dental research, 15, 51–54. 10.1177/08959374010150011301 [DOI] [PubMed] [Google Scholar]
- Smith AJ, Scheven BA, Takahashi Y, Ferracane JL, Shelton RM, & Cooper PR (2012). Dentine as a bioactive extracellular matrix. Archives of oral biology, 57(2), 109–121. 10.1016/j.archoralbio.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Smith AJ, Tobias RS, Cassidy N, Plant CG, Browne RM, Begue-Kirn C, Lesot H (1994). Odontoblast stimulation in ferrets by dentine matrix components. Archives of oral biology, 39(1), 13–22. 10.1016/0003-9969(94)90029-9 [DOI] [PubMed] [Google Scholar]
- Smith AJ, Tobias RS, Plant CG, Browne RM, Lesot H, & Ruch JV (1990). In vivo morphogenetic activity of dentine matrix proteins. Journal de biologie buccale, 18(2), 123–129. [PubMed] [Google Scholar]
- Stankoska K, Sarram L, Smith S, Bedran-Russo AK, Little CB, Swain MV, & Bertassoni LE (2016). Immunolocalization and distribution of proteoglycans in carious dentine. Australian dental journal, 61(3), 288–297. 10.1111/adj.12376 [DOI] [PubMed] [Google Scholar]
- Sui T, Dluhoš J, Li T, Zeng K, Cernescu A, Landini G, & Korsunsky AM (2018). Structure-function correlative microscopy of peritubular and intertubular dentine. Materials (Basel), 11(9). 10.3390/ma11091493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomson PL, Grover LM, Lumley PJ, Sloan AJ, Smith AJ, Cooper PR (2007). Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. Journal of Dentstry. 35(8), 636–642. https://doi:10.1016/j.jdent.2007.04.008 [DOI] [PubMed] [Google Scholar]
- Tomson PL, Lumley PJ, Smith AJ, & Cooper PR (2017). Growth factor release from dentine matrix by pulp-capping agents promotes pulp tissue repair-associated events. International endodontic journal, 50(3), 281–292. 10.1111/iej.12624 [DOI] [PubMed] [Google Scholar]
- Tziafas D, Alvanou A, Panagiotakopoulos N, Smith AJ, Lesot H, Komnenou A, & Ruch JV (1995). Induction of odontoblast-like cell differentiation in dog dental pulps after in vivo implantation of dentine matrix components. Archive of oral biology, 40(10), 883–893. 10.1016/0003-9969(95)00069-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


