Abstract
Currently, there is no specific antiviral treatment for COVID-19. However, drugs previously developed to treat other viral infections are being tested to verify if they might also be effective against SARS-CoV-2, the virus that causes COVID-19. Twenty years ago, the F.D.A. approved Lopinavir/ritonavir (LPV/r) to treat HIV infection. LPV and ritonavir were initially purposed to inhibit 3-chymotrypsin-like protease (3CLpro) of SARS-CoV and MERS-CoV and preliminary promising data on its efficacy for treating people infected with those viruses were available. Therefore, due to the high genetic similarities among those viruses and SARS-CoV-2, early during COVID-19 pandemic LPV/r was also proposed as one emergency treatment. We reviewed data from the literature about LPV/r treatment and SARS-CoV-2 infection, mainly focused on the efficacy and safety of this drugs for COVID-19 treatment. We can conclude that although up to date no clear benefit has been observed with the LPV/r treatment beyond standard care, its efficacy against SARS-COV-2 infection deserves further evaluations, particularly during the very early phase of the disease.
Keywords: Lopinavir, Ritonavir, Protease inhibitor, SARS-CoV-2, COVID-19 treatment
By the end of December 2019, the emergence of an outbreak of pneumonia of unknown etiology in Wuhan, Hubei Province of China, was communicated to the World Health Organization (WHO) China Country Office [1]. The RNA sequencing from the bronchoalveolar lavage fluid of an infected patient identified a new virus from the Coronaviridae family as the causative agent of this new disease [2]. The virus was successively named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), and the new pneumonia Coronavirus Disease (COVID-19) [3]. Similarly to other two coronaviruses which caused diseases outbreak in recent times [4,5], the Severe Acute Respiratory Syndrome (SARS-CoV) and the Middle-East Respiratory Syndrome Coronavirus (MERS-CoV), SARS-CoV-2 seems to have originated from bats [6], being mostly similar to the RaTG13 virus, isolated from Rhinolophus affinis [7]. It shares 79.6% sequence identity with SARS-CoV [7] and 50% with MERS-CoV [8]. SARS-CoV was the responsible for the Severe Acute Respiratory Syndrome (SARS) in the Guandong Province of China in 2003, causing up to 813 deaths among 8437 cases reported [9]. The MERS-CoV outbreak occurred in 2012 in Saudi Arabia, reaching 2494 confirmed cases and 858 deaths worldwide [10]. In mid-February, SARS-CoV-2 spread in 188 countries, reaching 108,822,960 people and resulting in 2,403,641 deaths worldwide [11]. The spectrum of clinical manifestations of COVID-19 can vary from a mild respiratory syndrome to an Acute Respiratory Distress Syndrome (ARDS) requiring mechanical ventilation [[12], [13], [14], [15]], where older age and comorbidities increase the risk of a more severe disease [16,17]. Case fatality rate has been reported to be around 4–4.5%, anyway varying widely among different countries and different testing strategies [[18], [19], [20]]. Up to date, only remdesivir and dexamethasone proved to be effective in clinical trials: remdesivir shortened the length of hospitalization, anyway without a meaningful impact on mortality [21], while dexamethasone significantly reduced mortality in patients requiring supplemental oxygen [22].
Lopinavir/ritonavir (LPV/r) is a fixed dose combination of two protease inhibitors, widely used as antiretroviral drug for Human Immunodeficiency Virus (HIV) second-line treatment [23]. Lopinavir is a potent inhibitor of the HIV-1 protease, thus producing immature, non-infectious virions. Anyway, lopinavir shows poor bioavailability, therefore it comes co-formulated with ritonavir, a potent inhibitor of the cytochrome P450 3A4 [24], which dramatically increase lopinavir blood levels.
Both lopinavir and ritonavir were initially proposed to inhibit 3-chymotrypsin-like protease (3CLpro) of SARS-CoV and MERS-CoV [25]. In a study of 2004 from Chu et al. [26] in patients affected by SARS, the association of LPV/r with ribavirin showed a lower occurrence of adverse clinical outcomes (ARDS or death) in the treatment group in comparison with the historical controls treated with ribavirin only (2.4% v 28.8%, p < 0.001) at day 21 after the onset of symptoms. In the initial treatment group, a reduction in steroid usage and nosocomial infections, as well as a decreasing viral load and a rising peripheral lymphocyte count were observed. Similarly, the study from Chan et al. [27] found that the early addition of LPV/r to the standard therapy (broad spectrum antibiotics, ribavirin and corticosteroids) was associated with a significant reduction in the overall death and intubation rate (2.3% and 0%, respectively), when compared to standard treatment only (15.6% and 11.0% respectively, p < 0.05). Interestingly, in the subgroup who had received LPV/r later as rescue therapy, no differences in the overall death, intubation and oxygen desaturation rates were observed in comparison with the matched cohort. In MERS-CoV infection, a study on twelve common marmosets [28] showed that the LPV/r and the interferon-1β (IFN-1β)-treated animals had better outcomes than the untreated animals. Moreover, LPV/r and interferon-β1b-treatment groups showed lower mean viral loads in necropsied lung and in extrapulmonary tissues and lower mortality rates (0–33%) in comparison with the untreated and the mycophenolate mofetil-treated groups (67%). Based on those preliminary clinical evidences and on genetic similarities of these two viruses with SARS-CoV-2, early during COVID-19 pandemic, LPV/r was also proposed as one emergency treatment.
Whether LPV/r may effectively target SARS-CoV-2 3CLpro is still under debate. Indeed, the HIV protease on which LPV/r is effective is from the aspartic protease family, whereas SARS-CoV-2 3CLpro from the cysteine protease family.
Drugs repositioning is an important strategy, especially in an emergency context such as a pandemic, because of lower costs of production and reduced times for drug availability and distribution [29]. Among those drugs which have been proposed in the therapy of COVID-19, the combination of lopinavir/ritonavir is still under evaluation.
Therefore, the aim of this review is to evaluate and make available the state of the art about the use of this drug in the treatment of COVID-19, in order to help clinicians in the management of affected patients.
Methodology and literature search strategy
Literature search
A comprehensive searching of PubMed, EMBASE and Cochrane Library was performed from June 1st up to September 1st 2020 with restriction to English, Italian, Spanish and Portuguese language papers. Unpublished trials were also identified from the clinical trial registry platforms (http://clinicaltrials.gov/). Preprint articles were retrieved from the websites MedRxiv (https://www.medrxiv.org) and BioRxiv (https://www.biorxiv.org). Moreover, manual search was conducted by screening the reference lists of inclusive studies. Our search strategy included the following relevant terms: lopinavir AND COVID-19, lopinavir AND COVID 19, lopinavir AND coronavirus, protease inhibitors AND COVID-19, protease inhibitors AND COVID 19, protease inhibitors AND coronavirus, protease inhibitors AND covid-19 AND pre-clinic, animal model AND SARS-CoV-2 AND lopinavir, SARS-CoV-2 AND lopinavir AND ritonavir.
Studies selection
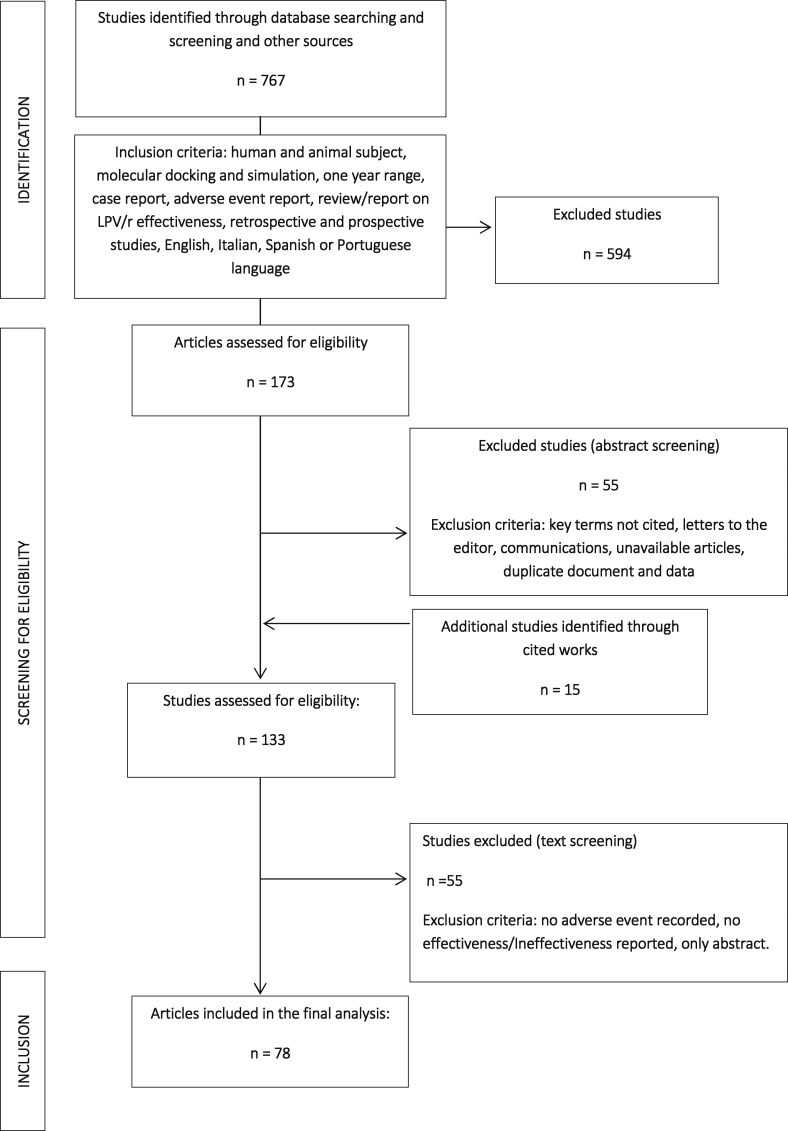
Three authors (P.M., E.Q.R. and M.P.) independently screened the titles, abstracts and full texts of retrieved articles to evaluate their eligibility [Fig. 1].
Fig. 1.
Flow chart of database searching and screening of studies for the systematic review.
In vitro and in vivo pre-clinical studies, randomized controlled trials, prospective and retrospective cohort studies, case series and clinical cases performed among adults with COVID-19 were included in the current literature review. We reviewed studies from the literature about protease inhibitors use in COVID-19 mainly focusing on in vitro and in vivo efficacy, on clinical outcome, mortality rate, virological eradication, safety and tolerability.
Pre-clinical studies
Collected data are summarized in Table 1 and described more in detail below.
Table 1.
Details of revised studies.
| References | Method | Drugs | Results |
|---|---|---|---|
| Park S.J. et al., 2020 [30] |
In vivo (animal model) |
Lopinavir/ritonavir, Hydroxychloroquine sulfate, Emtricitabine-Tenofovir | Reduced overall clinical symptoms and not significantly diminished respiratory or gastrointestinal SARS-CoV-2 titers |
| Arshad U. et al., 2020 [31] |
In vitro Vero cells in available literature |
Lopinavir, Ritonavir | In lung tissue: Cmax/EC50 > 10: Hydroxychloroquine, Atazanavir, Chloroquine, Tipranavir, Mefloquine, Ivermectin, Azithromycin, Lopinavir; In plasma: Cmax/EC50 > 1: Nelfinavir, Chloroquine, Remdesivir, Lopinavir (Ritonavir boosted), Eltrombopag, Hydroxychloroquine, Atazanavir (Ritonavir boosted), Indomethacin, Favipiravir, Sulfadoxine, Niclosamide, Mefloquine, Tipranavir (Ritonavir boosted), Ritonavir, Merimepodib, Anidulafungin, Nitazoxanide 1< Cmax/EC90 < 2: Anidulafungin, Lopinavir, Chloroquine, Ritonavir |
| Cattaneo D. et al., 2020 [33] |
In vitro IC90 estimation |
Lopinavir | Lopinavir IC50: 26 μM Lopinavir IC90:234 μM Lopinavir Protein-adjust IC90in plasma: 4680 μM Lopinavir Protein-adjust IC90 in epithelial lining fluid: 393 μM Lopinavir Protein-adjust IC90 in cerebrospinal fluid: 58,500 μM |
| Choy K.T. et al., 2020 [32] |
In vitro Vero E6 cells |
Remdesivir, Lopinavir | To the reduction in viral RNA copy: EC50Lopinavir: 26.1 μM |
| Kang C.K. et al., 2020 [34] |
In vitro Kidney Vero cells |
Lopinavir, Ritonavir | Concentration groups tested:
|
| De Meyer S. et al., 2020 [35] |
In vitro Caco-2 cells |
Darunavir | Visual Cytopathogenic Effect-read out EC50: Darunavir: > 100 μM 3-(4,5-dimethyl-2- thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) method EC50: Darunavir: > 100 μM Cytotoxic concentration causing death to 50% (CC50): Darunavir: >100 μM |
| Das S. et al., 2020 [36] |
In silico Blind molecular docking analyses with Mpro |
Commercially available compounds | Free binding energy for SARS-CoV-2 protease: Lopinavir ΔG: – 9.00 kcal/mol Ritonavir ΔG: - 9.52 kcal/mol |
| Beck B.R. et al., 2020 [44] |
In silico Molecular dynamic simulation |
Commercially available compounds | Drug-target interaction (DTI) prediction results against COVID-19's helicase: Lopinavir: 78.49 nM Ritonavir: 41.60 nM Darunavir: 90.38 nM |
| Khan S.A. et al., 2020 [49] |
In silico Molecular dynamic simulation |
Commercially available compounds | Free binding energy for SARS-CoV-2 protease by MM (GB/PB)SA method: Darunavir: - 48.1041 kcal/mol |
| Muralidharan N. et al., 2020 [37] |
In silico Molecular dynamic simulation |
Commercially available compounds | Free binding energy for SARS-CoV-2 protease: Lopinavir: - 4.1 kcal/mol Oseltamivir: - 4.65 kcal/mol Ritonavir: - 5.11 kcal/mol Lopinavir/Oseltamivir: - 5.4 kcal/mol Lopinavir/Oseltamivir/Ritonavir: - 8.32 kcal/mol |
| Nutho B. et al., 2020 [38] | In silico - Molecular dynamic simulation | Lopinavir and Ritonavir | Free binding free energy (ΔGbind) for SARS-CoV-2 protease by MM/PBSA: Lopinavir: - 10.89 ± 1.89 kcal/mol Ritonavir: - 14.93 ± 1.83 kcal/mol Binding free energy (ΔGbind) for SARS-CoV-2 protease by MM/GBSA: Lopinavir: - 13.83 ± 1.91 kcal/mol Ritonavir: - 27.78 ± 1.82 kcal/mol |
| Ortega J.T. et al., 2020 [45] |
In silico Molecular dynamic simulation |
Commercially available compounds | Free binding energy for SARS-CoV-2 protease: Lopinavir: −9.1 kcal/mol |
| Pant S. et al., 2020 [39] |
In silico Molecular dynamic simulation |
Commercially available compounds | Binding free energy (ΔGbind) for SARS-CoV-2 protease by MM/GBSA: Ritonavir: −87.24 kcal/mol Lopinavir: −73.33 kcal/mol Darunavir: −69.20 kcal/mol Docking Score: Ritonavir: −8.878 Lopinavir: −8.358 Darunavir: −7.208 |
| Peele K.A. et al., 2020 [40] |
In silico Molecular dynamic simulation |
Commercially available compounds | Docking score using GLIDE module: Lopinavir: −9.918 Darunavir: −8.843 |
| Mahanta S. et al., 2020 [48] |
In silico Molecular dynamic simulation |
Commercially available compounds | -CDocker Energy for SARS-CoV-2 protease: Lopinavir −62.07 kcal/mol Ritonavir −65.73 kcal/mol |
| Shah B. et al., 2020 [41] |
In silico Molecular dynamic simulation |
Commercially available compounds | Docking score from interaction with different COVID-19 structures as 5R7Y, 5R7Z, 5R80, 5R81 5R82: Lopinavir: - 6.834, −6.968, −7.331, −8.44, −7.58 Ritonavir: −7.621, -, −6.736, −6.764, −7.316 |
| Chen Y.W. et al., 2020 [46] |
In silico Molecular dynamic simulation |
Commercially available compounds | Binding energy for chain A active site of SARS-CoV-2 protease: Mean score: −8.2 kcal/mol Ritonavir: −7.9 kcal/mol Lopinavir: −8.0 kcal/mol Binding energy for chain B active site of SARS-CoV-2 protease: of SARS-CoV-2 protease: Mean score: −7.1 kcal/mol Ritonavir: −6.9 kcal/mol Lopinavir: −6.8 kcal/mol |
| Wang Q. et al., 2020 [47] |
In silico Molecular dynamic simulation |
Commercially available compounds | Free binding energy for SARS-CoV-2 protease: Darunavir: - 11.1 kcal/mol Ritonavir: −11.8 kcal/mol Lopinavir: −11.9 kcal/mol |
| Mamidala E. et al., 2020 [42] |
In silico Molecular dynamic simulation |
Commercially available compounds | Free binding energy for SARS-CoV-2 protease: Lopinavir: −6.11 kcal/mol Ritonavir: −8.25 kcal/mol |
| Gupta S. et al., 2020 [43] |
In silico Molecular dynamic simulation |
Literature available compounds | Binding energy raging: Lopinavir: > - 5.70 kcal/mol Range of predicted IC50 values: Standard compound(Lopinavir): 15.7–21.3 μM The total interaction energy of protein-ligand complex: Lopinavir- Mpro: −181.795 ± 13.5 kJ/mol Toxicological profile (PLD50): Lopinavir: 5000 mg/kg body weight |
|
In silico Molecular dynamic simulation |
Darunavir | Docking score range of Darunavir: – 8.6 to - 8.2 Very few interactions of Darunavir with the active site of the protease |
In vivo studies
We retrieved only one study that analyzed the efficacy of LPV/r in an animal model [30]. Park et al., inoculated ten ferrets with infective doses of a SARS-CoV-2 strain (NMC-nCoV02) through the intranasal route. At day-1 post-infection with SARS-CoV-2, ferrets were administered LPV/r, hydroxychloroquine sulfate or emtricitabine-tenofovir daily for 14 days. In addition, ten ferrets were treated with phosphate-buffered saline (PBS) or azathioprine for 7 days prior to SARS-CoV-2 infection. In this study, despite an overall reduction of clinical symptom intensity, treatment with LPV/r showed no improvement in disease duration in comparison with the control group. None of the antiviral candidates significantly diminished respiratory or gastrointestinal viral titers. Moreover, antiviral-treated groups had lower serum neutralization antibody titers than the control group, which was an interesting finding needing further studies.
In vitro studies
Another important aspect to consider is whether a drug can reach the appropriate concentration in vivo. In vitro studies have showed that many putative agents, among those lopinavir and ritonavir, likely never reach the target concentrations necessary to adequately suppress SARS-CoV-2 in plasma. These findings were possible thanks to the prediction of the Cmax/EC50 and Cmax/EC90 [31]. Lopinavir, hydroxychloroquine, chloroquine, mefloquine, atazanavir (ritonavir boosted), tipranavir (ritonavir boosted), azithromycin and ivermectin achieved concentrations in the lungs 10-fold higher than their EC50 and exceeded by 3.4 fold their EC90. In contrast, LPV/r, chloroquine and hydroxychloroquine reached a concentration in plasma lower than that required for antiviral activity [31]. Considering that the virus accumulates in the lung tissue of COVID-19 patients, the high concentration of antiviral drugs in the lung rather than plasma could be useful in pneumonia treatment during COVID-19.
The estimation of the protein-adjusted EC90 value of lopinavir revealed also that the dose required to provide optimal inhibition in plasma was unfeasible due to un-acceptable risk of toxicity. A recent study from Choy et al., tested the in vitro antiviral effect of lopinavir against SARS-CoV-2 which resulted in a half maximal inhibitory concentration (EC50) of 26 μM [32], 4000-to-8000 folds higher than that able to inhibit HIV (0.006 μM) [33].
To bypass the high concentrations of lopinavir required in plasma, combinational therapy with other effective compounds against SARS-CoV-2 was considered [32,34]. When combinational drugs were used, the plasmatic viral load was lower than the single-drug treatment or placebo [34]. In this way, the formed complexes may increase synergy and reduce needed concentration of lopinavir under the maximal therapeutic plasma concentration [32].
Finally, another inhibitor of HIV-protease, darunavir, showed no in vitro antiviral activity against SARS-CoV-2 at clinical concentrations [35].
In silico studies
Data on molecular dynamic simulation available in the literature reported two contrasting results. On one hand, LPV/r had high stability in the binding domain of the SARS-CoV-2 protease in terms of electrostatic and van deer Waals interactions [[36], [37], [38], [39], [40], [41], [42], [43]]. On other hand, the binding affinity was found higher for drugs or natural compounds like ledipasvir, velpatasvir, carfilzomib, saquinavir and thymopentin, viomycin and lead phytochemicals than lopinavir and ritonavir [[43], [44], [45], [46], [47], [48]].
Actually, the active site of SARS-CoV-2 protease is characterized by the presence of a cysteine residue, but lopinavir and ritonavir were designed to better interact with an aspartate belonging to the HIV-protease [47]. Using a drug-target interaction (DTI), lopinavir and ritonavir were predicted to have a potential affinity (Kd 78.49 and 41.60 nM, respectively) to SARS-CoV-2 helicase [44]. This evidence could explain why the inhibition efficiency of lopinavir and ritonavir is not favorable against the main protease of SARS-CoV-2.
According with the in vitro studies, the combination of drugs seems to be also more effective. In these terms, the binding energy of lopinavir, ritonavir and oseltamivir docked together against the SARS-CoV-2 protease improved over the binding energy of each drug separately [37].
Finally, darunavir was predicted to have the best docking score among some FDA approved antiviral drugs (e.g. lopinavir, ritonavir, remdesivir, saquinavir, amprenavir, rupintrivir) with higher binding affinity to the SARS-CoV-2 protease than the others antiviral drugs [39,40,49]. Nevertheless, De Meyer S. et al. suggested that such promising docking results were indicative of suboptimal binding to this protein and therefore darunavir was not to be considered the best candidate in the treatment of SARS-CoV-2 [35].
In conclusion, the diversity of outcomes from these in silico studies are probably due to different approaches applied to the molecular dynamic simulation.
Clinical studies
Efficacy
Clinical trials
An exploratory randomized controlled trial aimed to study efficacy and safety of LPV/r or arbidol in adult patients with mild/moderate COVID-19 (included 86 patients: 34 received LPV/r, 35 arbidol, and 17 with no antiviral medication) found that both monotherapies present little benefit in improving the clinical outcome compared with control group [50].
Hung et al. [51] performed a multicenter, prospective, open-label, randomized, phase 2 trial comparing monotherapy with LPV/r with a combination treatment including LPV/r, ribavirin and IFN-1β (the latter was administered only if treatment was started < 7 days after symptoms onset). Eighty-six (86) patients were randomly assigned to the combination group and 41 were assigned to LPV/r monotherapy. Overall, the analysis reported a better performance of the combination therapy, that showed shorter times to negative nasopharyngeal swab and clinical improvement and shorter hospital stays. It is interesting to notice that in the post-hoc analysis, there were no differences in the outcomes between the two groups.
In the open-label individually randomized controlled study from Cao et al. [52], 99 patients were treated with LPV/r for 14 days and 100 patients received standard of care (SOC). Median time between symptoms onset and treatment was 13 days. There was no improvement in time to clinical improvement in the LPV/r group, where the 28-day mortality was numerically lower in the LPV/r group (14/99) in comparison with the SOC group (25/100), anyway this difference was not statistically significant in the intention-to-treat analysis. In those treated within 12 days from symptom onset mortality was numerically lower.
Cai et al. [53] performed an open-label, non-randomized, before-after controlled study. Patients were treated with oral LPV/r or favipiravir plus IFN-1β by aerosol inhalation for 14 days (or less if viral clearance occurred). Overall, 35 patients were treated with favipiravir and 45 with LPV/r. The favipiravir group showed shorter time of viral clearance (4 d vs 11 d, p < 0.001) and better results at chest computed tomography (CT) at day 14 (91.4% vs 62.2%, p = 0.004).
On March 2020, WHO launched the SOLIDARITY study, a multinational randomized trial to facilitate the rapid worldwide comparison of COVID-19 unproved treatment (four existing antivirals and anti-inflammatory drugs including LPV/r, are compared with standard of care) [54]. A European satellite of SOLIDARITY trial program, DisCoVeRy intended to analyze the efficacy and safety of treatment options for patients in a limited time frame [55].
On July 4th, WHO discontinued the LPV/r treatment arm from the SOLIDARITY trial after an interim results analysis and after a review of the evidences from all trials presented at the July 1st-2nd WHO Summit on “COVID-19 research and innovation” showing how LPV/r produces little or no reduction in the mortality of hospitalized COVID-19 patients [56]. Also data from DisCoVeRy trial (NCT04315948) [55], announced at the beginning of July, showed that, at 28 days, the death rate was not significantly different in patients randomly allocated to receive LPV/r compared with those randomly allocated to usual hospital care only (22.1% versus 21.3%, 95% confidence interval 0.98 to 1.26, p = 0.10). There was also no evidence of beneficial effects on risk of progression to mechanical ventilation or length of hospital stay [57].
Retrospective studies
In a small retrospective study from Deng et al., the combination of arbidol plus LPV/r was found to be more effective in viral clearance at day 7 and 14, and in chest CT scans at day 7, in comparison with LPV/r monotherapy [58]. Interestingly, 41% of patients in the LPV/r versus 6% in the combination group received corticosteroids. In the preliminary results from the RECOVERY study, dexamethasone administration showed a 20% reduction of 28-day mortality in patients receiving oxygen supplementation [22]. Therefore, whether the combination of antiviral and steroids, or whether the time of administration of one or both agents may have a role in these results, needs further studies.
The study from Jiang et al. reported a median of 14 days to viral clearance in a cohort of 60 patients treated with three different combinations of antiviral therapies (IFN-1β + LPV/r, IFN-1β + LPV/r + arbidol and IFN-1β + LPV/r + oseltamivir) [59]. Anyway, no analysis of differences among the three treatment groups or severity of disease was performed and the number of days between symptoms onset and start of therapy was not reported.
Hraiech et al. [60] reported the findings of a cohort of severe patients admitted in four different Intensive Care Units (ICUs) in France. Seventeen patients were treated with azithromycin plus hydroxychloroquine, 13 with LPV/r and 15 received SOC only. No differences among the three different groups were observed at 6 days post-treatment in viral clearance at the nasopharyngeal swab. Interestingly, in the subgroup of patients that started antiviral treatment <5 days from symptoms onset, the LPV/r group had higher viral clearance than the azithromycin + hydroxychloroquine group (3/7 vs 0/7, p = 0.05). Data from a retrospective study that included 122 patients [61] showed that median duration of viral shedding was longer in patients who did not receive LPV/r (28.5 days) in comparison with LPV/r treatment group (22 days) (log-rank p = 0.009), where the difference was bigger for those who started LPV/r early during COVID-19 (19 days, log-rank p < 0.001) while no difference at all was observed in those who started LPV/r late (27.5 days, log-rank p = 0.51). Conversely, length of hospitalization was higher for patients in patients treated with LPV/r, thus probably reflecting the higher rates of severe disease in this group.
In the study from Ye et al. [62], patients treated with LPV/r in combination therapy showed lower times to negative swabs results in comparison with those not treated with LPV/r. Wang et al. found a median duration of viral spread of 10 days in patients treated with LPV/r diagnosed with COVID-19, while asymptomatic [63]. No difference was found in length of hospital stay and time to negative PCR in the study from Yuan et al. in patients treated with interferon-α (IFN-α) + LPV/r ± ribavirin [64]. In the study from Ding et al. [65], median time to RNA negative result was 11 days with no difference between the two antiviral regimens (LPV/r + IFNα ± arbidol). Interestingly, the only factor that was independently associated with a rapid viral clearance was a high lymphocyte count (≥1.3 × 109/l). A similar median time to negative RNA results was found in the study from Zhang et al. [66]. The study of Zuo et al. [67] found that the association of LPV/r + IFNα was associated with a short viral shedding duration (defined as <20 days) (p = 0.007), in comparison with LPV/r + IFNα + arbidol and LPV/r alone. Interestingly, patients who started treatment <5 days since symptoms onset displayed a faster RNA clearance in comparison with those who started >5 days after symptoms appearance (p = 0.001).
Case series
Cheng et al. [68] found no difference in the duration of viral shedding in patients with mild pneumonia who were treated with LPV/r. Anyway, the study population was very small (2 patients treated with LPV/r and three with SOC only) and one of the two patients treated with LPV/r interrupted treatment after four days.
Liu et al. [69] reported a median of 13 days of hospital stay in patients treated with LPV/r alone or in combination with interferon-α2b (IFN-α2b) in a cohort of 10 patients that started therapy 5 days after symptoms onset on average. Anyway, sample size was very small and four out of 10 patients changed their therapies during the observation period for adverse events and/or clinical deterioration. In another case serie, two patients started treatment two days after symptoms onset (one was asymptomatic) with a combination of LPV/r, arbidol, IFN-α2b and lianhuaqingwen granules. Negative swabs were obtained after 8 days for patient 1 and after 15 days for patient 2. Both had a mild pneumonia that improved at chest XR at day 8 of therapy [70].
Finally, several case series describing outcomes of SARS-CoV-2 and HIV co-infected patients showed as HIV-patients have been infected with SARS-Cov-2 spite of efficient antiretroviral therapy including LPV/r (data not shown).
Case report
One case report showed no detectable or low SARS-CoV-2 titers in a patient treated with LPV/r since the first day after LPV/r administration. Anyway, treatment was started 10 days after symptoms onset and the finding may be due to the natural course of the healing process from COVID-19 [71]. In another case report, where LPV/r was started at day 9 from symptoms onset, RNA titers at nasopharyngeal swabs decreased gradually until resulting negative in day 15. Anyway, a rebound in viral titers was observed at day 11 and 12, despite LPV/r administration [72].
Adverse events (AE)
As far as is known, the most common adverse events (AE) observed with LPV/r combination in HIV-infected patients have been diarrhoea, nausea, vomiting, hypertriglyceridaemia and hypercholesterolemia. Cases of pancreatitis and rare increases in the PR interval on electrocardiogram (ECG) have been reported in patients receiving LPV/r [73].
In the report from Cao et al., almost half of patients with COVID-19 in the LPV/r and in the control group (48.4% vs. 46.7%, respectively) reported one or more adverse events: gastrointestinal-related complaints including nausea, vomiting and diarrhea were more common in the LPV/r group [52]. In the study of Ly et al. [50] the percentage of patients with adverse events were lower but higher than in the arbidol group (35.3% patients in the LPV/r group experienced adverse events vs 14.3% patients in the arbidol group).
Another cases serie collected in China confirm that half of patients suffered digestive adverse effect and 7/10 patients showed hypokalemia [68,69]. Two recent meta-analyses [74,75] evaluating efficacy and safety of the most frequently used therapies for COVID-19 show as most of the AEs rate were comparable across the intervention and control groups. Conversely, LPV/r alone showed trend to more diarrhea events, while the combination of LPV/r, ribavirin and corticosteroids reduced incidence of diarrhea significantly.
Cheng et al. reported psychiatric symptoms such as depression, insomnia and suicidal thoughts in one patient treated with LPV/r, anyway isolation and anxiety for the disease may have played an important role in this case [68].
Cases of bradycardia-tachycardia syndrome and bradyarrythmia were reported in HIV-infected patients on LPV/r therapy, but the underlining pathophysiological mechanism remains unclear [76,77]. Few cases were reported in COVID-19 patients. A prospective study including 41 COVID-19 patients who received LPV/r treatment describes as nine (22%) patients experienced bradycardia. No patient had preexisting nodal pathology on the ECG on admission. Authors considered causality as bradycardia occurred at least 48 h after LPV/r initiation, bradycardia resolved after discontinuation or dose reduction of LPV/r, and no alternative cause was found. No correlation was found between LPV/r plasma concentrations and mean heart rate [78]. Cao et al. did not report any case of bradycardia in their randomized study [52] like in the two meta-analysis above mentioned.
Acute kidney injury (AKI) is a rare adverse event described in HIV-infected patients in treatment with LPV/r and it has been rarely described during COVID-19 treatment with this drug [79].
Conclusions
As we have described above, there are no conclusive results about LPV/r efficacy for treating COVID-19 and its use is currently only permitted in the course of clinical experimentation. On September 1st 2020, 67 studies registered in the database of the U.S National Library of Medicine, currently ongoing, include LPV/r for the treatment of COVID-19 (https://clinicaltrials.gov).
Although up to date no clear benefit was observed with the LPV/r treatment beyond standard care, its efficacy against SARS-COV-2 infection is still under evaluation.
In consideration of the actual biphasic models of the pathogenesis of COVID-19, which hypothesize a virologic-driven damage in the first days of disease and a second phase of inflammation-driven pathology [80], studies of LPV/r efficacy focused on patients during very early phase of disease may help to discern the usefulness of this drug in the actual emergency context.
Up to date neither of “old” (and probably neither of any “new”) drugs are certainly able to cure “all” patients with COVID-19 by reversing the disease or halting its worsening. However, more complex therapeutic approaches based on using combinations of drugs that have different targets and thus affect different pathways and mechanisms compromised by the disease might significantly improve patients' conditions and slow down the disease progression.
Funding support
This research did not receive any financial support.
Conflicts of interest
All authors disclose that they have no conflicts of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.World Health Organization . 2020. Pneumonia of unknown cause - China. Disease outbreak news.https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ [Google Scholar]
- 2.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2020. Naming the coronavirus disease (COVID-19) and the virus that causes it. Technical guidance.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/ [Google Scholar]
- 4.Lau S.K.P., Woo P.C.Y., Li K.S.M., Huang Y., Tsoi H.W., Wong B.H.L. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Boheemen S., de Graaf M., Lauber C., Bestebroer T.M., Raj V.S., Zaki A.M. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. Biofabrication. 2018;11:015002. doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . 2020. Cumulative number of reported probable cases of SARS.https://www.who.int/csr/sars/country/2003_07_11/en/ [Google Scholar]
- 10.World Health Organization . 2020. MERS situation update.https://applications.emro.who.int/docs/EMRPUB-CSR-241-2019-EN.pdf?ua=1&ua=1&ua=1&ua=1&ua=1&ua=1 [Google Scholar]
- 11.World Health Organization . 2020. Coronavirus Disease (COVID-19) dashboard update 8th september.https://covid19.who.int/ [Google Scholar]
- 12.Cevik M., Bamford C.G.G., Ho A. COVID-19 pandemic: a focused review for clinicians. Clin Microbiol Infect. 2020;26:842–847. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovato A., de Filippis C. Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020;99:569–576. doi: 10.1177/0145561320920762. [DOI] [PubMed] [Google Scholar]
- 15.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain V., Yuan J.M. Predictive symptoms and co-morbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Publ Health. 2020;65:533–546. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciceri F., Castagna A., Rovere-Querini P., De Cobelli F., Ruggeri A., Galli L. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509. doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karadag E. Increase in COVID-19 cases and case-fatality and case-recovery rates in Europe: a cross-temporal meta-analysis. J Med Virol. 2020;92:1511–1517. doi: 10.1002/jmv.26035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palem S.P., Palem H.P. The effect of COVID-19 on global population and its fatality rate: retrospective study by online database. Indian J Med Sci. 2020;72:13–16. [Google Scholar]
- 20.Sahu K.K., Mishra A.K., Lal A. Comprehensive update on current outbreak of novel coronavirus infection (2019-nCoV) Ann Transl Med. 2020;8:393. doi: 10.21037/atm.2020.02.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of covid-19 — preliminary report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. :NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. https://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf;jsessionid=C15E303F176AEC6CCF15210950CA015C?sequence=1
- 24.Chandwani A., Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag. 2008;4:1023–1033. doi: 10.2147/tcrm.s3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nukoolkarn V., Lee V.S., Malaisree M., Aruksakulwong O., Hannongbua S. Molecular dynamic simulations analysis of ritonavir and lopinavir as SARS-CoV 3CL(pro) inhibitors. J Theor Biol. 2008;254:861–867. doi: 10.1016/j.jtbi.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan K.S., Lai S.T., Chu C.M., Tsui E., Tam C.Y., Wong M.M.L. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399–406. [PubMed] [Google Scholar]
- 28.Chan J.F.-W., Yao Y., Yeung M.-L., Deng W., Bao L., Jia L. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Públic. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S.J., Yu K.M., Kim Y.I., Kim S.M., Kim E.H., Kim S.G. Antiviral efficacies of FDA-approved drugs against SARS-CoV-2 infection in ferrets. mBio. 2020;11 doi: 10.1128/mBio.01114-20. :e01114-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arshad U., Pertinez H., Box H., Tatham L., Rajoli R.K.R., Curley P. Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics. Clin Pharmacol Ther. 2020;108:775–790. doi: 10.1002/cpt.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choy K.T., Wong A.Y.L., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cattaneo D., Cattaneo D., Gervasoni C., Corbellino M., Galli M., Riva A. Does lopinavir really inhibit SARS-CoV-2? Pharmacol Res. 2020;158:104898. doi: 10.1016/j.phrs.2020.104898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang C.K., Seong M.W., Choi S.J., Kim T.S., Choe P.G., Song S.H. In vitro activity of lopinavir/ritonavir and hydroxychloroquine against severe acute respiratory syndrome coronavirus 2 at concentrations achievable by usual doses. Korean J Intern Med. 2020;35:728–787. doi: 10.3904/kjim.2020.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Meyer S., Bojkova D., Cinatl J., Van Damme E., Buyck C., Van Loock M. Lack of antiviral activity of darunavir against SARS-CoV-2. Int J Infect Dis. 2020;97:7–10. doi: 10.1016/j.ijid.2020.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das S., Sarmah S., Lyndem S., Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1763201. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muralidharan N., Sakthivel R., Velmurugan D., Gromiha M.M. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 protease against COVID-19. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1752802. in press. [DOI] [PubMed] [Google Scholar]
- 38.Nutho B., Mahalapbutr P., Hengphasatporn K., Pattaranggoon N.C., Simanon N., Shigeta Y. Why are lopinavir and ritonavir effective against the newly emerged coronavirus 2019? Atomistic insights into the inhibitory mechanisms. Biochemistry. 2020;59:1769–1779. doi: 10.1021/acs.biochem.0c00160. [DOI] [PubMed] [Google Scholar]
- 39.Pant S., Singh M., Ravichandiran V., Murty U.S.N., Srivastava H.K. Peptide-like and small-molecule inhibitors against Covid-19. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1757510. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peele K.A., Chandrasai P., Srihansa T., Krupanidhi S., Sai A.V., Babu D.J. Molecular docking and dynamic simulations for antiviral compounds against SARS-CoV-2: a computational study. Informatics Med. 2020;19:100345. doi: 10.1016/j.imu.2020.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah B., Modi P., Sagar S.R. In silico studies on therapeutic agents for COVID-19: drug repurposing approach. Life Sci. 2020;252:117652. doi: 10.1016/j.lfs.2020.117652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mamidala E. An In silico approach for identification of inhibitors as a potential therapeutics targeting SARS-Cov-2 protease. Asian J Pharmaceut Res Health Care. 2020;12:3–9. [Google Scholar]
- 43.Gupta S., Singh A.K., Kushwaha P.P., Prajapati K.S., Shuaib M., Senapati S. Identification of potential natural inhibitors of SARS-CoV2 main protease by molecular docking and simulation studies. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1776157. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beck B.R., Shin B., Choi Y., Park S., Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Struct Biotechnol J. 2020;18:784–790. doi: 10.1016/j.csbj.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortega J.T., Serrano M.L., Pujol F.H., Rangel H.R. Unrevealing sequence and structural features of novel coronavirus using in silico approaches: the main protease as molecular target. EXCLI J. 2020;19:400-409. doi: 10.17179/excli2020-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y.W., Yiu C.B., Wong K.Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL pro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Res. 2020;9:129. doi: 10.12688/f1000research.22457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q., Zhao Y., Chen X., Hong A. Virtual screening of approved clinic drugs with main protease (3CLpro) reveals potential inhibitory effects on SARS-CoV-2. J Biomol Struct Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1817786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahanta S., Chowdhury P., Gogoi N., Goswami N., Borah D., Kumar R. Potential anti-viral activity of approved repurposed drug against main protease of SARS-CoV-2: an in silico based approach. J Biomol Struct Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1768902. [DOI] [PubMed] [Google Scholar]
- 49.Khan S.A., Zia K., Ashraf S., Uddin R., Ul-Haq Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J Biomol Struct Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1751298. [DOI] [PubMed] [Google Scholar]
- 50.Li Y., Xie Z., Lin W., Cai W., Wen C., Guan Y. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial. Med. 2020;1:105–113. doi: 10.1016/j.medj.2020.04.001. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hung I.F.N., Lung K.C., Tso E.Y.K., Liu R., Chung T.W.H., Chu M.Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020;6:1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization . 2020. “Solidarity” clinical trial for COVID-19 treatments.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments [Google Scholar]
- 55.Trial of treatments for COVID-19 in Hospitalized Adults (DisCoVeRy) 2020. https://clinicaltrials.gov/ct2/show/NCT04315948 [Google Scholar]
- 56.World Health Organization . 2020. "Solidarity" clinical trial for COVID-19 treatments.https://www.who.int/news-room/feature-stories/detail/global-scientific-community-unites-to-track-progress-oncovid-19-r-d-identifies-new-research-priorities-and-criticalgaps/ [Google Scholar]
- 57.Covid-19: Lopinavir-ritonavir does not benefit hospitalised patients, UK trial finds. BMJ 2020;370:m2650. [DOI] [PubMed]
- 58.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. 2020;81:1–5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang Y., He S., Zhang C., Wang X., Chen X., Jin Y. Clinical characteristics of 60 discharged cases of 2019 novel coronavirus-infected pneumonia in Taizhou, China. Ann Transl Med. 2020;8:547. doi: 10.21037/atm.2020.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hraiech S., Bourenne J., Kuteifan K., Helms J., Carvelli J., Gainnier M. Lack of viral clearance by the combination of hydroxychloroquine and azithromycin or lopinavir and ritonavir in SARS-CoV-2-related acute respiratory distress syndrome. Ann Intensive Care. 2020;10:63. doi: 10.1186/s13613-020-00678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan D., Liu X.Y., Zhu Y.N., Huang L., Dan B.T., Zhang G.J. Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in hospitalised non- critically ill patients with SARS-CoV-2 infection. Eur Respir J. 2020;56:2000799. doi: 10.1183/13993003.00799-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye X., Luo Y., Xia S., Sun Q., Ding J., Zhou Y. Clinical efficacy of lopinavir/Ritonavir in the treatment of Coronavirus disease 2019. Eur Rev Med Pharmacol Sci. 2020;24:3390–3396. doi: 10.26355/eurrev_202003_20706. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Liu Y., Liu L., Wang X., Luo N., Li L. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in shenzhen, China. J Infect Dis. 2020;221:1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan J., Zou R., Zeng L., Kou S., Lan J., Li X. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res. 2020;69:599–606. doi: 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding J.G., Li J., Hong L., Yu X.Q., Ye E.L., Sun G.Q. Viral kinetics and factors associated with rapid viral clearance during lopinavir/ritonavir-based combination therapy in non-severe COVID-19 patients. Eur Rev Med Pharmacol Sci. 2020;24:5788–5796. doi: 10.26355/eurrev_202005_21373. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Z., Wang S., Tu X., Peng X., Huang Y., Wang L. A comparative study on the time to achieve negative nucleic acid testing and hospital stays between danoprevir and lopinavir/ritonavir in the treatment of patients with COVID-19. J Med Virol. 2020;92:2631–2636. doi: 10.1002/jmv.26141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuo Y., Liu Y., Zhong Q., Zhang K., Xu Y., Wang Z. Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID-19: a retrospective study in two designated hospitals in Anhui, China. J Med Virol. 2020;92:2666–2674. doi: 10.1002/jmv.26127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng C.Y., Lee Y.L., Chen C.P., Lin Y.C., Liu C.E., Liao C.H. Lopinavir/Ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan. J Microbiol Immunol Infect. 2020;53:488–492. doi: 10.1016/j.jmii.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K. Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L., Xu X., Ruan J., Lin S., Jiang J., Ye H. Quadruple therapy for asymptomatic COVID-19 infection patients. Expert Rev Anti Infect Ther. 2020;18:617–624. doi: 10.1080/14787210.2020.1758066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klement-Frutos E., Burrel S., Peytavin G., Marot S., Lê M.P., Godefroy N. Early administration of ritonavir-boosted lopinavir could prevent severe COVID-19. J Infect. 2020;82:159–198. doi: 10.1016/j.jinf.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.European Medicine Agency . 2020. Kaletra EPAR: product information.https://www.ema.europa.eu/en/documents/product-information/kaletra-epar-product-information_en.pdf [Google Scholar]
- 74.Zhong H., Wang Y., Zhang Z.L., Liu Y.X., Le K.J., Cui M. Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: a systematic review and meta-analysis. Pharmacol Res. 2020;157:104872. doi: 10.1016/j.phrs.2020.104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu W., Zhou P., Chen K., Ye Z., Liu F., Li X. Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARS-CoV-2 and other acute viral infections: a systematic review and meta-analysis. CMAJ. 2020;192:E734–E744. doi: 10.1503/cmaj.200647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kikuchi Y., Genka I., Ishizaki A., Sunagawa K., Yasuoka A., Oka S. Serious bradyarrhythmia that was possibly induced by lopinavir-ritonavir in 2 patients with acquired immunodeficiency syndrome. Clin Infect Dis. 2002;35:488–490. doi: 10.1086/341975. [DOI] [PubMed] [Google Scholar]
- 77.Yotsumoto M., Kitano K., Saito H. Bradycardia-tachycardia syndrome induced by lopinavir-ritonavir in a patient with AIDS. AIDS. 2005;19:1547–1548. doi: 10.1097/01.aids.0000183942.05849.1b. [DOI] [PubMed] [Google Scholar]
- 78.Beyls C., Martin N., Hermida A., Abou-Arab O., Mahjoub Y. Lopinavir-ritonavir treatment for COVID-19 infection in intensive care unit: risk of bradycardia. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Binois Y., Hachad H., Salem J.E., Charpentier J., Lebrun-Vignes B., Pène F. Acute kidney injury associated with lopinavir/ritonavir combined therapy in patients with Covid-19. Kidney Int Rep. 2020;5:1787–1790. doi: 10.1016/j.ekir.2020.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao W., Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020;30:367–369. doi: 10.1038/s41422-020-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]