Abstract
Recent evidence suggests that oxidative stress contributes to the pathogenesis of prostate cancer. The present study focused on the effect of apocynin, an inhibitor of NADPH oxidase, on prostate carcinogenesis using the transgenic rat for adenocarcinoma of prostate (TRAP) model. There were no toxic effects with apocynin treatment. The percentages and numbers of carcinomas in both the ventral and lateral prostate were significantly reduced by apocynin treatment, with dose dependence. Reduction of reactive oxygen species by apocynin was confirmed by immunohistochemistry of 8‐OHdG and dihydroethidium staining. Positivity of Ki67 was significantly reduced by apocynin treatment, and downregulation of clusterin expression, as well as inactivation of the MEK‐ERK1/2 pathway, was a feature of the apocynin treated groups. In human prostate cancer cell line LNCaP, apocynin also inhibited reactive oxygen species production and blocked cell growth by inducing G0/G1 arrest with downregulation of clusterin and cyclin D1. These data suggest that apocynin possesses chemopreventive potential against prostate cancer.
Apocynin, a NADPH oxidase inhibitor, suppressed prostate carcinogenesis in TRAP model with reduction of reactive oxygen species (ROS) generation and cell proliferation in vivo. it also inhibited ROS production and blocked cell growth by inducing G0/G1 arrest with down‐regulation of clusterin and cyclin D1 in LNCaP cells. These data suggest that apocynin possesses a chemopreventive potential for prostate cancer.
Prostate cancer is the second most frequently diagnosed cancer in men in the world, with particularly high incidences in Oceania, Europe and North America. In Japan, incident and mortality rates for prostate cancer are relatively low are but increasing.1, 2 There are potentially curative options, such as radical prostatectomy or radiotherapy, but once the disease is metastatic, the outlook is poor. Therefore, research into chemoprevention of prostate cancer is critical.
Reactive oxygen species (ROS) can be important factors for carcinogenesis and tumor progression, not only inducing DNA damage but also producing cellular alterations, such as upregulation of MAPK and protein kinase C.3, 4 Recently, oxidative stress has been reported to contribute to cancer and progression in the prostate.5, 6 Therefore, we have focused on inhibition of ROS production as an anti‐carcinogenic approach. ROS is produced by mitochondria, peroxisomes, cytochrome P‐450 and other cellular elements as a byproduct, and is generated by NADPH oxidase, which is also implicated in a variety of signaling events, including cell growth, cell survival and cell death.7 Apocynin, which belongs to the methoxy‐substituted catechol family, inhibits NADPH oxidase activity by blocking the formation of NADPH oxidase complex8 and is commonly used as a standard NOX inhibitor for research purposes.7 In addition, apocynin can be converted by peroxidase‐mediated oxidation to a dimer, which has been shown to be a more efficient inhibitor than apocynin itself.9 We previously presented evidence that apocynin reduced oxidative stress induced by arsenite treatment of rat urothelium in vivo.10
In the present study we focus on NADPH oxidase and test whether its inhibitor, apocynin, is able to suppress prostate carcinogenesis in the transgenic rat for adenocarcinoma of prostate (TRAP) model, which was generated in our laboratory and features development of well‐differentiated prostate adenocarcinomas in prostatic lobes within a short period. The TRAP rat has a transgene that encodes SV40 T antigen under probasin promoter, so that the carcinomas that develop are androgen‐dependent.11, 12
Materials and Methods
Animal experiment
Male heterozygous TRAP rats established in our laboratory with a Sprague–Dawley genetic background were used in the present study. All animals were housed in plastic cages on wood‐chip bedding in an air‐conditioned specific pathogen‐free animal room at 22 ± 2°C and 55 ± 5% humidity with a 12 h light/dark cycle, and fed a basal diet (Oriental MF, Oriental Yeast, Tokyo, Japan) and provided water, with or without apocynin, ad libitum. All animal experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of Nagoya City University School of Medical Sciences.
Six‐week‐old TRAP rats were divided into three groups of 11 rats each. The animals were given drinking water containing 0, 100 and 500 mg/L apocynin for 8 weeks and body weight and water consumption were estimated every week. At experimental week 8, under deep isoflurane anesthesia, blood was collected from 9.00 to 11.00 h to measure testosterone and estradiol hormone levels using radioimmunoassays from a commercial laboratory (SRL, Tokyo, Japan). Adiponectin concentrations in serum were determined by ELISA (adiponectin ELISA kit; Otsuka Pharmaceutical, Tokyo, Japan). The urogenital complex of each rat was removed as a whole together with the seminal vesicles, then the ventral prostate was weighed. A part of the prostate glands was immediately frozen in liquid nitrogen and stored at −80°C until processed. After that, the remaining tissue was fixed. Livers, kidneys and testes were also removed, weighed and fixed. For clusterin immunostaining, normal prostate glands from a 14‐week‐old male Sprague–Dawley rat were fixed. The tissues were routinely processed into paraffin‐embedded sections and stained with H&E.
Histopathology and immunohistochemistry
Neoplastic lesions in the prostate gland of TRAP rats were evaluated as previously described.13, 14 Briefly, neoplastic lesions were classified into three types: low grade prostatic intraepithelial neoplasia (LG‐PIN), high grade PIN (HG‐PIN) and adenocarcinoma. The relative numbers of acini with the histological characteristics of each type, that is, LG‐PIN, HG‐PIN and adenocarcinoma, were quantified by counting the total acini in each prostatic lobe. Deparaffinized sections were incubated with diluted antibodies for Ki‐67 (Novocastra Laboratories, Newcastle, UK), anti‐8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) antibody (Japan Institute for the Control of Aging, Fukuroi, Japan), clusterin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), androgen receptor (AR) and SV40 T antigen (Santa Cruz Biotechnology). The number of Ki‐67‐labeled or 8‐OHdG‐labeled cells in at least 1000 cells was counted to determine the labeling indices of HG‐PIN and adenocarcinoma.
Detection of reactive oxygen species production
Six‐micron frozen serial sections cut on a standard cryostat with clean blades were mounted on slides, then incubated with 5 μM dihydroethidium (Life Technologies, Carlsbad, CA, USA) in PBS for 15 min in the dark. The slides were washed two times with warm PBS and the fluorescence intensity was assessed at 518/605 nm with a spectrofluorometer. Images were also recorded with a fluorescence microscope (BZ‐9000; Keyence, Osaka, Japan).
Microarray analysis
Total RNA was isolated from ventral prostate tissues en bloc by phenol–chloroform extraction (ISOGEN, Nippon Gene, Toyama, Japan). Gene expression analysis was performed using a Rat Oligo chip 20k (Toray Industries, Tokyo, Japan) according to the manufacturer's instructions. The RNA ventral prostate expression from 500 mg/L apocynin‐treated rats was compared with that from control rats. After global median normalization, data cleansing was performed to remove the values for which fluorescence intensity was <100. The genes for which expression were more than twofold increased or reduced to less than half in 500 mg/L apocynin‐treated rats as compared to control rats were selected.
Real‐time RT‐PCR
Total RNAs from ventral prostate tissues were reverse‐transcribed with the ThermoScript first‐strand synthesis system (Life Technologies), and real‐time RT‐PCR was performed using a LightCycler (Roche Diagnostics GmbH, Penzberg, Germany). The quantitative value of clusterin was normalized to endogenous cyclophilin. Clusterin RT‐PCR primers were 5′‐TTATGGACACAGTGGCAGAG‐3′ and 5′‐TACAGAACCCAGAGGAAGGA‐3′. Cyclophilin RT‐PCR primers were 5′‐TGCTGGACCAAACACAAATG‐3′ and 5′‐GAAGGGGAATGAGGAAAATA‐3′.
Immunoblot analyses
Ventral prostate tissues were homogenized with RIPA buffer (Pierce Biotechnology, Rockford, IL, USA) containing a protease inhibitor (Pierce Biotechnology) on ice. The insoluble matter was removed by centrifugation at 10 000g for 20 min at 4°C and supernatants were collected. Protein concentrations were determined with a Coomassie Plus – The Better Bradford Assay Kit (Pierce Biotechnology). Samples were mixed with 2× sample buffer (Bio‐Rad Laboratories, Hercules, CA, USA) and heated for 5 min at 95°C and then subjected to SDS‐PAGE. The separated proteins were transferred onto nitrocellulose membranes followed by blocking with SuperBlock Blocking Buffer (Thermo Fisher Scientific, Waltham, MA, USA) for 1 h at room temperature. Membranes were probed with antibodies for cyclin D1, clusterin, cleaved caspase‐3, MEK, phospho‐MEK, p44/42 MAPK (ERK1/2), phopho‐ERK1/2, p38 MAPK, phospho‐p38 MAPK, and caspase 3 and 7 (Cell Signaling Technology, Danvers, MA, USA) in 1× TBS with 0.1% Tween 20 at 4°C overnight, followed by exposure to peroxidase‐conjugated appropriate secondary antibodies and visualization with an enhanced chemiluminescence detection system (GE Healthcare Bio‐sciences, Buckinghamshire, NA, UK). To confirm equal protein loading, each membrane was stripped and reprobed with anti‐β‐actin (Sigma‐Aldrich, St. Louis, MO, USA).
Cell line
The human androgen‐dependent prostate cancer cell line LNCaP was obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were grown in RPMI1640 medium with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin (all from Life Technologies) under an atmosphere of 95% air and 5% CO2 at 37°C.
Cell proliferation assay
Cell proliferation of prostate cancer cell lines was assessed by 4‐[3‐(4‐iodophenyl)‐2‐(4‐nitrophenyl)‐2H‐5‐tetrazolio]‐1,3‐benzene disulfonate tetrazolium salt (WST‐1) assay (Roche Applied Science, Mannheim, Germany). Briefly, cells were seeded in 96‐well plates at 500 cells/well in 200 μL of culture medium. Apocynin was added 24 h after seeding and incubated for 3 days. WST‐1 reagent was added to each well with incubation for 60 min at 37°C, and then each well was measured for absorbance at 430 nm.
Detection of reactive oxygen species production in LNCaP cells
The culture supernatant was removed from all wells 24 h after apocynin treatment, and the cells were washed twice with warm PBS, then 2′,7′‐dichlorofluorescin‐diacetate (100 μg/mL, DCFH‐DA, Sigma‐Aldrich) was added with further incubation at 37°C for 20 min, in the dark. The cells were washed two times with warm RPMI1640 medium, and images were recorded using a fluorescence microscope (BZ‐9000; Keyence).
Cell cycle analysis
Cells were treated with apocynin for 24 h, then suspensions were prepared and stained with propidium iodide (Guava Cell Cycle Reagent, Guava Technologies, Hayward, CA, USA) according to the Guava Cell Cycle Assay protocol. Cell cycle phase distributions were determined on a Guava PCA Instrument using CytoSoft Software.
Statistical analysis
All in vitro experiments were performed at least in triplicate to confirm reproducibility. Statistical analyses were performed with mean ± SD values using one‐way anova and Dunnett's test. Statistical significance was concluded at *P < 0.05, **P < 0.01 or ***P < 0.001.
Results
Apocynin reduction of progression of prostate tumorigenesis as well as cell proliferation and reactive oxygen species in TRAP rats
During the experiments, water consumption by 500 mg/L apocynin‐treated rats was approximately 10% lower than in the controls (Table 1). However, administration of apocynin did not cause adverse effects (e.g. impacting the growth of rats) during the study. There were no significant differences in the final body weights or in absolute and relative liver, kidney, testes and prostate weights among the groups (Table 1). Histologically, there were no changes indicative of toxicity in the liver, kidneys, and testes with apocynin (data not shown). The serum level of testosterone in 500 mg/L apocynin treated rats (3.1 ± 1.6 ng/mL) was slightly higher than in other groups (control and 100 mg/L apocynin: 1.7 ± 1.0 and 1.9 ± 0.8 ng/mL, respectively) but that of estradiol was not affected (control, 100 and 500 mg/L apocynin: 3.2 ± 1.0, 3.4 ± 1.2 and 3.3 ± 0.7 pg/mL, respectively). Adiponection concentration in the serum did not differ among the groups (control, 100 and 500 mg/L apocynin: 3.5 ± 1.1, 3.2 ± 0.5 and 3.5 ± 0.8 ng/mL, respectively).
Table 1.
Body and organ weights, water consumption
| Treatment | Number of rats | Body weight (g) | Liver (g) | Kidneys (g) | Testes (g) | Ventral prostate (g) | Water consumption (mL/rat/day) |
|---|---|---|---|---|---|---|---|
| Control | 11 | 533 ± 58 | 18.5 ± 2.0 | 3.2 ± 0.2 | 3.5 ± 0.4 | 0.30 ± 0.06 | 45.5 ± 4.4 |
| 100 mg/L | 11 | 553 ± 49 | 20.1 ± 2.3 | 3.1 ± 0.3 | 3.6 ± 0.3 | 0.36 ± 0.09 | 46.5 ± 5.5 |
| 500 mg/L | 11 | 558 ± 65 | 20.2 ± 3.2 | 3.2 ± 0.4 | 3.6 ± 0.4 | 0.30 ± 0.04 | 39.6 ± 2.6a |
Significantly different from control group, P < 0.05.
Representative histopathological findings of ventral and lateral prostate in each group are presented in Figure 1(a). In the ventral prostate, there was a marked or partial pathologic response to apocynin treatment, as demonstrated by a significant reduction in the prostatic neoplastic lesions in TRAP rats; however, small foci of PIN remained. Decrease in the incidence of adenocarcinoma was also observed in the lateral prostate (Table 2). Quantitative evaluation of the proportion of preneoplastic and neoplastic lesions in the prostate gland showed significant suppression of progression from LG‐PIN or HG‐PIN to adenocarcinoma in rats treated with apocynin in a dose‐dependent manner (Table 2). To focus on adenocarcinoma, the number of foci per area in both the ventral and lateral prostate was significantly reduced by apocynin in a dose‐dependent manner (Fig. 1b,c). There was no difference in the average size of adenocarcinomas in both the ventral and lateral prostate among the groups (data not shown). There was a significant decrease in the labeling index of Ki‐67 and 8‐OHdG in HG‐PIN of the ventral and lateral prostates of TRAP rats given apocynin in a dose‐dependent manner (Table 3). Although the rate of significance was slightly less, the results were mostly the same in the adenocarcinoma of the ventral and lateral prostates (Table S1). Results for ROS detection in ventral prostate by dihydroethidium are presented in Figure 1(d), with significant reduction (11 and 22% reduction at 100 and 500 mg/L, respectively) documented (Fig. 1e). Androgen receptors and SV40 T antigen were diffusely detected immunohistochemically in areas of PIN and adenocarcinomas in the ventral and lateral prostate, with no differences among the groups (data not shown).
Figure 1.
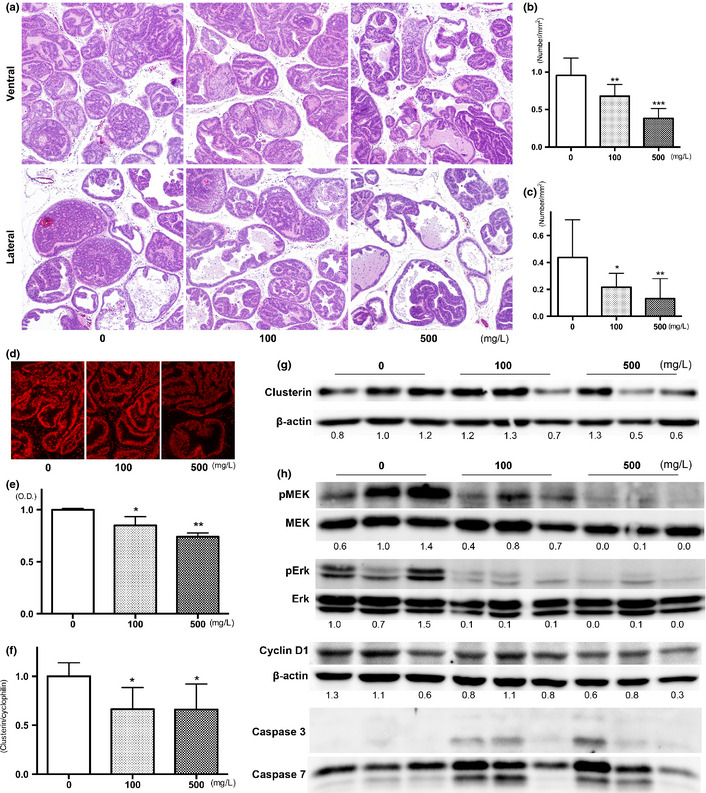
Effects of apocynin on prostatic lesions. Representative histopathological findings for lesions in the ventral and lateral prostates of the controls, 100 and 500 mg/L apocynin groups (a). Number of adenocarcinoma per area in ventral (b) and lateral (c) prostate of TRAP rats treated with apocynin. Photos (d) and data (e) for reactive oxygen species production detected by dihydroethidium staining in ventral prostate of TRAP rats treated with apocynin. Expression of clusterin detected by quantitative RT‐PCR (f) and western blotting (g). Results of immunoblot analysis of MAPK, cyclin D1 and caspases in ventral prostates of TRAP rats treated with apocynin. Data are mean ± SD values from 3 independent experiments. *,**,***P < 0.05, 0.01 and 0.001 compared to controls, respectively.
Table 2.
Incidence of carcinoma and quantitative evaluation of neoplastic lesions in ventral and lateral prostate
| Treatment | Number of rats | Ventral | Lateral | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Incidence of carcinoma | % of lesions in prostate | Incidence of carcinoma | % of lesions in prostate | ||||||
| LG‐PIN | HG‐PIN | Carcinoma | LG‐PIN | HG‐PIN | Carcinoma | ||||
| Control | 11 | 11 (100%) | 6.6 ± 2.5 | 81.8 ± 4.8 | 11.7 ± 4.5 | 10 (91%) | 11.8 ± 5.2 | 82.8 ± 6.8 | 5.4 ± 3.9 |
| 100 mg/L | 11 | 11 (100%) | 8.0 ± 2.5 | 83.6 ± 3.8 | 8.4 ± 2.8* | 10 (91%) | 17.4 ± 4.3 | 80.1 ± 4.3 | 2.5 ± 1.4* |
| 500 mg/L | 11 | 11 (100%) | 8.3 ± 1.6 | 87.3 ± 3.2** | 4.4 ± 1.9*** | 6 (55%) | 26.8 ± 9.9*** | 71.8 ± 9.0** | 1.5 ± 1.7** |
*,**,***Indicate significant difference from the control group, P < 0.05, 0.1, 0.001, respectively.
Table 3.
Labeling indices of Ki67 and 8‐OHdG in HG‐PIN of ventral and lateral prostate
| Treatment | Ventral prostate | Lateral prostate | ||
|---|---|---|---|---|
| Ki67 | 8‐OHdG | Ki67 | 8‐OHdG | |
| Control | 52.6 ± 1.1 | 2.9 ± 0.4 | 49.7 ± 1.6 | 2.7 ± 0.4 |
| 100 mg/L | 43.8 ± 2.2*** | 2.3 ± 0.4** | 35.2 ± 7.2*** | 2.0 ± 0.2*** |
| 500 mg/L | 24.4 ± 1.8*** | 1.5 ± 0.3*** | 18.4 ± 2.8*** | 1.5 ± 0.2*** |
**, ***Significantly different from control group, P < 0.01, 0.001, respectively.
Reduction of cell proliferation by apocynin treatment was associated with changes in clusterin and the MEK‐ERK1/2 pathway
To elucidate the mechanisms of anti‐carcinogenesis of apocynin, microarray analysis was performed (GEO: GSE47200). Genes that were upregulated or downregulated by apocynin are listed in Table 4. After selection, 9 upregulated and 14 downregulated genes were detected, some related to oxidative responses (NADH‐ubiquinone oxidoreductase chain 3, cytochrome c oxidase subunit 1 and 2) and others immunological responses (Ig lambda‐2 chain C region, Cfh protein, secretoglobin family 2A member 2 precursor, RT1 class I, CE1, CE2 and CE10, RT1 class Ia, locus A1). Because apocynin reduced rat prostate carcinogenesis, we focused on the clusterin precursor belonging to downregulated genes, which is known to be related to tumorigenesis in many sites. Reduction of clusterin expression in the ventral prostate of apocynin‐treated rats was confirmed by real‐time RT‐PCR (Fig. 1f) and also by western blotting (Fig. 1g). Immunohistochemical analyses revealed that clusterin expression was observed mainly in the epithelial membranes of the ventral prostate of TRAP rats, but no staining was observed in the epithelium of the prostate of Sprague–Dawley rats (Fig. S1a). Differences in the intensity of clusterin immunostaining determined by optical density measurement was relatively inconspicuous among LG‐PIN, HG‐PIN or adenocarcinoma in the ventral lobes of the control TRAP rats (Fig. S1b). However, clusterin expression in HG‐PIN of ventral prostate of apocynin‐treated TRAP rats was significantly reduced compared to the control (Fig. S1c; control, 100 and 500 mg/L apocynin: 50.1 ± 5.0, 43.2 ± 7.4 and 35.6 ± 4.3, respectively). Reduction of phosphorylation in MEK and p44/42 MAPK (ERK1/2), and cyclin D1 were also detected. Cleaved caspases 3 and 7 were detected in the ventral prostate of some apocynin‐treated rats (Fig. 1h).
Table 4.
Upregulated and downregulated genes by apocynin treatment in ventral prostate
| ID | Reference ID | Symbol | Description | Ratio |
|---|---|---|---|---|
| Upregulated genes | ||||
| ENSRNOG00000038093 | – | LAC2_RAT | Ig lambda‐2 chain C region | 5.62 |
| ENSRNOG00000033745 | – | Q5FVP9_RAT | Cfh protein | 3.10 |
| ENSRNOG00000005743 | NM_001108582.1 | NP_001102052.1 | LOC362136 (predicted) (RGD1309610_predicted), mRNA | 2.58 |
| ENSRNOG00000033615 | – | NU3M_RAT | NADH‐ubiquinone oxidoreductase chain 3 (EC 1.6.5.3) | 2.45 |
| ENSRNOG00000015155 | NM_001037351.1 | Tnnc2 | Troponin C2, fast | 2.11 |
| ENSRNOG00000008746 | NM_001108594.1 | NP_001102064.1 | Histidyl tRNA synthetase 2 | 2.07 |
| ENSRNOG00000001333 | NM_012826.1 | Azgp1 | Zinc‐alpha‐2‐glycoprotein precursor | 2.06 |
| ENSRNOG00000002871 | NM_001108984.1 | NP_001102454.1 | RNA binding motif protein 25 | 2.05 |
| ENSRNOG00000023151 | NM_203333.2 | Scgb2a2 | Secretoglobin family 2A member 2 precursor | 2.01 |
| Downregulated genes | ||||
| ENSRNOG00000021604 | NM_001008835.1 | RT1‐CE1 | RT1 class I, CE1 | 0.28 |
| ENSRNOG00000002343 | NM_017237.2 | Uchl1 | Ubiquitin carboxyl‐terminal hydrolase isozyme L1 (EC 3.4.19.12) | 0.36 |
| ENSRNOG00000038999 | NM_001008827.1 | RT1‐A1 | RT1 class Ia, locus A1 | 0.36 |
| ENSRNOG00000029579 | NM_001008840.1 | RT1‐CE2 | RT1 class I, CE2 | 0.41 |
| ENSRNOG00000030251 | – | RT1‐CE10 | RT1 class I, CE10 | 0.41 |
| ENSRNOG00000032872 | – | UBIQ_RAT | Ubiquitin | 0.43 |
| ENSRNOG00000034234 | – | COX1_RAT | Cytochrome c oxidase subunit 1 (EC 1.9.3.1) | 0.43 |
| ENSRNOG00000037414 | – | Q5I1B4_RAT | ABP beta (Fragment) | 0.44 |
| ENSRNOG00000030371 | – | COX2_RAT | Cytochrome c oxidase subunit 2 | 0.45 |
| ENSRNOG00000039668 | NM_001107100.1 | NP_001100570.1 | Procollagen, type VIII, alpha 1 | 0.46 |
| ENSRNOG00000016460 | NM_053021.2 | Clu | Clusterin precursor | 0.49 |
| ENSRNOG00000011647 | NM_053485.2 | S100a6 | Protein S100‐A6 | 0.50 |
| ENSRNOG00000007539 | NM_138881.1 | Best5 | Radical S‐adenosyl methionine domain‐containing protein 2 | 0.50 |
| ENSRNOG00000002052 | NM_022543.2 | Ssg1 | Coiled‐coil domain‐containing protein 80 precursor | 0.50 |
Apocynin affected reduction of cell growth via the same pathways in a human prostate cancer cell line
In the human prostate cell line, significant reduction of cell growth and ROS generation was detected after apocynin treatment at concentrations higher than 250 μM (Fig. 2a,b). Apocynin‐treated cells appeared to accumulate in G1 phase at 500 μM, compared to the controls (P < 0.05), with concomitant decrease in the percentage of cells in the S phase (Fig. 2c). Downregulation of clusterin around 70 kDa and cyclin D1 expression were detected by western blotting (Fig. 2d).
Figure 2.
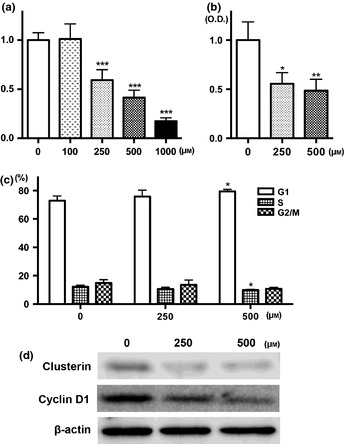
Anti‐growth effects of apocynin in LNCaP cells. Cells were incubated with apocynin for 72 h and then cytotoxicity was assessed by WST‐1 assay (a). Reactive oxygen species production data detected by DCFH‐DA after apocynin treatment for 24 h (b). Cell cycle distribution of LNCaP cells after treatment with apocynin for 24 h (c). Immunoblot analysis of the protein levels of clusterin, cyclin D1 and ß‐actin with apocynin treatment (d). *P < 0.05, **P < 0.01 and ***P < 0.001 compared to controls. O.D, optical density.
Discussion
In the present study, we demonstrated suppressive effects of apocynin treatment on prostatic carcinogenesis in the TRAP rat model. Because apocynin is known to be an NADPH oxidase inhibitor, we focused on ROS conditions in prostate tissue using immunohistochemistry for 8‐OHdG and dihydroethidium staining. Some reports have indicated that antioxidants such as N‐acetylcysteine and vitamin C clearly reduce oxidative stress in vitro, but exert less effects with oral dosing in vivo.15, 16 ROS is generally produced from mitochondria, peroxisomes, cytochrome P450 and NADPH oxidase.4, 6, 7 Apocynin only affects ROS reduction from NADPH oxidase, but it would be expected to be a good antioxidant in vivo. In addition, we did not detect any toxic effects of apocynin in this study.
Reactive oxygen species generation is not only considered associated with tissue injury and/or DNA damage, but also neoplastic transformation, aberrant growth and/or proliferation.4, 7 Indeed, ROS may play broad roles in cellular processes associated with initiation and development of many cancers, including prostate cancer.6 In this study, apocynin reduced the Ki‐67 labeling index and cyclin D1 expression, which indicates that ROS generation is related to cell proliferation in prostate lobes of TRAP rats. Furthermore, NADPH oxidase is reported to regulate plasma adiponectin,17 whose concentration may be negatively correlated with prostate cancer progression.18 Therefore, we investigated adiponectin concentration in serum, but did not find any differences among the groups.
In TRAP rats, we previously detected activation of p38 MAPK and ERK1/2 in prostate tissue, and inactivation by chemopreventive agents such as angiotensin II receptor blocker or purple corn color.14, 19 These also reduced cell proliferation and/or induced apoptosis in prostate tissue. In this study, we also detected dephosphorylation of ERK1/2 in the prostate after apocynin treatment. To assess the relationship between ROS generation and phosphorylation of ERK1/2, microarray analysis was employed. The data obtained for upregulated and downregulated genes indicated associations with ROS generation, immune function and translation by gene ontology.20 We focused on clusterin, dysregulated in many types of cancer, including prostate cancer,21 with cellular functions such as a sulfated glycoprotein in regulation of apoptosis, cell cycle control, DNA repair, cell adhesion and tissue remodeling.22, 23 It has two isoforms. Secretory clusterin (sCLU) has a chaperone action like that of small heat shock proteins, and is sized around 70–80 kDa. The other clusterin isoform is the nuclear form (nCLU) of 55 kDa, associated with cell death.21, 22, 23 In this study, we detected significant reduction of clusterin protein around 70 kDa. Because sCLU is reported to be downregulated by p53 in some tumor cells,22 high expression of clusterin in the prostate would be expected given dysfunction of p53 under the influence of SV40 T antigen in TRAP rats.24
In human prostate cancer, clusterin expression is initially low in contrast with the prostate cancer cells resistant to conventional chemotherapy or hormonal therapy which show upregulation.21, 25 Meanwhile, we detected rather high expression of clusterin in all stages of prostate cancer development in TRAP rats. In te transgenic adenocarcinoma of mouse prostate (TRAMP) model, higher expression of clusterin in the prostate glands was detected compared to that in non‐transgenic mice; however, analyses of overt carcinoma revealed no expression.26 These data suggest that SV40 T antigen supports to induce clusterin expression among the carcinogenic process in the prostate of both mice and rats. The difference in clusterin expression in carcinoma may be related to the different histology between mice (poorly‐differentiated neuroendocrine‐like carcinomas) and rats (moderately differentiated adenocarcinomas), because clusterin is mainly stained in the membrane.
Recently, sCLU was reported to induce phosphorylation of ERK1/2 in lung adenocarcinoma and pancreatic cancer cells.27, 28 Therefore, we considered that apocynin reduced prostate carcinogenesis via clusterin, acting on the ERK1/2 pathway and reducing cell proliferation in TRAP rats. We also found that apocynin reduced cell growth and ROS generation, along with expression of clusterin and cyclin D1 in LNCaP cells (human prostate cell line). Although there is a possibility that the exact intracellular molecular pathways affected by apocynin treatment may be different between humans and rats, we suggest that there may be similar anti‐neoplastic effects caused by apocynin, especially related to clusterin expression, in rat and human prostate.
Chemoprevention attempts are comprehensive in prostate cancer, and various agents are reported to prevent, reverse or delay tumor deveolpment and progression.29 In this study, we detected lower incidence of adenocarcinoma in the lateral lobes of 500 mg/L apocynin‐treated rats, and reduction of cell proliferation was observed in both PIN and adenocarcinoma. Thus, we consider that apocynin has potential to prevent and delay the carcinogenic process, and is suitable as a chemopreventive drug. Recently, custirsen (OGX‐011), a clusterin inhibitor, was selected for use as an anti‐cancer drug in a randomized phase II study of patients with metastatic castration‐resistant prostate cancer.30, 31 We earlier reported that apocynin inhibited growth of androgen‐independent prostate cancer with inhibition of phosphorylation of Rac1 and NF‐κB and angiogenesis.32 Together with the present data, we confirm that apocynin has the potential to be a candidate anti‐cancer drug for androgen‐dependent/independent prostate cancer.
In conclusion, apocynin, an NADPH oxidase inhibitor, suppressed prostate carcinogenesis while reducing ROS in the TRAP rat model. The mechanism of prevention appears to involve regulation of cell proliferation via clusterin and the MEK‐ERK1/2 pathway. Apocynin warrants further attention as a promising chemopreventive drug for prostate cancer.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Expression of clusterin in ventral lobe of prostate. Pictures of H&E and immunohistochemistry of clusterin in normal epithelium in Sprague–Dawley rat, LG‐PIN, HG‐PIN and carcinoma in TRAP rats (a) and expression of clusterin (b). Expression of clusterin in HG‐PIN of ventral lobe of control, 100 and 500 mg/L apocynin treated rats (c). Carcinoma; Adenocarcinoma. *,***P < 0.05 and 0.001 compared to Normal epithelium or control, respectively.
Table S1. Labeling indices of Ki67 and 8‐OHdG in adenocarcinoma of ventral and lateral prostate.
Acknowledgments
This work was supported by a Grant‐in‐Aid for the 3rd Term Comprehensive 10‐Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan and grants from Ono Pharmaceutical, the Society for Promotion of Pathology in Nagoya, and the Research Foundation for Oriental Medicine. The authors would like to thank Dr Malcolm Moore for his help in reviewing this manuscript.
(Cancer Sci 2013; 104: 1711–1717)
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Damber JE, Aus G. Prostate cancer. Lancet 2008; 371: 1710–21. [DOI] [PubMed] [Google Scholar]
- 3. Lee YJ, Lee JH, Han HJ. Extracellular adenosine triphosphate protects oxidative stress‐induced increase of p21(WAF1/Cip1) and p27(Kip1) expression in primary cultured renal proximal tubule cells: role of PI3K and Akt signaling. J Cell Physiol 2006; 209: 802–10. [DOI] [PubMed] [Google Scholar]
- 4. Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev 2006; 25: 695–705. [DOI] [PubMed] [Google Scholar]
- 5. Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 2008; 68: 1777–85. [DOI] [PubMed] [Google Scholar]
- 6. Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett 2009; 282: 125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bedard K, Krause KH. The NOX family of ROS‐generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007; 87: 245–313. [DOI] [PubMed] [Google Scholar]
- 8. Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy‐substituted catechol. Am J Respir Cell Mol Biol 1994; 11: 95–102. [DOI] [PubMed] [Google Scholar]
- 9. Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm 2008; 2008: 106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki S, Arnold LL, Pennington KL, Kakiuchi‐Kiyota S, Cohen SM. Effects of co‐administration of dietary sodium arsenite and an NADPH oxidase inhibitor on the rat bladder epithelium. Toxicology 2009; 261: 41–6. [DOI] [PubMed] [Google Scholar]
- 11. Asamoto M, Hokaiwado N, Cho YM et al Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res 2001; 61: 4693–700. [PubMed] [Google Scholar]
- 12. Cho YM, Takahashi S, Asamoto M et al Age‐dependent histopathological findings in the prostate of probasin/SV40 T antigen transgenic rats: lack of influence of carcinogen or testosterone treatment. Cancer Sci 2003; 94: 153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seeni A, Takahashi S, Takeshita K et al Suppression of prostate cancer growth by resveratrol in the transgenic rat for adenocarcinoma of prostate (TRAP) model. Asian Pac J Cancer Prev 2008; 9: 7–14. [PubMed] [Google Scholar]
- 14. Long N, Suzuki S, Sato S et al Purple corn color inhibition of prostate carcinogenesis by targeting cell growth pathways. Cancer Sci 2013; 104: 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Z, Zhou J, Lu X, Gong Z, Le XC. Arsenic speciation in urine from acute promyelocytic leukemia patients undergoing arsenic trioxide treatment. Chem Res Toxicol 2004; 17: 95–103. [DOI] [PubMed] [Google Scholar]
- 16. Gibson KR, Neilson IL, Barrett F et al Evaluation of the antioxidant properties of N‐acetylcysteine in human platelets: prerequisite for bioconversion to glutathione for antioxidant and antiplatelet activity. J Cardiovasc Pharmacol 2009; 54: 319–26. [DOI] [PubMed] [Google Scholar]
- 17. Furukawa S, Fujita T, Shimabukuro M et al Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114: 1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology 2005; 65: 1168–72. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi S, Uemura H, Seeni A et al Therapeutic targeting of angiotensin II receptor type 1 to regulate androgen receptor in prostate cancer. Prostate 2012; 72: 1559–72. [DOI] [PubMed] [Google Scholar]
- 20. Ashburner M, Ball CA, Blake JA et al Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000; 25: 25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rizzi F, Bettuzzi S. The clusterin paradigm in prostate and breast carcinogenesis. Endocr Relat Cancer 2010; 17: R1–17. [DOI] [PubMed] [Google Scholar]
- 22. Shannan B, Seifert M, Leskov K et al Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ 2006; 13: 12–9. [DOI] [PubMed] [Google Scholar]
- 23. Shannan B, Seifert M, Boothman DA, Tilgen W, Reichrath J. Clusterin and DNA repair: a new function in cancer for a key player in apoptosis and cell cycle control. J Mol Histol 2006; 37: 183–8. [DOI] [PubMed] [Google Scholar]
- 24. Ahuja D, Saenz‐Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 2005; 24: 7729–45. [DOI] [PubMed] [Google Scholar]
- 25. Rizzi F, Bettuzzi S. Clusterin (CLU) and prostate cancer. Adv Cancer Res 2009; 105: 1–19. [DOI] [PubMed] [Google Scholar]
- 26. Caporali A, Davalli P, Astancolle S et al The chemopreventive action of catechins in the TRAMP mouse model of prostate carcinogenesis is accompanied by clusterin over‐expression. Carcinogenesis 2004; 25: 2217–24. [DOI] [PubMed] [Google Scholar]
- 27. Chou TY, Chen WC, Lee AC, Hung SM, Shih NY, Chen MY. Clusterin silencing in human lung adenocarcinoma cells induces a mesenchymal‐to‐epithelial transition through modulating the ERK/Slug pathway. Cell Signal 2009; 21: 704–11. [DOI] [PubMed] [Google Scholar]
- 28. Tang Y, Liu F, Zheng C, Sun S, Jiang Y. Knockdown of clusterin sensitizes pancreatic cancer cells to gemcitabine chemotherapy by ERK1/2 inactivation. J Exp Clin Cancer Res 2012; 31: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta S. Prostate cancer chemoprevention: current status and future prospects. Toxicol Appl Pharmacol 2007; 224: 369–76. [DOI] [PubMed] [Google Scholar]
- 30. Saad F, Hotte S, North S et al Randomized phase II trial of Custirsen (OGX‐011) in combination with docetaxel or mitoxantrone as second‐line therapy in patients with metastatic castrate‐resistant prostate cancer progressing after first‐line docetaxel: CUOG trial P‐06c. Clin Cancer Res 2011; 17: 5765–73. [DOI] [PubMed] [Google Scholar]
- 31. Chi KN, Hotte SJ, Yu EY et al Randomized phase II study of docetaxel and prednisone with or without OGX‐011 in patients with metastatic castration‐resistant prostate cancer. J Clin Oncol 2010; 28: 4247–54. [DOI] [PubMed] [Google Scholar]
- 32. Suzuki S, Pitchakarn P, Sato S, Shirai T, Takahashi S. Apocynin, an NADPH oxidase inhibitor, suppresses progression of prostate cancer via Rac1 dephosphorylation. Exp Toxicol Pathol 2013; 65: 1035–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Expression of clusterin in ventral lobe of prostate. Pictures of H&E and immunohistochemistry of clusterin in normal epithelium in Sprague–Dawley rat, LG‐PIN, HG‐PIN and carcinoma in TRAP rats (a) and expression of clusterin (b). Expression of clusterin in HG‐PIN of ventral lobe of control, 100 and 500 mg/L apocynin treated rats (c). Carcinoma; Adenocarcinoma. *,***P < 0.05 and 0.001 compared to Normal epithelium or control, respectively.
Table S1. Labeling indices of Ki67 and 8‐OHdG in adenocarcinoma of ventral and lateral prostate.


