Abstract
Dysregulation of p27Kip1 due to proteolysis that involves the ubiquitin ligase (SCF) complex with S‐phase kinase‐associated protein 2 (Skp2) as the substrate‐recognition component (SCF Skp2) frequently results in tumorigenesis. In this report, we developed a high‐throughput screening system to identify small‐molecule inhibitors of p27Kip1 degradation. This system was established by tagging Skp2 with fluorescent monomeric Azami Green (mAG) and CDK subunit 1 (Cks1) (mAGSkp2–Cks1) to bind to p27Kip1 phosphopeptides. We identified two compounds that inhibited the interaction between mAGSkp2–Cks1 and p27Kip1: linichlorin A and gentian violet. Further studies have shown that the compounds inhibit the ubiquitination of p27Kip1 in vitro as well as p27Kip1 degradation in HeLa cells. Notably, both compounds exhibited preferential antiproliferative activity against HeLa and tsFT210 cells compared with NIH3T3 cells and delayed the G 1 phase progression in tsFT210 cells. Our approach indicates a potential strategy for restoring p27Kip1 levels in human cancers.
Cyclin‐dependent kinases (CDK), which function upon activation by cyclin binding, are known to be an active key molecule complexes that regulate the progression of cell cycles. The activities of CDK are constrained by CDK inhibitors (CKI).1, 2, 3 One CKI, p27Kip1, a 198‐amino‐acid protein that was discovered in cells arrested by TGF‐β,4, 5 has significant functions in governing cell proliferation, cell motility, senescence and apoptosis.1, 2, 3 Unlike other tumor suppressors, for instance p53, p27Kip1 is rarely mutated in human cancers. It is reported that p27Kip1 is usually dysregulated in cancers, despite high or constant p27Kip1 mRNA levels.3, 6, 7 During the cell cycle, p27Kip1 negatively regulates the G1‐S transition, and its levels peak during G1 phase, causing arrest in G1. 1, 3, 8, 9 These fluctuations are not mirrored in its mRNA levels,9, 10 suggesting that downregulation of p27Kip1 in human cancers, which is associated with many aggressive phenotypes and a poor prognosis in various cancers (e.g. breast, colon, prostate, lung and gastric cancers), is caused primarily by post‐transcriptional events.3, 9
It is well known that p27Kip1 is degraded through a sequential degradation system, called the ubiquitin‐proteasome system (UPS).11 This process begins with the transfer and covalent attachment of ubiquitin to target proteins through a cascade of enzymatic reactions, followed by degradation of the marked target proteins by the proteasome.12 Biochemical studies have shown that p27Kip1 is ubiquitinated in vitro and in vivo primarily by SCFSkp2, a ubiquitin ligase complex that contains S‐phase kinase‐associated protein 2 (Skp2).13, 14, 15, 16 p27Kip1 is recognized by Skp2 only when it is phosphorylated by CDK2/cyclin E on Thr‐187.17, 18, 19 Moreover, recognition of p27Kip1 by SCFSkp2 requires an accessory protein, CDK subunit 1 (Cks1), which binds to phosphorylated p27Kip1 and Skp2.20, 21 Thus, Skp2 and Cks1 are equally important for the recognition of and binding to p27Kip1.
Several published studies suggest that Skp2 has oncogenic activity.22, 23 Notably, transformed cell lines and human cancers are frequently associated with overexpression of Skp2 and p27Kip1 downregulation.6, 24, 25 With activated Ras, Skp2 transforms cells26 and induces lymphomas in transgenic mice,27 and transgenic expression of Skp2 in mouse prostate causes hyperplasia, dysplasia and low‐grade prostate carcinoma with significant p27Kip1 downregulation.28 In contrast, Skp2 knockout mice are fertile and viable and have elevated p27Kip1 levels.16 Moreover, siRNA‐mediated silencing of Skp2 in oral and lung carcinomas inhibits and suppresses tumor proliferation.29, 30 Thus, the involvement of Skp2 in many aggressive cancers suggests that targeting it using small‐molecule inhibitors is a promising cancer therapy.
Recent advances in chemical biology studies have allowed us to identify such biologically active small molecule inhibitors of various targets by high‐throughput compound screening. Several compounds able to prevent p27Kip1 degradation are reported.31, 32, 33 In Rico‐Bautista et al., 31 cell‐based high‐throughput screening using Skp2 overexpressing cells identified two compounds that restored the levels of nuclear p27Kip1 efficiently. While the direct molecular targets of these compounds were yet to be unveiled, one of the compounds turned out to induce the downregulation of Skp2 in cells. The other compounds of this study31 and a compound found in another study32 have an activity to increase the mRNA level of p27Kip1. Using an in vitro‐reconstituted electrophoresis‐based ubiquitination assay as a screening system, a compound that inhibits ubiquitination dependent proteolysis of p27Kip1 is isolated.33
In this report, we developed a high‐throughput screening (HTS) system to identify small molecule inhibitors of protein–protein interaction between Skp2–Cks1 and p27Kip1. We identified two small molecule inhibitors of p27Kip1 degradation. These small molecule inhibitors inhibited the in vitro ubiquitination of p27Kip1 by SCFSkp2, stabilized p27Kip1 levels in HeLa cells and inhibited the growth of human and mouse cancer cells.
Materials and Methods
Recombinant baculoviruses
To construct recombinant baculoviruses that express mAGSkp2 (mAG, N‐terminally fused fluorescent monomeric Azami Green)34 and Cks1, hemagglutinin (HA)‐tagged mAGSkp2 and Cks1 DNA fragments were inserted into the EcoRI‐NotI and SalI‐NotI sites of pFast‐Bac. The recombinant plasmids were then transformed into DH10Bac cells followed by transfection of the obtained recombinant bacmids into ExpressSF+ Serum‐Free Insect Cells (Protein Sciences) using Cellfectin II (Invitrogen, Carlsbad, CA, USA). Recombinant baculovirus that expressed Skp1 was obtained from Dr Keiichi Nakayama (Kyushu University, Fukuoka‐shi, Japan).
Antibodies
For western blot analysis to detect endogenous protein levels, commercial antibodies against Skp2 (Santa Cruz), p130 (Santa Cruz) and p21 (Abcam, Cambridge, UK) were used at concentrations recommended by these manufacturers.
Cell culture conditions
Insect cells were grown in Sf‐900 II SFM (serum‐free medium complete 1×, GIBCO) and cultured at 27°C with constant stirring at 130 rpm. A total of 150 mL of insect cell culture was coinfected with 3 mL of each recombinant baculovirus (mAGSkp2, Cks1 and Skp1) for 72 h before the cells were harvested. HeLa cells were maintained in DMEM (Invitrogen) that contained 10% fetal calf serum, and NIH3T3 cells were maintained in MEM (SIGMA, St. Louis, MO, USA) that contained 10% calf serum; both media were supplemented with 0.5% penicillin/streptomycin (Invitrogen). Cells were maintained in a humidified incubator at 37°C and 5% CO2. The tsFT210 cell line, which is a temperature‐sensitive mutant that has been isolated from the mouse mammary carcinoma cell line FM3A, was maintained in RPMI‐1640 medium (Invitrogen) that was supplemented with 5% calf serum in a suspension culture at 32°C and 5% CO2. tsFT210 cells can be arrested at G2 phase and are large in size when cultured at 39°C at 5% CO2.35, 36, 37
Development of binding assay and screening for inhibitors of Skp2–Cks1 and p27Kip1 interaction
A 24‐residue (residue 175–197) p27Kip1 phosphopeptide that harbored the target sequence (C‐SDGSPNAGSVEQpTPKKPGLRRRQT), which binds Skp1–Skp2–Cks1 with equal affinity compared with the full‐length phosphorylated p27Kip1 peptide38 and its derivatives without phosphorylation, was chemically synthesized, purified and bound covalently to maleimide‐activated 96‐well plates (Pierce) at 100 μL/well of a 10 μM solution according to the manufacturer's protocol.
Insect cells expressing mAGSkp2, Cks1 and Skp1 were lysed by sonication in lysis buffer (20 mM Tris, pH 7.5, 125 mM NaCl, 0.5% Nonidet P‐40, 50 mM NaF, 1 mM EDTA, 200 μM Na3VO4 and 1 mM dithiothreitol), containing Complete Protease Inhibitors (Roche Applied Science, Penzberg, Germany). Screening for inhibitors of the Skp2–Cks1 and p27Kip1 interaction from RIKEN NPDepo (Natural Product Depository) chemical library39 was performed at high‐throughput manner on a Biomek 2000 liquid handling system (Beckman‐Coulter, Brea, CA, USA), essentially as described previously.40 Briefly, 50 μL of insect cell lysate was mixed with chemical compounds at a final concentration of 60 μg/mL. The mixtures were added to 96‐well phosphopeptide‐bound plates and incubated overnight at 4°C on a shaker. After five rinses with 0.05% Nonidet P‐40 in PBS and two rinses with PBS, the reaction was quantified spectrophotometrically at excitation 488 nm and emission 546 nm.
Cell proliferation assay
A total of 4 × 103 HeLa cells/200 μL/well, 1 × 104 NIH3T3 cells/200 μL/well or 1.6 × 104 tsFT210 cells/200 μL/well were seeded into the wells of 96‐well plates and were incubated for 24 h in a humidified 37°C incubator with 5% CO2 for HeLa and NIH3T3 cells and in a 32°C incubator for tsFT210 cells. Then, the cells were treated with increasing concentrations of compounds for 48 h, cell growth was measured using Cell Count Reagent SF (Nacalai Tesque, Kyoto, Japan), and IC50 values were calculated, based on the absorbance at 450 nm (Wallac 1420 ARVO; PerkinElmer, Waltham, MA, USA).
Protein stability assay
HeLa cells were treated with compounds at their IC50 concentrations for 18 h, and 20 μg/mL cycloheximide (CHX) was added for the indicated times prior to the preparation of cell lysates. Total protein amounts were measured by Bio‐Rad protein assay (Bio‐Rad Laboratories, Hercules, CA, USA), with BSA as standard. Approximately 40 μg of cell lysate was subjected to SDS‐PAGE, followed by immunoblot analysis with anti‐p27Kip1 (BD Transduction Laboratory, Franklin Lakes, NJ, USA).
RNA extraction, cDNA synthesis and semi‐quantitative RT‐PCR
Total RNA of compound‐treated HeLa cells was extracted using TRizol (Invitrogen) according to the manufacturer's instructions. First‐strand cDNA was synthesized using the ImProm‐II Reverse Transcription System (Promega, Fitchburg, WI, USA) and was used for semi‐quantitative PCR employing intron‐spanning primers.41 Primer sequences for p27Kip1 are FW: 5′‐ACCTGCAACCGACGATTCTT‐3′ and RV: 5′‐CCCTTCCCCAAAATTGCTTC‐3′. Primer sequences for β‐actin are FW: 5′‐CTGGACTTCGAGCAAGA‐3′ and RV: 5′‐TCCTGCTTGCTGATCCA‐3′.
Purification of p27Kip1
The coding region of p27Kip1 was cloned into the pET‐28a(+) vector with 8 His‐Tag at the N‐terminal, using the BamHI and NcoI sites to produce recombinant p27Kip1. Recombinant p27Kip1 was transformed and expressed in BL21 (DE3) cells and purified with HisTrap affinity columns (GE Healthcare, Little Chalfont, UK) according to the manufacturer's protocols.
In vitro ubiquitination assay
The p27Kip1 in vitro ubiquitination assay was performed as previously described21, 42 using SCF complex expressed and immunoprecipitated from HEK293T cells.
Flow cytometric analysis
To obtain G2‐synchronized cells, tsFT210 cells were seeded into the wells of 24‐well plates at 10 × 104 cells/500 μL/well and preincubated at 39°C for 18 h. Then, the cells were treated with compounds at the indicated concentrations prior to incubation at 32°C for the indicated times. Cell suspensions were harvested, washed with PBS, and resuspended in 500 μL propidium iodide buffer, containing 50 μg/mL propidium iodide (Sigma‐Aldrich, St. Louis, MO, USA), 0.1% sodium citrate, 0.2% Nonidet P‐40 and 2 μg/mL RNase A (Nacalai Tesque), for at least 30 min in the dark. The DNA content of the cells was analyzed on a Cytomics FC500 (Beckman Coulter).
Results
Development of screening system to identify inhibitors of Skp2–Cks1 and p27Kip1 interaction
We developed a high‐throughput screening system using Skp1, Skp2 and Cks1 expressed in the baculovirus protein expression system and phosphopeptides of p27Kip1‐derived sequences. In this system, we fused mAG, a fluorescent protein from the stony coral Galaxeidae 34 to the N‐terminus of Skp2, allowing us to quantify the binding of mAGSkp2–Cks1 to p27Kip1 by spectrofluorometry (Fig. 1a).
Figure 1.
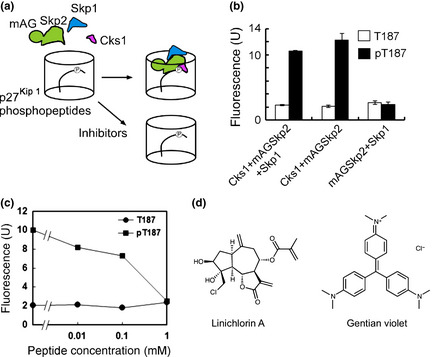
Screening system and high‐throughput screening for inhibitors of Skp2–Cks1 and p27Kip1 interaction. (a) Insect cell lysates expressing Skp1, mAGSkp2 and Cks1 were added to the wells of 96‐well plates that were covalently attached with p27Kip1 phosphopeptides. Bound Skp1–mAGSkp2–Cks1 to p27Kip1 phosphopeptides in a phosphorylation‐dependent manner was confirmed by spectrofluorometry. When inhibitors are present, the fluorescent signal by mAGSkp2 decreases. (b) p27Kip1 phosphopeptide binding sequence to Skp1–Skp2–Cks1 (pT187; C‐SDGSPNAGSVEQpTPKKPGLRRRQT) or its nonphosphorylated version (T187) was bound to 96‐well plates at 100 μL/well of 10 μM peptide solution. Binding of Skp1–mAGSkp2–Cks1 to p27Kip1 peptides was detected spectrofluorometrically and was only observed with pT187, indicated by the black bars. No binding was detected with T187 (white bars). Binding was observed when Cks1 was present, whereas no effect was seen with Skp1 in the binding assay. Data are representative of four replicates. (c) Peptide competitive binding assay. Insect cell lysates expressing mAGSkp2 and Cks1 mixed with p27Kip1 phosphopeptide solution at the indicated concentrations were added to 96‐well plates covalently bound with p27Kip1 phosphopeptides. Using a spectrophotometer, a concentration‐dependent decrease in binding was observed with the p27Kip1 phosphopeptide solution. At 1 mM phosphopeptide solution, binding was abolished. (d) Structures of linichorin A and gentian violet. These compounds were identified as small molecule inhibitors of Skp2–Cks1 and p27Kip1 interaction. [Correction added on 23 October 2013, after first online publication: In Figure 1d, the structure of linichlorin A is not correct in the first online publication. In this version of the article, it has been replaced with the correct structure.]
When insect cell lysates expressing Skp1, mAG‐Skp2 and Cks1 were incorporated into the 96‐well plates in which p27Kip1‐derived phosphopeptides were covalently bound, the binding of mAG‐Skp2 to p27Kip1 peptides was detected by spectrofluorometry. The binding was observed only to p27Kip1 phosphopeptides (Fig. 1b). The importance of Skp1 and Cks1 in p27Kip1 phosphorylation‐dependent binding was also evaluated and we noted that Skp1 is not necessary for p27Kip1 phosphorylation‐dependent binding. In contrast, the fluorescent signal was abolished in cell lysates that did not express Cks1. Thus, Cks1 but not Skp1 is essential for p27Kip1 phosphopeptide recognition (Fig. 1b).
To determine the reliability of our screening system, we performed a peptide competitive binding assay. Insect cell lysates expressing mAG‐Skp2 and Cks1, mixed with p27Kip1 phosphopeptide solution at the indicated concentrations, were added to 96‐well plates to which p27Kip1 phosphopeptides were bound (Fig. 1c). In the presence of 10 μM phosphopeptides, the interaction between mAG‐Skp2–Cks1 and well‐bound p27Kip1 phosphopeptides was inhibited by approximately 20%. This binding decreased further with higher concentrations of the p27Kip1 phosphopeptide solution, indicating the reliability and efficacy of our screening system. Thus, we used this system for small‐molecule inhibitor screening.
Identification of linichlorin A and gentian violet as specific inhibitors of Skp2–Cks1 and p27Kip1 interaction
We screened approximately 20 000 compounds in the RIKEN NPDepo chemical library at 60 μg/mL.39 We identified 258 compounds that reduced the fluorescence of mAGSkp2 to <80% of control levels as primary hits. In a secondary screening, all 258 hit compounds were re‐examined at the same concentration to confirm reproducibility of their inhibitory activities and, at the same time, compounds that inhibit other phosphorylation dependent protein interactions, such as polo box domain dependent interaction,40 were excluded. After these selections, 30 compounds were considered positive in our assay. Next, we measured the effects of these compounds on cell growth. Among the 30 compounds, 15 compounds showed strong growth inhibition on HeLa cells while the other 15 compounds exhibited weaker growth inhibition. Among the 15 stronger compounds, linichlorin A and gentian violet were found to show the strongest effect on cell growth (Fig. 1d). We also observed that the inhibitory activity of linichlorin A and gentian violet obtained from the pulled down assay (Fig. 2a) corresponds to the inhibitory activity obtained in HTS. Thus, these two compounds were selected for further study.
Figure 2.
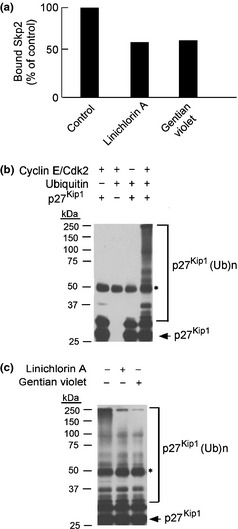
Compounds inhibit in vitro ubiquitination of p27Kip1. (a) Effect of compounds on the interaction between Skp2–Cks1 complex and phosphorylated p27Kip1. Skp2–Cks1 complex was pulled down by sepharose beads conjugated with p27‐derived phosphopeptides or non‐phophopeptides. Bound complex was examined by western blot with anti‐HA. Binding was reduced in the presence of compounds. (b) Ubiquitination reactions were subjected to SDS‐PAGE, followed by immunoblot analysis with anti‐p27Kip1, to examine the effect of the indicated reagents and enzymes on in vitro ubiquitination of p27Kip1. *Signal derived from IgG heavy chain. (c) Effect of compounds on in vitro ubiquitination of p27Kip1. In the presence of linichlorin A at 3.2 μM and gentian violet at 0.4 μM, in vitro ubiquitination of p27Kip1 was inhibited (concentrations represent the IC 50 of each compound in HeLa cells; refer to Fig. 4a for details). Quantification by ImageQuant TL showed a significant reduction of 70–80% in the amount of in vitro ubiquitinated p27Kip1 by both compounds. *Signal derived from IgG heavy chain.
Linichlorin A and gentian violet inhibit in vitro ubiquitination of p27Kip1
As shown in Figure 2(b), we have constructed the in vitro ubiquitination reaction of p27Kip1. The ubiquitination was observed only when the reagents and enzymes (ubiquitin, p27Kip1 and CDK2/Cyclin E) were present in the reaction. After adding linichlorin A or gentian violet to the ubiquitination reaction, in vitro p27Kip1 ubiquitination declined by 70–80% (Fig. 2c), as quantified on an ImageQuant TL (GE Healthcare). Thus, linichlorin A and gentian violet inhibited p27Kip1 ubiquitination in vitro.
p27Kip1 stabilization by linichlorin A and gentian violet
To examine the effect of these compounds on the stability of p27Kip1, exponentially growing HeLa cells were treated with the compounds for 18 h. Then, 20 μg/mL CHX was administered to the cells to block the protein translation, and p27Kip1 levels were measured by immunoblotting. As shown in Figure 3(a) and (b), p27Kip1 was degraded rapidly after the addition of CHX in control cells (DMSO). In contrast, the degradation of p27Kip1 was almost completely inhibited in the presence of either compound, demonstrating that linichlorin A and gentian violet stabilize p27Kip1 in HeLa cells after the protein translation. In fact, as expected, no significant change in the p27Kip1 mRNA expression level was observed in the compound treated cells (Fig. 3c). In addition, the expression level of Skp2 protein was not affected under these conditions, although it was decreased in the presence of a higher concentration of gentian violet (Fig. S1).
Figure 3.
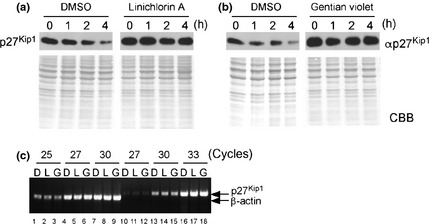
Stabilization of p27Kip1 in HeLa cells by the compounds. Exponentially growing HeLa cells were treated with linichlorin A at 3.2 μM (a) or gentian violet at 0.4 μM (b) for 18 h (concentrations represent the IC 50 of each compound in HeLa cells; refer to Fig. 4a for details); 20 μg/mL CHX was then added, and p27Kip1 levels were measured at the indicated times by western blot with anti‐p27Kip1. Coomassie Brilliant Blue (CBB) stain is also shown in the lower panel of each immunoblot. (c) Expression levels of mRNA after compound treatment were examined by RT‐PCR (25, 27 and 30 cycles for mRNA detection of β‐actin [lanes 1–9] and 27, 30 and 33 cycles for that of p27Kip1 [lanes 10–18], respectively). HeLa cells were treated with DMSO (D), linichlorin A (L) (3.2 μM) and gentian violet (G) (0.4 μM) for 18 h, and RNA was extracted and processed for semi‐quantitative RT‐PCR.
Linichlorin A and gentian violet inhibit cell growth and delay cell cycle progression
Next, we examined the effect of these compounds on the growth of tumor and non‐tumor cells. HeLa (human tumor cells), tsFT210 (mouse tumor cells) and NIH3T3 (mouse immortalized cells) were exposed to increasing concentrations of linichlorin A and gentian violet for 48 h and their viability was measured by WST‐8 assay (Fig. 4a). The IC50 values for linichlorin A in HeLa, tsFT210 and NIH3T3 cells were 3.2, 1.6 and 12.7 μM, respectively, and 0.4, 0.6 and 5.3 μM for gentian violet, respectively. This shows that the compounds inhibited growth to a greater extent in HeLa and tsFT210 cells compared with NIH3T3 cells. When we compared the expression level of p27Kip1 between two mouse cells (tsFT210 cells and NIH3T3 cells) by immunoblotting, the endogenous expression level of p27Kip1 in growing cells was higher in NIH3T3 cells (Fig. 4b). However, in the range of the compound concentration examined, p27Kip1 expression level was increased by the compounds dose‐dependently only in tsFT210 cells, as it is in HeLa cells (Fig. 4b). Although the mechanism of this difference in the drug sensitivity remains to be elucidated, this difference may explain the stronger growth inhibitory effect of these compounds in cancer cell lines.
Figure 4.

Compounds induce cell growth and delay cell cycle progression. (a) Effect of linichlorin A and gentian violet on HeLa, tsFT210 and NIH3T3 cell growth by WST‐8 assay at the indicated concentrations for 48 h. Data are representative of three replicates. (b) Dose‐dependent effects of linichlorin A and gentian violet on the expression level of p27Kip1 in HeLa, tsFT210 and NIH3T3 cells. Cells were treated with compounds at indicated concentrations for 18 h. Total proteins (20 μg each) from these cells were loaded on SDS‐PAGE and processed for the detection of p27Kip1 by immunoblotting. (c) Effect of compounds on cell cycle progression in tsFT210 cells. Cells were synchronized at G 2 phase at 39°C for 18 h, and the compounds were added at their respective IC 50 values (linichlorin A 1.6 μM; gentian violet 0.6 μM) before the synchronized cells were released at 32°C at 0 h. Cells were harvested at the indicated times and stained with propidium iodide prior to flow cytometric analysis. DNA contents of G 1 (2C), S (2C–4C) and G 2/M (4C) cells are indicated by triangles. Analyses were repeated at least twice and the quantitative data in each panel represents the percentages of each phase. (d) Effect of compounds on p27Kip1. tsFT210 cells treated with compounds as in b were harvested at the indicated times and the endogenous p27Kip1 levels were examined by western blot with anti‐p27Kip1. The intensity of the bands was quantified using Image‐J software (Wayne Rasband, Bethesda, MD, USA) and is indicated in multiple of that of 0 h control. CBB stain is also shown in the lower panel of the immunoblot.
It is predicted that the G1–S transition will be retarded when p27Kip1 is stabilized. To confirm this, we examined the compounds' effects on the cell cycle progression using tsFT210 cells that have temperature sensitive Cdc2 mutation. After tsFT210 cells were synchronized at G2 phase by incubation at 39°C (0 h), the cells were treated with compounds at their respective IC50 values (linichlorin A 1.6 μM and gentian violet 0.6 μM) prior to incubation at 32°C for 8 and 12 h. As a result, the compounds delayed the initiation of S phase (Fig. 4c) and the levels of p27Kip1 were significantly increased in the compound‐treated cells (Fig. 4d).
Discussion
Low levels of p27Kip1 and overexpression of Skp2 in many human carcinomas and lymphomas are caused primarily by the increased proteolysis of p27Kip1 through ubiquitination by SCFSkp2 E3 ligase.43, 44, 45, 46 Thus, discovery of small molecule inhibitors through the development of screening systems that specifically target SCFSkp2 E3 ligase by blocking the interaction between the F‐box protein (Skp2) and its substrate (p27Kip1) presents an ideal cancer therapeutic.
We developed an HTS system for inhibitors of the interaction of Skp2–Cks1 and p27Kip1. Through HTS and further study, we identified linichlorin A and gentian violet as the most potent compounds that inhibited the ubiquitin‐proteasome dependent degradation of p27Kip1 (Fig. 1d). Linichlorin A is a sesquiterpene lactone that was first isolated from Centaurea linifolia Vahl47 and does not have any reported biological activity, whereas gentian violet (also known as crystal violet) is a triphenylmethane‐classed dye that has antifungal, antibacterial and antiparasitic activities.48 While both compounds have inhibitory effects on the binding between mAGSkp2–Cks1 and p27Kip1 phosphopeptides, these compounds did not show any effect on the phosphorylation‐dependent binding of other F‐box proteins, β‐TrCP1 and β‐TrCP2. In addition, these compounds also did not show any effect on other phosphorylation dependent protein–protein interactions of PBD,40 Pin1, 14‐3‐3 and Mdc1 (Watanabe N and Osada H, unpublished results). Therefore, we consider that the inhibitory effect of these compounds is specific to Skp2–p27Kip1 interaction.
From the structure of Skp2 and Cks1 complexed with p27Kip1 phosphopeptides,38 it is predicted that these compounds bind to the pocket created by Skp2 and Cks1 rather than binding to the phosphorylated peptides. In fact, no inhibition on binding was observed in our screening system when the compounds were pre‐mixed with phosphorylated peptides in 96‐well plates and washed before the addition of Skp2–Cks1 containing lysates, indicating that the compounds do not bind to the phosphopeptides but to the Skp2–Cks1 complex, as predicted. In this regard, the increase in the expression level of two other known Skp2–Cks1 target proteins, p21Cip1( 49 ) and p130,50 by these compounds supports this idea (Fig. S2).
According to earlier findings,13, 14, 17, 18, 20 the ubiquitination of p27Kip1 is triggered by its phosphorylation by CDK2/cyclin E, followed by recognition of phosphorylated p27Kip1 by SCFSkp2 E3 ligase and an adapter protein, Cks1, which polyubiquitinates p27Kip1 and targets it for proteasomal degradation. Here, we found that linichlorin A and gentian violet had actually inhibited p27Kip1 ubiquitination in vitro (Fig. 2c), as predicted by their Skp2–p27Kip1 binding inhibitory activities, followed by stabilization of p27Kip1 level in the compound‐treated HeLa cells (Fig. 3a,b).
We have also demonstrated that linichlorin A and gentian violet have substantial, selective antiproliferative activity against cancer and transformed cells in the micromolar range (Fig. 4a). In many types of tumor cells, it is reported that Skp2 is overexpressed and the level of p27Kip1 is downregulated. This downregulation of p27Kip1 is considered to be responsible for the accelerated growth of tumor cells. Thus, the inhibition on Skp2‐dependent degradation of p27Kip1 may exhibit larger effects on the growth of tumor cells.
Although further analyses using cells with reduced or overexpressed levels of p27Kip1 and/or Skp2 are necessary, our results strongly suggest that these compounds inhibit the growth of cancer cells at G1 phase of the cell cycle by stabilizing p27Kip1 through the inhibition of the interaction between Skp2–Cks1 and p27Kip1. Taken together, our study demonstrates a potential strategy for restoring p27Kip1 levels in cancers using small molecule inhibitors.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Dose‐dependent effect of linichlorin A and gentian violet on the expression level of SKP2 in HeLa cells.
Fig. S2. Effect of compounds on the level of p21Cip1 and p130.
Acknowledgments
This work was supported in part by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We thank the members of the RIKEN Chemical Biology Core Facility (especially Emiko Sanada) for technical assistance, support and discussions. L‐C. Ooi is grateful for financial support from the RIKEN International Program Associate and from the Universiti Sains Malaysia Fellowship Scheme.
(Cancer Sci 2013; 104: 1461–1467)
References
- 1. Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin‐dependent kinases. Genes Dev 1995; 9: 1149–63. [DOI] [PubMed] [Google Scholar]
- 2. Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1‐phase progression. Genes Dev 1999; 13: 1501–12. [DOI] [PubMed] [Google Scholar]
- 3. Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer 2008; 8: 253–67. [DOI] [PubMed] [Google Scholar]
- 4. Polyak K, Kato JY, Solomon MJ et al p27Kip1, a cyclin‐Cdk inhibitor, links transforming growth factor‐β and contact inhibition to cell‐cycle arrest. Genes Dev 1994; 8: 9–22. [DOI] [PubMed] [Google Scholar]
- 5. Slingerland JM, Hengst L, Pan CH, Alexander D, Stampfer MR, Reed SI. A novel inhibitor of cyclin‐Cdk activity detected in transforming growth factor β‐arrested epithelial cells. Mol Cell Biol 1994; 14: 3683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol 2000; 183: 10–7. [DOI] [PubMed] [Google Scholar]
- 7. Catzavelos C, Bhatacharya N, Ung YC et al Decreased levels of the cell‐cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med 1997; 3: 227–30. [DOI] [PubMed] [Google Scholar]
- 8. Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 1996; 272: 877–80. [DOI] [PubMed] [Google Scholar]
- 9. Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science 1996; 271: 1861–4. [DOI] [PubMed] [Google Scholar]
- 10. Alessandrini A, Chiaur DS, Pagano M. Regulation of the cyclin‐dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia 1997; 11: 342–5. [DOI] [PubMed] [Google Scholar]
- 11. Pagano M, Tam SW, Theodoras AM et al Role of the ubiquitin‐proteasome pathway in regulating abundance of the cyclin‐dependent kinase inhibitor p27. Science 1995; 269: 682–5. [DOI] [PubMed] [Google Scholar]
- 12. Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998; 67: 425–79. [DOI] [PubMed] [Google Scholar]
- 13. Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin‐mediated degradation of the CDK inhibitor p27. Nat Cell Biol 1999; 1: 193–9. [DOI] [PubMed] [Google Scholar]
- 14. Sutterluty H, Chatelain E, Marti A et al p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol 1999; 1: 207–14. [DOI] [PubMed] [Google Scholar]
- 15. Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr Biol 1999; 9: 661–4. [DOI] [PubMed] [Google Scholar]
- 16. Nakayama K, Nagahama H, Minamishima YA et al Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J 2000; 19: 2069–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vlach J, Hennecke S, Amati B. Phosphorylation‐dependent degradation of the cyclin‐dependent kinase inhibitor p27Kip1. EMBO J 1997; 16: 5334–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E‐CDK2 is a regulator of p27Kip1. Genes Dev 1997; 11: 1464–78. [DOI] [PubMed] [Google Scholar]
- 19. Montagnoli A, Fiore F, Eytan E et al Ubiquitination of p27 is regulated by Cdk‐dependent phosphorylation and trimeric complex formation. Genes Dev 1999; 13: 1181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ganoth D, Bornstein G, Ko TK et al The cell‐cycle regulatory protein Cks1 is required for SCFSkp2‐mediated ubiquitinylation of p27. Nat Cell Biol 2001; 3: 321–4. [DOI] [PubMed] [Google Scholar]
- 21. Spruck C, Strohmaier H, Watson M et al A CDK‐independent function of mammalian Cks1: targeting of SCFSkp2 to the CDK inhibitor p27Kip1. Mol Cell 2001; 7: 639–50. [DOI] [PubMed] [Google Scholar]
- 22. Nakayama K, Nagahama H, Minamishima YA et al Skp2‐mediated degradation of p27 regulates progression into mitosis. Dev Cell 2004; 6: 661–72. [DOI] [PubMed] [Google Scholar]
- 23. Hershko DD. Oncogenic properties and prognostic implications of the ubiquitin ligase Skp2 in cancer. Cancer 2008; 112: 1415–24. [DOI] [PubMed] [Google Scholar]
- 24. Yang G, Ayala G, De Marzo A et al Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin‐dependent kinase inhibitor p27 and PTEN and with reduced recurrence‐free survival. Clin Cancer Res 2002; 8: 3419–26. [PubMed] [Google Scholar]
- 25. Hershko D, Bornstein G, Ben‐Izhak O et al Inverse relation between levels of p27Kip1 and of its ubiquitin ligase subunit Skp2 in colorectal carcinomas. Cancer 2001; 91: 1745–51. [DOI] [PubMed] [Google Scholar]
- 26. Gstaiger M, Jordan R, Lim M et al Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci 2001; 98: 5043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Latres E, Chiarle R, Schulman BA et al Role of the F‐box protein Skp2 in lymphomagenesis. Proc Natl Acad Sci 2001; 98: 2515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shim EH, Johnson L, Noh HL et al Expression of the F‐box protein SKP2 induces hyperplasia, dysplasia, and low‐grade carcinoma in the mouse prostate. Cancer Res 2003; 63: 1583–8. [PubMed] [Google Scholar]
- 29. Sumimoto H, Yamagata S, Shimizu A et al Gene therapy for human small‐cell lung carcinoma by inactivation of Skp‐2 with virally mediated RNA interference. Gene Ther 2005; 12: 95–100. [DOI] [PubMed] [Google Scholar]
- 30. Kudo Y, Kitajima S, Ogawa I, Kitagawa M, Miyauchi M, Takata T. Small interferring RNA targeting of S phase kinase‐interacting protein 2 inhibits cell growth of oral cancer cells by inhibiting p27 degradation. Mol Cancer Ther 2005; 4: 471–6. [DOI] [PubMed] [Google Scholar]
- 31. Rico‐Bautista E, Yang CC, Lu L, Roth GP, Wolf DA. Chemical genetics approach to restoring p27Kip1 reveals novel compounds with antiproliferative activity in prostate cancer cells. BMC Biol 2010; 8: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roy S, Kaur M, Agarwal C, Tecklenburg M, Sclafani RA, Agarwal R. p21 and p27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Mol Cancer Ther 2007; 6: 2696–707. [DOI] [PubMed] [Google Scholar]
- 33. Chen Q, Xie W, Kuhn DJ et al Targeting the p27 E3 ligase SCFSkp2 results in p27‐and Skp2‐mediated cell‐cycle arrest and activation of autophagy. Blood 2008; 111: 4690–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karasawa S, Araki T, Yamamoto‐Hino M, Miyawaki A. A green‐emitting fluorescent protein from Galaxeidae coral and its monomeric version for use in fluorescent labeling. J Biol Chem 2003; 278: 34167–71. [DOI] [PubMed] [Google Scholar]
- 35. Mineo C, Murakami Y, Ishimi Y, Hanaoka F, Yamada M. Isolation and analysis of a mammalian temperature‐sensitive mutant defective in G2 functions. Exp Cell Res 1986; 167: 53–62. [DOI] [PubMed] [Google Scholar]
- 36. Yasuda H, Kamijo M, Honda R, Nakamura M, Hanaoka F, Ohba Y. A point mutation in C‐terminal region of cdc2 kinase causes a G2‐phase arrest in a mouse temperature‐sensitive FM3A cell mutant. Cell Struct Funct 1991; 16: 105–12. [DOI] [PubMed] [Google Scholar]
- 37. Osada H, Cui CB, Onose R, Hanaoka F. Screening of cell cycle inhibitors from microbial metabolites by a bioassay using a mouse cdc2 mutant cell line, tsFT210. Bioorg Med Chem 1997; 5: 193–203. [DOI] [PubMed] [Google Scholar]
- 38. Hao B, Zheng N, Schulman BA et al Structural basis of the Cks1‐dependent recognition of p27Kip1 by the SCFSkp2 ubiquitin ligase. Mol Cell 2005; 20: 9–19. [DOI] [PubMed] [Google Scholar]
- 39. Tomiki T, Saito T, Ueki M et al RIKEN natural products encyclopedia.(RIKEN NPEdia), a chemical database of RIKEN natural products depository (RIKEN NPDepo). J Comp Aid Chem 2006; 7: 157–62. [Google Scholar]
- 40. Watanabe N, Sekine T, Takagi M et al Deficiency in chromosome congression by the inhibition of Plk1 polo box domain‐dependent recognition. J Biol Chem 2009; 284: 2344–53. [DOI] [PubMed] [Google Scholar]
- 41. Moreira MP, Silva LM, Martins WK. The role of GADD45A in resistance to oxidative stress‐mediated cell death in human colon tumor cell lines. Appl Cancer Res 2009; 29: 179–84. [Google Scholar]
- 42. Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI. Human F‐box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 2001; 413: 316–22. [DOI] [PubMed] [Google Scholar]
- 43. Kudo Y, Kitajima S, Sato S, Miyauchi M, Ogawa I, Takata T. High expression of S‐phase kinase‐interacting protein 2, human F‐box protein, correlates with poor prognosis in oral squamous cell carcinomas. Cancer Res 2001; 61: 7044–7. [PubMed] [Google Scholar]
- 44. Esposito V, Baldi A, DeLuca A et al Prognostic role of the cyclin‐dependent kinase inhibitor p27 in non‐small cell lung cancer. Cancer Res 1997; 57: 3381–5. [PubMed] [Google Scholar]
- 45. Loda M, Cukor B, Tam SW et al Increased proteasome‐dependent degradation of the cyclin‐dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med 1997; 3: 231–4. [DOI] [PubMed] [Google Scholar]
- 46. Chiarle R, Budel LM, Skolnik J et al Increased proteasome degradation of cyclin‐dependent kinase inhibitor p27 is associated with a decreased overall survival in mantle cell lymphoma. Blood 2000; 95: 619–26. [PubMed] [Google Scholar]
- 47. Gonzalez AG, Bermejo J, Amaro JM, Massanet GM, Galindo A, Cabrera I. Sesquiterpene lactones from Centaurea linifolia Vahl. Can J Chem 1978; 56: 491–4. [Google Scholar]
- 48. Docampo R, Moreno SNJ. The metabolism and mode of action of Gentian‐Violet. Drug Metab Rev 1990; 22: 161–78. [DOI] [PubMed] [Google Scholar]
- 49. Bornstein G, Bloom J, Sitry‐Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem 2003; 278: 25752–7. [DOI] [PubMed] [Google Scholar]
- 50. Tedesco D, Lukas J, Reed SI. The pRb‐related protein p130 is regulated by phosphorylation‐dependent proteolysis via the protein‐ubiquitin ligase SCF(Skp2). Genes Dev 2002; 16: 2946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Dose‐dependent effect of linichlorin A and gentian violet on the expression level of SKP2 in HeLa cells.
Fig. S2. Effect of compounds on the level of p21Cip1 and p130.


