Abstract
Vasohibin‐2 (VASH2) is a homolog of vasohibin‐1 and exhibits pro‐angiogenic activity. We recently reported that VASH2 is expressed in certain ovarian cancers and promotes tumor growth through angiogenesis. To further demonstrate the effectiveness of molecular targeting of VASH2 for anticancer treatment, we applied in vivo delivery of siRNA targeting VASH2 (siVASH2) using atelocollagen to a xenograft model of ovarian cancer. We inoculated mice s.c. with DISS and SKOV‐3, two representative human ovarian serous adenocarcinoma cell lines. When tumors were measurable, we initiated treatment with control or siVASH2 mixed with atelocollagen, which enveloped the whole tumor. Treatment with siVASH2 significantly inhibited s.c. tumor growth by abrogating tumor angiogenesis. We confirmed that expression of VASH2 mRNA in the tumor was downregulated by siVASH2 treatment. In addition, the siVASH2‐treated tumor contained more blood vessels covered with pericytes, indicating that knockdown of VASH2 contributes to the normalization of tumor blood vessels. Based on these results, VASH2 may be a promising molecular target for ovarian cancer treatment.
Administration of siRNA targeting a novel angiogenesis stimulator, vasohibin‐2, with atelocollagen biomaterial retarded ovarian tumor growth through decrease of angiogenesis and acceleration of vascular normalization. These results demonstrated the effectiveness of molecular targeting of vasohibin‐2 in ovarian cancer.

Ovarian cancer is the second most common gynecological malignancy and is the prime cause of death in industrialized countries.1 Debulking surgery and adjuvant combination chemotherapy with platinum and taxane are fundamental treatments, showing initial complete responses in 80% of patients.2, 3 However, many patients experience abdominal recurrence; numerous novel clinical strategies are being evaluated for treating ovarian cancers to improve outcomes compared to outcomes of standard chemotherapy.4 Angiogenesis is recognized as a principal hallmark of various cancers5 and is also thought to be a key process for ovarian cancer growth. A promising option for treating ovarian cancer may be anti‐angiogenic therapy.6 Indeed, a mAb against vascular endothelial growth factor (VEGF), bevacizumab, showed clinical activity against ovarian cancer in a phase 3 clinical trial.7 Although the effectiveness of anti‐angiogenic drugs is encouraging, resistance to anti‐angiogenic therapy has been reported in several review articles.8, 9, 10 To overcome these problems, novel strategies for anti‐angiogenic therapy must be developed.
The vasohibin family includes vasohibin‐1 (VASH1) and vasohibin‐2 (VASH2). Vasohibin‐1 is an intrinsic negative feedback regulator of angiogenesis, which is selectively induced in endothelial cells (ECs) by an angiogenesis stimulator, such as VEGF or basic fibroblast growth factor.11 Vasohibin‐2 is a homolog of VASH1 and exhibits pro‐angiogenic activity at the sprouting front of angiogenesis.12 To date, very few reports regarding the relationship between VASH2 and tumor angiogenesis are available in published work.13, 14, 15 In our previous study, the knockdown of VASH2 by the stable transfection of a shRNA vector showed notable inhibition of tumor growth, peritoneal dissemination, and tumor angiogenesis in a murine xenograft model of ovarian cancer.14 Therefore, we hypothesized that gene silencing by exogenous administration of siRNA should be effective in a therapeutic model of ovarian cancer. However, the rapid degradation of siRNA and short duration of its biological action in vivo require efficient delivery technology. To overcome this limited stability, several useful delivery systems, such as nanoparticles,7 liposomes,16 and adeno‐associated viral vectors,17 have been developed. Atelocollagen, a highly purified pepsin‐treated type I collagen of calf dermis, is a system for delivering siRNA. Collagen is a fibrous protein in the connective tissue, and plays an important role in maintaining tissue and organ morphology. A collagen molecule has an amino acid sequence known as the telopeptide at both the N‐ and C‐termini, which confers most of its antigenicity. When the telopeptide is removed by treatment with pepsin, atelocollagen with low antigenicity is obtained.18 Atelocollagen is already in clinical use for wound healing and vessel prosthesis, and as a hemostatic agent.19 It has been reported that atelocollagen increases cellular uptake and nuclease resistance, prolongs release of the siRNA in vivo, and displays low toxicity.19
In this study, we applied in vivo delivery of siRNA targeting VASH2 (siVASH2) by atelocollagen in an s.c. xenograft model of ovarian cancer cell lines to evaluate its therapeutic significance in terms of tumor growth, tumor angiogenesis, and tumor vessel maturation.
Material and Methods
Cell culture
The human ovarian serous adenocarcinoma cell line SKOV‐320 was purchased from ATCC (Manassas, VA, USA). The DISS cell line has been described previously.21 Cells were cultured in RPMI‐1640 medium (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10% heat‐inactivated FBS (BioWest SAS, Nuaille, France). All cells were cultured at 37°C in a humidified atmosphere with 5% CO2.
Preparation of siRNAs and in vitro transfection
Synthetic Stealth siRNAs were designed and purchased from Invitrogen Life Technologies (Carlsbad, CA, USA) in desalted and annealed form. Three different Stealth siRNAs targeting human VASH2 with the following sense and antisense sequences were used: siVASH2 A (sense, 5′‐GAAAUGACC‐CGAGAGUCCUUGCCUA‐3′; antisense, 5′‐UAGGCAAGGA‐CUCUCGGGUCAUUUC‐3′); siVASH2 B (sense, 5′‐GAGAAAUAUGCCAGGGACAUGAGAA‐3′; antisense, 5′‐UUCUCAUGUCCCUGGCAUAUUUCUC‐3′); and siVASH2 C (sense, 5′‐CACUCUGAAUGAAGUGGGCUAUCAA‐3′; antisense, 5′‐UUGAUAGCCCACUUCAUUCAGAGUG‐3′). Each freeze‐dried siRNA was reconstituted in RNase‐free water to make a 20 μM stock solution. Non‐specific Stealth RNAi Negative Control Medium GC Duplex #2 was also purchased from Invitrogen and used as control. Ovarian cancer cells were transfected with siRNAs by reverse transfection according to the manufacturer's instructions. Briefly, 60 pmol siRNA and 1 mL Opti‐MEM I Medium (Invitrogen) without serum were mixed in a 6‐cm cell culture dish. After swirling the dish, 10 μL Lipofectamine RNAiMAX reagent (Invitrogen) was added and the mixture was incubated for 20 min at room temperature to obtain siRNA‐Lipofectamine RNAiMAX complexes. Next, 5 mL of cell suspension including 2 × 106 cells in complete growth medium without antibiotics was added into the cell culture dish. This gives a final siRNA concentration of 10 nM. Forty‐eight hours after incubation at 37°C in a CO2 incubator, gene knockdown was evaluated by quantitative real‐time RT‐PCR.
Quantitative real‐time RT‐PCR
Forty‐eight hours after transfection of negative control siRNA or siVASH2 into DISS cells, total RNA was extracted from cell cultures with ISOGEN (Nippon Gene, Toyama, Japan) according to the manufacturer's instructions. The concentration of extracted RNA was determined using a Nanodrop 2000c spectrophotometer (Thermo Scientific, Waltham, MA, USA). First‐strand cDNA was generated using ReverTra Ace (Toyobo, Osaka, Japan). The specific primer pairs used were as follows: human GAPDH forward primer, 5′‐ACCACAGTCCATGCCATCAC‐3′ and reverse primer, 5′‐TCCACCACCCTGTTGCTGTA‐3′; human VASH2 forward primer, 5′‐ACGTCTCAAAGATGCTGAGG‐3′ and reverse primer, 5′‐TTCTCACTTGGGTCGGAGAG‐3′; human VEGF‐A forward primer, 5′‐ CTTGCCTTGCTGCTCTACC‐3′ and reverse primer, 5′‐CACACAGGATGGCTTGA‐AG‐3′; human transforming growth factor (TGF) β‐1 forward primer, 5′‐TTGATGTCACCGGAGTTGTG‐3′ and reverse primer, 5′‐GTTCATGCCATGAATGGTGG‐3′; and human platelet‐derived growth factor (PDGF)‐BB forward primer, 5′‐AGTTGGACCTGAACATGACC‐3′ and reverse primer, 5′‐ TCTCGATCTTTCTCACCTGG‐3′. The CFX96 real‐time PCR detection system was used with SYBR Premix Ex Taq (Takara Bio, Tokyo, Japan) for real‐time PCR analysis. Polymerase chain reaction conditions were as follows: initial denaturation at 95°C for 3 min followed by 50 cycles of a 10‐s phase at 95°C; a 10‐s phase at 56°C; and a 30‐s phase at 72°C. Amplification of GAPDH was used as an internal control. Relative VASH2 mRNA expression levels were calculated using the comparative C t method.
Proliferation of tumor cells
Proliferation of tumor cells was measured by carrying out the Tetra COLOR One cell proliferation assay (Seikagaku, Tokyo, Japan). Briefly, cells were seeded at a density of 2 × 103 cells/well in a 96‐well plate at 24 h after transfection of siRNAs and were incubated at 37°C. After 48 and 72 h, 5 μL Tetra COLOR One was added to each well. The mixture was subsequently incubated for an additional 3 h, and absorbance at 450 nm was monitored.
Preparation of siRNA and atelocollagen complex
The siRNAs and atelocollagen complexes were prepared and given to mice using an in vivo siRNA transfection kit (AteloGene Local Use; Koken, Tokyo, Japan) according to the manufacturer's instructions. Briefly, 600 μL atelocollagen and the same volume of siRNA solution (10 μM) were mixed by rotation at 4°C for 20 min. After centrifugation for 1 min at 6000 g, siRNA and this atelocollagen mixture was used for in vivo administration. By using this method, 1 nmol siRNAs can be delivered into mice with one inoculation.
In vivo delivery of siRNAs using atelocollagen into mice
Female 6–8‐week‐old BALB/c nude mice were obtained from CLEA Japan (Tokyo, Japan). Animal experiment protocols were approved and carried out according to the guidelines for animal experimentation of Jichi Medical University (Tochigi, Japan). A total of 2 × 106 DISS cells or 1 × 106 SKOV‐3 cells were inoculated s.c. into the back of nude mice. When s.c. tumors were measurable, approximately 5 mm in diameter, we initiated treatment with siRNAs mixed with atelocollagen, which enveloped the whole tumor. Tumor size was measured using digital calipers twice per week and the tumor volume was estimated using the following formula: volume = 1/2 × (long diameter) × (short diameter)2. After the treatment with siRNAs was repeated four times, mice were killed and s.c. tumors were harvested for subsequent analyses.
VASH2 gene expression in s.c. tumor
To obtain total RNA from in vivo tissue samples, freshly harvested tumor tissues were homogenized and lysed with TissueRuptor (Qiagen, Hilden, Germany) in QIAzol Lysis Reagent (Qiagen). Next, total RNA was extracted and precipitated using chloroform, isopropanol, and ethanol according to the manufacturer's instructions. After dissolving the RNA in RNase‐free water, we carried out quantitative real‐time RT‐PCR to evaluate VASH2 gene expression.
Assessment of tumor angiogenesis
For immunohistochemical analysis of tumor angiogenesis, harvested tumors were frozen in optimal cutting temperature compound (Sakura, Tokyo, Japan), cut into 7‐μm‐thick sections, fixed in methanol for 20 min at −20°C, and blocked with 1% BSA in PBS for 1 h at room temperature. Primary antibody reactions were carried out overnight at 4°C with rat mAb against mouse CD31 (BD Biosciences, San Diego, CA, USA) at a dilution of 1:500, and with mouse mAb against mouse α‐smooth muscle actin (α‐SMA; Sigma‐Aldrich, St. Louis, MO, USA) at a dilution of 1:800. Secondary antibody reactions were carried out for 1 h at room temperature with Alexa Fluor 488‐conjugated donkey anti‐rat IgG or Alexa Fluor 594‐conjugated goat anti‐mouse IgG (Molecular Probes, Eugene, OR, USA) at a dilution of 1:500. After the sections were washed three times with PBS, they were covered with fluorescent mounting medium (Dako, Carpinteria, CA, USA). All samples were analyzed using a BZ‐9000 fluorescence microscope (Keyence, Osaka, Japan) at room temperature. To assess tumor angiogenesis, the vascular luminal area was calculated using five different fields of each tumor section with BZ‐H2A software (Keyence).
Statistical analysis
Student's t‐test was used to test for significant differences between the two groups. A P‐value of <0.05 was considered significant.
Results
Efficacy of siVASH2 on ovarian cancer cell lines in vitro
To date, at least seven splicing variant transcripts of human VASH2 are registered in the database.14 We designed human‐specific siVASH2 to knock down most of the splicing variants and carried out knockdown assays. To confirm the knockdown efficacy of siVASH2, DISS cells were transfected with three types of siVASH2. As shown in Figure 1, the efficacy of knockdown was more than 90% in each siVASH2. Among these, siVASH2 C showed the highest knockdown activity. Therefore, we used siVASH2 C for the following experiments. In addition, knockdown of VASH2 did not affect the in vitro proliferation of DISS cells (Fig. 1).
Figure 1.
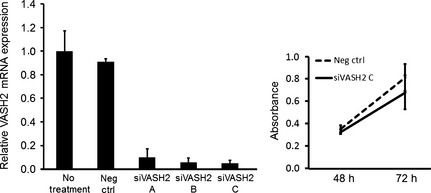
Knockdown efficacy of a siRNA targeting vasohibin‐2 (VASH2) and the effects on in vitro proliferation of cancer cells. Three types of human specific siRNAs targeting VASH2 (siVASH2 A–C) were designed and the knockdown efficacy was evaluated in vitro. Left, the column graph shows VASH2 mRNA expression in DISS cells, evaluated using quantitative RT‐PCR analysis at 48 h after transfection of negative control siRNA or siVASH2. Expression of VASH2 in the no‐treatment group was defined as 1. Right, the line graph shows the proliferation of DISS cells at 48 and 72 h after transfection. Data are presented as the mean and SDs (n = 4). Neg ctrl, negative control siRNA.
In vivo treatment with siRNA and atelocollagen mixture in ovarian cancer xenograft model
After confirming the knockdown efficacy of siVASH2 in vitro, we applied the in vivo delivery technique of siRNAs to an s.c. xenograft model of ovarian cancer. As shown in Figure 2, an siRNA and atelocollagen mixture was applied to the s.c. tumor so that the whole tumor was exposed to treatment. Treatment with siVASH2 significantly inhibited DISS s.c. tumor growth compared with the negative control siRNA‐treated group. We also confirmed the downregulation of VASH2 mRNA within the tumor treated with siVASH2 (Fig. 3). A similar in vivo antitumor effect of siVASH2 and atelocollagen mixture was observed in the human ovarian serous adenocarcinoma cell line SKOV‐3 (Fig. 4).
Figure 2.
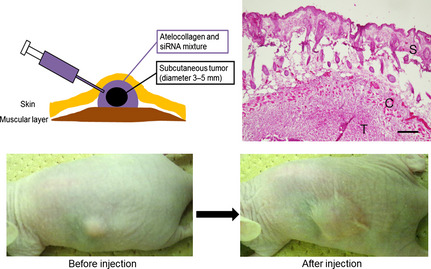
Treatment of a xenograft model of ovarian cancer using atelocollagen and siRNA mixture in vivo. When s.c. tumors were measurable (approximately 5 mm in diameter), we initiated treatment with negative control siRNA or siRNAs targeting vasohibin‐2 mixed with atelocollagen, which covered the entire tumor. Top left, schematic illustration modified from the manufacturer's instructions. Top right, staining with H&E shows the tumor (T) covered by the atelocollagen (C) and siRNA mixture in the s.c. space as shown in the illustration. S, skin. Bar = 200 μm. Bottom, photographs show the s.c. tumor before and after treatment.
Figure 3.
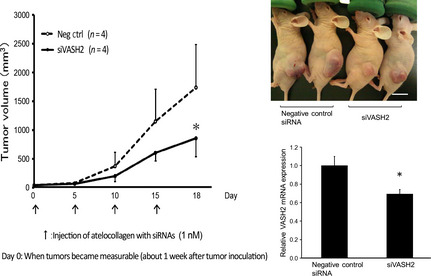
Antitumor effect of in vivo delivery of siRNAs targeting vasohibin‐2 (siVASH2) mixed with atelocollagen in an s.c. tumor model of ovarian cancer (DISS cells). Each siRNA was injected every 4 days and four times in total to s.c. tumors. Left, line graph showing the serial tumor growth of the two groups. Top right, photographs taken 18 days after initiation of siRNA treatment. Bottom right, column graph showing the expression of VASH2 mRNA in the DISS s.c. tumor determined using quantitative RT‐PCR. Data are presented as the mean and SDs (n = 4). Bar = 1 cm. *P < 0.05 versus negative control siRNA (Neg ctrl).
Figure 4.
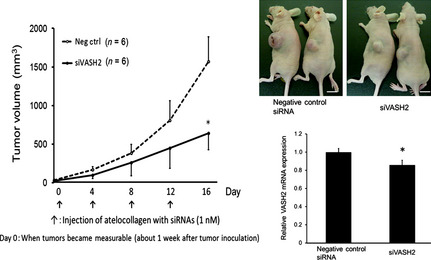
Antitumor effect of in vivo delivery of siRNAs targeting vasohibin‐2 (siVASH2) mixed with atelocollagen in an s.c. tumor model of ovarian cancer (SKOV‐3 cells). Each siRNA was injected every 3 days and four times in total into s.c. tumors. Left, line graph showing the serial tumor growth of the two groups. Top right, photographs taken 16 days after initiation of siRNA treatment. Bar = 1 cm. Bottom right, column graph showing the expression of VASH2 mRNA in the SKOV‐3 s.c. tumor determined using quantitative RT‐PCR. Data are presented as the mean and SDs (n = 6). *P < 0.05 versus negative control siRNA (Neg ctrl).
Tumor angiogenesis and vessel maturation in vivo
Next, we analyzed angiogenesis in the DISS and SKOV‐3 s.c. tumors of a murine xenograft model. Using immunofluorescent staining of CD31, we observed significant inhibition of tumor angiogenesis in the siVASH2‐treated group compared with the negative control siRNA‐treated group (Fig. 5). Moreover, we evaluated the pericyte coverage of tumor blood vessels of DISS and SKOV‐3 s.c. tumors. As shown in Figure 6, siVASH2‐treated tumors contained a larger number of blood vessels covered with pericytes, indicating that these tumors contained more mature blood vessels.
Figure 5.
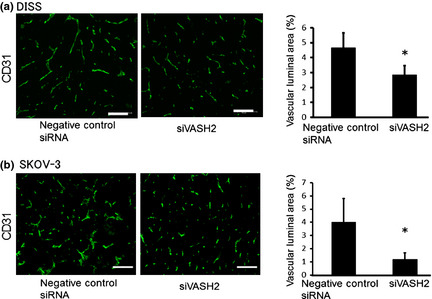
Quantification of tumor angiogenesis in a murine model of ovarian cancer. (a) Tumor blood vessels of s.c. tumors (DISS cells) treated with negative control siRNA or siRNAs targeting vasohibin‐2 (siVASH2) were immunostained with anti‐CD31 antibody. Column graph shows the vascular luminal area, which was calculated based on areas in five different fields. (b) Tumor blood vessels of s.c. tumors (SKOV‐3 cells) treated with negative control siRNA or siVASH2 were immunostained with anti‐CD31 antibody. Column graph shows the vascular luminal area calculated as in (a). Mean and SDs are shown (n = 4). Bar = 100 μm. *P < 0.05 versus negative control siRNA.
Figure 6.
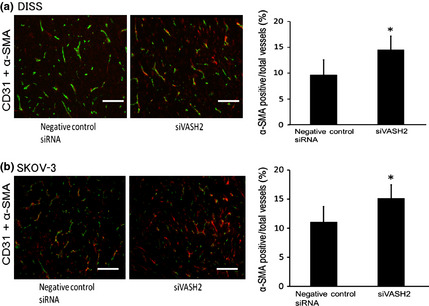
Pericyte coverage of tumor blood vessels in a murine model of ovarian cancer. (a) Tumor blood vessels of s.c. tumors (DISS cells) treated with negative control siRNA or siRNAs targeting vasohibin‐2 (siVASH2) were co‐immunostained with anti‐CD31 (green) and anti‐α‐smooth muscle actin (α‐SMA; red) antibodies. Merged vessels were regarded as the vessels covered with pericytes. Column graph shows the positivity rate for α‐SMA in tumor vessels, based on areas in five different fields. (b) Tumor blood vessels of s.c. tumors (SKOV‐3 cells) treated with negative control siRNA or siVASH2 were co‐immunostained with anti‐CD31 (green) and α‐SMA (red) antibodies. The positivity rate for α‐SMA in tumor vessels was calculated as described in (a). Data are presented as the mean and SDs (n = 4). Bar = 100 μm. *P < 0.05 versus negative control siRNA.
Changes in angiogenesis‐related factors due to siVASH2 treatment
We examined the expression of various angiogenesis‐related factors in ovarian cancer cell lines treated with negative control siRNA or siVASH2. As shown in Figure 7, the expression of angiogenesis‐related factors, including VEGF, TGFβ‐1, and PDGF‐BB did not change after siVASH2 treatment under in vitro conditions. Expression of angiopoietins related to pericyte attachment and detachment was primarily low; siVASH2 treatment did not affect angiopoietin expression in in vitro cultured ovarian cancer cells (data not shown).
Figure 7.
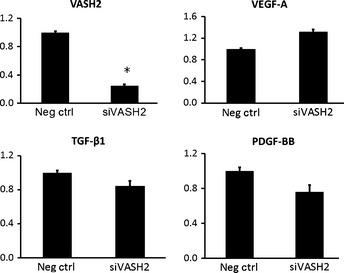
Changes in mRNA expression of angiogenesis‐related factors following knockdown of vasohibin‐2 (VASH2) in a murine model of ovarian cancer. Total RNA was extracted from DISS cells treated with negative control siRNA or siRNAs targeting VASH2 (siVASH2), and the mRNA expression of angiogenesis‐related factors was examined by quantitative RT‐PCR. Data are presented as the mean and SDs (n = 4). The mRNA expression of the negative control siRNA‐treated group in each target gene was defined as 1. *P < 0.05 versus negative control siRNA (Neg ctrl). PDGF, platelet‐derived growth factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
Discussion
In this study, we showed that exogenous treatment with siVASH2 and atelocollagen biomaterial has a significant knockdown effect on the VASH2 gene and an antitumor effect by decreasing tumor angiogenesis in vivo. In addition, siVASH2‐treated tumors contained a larger number of mature blood vessels. These results suggest that VASH2 can be used as a novel molecular target for anti‐angiogenic cancer therapy in ovarian cancer.
RNA interference technology using siRNAs has been experimentally introduced worldwide as a cancer therapy and is expected to be developed as a nucleic acid‐based medicine.22 To obtain a sufficient anticancer effect, it is important not only to select appropriate gene targets, but also to develop suitable drug delivery systems (DDS). Atelocollagen is one such DDS of siRNA, and its usefulness in in vivo cancer models has been reported previously.23, 24, 25 Several clinical trials for cancer therapy using RNAi techniques are also ongoing.22 However, to date, there has been no report on the use of DDS of siRNAs in gynecological malignancies, including ovarian cancer. Here, we showed the in vivo antitumor effect of RNAi technology targeting VASH2 with atelocollagen biomaterial in ovarian cancer for the first time.
Vasohibin‐2 has been reported to be expressed in several types of cancers and acts on neighboring ECs as an angiogenesis stimulator in a paracrine manner.14 Specific knockdown of VASH2 from cancer cells retarded tumor growth by decreasing tumor angiogenesis, the mechanism of which is to inhibit proliferation and migration of ECs.13, 14, 15 In this study, knockdown of endogenous VASH2 by treatment with siVASH2 and atelocollagen biomaterial retarded s.c. tumor growth by decreasing angiogenesis; a previous study used stable VASH2 knockdown clones to achieve this effect.13, 14, 15 Treatment with siVASH2 alone did not significantly alter the in vitro proliferation of cancer cells. Expression of angiogenesis‐related factors, including VEGF, TGFβ‐1, and PDGF‐BB, did not change following siVASH2 treatment. Therefore, knockdown of VASH2 may have indirectly affected tumor cells by inhibiting EC function. Our results showed that the knockdown efficacy of VASH2 considerably differed between in vitro and in vivo treatments. The in vivo knockdown effect may vary among cancer cells because in vivo tumors are heterogeneous; additionally, contamination with cells other than the cancer cells present in the tumor microenvironment may have occurred. Therefore, the discrepancy between the knockdown efficacy of VASH2 and angiogenesis inhibition may have been observed in in vivo experimental models, as shown in Figures 3, 4, 5.
vNewly formed tumor blood vessels are immature and show high vascular permeability, high interstitial pressure, and impaired blood flow. These characteristics cause resistance to conventional chemotherapy.26 Vascular normalization by pericyte coverage improves the tumor microenvironment, including blood flow, and facilitates chemotherapeutic drug delivery to the tumor tissue. Although anti‐VEGF therapy such as bevacizumab normalizes tumor vessels, this effect is transient and tumor vessels eventually regress. Tumor vascular regression causes hypoxia within the tumor, followed by recurrence of tumor angiogenesis due to the development of compensatory mechanisms for producing angiogenic factors other than VEGF or the recruitment of bone marrow‐derived angiogenic cells.27 Hypoxia due to the regression of tumor vessels could also make cancer cells more invasive and metastatic by inducing hypoxia‐inducible factor‐1.28 Therefore, persistent normalization of tumor vessels is required for improving the tumor microenvironment and inhibiting metastasis.29 Our results indicate that siVASH2‐treated tumors contained more blood vessels covered with pericytes, suggesting that inhibition of VASH2 contributes to tumor vessel normalization. Although angiopoietins are strongly related to pericyte coverage, their primary expression level was low and siVASH2 treatment did not affect their expression level in cultured ovarian cancer cells. Thus, VASH2 might contribute to the destabilization of tumor blood vessels, which requires further investigation.
Vasohibin‐2 is a promising molecular target for anti‐angiogenic therapy that can be used to avoid the toxic side‐effects of anti‐VEGF therapy, such as hypertension and proteinuria, as it is a VEGF‐independent and EC‐extrinsic angiogenesis regulator and is expressed at low levels in normal adult tissues, except the brain and genital organs.12 Moreover, atelocollagen itself shows neither toxicity nor antigenicity in animals because antigenic telopeptides are removed through pepsin digestion.30 Collectively, RNAi therapy targeting VASH2 with atelocollagen biomaterial may be a powerful strategy against ovarian cancer and may offer safer and more favorable therapeutic outcomes. In addition, VASH1 inhibits tumor angiogenesis and makes tumor blood vessels mature. Moreover, VASH1 maintains the tumor vessels and vascular regression does not occur.31, 32 Thus, a combination of VASH2 inhibition and VASH1 upregulation would be an attractive therapeutic strategy for cancer treatment.
To date, it has been shown that VASH1 inhibits tumor angiogenesis,32, 33, 34, 35, 36 whereas VASH2 stimulates this process.13, 14, 15 However, the putative vasohibin receptor and its downstream signaling have not been identified. It has been hypothesized that these two factors share a vasohibin receptor: one acts agonistically, and the other acts antagonistically. Studies are currently underway to resolve this question. Additionally, we are now searching for the bioactive center of VASH2 and attempting to develop a novel targeted molecular therapy such as a neutralizing mAb therapy. The significance and functional differences among splicing variants of VASH2 are also under investigation.
In conclusion, exogenous treatment using siVASH2 with atelocollagen biomaterial exerted a significant antitumor effect on ovarian cancer in vivo against tumor growth and tumor angiogenesis. Knockdown of VASH2 may also help tumor vascular normalization. Targeting VASH2 can be applied as a novel molecular target therapy for ovarian cancer.
Disclosure Statement
The authors have no conflict of interests.
Acknowledgment
This work was supported by The Research Award to Jichi Medical University Graduate Students (T.K.).
(Cancer Sci 2013; 104: 1705–1710)
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 2. Cannistra SA. Cancer of the ovary. N Engl J Med 2004; 351: 2519–29. [DOI] [PubMed] [Google Scholar]
- 3. Banerjee S, Gore M. Recent advances in systemic treatments for ovarian cancer. Cancer Imaging 2012; 12: 305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozols RF. Challenges for chemotherapy in ovarian cancer. Ann Oncol 2006; 17: v181–7. [DOI] [PubMed] [Google Scholar]
- 5. Hanahan D, Weinberg RA. Hallmarks of Cancer: the Next Generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 6. Liu J, Matulonis UA. Anti‐angiogenic agents in ovarian cancer: dawn of a new era? Curr Oncol Rep 2011; 13: 450–8. [DOI] [PubMed] [Google Scholar]
- 7. Burger RA. Overview of anti‐angiogenic agents in development for ovarian cancer. Gynecol Oncol 2011; 121: 230–8. [DOI] [PubMed] [Google Scholar]
- 8. Koutras AK, Fountzilas G, Makatsoris T, Peroukides S, Kalofonos HP. Bevacizumab in the treatment of breast cancer. Cancer Treat Rev 2010; 36: 75–82. [DOI] [PubMed] [Google Scholar]
- 9. Tamaskar I, Pili R. Update on novel agents in renal cell carcinoma. Expert Rev Anticancer Ther 2009; 9: 1817–27. [DOI] [PubMed] [Google Scholar]
- 10. Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high‐grade glioma. Nat Rev Neurol 2009; 5: 610–20. [DOI] [PubMed] [Google Scholar]
- 11. Watanabe K, Hasegawa Y, Yamashita H et al Vasohibin as an endothelium‐derived negative feedback regulator of angiogenesis. J Clin Invest 2004; 114: 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kimura H, Miyashita H, Suzuki Y et al Distinctive localization and opposed roles of vasohibin‐1 and vasohibin‐2 in the regulation of angiogenesis. Blood 2009; 113: 4810–8. [DOI] [PubMed] [Google Scholar]
- 13. Xue X, Gao W, Sun B et al Vasohibin 2 is transcriptionally activated and promotes angiogenesis in hepatocellular carcinoma. Oncogene 2013; 32: 1724–34. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi Y, Koyanagi T, Suzuki Y et al Vasohibin‐2 expressed in human serous ovarian adenocarcinoma accelerates tumor growth by promoting angiogenesis. Mol Cancer Res 2012; 10: 1135–46. [DOI] [PubMed] [Google Scholar]
- 15. Koyanagi T, Saga Y, Takahashi Y, Suzuki Y, Suzuki M, Sato Y. Downregulation of vasohibin‐2, a novel angiogenesis regulator, suppresses tumor growth by inhibiting angiogenesis in endometrial cancer cells. Oncol Lett 2013; 5: 1058–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bertrand JR, Pottier M, Vekris A, Opolon P, Maksimenko A, Malvy C. Comparison of antisense oligonucleotides and siRNAs in cell culture and in vivo. Biochem Biophys Res Commun 2004; 296: 1000–4. [DOI] [PubMed] [Google Scholar]
- 17. Tomar RS, Matta H, Chaudhary PM. Use of adeno‐associated viral vector for delivery of small interfering RNA. Oncogene 2003; 22: 5712–5. [DOI] [PubMed] [Google Scholar]
- 18. Stenzel KH, Miyata T, Rubin AL. Collagen as a biomaterial. Annu Rev Biophys Bioeng 1974; 3: 231–53. [DOI] [PubMed] [Google Scholar]
- 19. Ochiya T, Nagahara S, Sano A, Itoh H, Terada M. Biomaterials for gene delivery: atelocollagen‐mediated controlled release of molecular medicines. Curr Gene Ther 2001; 1: 31–52. [DOI] [PubMed] [Google Scholar]
- 20. Fogh J, Wright WC, Loveless JD. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J Natl Cancer Inst 1977; 58: 209–14. [DOI] [PubMed] [Google Scholar]
- 21. Yokoyama Y, Xin B, Shigeto T et al Clofibric acid, a peroxisome proliferator‐activated receptor alpha ligand, inhibits growth of human ovarian cancer. Mol Cancer Ther 2007; 6: 1379–86. [DOI] [PubMed] [Google Scholar]
- 22. Ashihara E, Kawata E, Maekawa T. Future prospect of RNA interference for cancer therapies. Curr Drug Targets 2010; 11: 345–60. [DOI] [PubMed] [Google Scholar]
- 23. Minakuchi Y, Takeshita F, Kosaka N et al Atelocollagen‐mediated synthetic small interfering RNA delivery for effective gene silencing in vitro and in vivo. Nucleic Acids Res 2004; 32: e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takeshita F, Minakuchi Y, Nagahara S et al Efficient delivery of small interfering RNA to bone‐metastatic tumors by using atelocollagen in vivo. Proc Natl Acad Sci U S A 2005; 102: 12177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawata E, Ashihara E, Kimura S et al Administration of PLK‐1 small interfering RNA with atelocollagen prevents the growth of liver metastases of lung cancer. Mol Cancer Ther 2008; 7: 2904–12. [DOI] [PubMed] [Google Scholar]
- 26. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–57. [DOI] [PubMed] [Google Scholar]
- 27. Bergers G, Hanahan D. Modes of resistance to anti‐angiogenic therapy. Nat Rev Cancer 2008; 8: 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Semenza GL. Targeting HIF‐1 for cancer therapy. Nat Rev Cancer 2003; 3: 721–32. [DOI] [PubMed] [Google Scholar]
- 29. Sato Y. Persistent vascular normalization as an alternative goal of anti‐angiogenic cancer therapy. Cancer Sci 2011; 102: 1253–6. [DOI] [PubMed] [Google Scholar]
- 30. Ochiya T, Takahama Y, Nagahara S et al New delivery system for plasmid DNA in vivo using atelocollagen as a carrier material: the Minipellet. Nat Med 1999; 5: 707–10. [DOI] [PubMed] [Google Scholar]
- 31. Heishi T, Hosaka T, Suzuki Y et al Endogenous angiogenesis inhibitor vasohibin1 exhibits broad‐spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am J Pathol 2010; 176: 1950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hosaka T, Kimura H, Heishi T et al Vasohibin‐1 expression in endothelium of tumor blood vessels regulates angiogenesis. Am J Pathol 2009; 175: 430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoshinaga K, Ito K, Moriya T et al Expression of vasohibin as a novel endothelium‐derived angiogenesis inhibitor in endometrial cancer. Cancer Sci 2008; 99: 914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tamaki K, Moriya T, Sato Y et al Vasohibin‐1 in human breast carcinoma: a potential negative feedback regulator of angiogenesis. Cancer Sci 2009; 100: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tamaki K, Sasano H, Maruo Y et al Vasohibin‐1 as a potential predictor of aggressive behavior of ductal carcinoma in situ of the breast. Cancer Sci 2010; 101: 1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoshinaga K, Ito K, Moriya T et al Roles of intrinsic angiogenesis inhibitor, vasohibin, in cervical carcinomas. Cancer Sci 2011; 102: 446–51. [DOI] [PubMed] [Google Scholar]


