Abstract
The combination of docetaxel, cisplatin, and 5‐fluorouracil (DCF) as preoperative treatment for esophageal squamous cell carcinoma (ESCC) has not been investigated. We carried out a multicenter phase II feasibility study of preoperative chemotherapy with DCF for ESCC. Patients with clinical stage II/III ESCC (International Union Against Cancer TNM classification system, 6th edition) were eligible. Chemotherapy consisted of i.v. docetaxel (70–75 mg/m2) and cisplatin (70–75 mg/m2) on day 1, and continuous infusion of fluorouracil (750 mg/m2/day) on days 1–5. Antibiotic prophylaxis on days 5–15 was mandatory. This regimen was repeated every 3 weeks with a maximum of three cycles allowed. After completion of chemotherapy, esophagectomy with extended lymphadenectomy was carried out. The primary endpoint was the completion rate of protocol treatment. Forty‐two eligible patients were enrolled. During chemotherapy, the most common grade 3 or 4 toxicities were neutropenia (83%), anorexia (7%), and stomatitis (5%). Forty‐one (98%) patients underwent surgery. The completion rate of protocol treatment was 90.5% (38/42). No treatment‐related death was observed and the incidence of operative morbidity was tolerable. According to RECIST, the overall response rate after the completion of DCF was 64.3%. Pathological complete response was achieved in 17%. The estimated 2‐year progression‐free survival and overall survival were 74.5% and 88.0%, respectively. Although these data are preliminary, preoperative DCF was well tolerated. Antitumor activity was highly promising and warrants further investigation. This trial was registered with University Hospital Medical Information Network (no. UMIN000002396).
Treatment of resectable esophageal cancer remains unsatisfactory. Among approaches, various multimodality combined therapies to improve local and distant relapse rates with preoperative chemotherapy or chemoradiotherapy have been investigated. Preoperative chemoradiotherapy was shown to increase disease‐free and overall survival (OS) compared to the surgery‐alone arm in some randomized trials, but was associated with a significantly increased rate of perioperative complications.1, 2 Furthermore, treatment strategies for specific histologic types are controversial, particularly with regard to adenocarcinoma and squamous cell carcinoma, given the general consensus that these two histological cancers should be considered as having different biologies.3 Esophageal squamous cell carcinoma (ESCC) is a major histological type in Asia, including Japan, but most clinical trials have investigated treatment of resectable esophageal cancer without regard to type.
One alternative approach to the adjuvant strategy is perioperative chemotherapy without radiation. In a Japan Clinical Oncology Study Group trial (JCOG 9907) which compared preoperative chemotherapy with postoperative chemotherapy in the treatment of stage II/III ESCC, preoperative chemotherapy with cisplatin and 5‐fluorouracil (5‐FU) (CF) followed by surgery was shown to improve OS without additional serious adverse events.4 In this trial, preoperative CF resulted in an OS of 55% at 5 years, a relatively good result in comparison with preoperative chemoradiotherapy in Western countries.1, 2 Preoperative chemotherapy with CF is accordingly regarded as a standard treatment for stage II/III ESCC. Survival was limited, however, indicating the need further therapeutic intervention.
Docetaxel has shown activity against many solid tumors as both monotherapy and in combination with other agents. Two phase III trials showed survival benefits for induction chemotherapy with docetaxel plus cisplatin and 5‐FU (DCF) compared to CF in locally advanced squamous cell carcinoma of the head and neck (SCCHN).5, 6 Furthermore, DCF has also shown a survival benefit for advanced gastric cancer (AGC) compared to CF, and is now considered one of the standard regimens for AGC.7 Docetaxel plus cisplatin and 5‐FU showed improvement not only tumor shrinkage but also survival benefit. We could achieve an improvement of not only the local tumor control but also suppression of the micrometastasis by introducing preoperative chemotherapy with DCF.
Based on these promising results, we speculated that the addition of docetaxel to CF would improve efficacy for ESCC. To our knowledge, however, combination therapy with DCF in the treatment of ESCC has not been investigated in the preoperative setting, and has not been nominated for investigation in planned future preoperative chemotherapy trials.
Here, we carried out a feasibility study of preoperative chemotherapy with DCF in patients with clinical stage II/III squamous cell carcinoma of the thoracic esophagus.
Patients and Methods
Eligibility criteria
Patients entered into this study met the following eligibility criteria: histologically proven squamous cell carcinoma of the thoracic esophagus; clinical stage IIA, IIB, or III (International Union Against Cancer TNM classification system, 6th edition); Eastern Cooperative Oncology Group performance status (PS) of 0 to 1; age 20–70 years (first cohort), or 20–75 years (expansion cohort); and adequate organ function. Written informed consent was required from all patients before the start of study therapy. Patients who met any of the following conditions were excluded: prior chemotherapy; concurrent active malignancy; active infection; serious medical problems that might interfere with the achievement of study objectives; and pregnancy or lactation.
The study was approved by the Institutional Review Board at each site.
Chemotherapy
Chemotherapy consisted of a 1‐h i.v. infusion of docetaxel at 70 mg/m2; 2‐h infusion of cisplatin at 70 mg/m2 on day 1; and continuous i.v. infusion of 5‐FU at 750 mg/m2/day on days 1–5 (DCF: 70/70/750). This regimen was repeated every 3 weeks until unacceptable toxicity, patient refusal, or disease progression was observed, up to a maximum of three cycles. Prophylactic use of granulocyte colony‐stimulating factor (G‐CSF) was not allowed, but prophylactic use of ciprofloxacin on days 5–15 was mandatory.
Surgery
Surgery was carried out within 42 days of the start date of the last chemotherapy cycle. Patients underwent right or left thoracotomy for curative resection by total or subtotal thoracic esophagectomy. A laparoscopic procedure for esophagectomy was permitted. Regional lymphadenectomy consisted of two‐ or three‐field extended lymphadenectomy. Evaluations of residual tumor (R) were classified as follows: R0, no residual tumor; R1, suspicious of residual tumor or microscopic residual tumor; or R2, macroscopic residual tumor. Surgical specimens were evaluated pathologically and graded according to the proportion of tumor affected by degeneration or necrosis using a grading system by the Japanese Classification of Esophageal Carcinoma:8 grade 0, no part of tumor affected; grade 1, less than two‐thirds affected; grade 2, between two‐thirds and entire tumor affected; and grade 3, no residual tumor (pathologically complete response [pCR]).
Treatment assessment and dose modifications
Baseline evaluation consisted of history, physical examination, radiographic imaging, routine laboratory studies, and electrocardiogram. Safety assessments were repeated weekly after the start of chemotherapy according to the US National Cancer Institute's Common Terminology Criteria for Adverse Events (version 3.0). Close follow‐up using both endoscopy and computed tomography was mandatory in the third week of every cycle. If disease progression or new metastasis was detected, the subsequent cycle was not permitted and immediate surgery was mandated.
As patients received three chemotherapeutic agents, dose adjustment was carried out for each individual agent based on its estimated causal relationship to the toxicity. Doses of 5‐FU, cisplatin, and docetaxel were reduced by 20% in the subsequent course if grade 4 neutropenia, anemia, or thrombocytopenia was observed. Doses of 5‐FU and docetaxel were reduced by 20% in the subsequent course if grade 3 or 4 stomatitis, esophagitis, or diarrhea was observed. The dose of cisplatin was reduced by 20% in the subsequent course if creatinine clearance (CCr) was 50 ≤ CCr < 60, by 40% if CCr was 40 ≤ CCr <50, and then stopped if CCr was <40 mL/min, or ototoxicity of grade 2 or higher was observed.
We determined to stop protocol treatment if: (i) the patient could not receive chemotherapy due to unacceptable toxicities; (ii) tumor was defined as unresectable before surgery or the patient did not undergo surgery for any reason; or (iii) tumor was defined as R1/2 after surgery.
End‐points and statistical methods
The primary end‐point was the completion rate of protocol treatment, with complete delivery defined as completion of at least two cycles of preoperative chemotherapy, and pathologically proven complete resection (R0). Secondary end‐points included: the safety and tolerability of this chemotherapy; evaluation of operative morbidity and mortality; and evaluation of efficacy, including response rate, progression‐free survival (PFS), and OS. Tumor response was evaluated for patients who had measurable lesions according to RECIST version 1.0.
In the present trial, we expected that the incidence of toxicities with DCF would increase above that with CF in the preoperative setting, and that the rate of treatment completion would be lower than that in JCOG 9907 (89.6%). Accordingly, we assumed a null hypothesis with a 75% completion rate for protocol treatment, and expected a completion rate of protocol treatment of 90%. Given a one‐sided alpha of 0.1 and statistical power of 80%, a minimum of 28 patients was needed. The projected sample size was 30 patients in total. In the first cohort we enrolled patients aged 70 years or younger in consideration of safety. As the protocol was well‐tolerated in the first cohort (DCF: 70/70/750), however, we amended the protocol as follows: (i) dose escalation of docetaxel from 70 mg/m2 to 75 mg/m2; (ii) dose escalation of cisplatin from 70 mg/m2 to 75 mg/m2; and (iii) raising of the upper age limit from 70 to 75 years old. We then planned that at least 10 patients should be enrolled in an expansion cohort (DCF: 75/75/750).
The survival curve was estimated using the Kaplan–Meier method. Safety and efficacy analyses were both carried out on a per protocol set population, defined as all patients enrolled in the study who received at least one dose of chemotherapy. A subject's PFS was defined as the time from the date of registration to the first documentation of disease progression, subsequent therapy, or death. Overall survival was determined from the date of registration to the date of death from any causes or the last confirmation of survival. Statistical data were obtained using the spss 20.0 software package (SPSS Inc., Chicago, IL, USA).
This trial was registered with University Hospital Medical Information Network (no. UMIN000002396).
Results
Patient characteristics
From July 2009 to May 2010, 42 eligible patients were enrolled from five institutions (first cohort, 32 patients; expansion cohort, 10 patients). The following analyses were carried out in all 42 patients. Baseline characteristics of the study population are listed in Table 1. Most patients were men (95%), and the median age was 62 years (range, 36–73 years). All treated patients had an Eastern Cooperative Oncology Group PS of 0 or 1 (PS 0_67%; PS 1_33%). More than half of the patients were T3 status and clinical stage III.
Table 1.
Characteristics of patients
| Characteristic | No. of patients | ||
|---|---|---|---|
| DCF (70/70/750) (n = 32) | DCF (75/75/750) (n = 10) | Total (n = 42) | |
| Sex | |||
| Male | 30 | 10 | 40 |
| Female | 2 | 0 | 2 |
| Age, years | |||
| Median | 61 | 67 | 62 |
| Range | 36–70 | 49–73 | 36–73 |
| ECOG performance status | |||
| 0 | 20 | 8 | 28 |
| 1 | 12 | 2 | 14 |
| Site of primary tumor | |||
| Ut | 6 | 2 | 8 |
| Mt | 19 | 5 | 24 |
| Lt | 7 | 3 | 10 |
| Clinical T stage | |||
| cT1 | 6 | 0 | 6 |
| cT2 | 4 | 2 | 6 |
| cT3 | 22 | 8 | 30 |
| Clinical N stage | |||
| cN0 | 6 | 3 | 9 |
| cN1 | 26 | 7 | 33 |
| Clinical stage | |||
| IIA | 6 | 3 | 9 |
| IIB | 8 | 1 | 9 |
| III | 18 | 6 | 24 |
ECOG, Eastern Cooperative Oncology Group.; Lt, lower thoracic esophagus; Mt, middle thoracic esophagus; Ut, upper thoracic esophagus.
Treatment profile
An accrual and treatment profile is shown in Figure 1. With regard to treatment failure, protocol treatment was terminated in seven patients due to treatment‐related toxicity, as follows. One patient discontinued the subsequent second cycle of chemotherapy because he did not achieve a response. Two patients did not receive subsequent chemotherapy due to severe toxicities, one due to encephalopathy induced by 5‐FU and one due to febrile neutropenia occurring in each of two courses. The remaining four patients refused to continue subsequent chemotherapy, mainly because of gastrointestinal toxicities.
Figure 1.
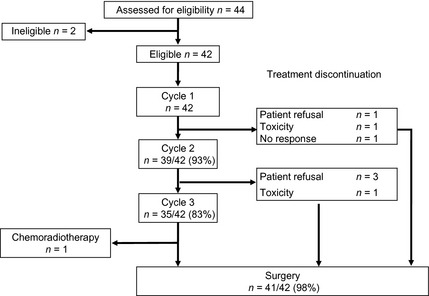
Accrual and treatment summary.
Eighteen patients required a dose reduction in the second cycle and nine in the third, mainly due to neutropenia. Almost half of the patients had to have their doses reduced in cycle 2. However, most patients were able to receive subsequent chemotherapy due to appropriate dose reduction, and 83% of patients completed three cycles of chemotherapy after a reduction. Among all courses, two patients required a delay of 7 days or more, due to prolonged neutropenia and elevation of liver enzymes in one patient each. The number of patients who received G‐CSF was six in the first cycle, five in the second, and none in the third, due to persistent grade 3–4 neutropenia or febrile neutropenia.
Toxicity
Overall toxicities during chemotherapy are listed in Table 2. As anticipated from previous studies of DCF, the major toxicities were leukopenia and neutropenia. However, only one (4%) patient with febrile neutropenia was seen. Common non‐hematological adverse events over grade 3 or 4 were anorexia (7%), stomatitis (5%), and nausea (2%). Although adverse events were most severe in the first cycle in most patients, all toxicities were within expectations and were manageable. No treatment‐related deaths were observed.
Table 2.
Adverse events of preoperative chemotherapy
| Adverse events | No. of patients | |||||
|---|---|---|---|---|---|---|
| DCF (70/70/750) n = 32 | DCF (75/75/750) n = 10 | Total n = 42 | ||||
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | All grades (%) | Grades 3/4 (%) | |
| Leukopenia | 15 | 1 | 3 | 0 | 42 (100) | 19 (45.2) |
| Neutropenia | 12 | 16 | 4 | 3 | 42 (100) | 35 (83.3) |
| Anemia | 0 | 0 | 0 | 0 | 41 (98) | 0 (0.0) |
| Thrombocytopenia | 1 | 0 | 0 | 0 | 10 (14) | 1 (2.4) |
| Febrile neutropenia | 1 | 0 | 0 | 0 | 1 (2) | 1 (2.4) |
| Anorexia | 3 | 0 | 0 | 0 | 39 (93) | 3 (7.1) |
| Stomatitis | 2 | 0 | 0 | 0 | 27 (64) | 2 (4.8) |
| Nausea | 1 | 0 | 0 | 0 | 28 (67) | 1 (2.4) |
| Diarrhea | 0 | 0 | 0 | 0 | 16 (38) | 0 (0.0) |
| Vomiting | 0 | 0 | 0 | 0 | 10 (24) | 0 (0.0) |
| Creatinine | 0 | 0 | 0 | 0 | 9 (21) | 0 (0.0) |
| Hyperbilirubinemia | 0 | 0 | 0 | 0 | 8 (19) | 0 (0.0) |
| Elevation of AST | 0 | 0 | 0 | 0 | 16 (38) | 0 (0.0) |
| Elevation of ALT | 2 | 0 | 0 | 0 | 19 (45) | 2 (4.8) |
| Encephalopathy due to 5‐FU | 1 | 0 | 0 | 0 | 1 (2) | 1 (2.4) |
DCF (70/70/750) treatment consisted of a 1‐h i.v. infusion of docetaxel at 70 mg/m2, 2‐h infusion of cisplatin at 70 mg/m2 on day 1, and continuous i.v. infusion of 5‐FU at 750 mg/m2/day on days 1–5, with an upper age limit of 70 years; DCF (75/75/750) treatment consisted of a 1‐h i.v. infusion of docetaxel at 75 mg/m2, 2‐h infusion of cisplatin at 75 mg/m2 on day 1, and continuous i.v. infusion of 5‐FU at 750 mg/m2/day on days 1–5, with an upper age limit of 75 years. 5‐FU, 5‐fluorouracil.
Surgery and postoperative complications
Table 3 gives an overview of surgical outcomes and complications. Forty‐one patients received subsequent surgery after completion of preoperative DCF. One patient for whom a good response was documented after three cycles of DCF refused surgery and received definitive chemoradiotherapy with CF.
Table 3.
Operative details and postoperative outcomes
| No. of patients | % | |
|---|---|---|
| Surgical approach | 41 | 100 |
| Right thoractomy | 29 | 71 |
| Arthroscopic | 11 | 27 |
| Left thoractomy | 1 | 2 |
| Type of lymphadenectomy | ||
| D3 | 39 | 95 |
| D2 | 2 | 5 |
| Type of resection | ||
| R0 | 40 | 98 |
| R1 | 1 | 2 |
| Postoperative complications | ||
| Recurrent nerve palsy | 9 | 22 |
| Pneumonia | 7 | 17 |
| Anastomotic leakages | 5 | 12 |
| Wound infection | 9 | 22 |
| Pyothorax | 3 | 7 |
| Pneumothorax | 1 | 2 |
| Lymphorrhea | 1 | 2 |
| Acute circulatory failure | 1 | 2 |
| Chylothorax | 1 | 2 |
| Postoperative mortality | 0 | 0 |
R0, no residual tumor; R1, suspicious of residual tumor or microscopic residual tumor.
All patients who underwent surgery received extended lymphadenectomy, and 40 of 41 patients underwent R0 resection pathologically. Median operation time was 452 min (range, 263–725 min) and median blood loss was 481 mL (range, 63–2342 mL). Median duration time from the beginning of the last cycle to surgery was 39 days (range, 28–65 days). Most cases of infectious events such as pneumonia or wound infection were manageable. Overall, there was no mortality and no serious complications.
Treatment outcomes
Of all 42 patients, two patients failed to complete two cycles of chemotherapy due to adverse events. After completion of one cycle, both underwent surgery with R0 resection without remarkable postoperative complications. Of patients who received two or more cycles of chemotherapy, one patient was shown to require R1 resection pathologically after esophagectomy and one patient refused to undergo surgery. Thus, 38 patients fulfilled the requirements for primary analysis for feasibility according to the definition in the protocol (completion of at least two courses of preoperative chemotherapy and R0 surgery), giving a completion rate of protocol treatment of 90.5%.
Of 28 patients who had measurable lesions, one complete and 17 partial responses were observed, giving an overall response rate of 64.3% (95% confidence interval, 44.1–81.4%). Histopathological complete response (grade 3) was achieved in 7/41 (17%) of patients. Grade 2, 1, and 0 responses were seen in 14/41 (34%), 18/41 (44%), and 2/41 (5%) of patients, respectively. Most patients were pathologically proved to show down‐staging after surgery.
With a median follow‐up time of 27 months (range, 12–34 months), the estimated 2‐year PFS and OS were 74.5% and 88.0%, respectively (Fig. 2). Median PFS was not reached.
Figure 2.
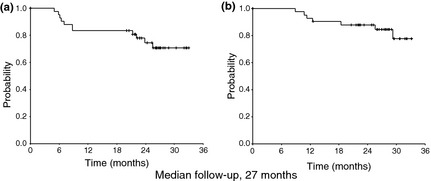
Kaplan–Meier estimates of (a) progression‐free survival and (b) overall survival. The estimated 2‐year progression‐free survival and overall survival were 74.5% and 88.0%, respectively.
Discussion
In this multicenter trial of preoperative chemotherapy, a combination regimen with DCF was shown to be safe and tolerable in patients with clinical stage II–III ESCC. Furthermore, this combination treatment showed high activity in terms of both clinical and histopathological response.
Two randomized phase III trials with induction chemotherapy for locally advanced SCCHN indicated a clear benefit in OS, PFS, and response rate for DCF compared to CF.5, 6 We therefore speculated that combination chemotherapy with DCF might prove promising when used in the preoperative setting against ESCC, and planned the present study accordingly.
Among our findings, toxicity profiles during chemotherapy were comparable to safety data from other phase III trials with locally advanced SCCHN.5, 6 As expected, DCF induced severe neutropenia but did not lead to an increased frequency of complications. This might have been due to the prophylactic use of antibiotic therapy during chemotherapy. Prophylactic use of fluoroquinolone is widely carried out if patients appear to be suffering from intensive chemotherapy.9, 10 Although non‐hematological adverse events were mainly gastrointestinal toxicities such as anorexia, stomatitis, and nausea, these were comparable to adverse events reported in patients treated with preoperative CF in the JCOG 9907 study. Although the incidence of postoperative complications was slightly higher than that in JCOG 9907, they were considered to be clearly acceptable, suggesting that three cycles of DCF would not compromise surgical intervention.
Although efficacy was not a primary end‐point of the current study, antitumor activity was highly promising. The pCR rate was 17%, which is markedly high compared with 5% reported for preoperative CF in JCOG 9907. This considerable activity may be the result of not only the addition of docetaxel, but also the high dose intensity of this combination regimen. In JCOG 9907, preoperative and postoperative adjuvant chemotherapy consisted of only two cycles of CF. However, induction chemotherapy with DCF in locally advanced SCCHN usually consists of three or four cycles. We therefore allowed this DCF regimen a maximum of three cycles, and specified that protocol treatment should be delivered for at least two cycles unless disease progression was observed. Another phase II trial reported preoperative chemotherapy with DCF for locally advanced ESCC;11 although patient backgrounds were closely similar to those in our present trial, the regimen consisted of docetaxel 60 mg/m2 and cisplatin 60 mg/m2 on day 1, and 5‐FU 800 mg/m2/day continuously on days 1–5 every 4 weeks, for only two cycles. Although this regimen was feasible and the overall response rate was 62.5%, the pCR rate was only 4%. Our treatment schedule consisted of a higher dose intensity and more chemotherapy cycles than this schedule, which may have led to its high pCR rate.
With a median follow‐up time of 27 months, 2‐year PFS was 75%. Even allowing for this relatively short duration, the 3‐year PFS rate of 50% from historical data in JCOG 9907 suggests that combination chemotherapy with DCF is a promising preoperative regimen for ESCC.
At the time the present study was planned, no study had yet reported the feasibility and safety of combination therapy with DCF as preoperative chemotherapy in the treatment of ESCC. Although conventional DCF regimens in SCCHN and AGC with docetaxel and cisplatin doses over 70 mg/m2 had been reported, 70 mg/m2 is the upper regulatory limit for both docetaxel and cisplatin for the treatment of esophageal carcinoma in Japan, and we were accordingly obliged to start the present trial at no higher than the approved dose. Furthermore, in the TAX 323 trial in locally advanced SCCHN, eligibility criteria were limited to age less than 70 years and PS of 0–1.5
Following the completion of treatment in the first cohort, we considered that combination chemotherapy with DCF (70/70/750) was well tolerated in the preoperative setting in patients with ESCC aged 70 years and younger. We then planned a protocol amendment to examine the feasibility of parent DCF (both docetaxel and cisplatin at a dose of 75 mg/m2) at age 75 years and younger on the basis that eligibility criteria regarding age in our next phase III trial will be the same as in JCOG 9907, a previous phase III trial.
In the expansion cohort, no increase was seen in the incidence of toxicity during chemotherapy or in that of morbidity and mortality after surgery. Furthermore, little difference was seen in antitumor effect between the two cohorts (data not shown).
Recently, the results of a phase II trial of perioperative chemotherapy with DCF for esophageal and gastric adenocarcinoma were reported. The treatment schedule consisted of DCF (75/75/750) every 3 weeks for three cycles before and after surgery.12 In contrast to our study, prophylactic use of G‐CSF was mandatory for all cycles of chemotherapy. Of 43 patients enrolled, 41 (95%) and 37 (87%) completed the second and third preoperative cycles, respectively. Surgery was carried out in 41 patients, consisting of transthoracic esophagectomy in 30 (73%). The pathological CR rate was 10% (4/41). Although several differences in treatment schedule or histologic background between this previous and our present study were noted, these two studies suggest that perioperative DCF, particularly preoperative DCF, is a well‐tolerated regimen for the treatment of esophageal carcinoma.
Despite recent advances in chemotherapy and extended surgery, treatment outcomes in esophageal cancer have remained markedly poor. This failure to improve survival has resulted in a multimodal treatment approach to patients with resectable esophageal cancer. To date, two large phase III trials have compared preoperative chemotherapy with CF with surgery alone, but reached completely different conclusions, and the efficacy of preoperative chemotherapy against esophageal cancer is still under debate.13, 14 On the basis of the results of JCOG 9907, preoperative chemotherapy with CF is widely used in Japan. In other countries, in contrast, preoperative chemoradiotherapy is standard treatment, particularly in Western countries, on the basis of several recent meta‐analyses that showed a survival benefit in patients with both squamous cell carcinoma and adenocarcinoma.15, 16 However, the locoregional recurrence rate was only 25% among all relapsed patients who received preoperative CF in JCOG 9907. This result was considered to be due to the intensive three‐field lymphadenectomy used in Japan. As the R0 resection rate in JCOG 9907 was more than 90%, further reinforcement of the surgical approach will be difficult, and we expect that ongoing development of systemic preoperative chemotherapy offers a greater opportunity to produce an overall improvement in treatment outcomes than preoperative chemoradiotherapy. Although the reports included in the recent meta‐analyses reported improvements in postoperative complications,15, 16 it is still commonly thought that mortality and morbidity are higher for preoperative chemoradiotherapy than for surgery alone.17, 18 Stahl et al.19 reported preoperative chemoradiotherapy compared with chemotherapy for locally advanced adenocarcinoma of esophagogastric cancer. Although this trial was closed early due to poor accrual and statistical significance was not achieved, 3‐year survival favored the chemoradiotherapy arm (47.4% vs 27.7%). However, hospital mortality was increased by adding preoperative radiotherapy (10.2% vs 3.8%, P = 0.26). A conclusive answer to this question will require a direct comparison of preoperative chemotherapy and chemoradiotherapy. The need to address this clinical question is particularly urgent in regions where squamous cell carcinoma is predominant.
In conclusion, we found that preoperative DCF was well tolerated and feasible in patients with resectable ESCC. As antitumor activity was highly promising, a three‐arm randomized phase III trial is in progress comparing CF, DCF, and chemoradiotherapy with CF as preoperative therapy for locally advanced ESCC.20 We are confident that the results of this confirmatory phase III trial will be clinically significant and valuable, and will provide an answer to the controversy surrounding preoperative chemotherapy versus chemoradiotherapy.
Disclosure Statement
M.T. has received honoraria from Merck Serono Co. Ltd. and Bristol‐Myers K.K., and research funding from Eisai Co. Ltd., Boehringer Ingelheim Co. Ltd., and Yakult Honsha Co. Ltd.; K.K. has received research funding from Daiichi‐Sankyo Co. Ltd., and Ohtsuka Pharmaceutical Co. Ltd. This work was supported by the Foundation for Promotion of Cancer Research in Japan.
Acknowledgments
The authors would like to thank the patients and families who participated in this study. They also wish to acknowledge the support of their colleagues. This article is an original report that was presented in part at the 47th American Society of Clinical Oncology Annual Meeting June 3–7, 2011, Chicago, IL, USA.
(Cancer Sci 2013; 104: 1455–1460)
References
- 1. Tepper J, Krasna MJ, Niedzwiecki D et al Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008; 26: 1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Hagen P, Hulshof MC, van Lanschot JJ et al Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–84. [DOI] [PubMed] [Google Scholar]
- 3. Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol 2007; 17: 38–44. [DOI] [PubMed] [Google Scholar]
- 4. Ando N, Kato H, Igaki H et al A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5‐fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012; 19: 68–74. [DOI] [PubMed] [Google Scholar]
- 5. Vermorken JB, Remenar E, van Herpen C et al Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 2007; 357: 1695–704. [DOI] [PubMed] [Google Scholar]
- 6. Posner MR, Hershock DM, Blajman CR et al Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007; 357: 1705–15. [DOI] [PubMed] [Google Scholar]
- 7. Van Cutsem E, Moiseyenko VM, Tjulandin S et al Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first‐line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006; 24: 4991–7. [DOI] [PubMed] [Google Scholar]
- 8. Japanese Society for Esophageal Diseases . Guidelines for clinical and pathologic studies on carcinoma of the esophagus ninth edition: part II. Esophagus 2004; 1: 107–25. [Google Scholar]
- 9. Flowers CR, Seidenfeld J, Bow EJ et al Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2013; 31: 794–810. [DOI] [PubMed] [Google Scholar]
- 10. Cullen M, Steven N, Billingham L et al Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. N Engl J Med 2005; 353: 988–98. [DOI] [PubMed] [Google Scholar]
- 11. Ura T, Nagase M, Fujii S et al Feasibility study of preoperative docetaxel (D), cisplatin (C), and fluorouracil (F) in esophageal cancer. J Clin Oncol (Meeting Abstracts in ASCO) 2010. (Abstr 81). [Google Scholar]
- 12. Ferri LE, Ades S, Alcindor T et al Perioperative docetaxel, cisplatin, and 5‐fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol 2012; 23: 1512–7. [DOI] [PubMed] [Google Scholar]
- 13. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long‐term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009; 27: 5062–7. [DOI] [PubMed] [Google Scholar]
- 14. Kelsen DP, Winter KA, Gunderson LL et al Long‐term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol 2007; 25: 3719–25. [DOI] [PubMed] [Google Scholar]
- 15. Sjoquist KM, Burmeister BH, Smithers BM et al Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta‐analysis. Lancet Oncol 2011; 12: 681–92. [DOI] [PubMed] [Google Scholar]
- 16. Kranzfelder M, Schuster T, Geinitz H, Friess H, Buchler P. Meta‐analysis of neoadjuvant treatment modalities and definitive non‐surgical therapy for oesophageal squamous cell cancer. Br J Surg 2011; 98: 768–83. [DOI] [PubMed] [Google Scholar]
- 17. Kaklamanos IG, Walker GR, Ferry K, Franceschi D, Livingstone AS. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta‐analysis of randomized clinical trials. Ann Surg Oncol 2003; 10: 754–61. [DOI] [PubMed] [Google Scholar]
- 18. Fiorica F, Di Bona D, Schepis F et al Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta‐analysis. Gut 2004; 53: 925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stahl M, Walz MK, Stuschke M et al Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009; 27: 851–6. [DOI] [PubMed] [Google Scholar]
- 20. Nakamura K, Kato K, Igaki H et al Three‐arm Phase III Trial Comparing Cisplatin Plus 5‐FU (CF) Versus Docetaxel, Cisplatin Plus 5‐FU (DCF) Versus Radiotherapy with CF (CF‐RT) as Preoperative Therapy for Locally Advanced Esophageal Cancer (JCOG1109, NExT Study). Jpn J Clin Oncol 2013; 43: 752–5. [DOI] [PubMed] [Google Scholar]


