Abstract
Cancer cells require glucose to support their rapid growth through a process known as aerobic glycolysis, or the Warburg effect. As in ovarian cancer cells, increased metabolic activity and glucose concentration has been linked to aggressiveness of cancer. However, it is unclear as to whether targeting the glycolytic pathway may kill the malignant cells and likely have broad therapeutic implications against ovarian cancer metastasis. In the present research, we found that EF24, a HIF‐1α inhibitor, could significantly block glucose uptake, the rate of glycolysis, and lactate production compared with vehicle treatment in SKOV‐3, A2780 and OVCAR‐3 cells. These results might possibly contribute to the further observation that EF24 could inhibit ovarian cancer cell migration and invasion from wound healing and Transwell assays. Furthermore, as an important mediator of glucose metabolism, glucose transporter 1 (Glut1) was found to contribute to the function of EF24 in both energy metabolism and metastasis. To examine the effect of EF24 and the mediated role of Glut1 in vivo in a xenograph subcutaneous tumor model, intraperitoneal metastasis and lung metastasis model were introduced. Our results indicated that EF24 treatment could inhibit tumor growth, intraperitoneal metastasis and lung metastasis of SKOV‐3 cells, and Glut1 is a possible mediator for the role of EF24. In conclusion, our results highlight that an anti‐cancer reagent with an inhibiting effect on energy metabolism could inhibit metastasis, and EF24 is a possible candidate for anti‐metastasis therapeutic applications for ovarian cancer.
We report in this study the inhibition effect of EF24 against the energy metabolism in ovarian cancer cells and tumors through the down‐regulation of Glut1 expression. This effect may further lead to the inhibition of cell migration, invasion and tumor metastasis. Therefore, EF24 is likely a possible candidate for anti‐metastasis therapeutic applications for ovarian cancer.
Ovarian cancer incidence rates vary widely across the globe.1 Current treatment includes surgical resection (debulking), followed by multi‐agent chemotherapy, usually involving i.v. or i.p. injection of platin compounds or taxane. However, metastatic ovarian cancer carries such a poor prognosis that systemic therapy with cytotoxic agents provides almost no benefit. Thus, improving survival for patients with metastatic ovarian cancer requires development of effective systemic therapy.
Whereas normal cells use mitochondrial respiration to provide energy, the majority of tumor cells use aerobic glycolysis, also known as the Warburg effect.2 Recent studies strongly suggest that the Warburg effect is a key contributor to malignant progression and is a potential therapeutic target.3 Numerous studies demonstrate that glucose transporter 1 (Glut1) is a rate‐limiting transporter for glucose uptake, and its expression correlates with anaerobic glycolysis.4, 5 Elevated Glut1 expression is observed in most ovarian cancer cell lines, which is not the case with primary ovarian cells, and this elevation has also be found in a subset of ovarian cancer patients.6 Moreover, it is demonstrated that suppression of Glut1 expression by siRNA significantly impairs the tumorigenicity of ovarian cancer cells.7 Together, this evidence indicates that derivatives of the Glut1 inhibitors might generate a therapeutic effect in ovarian cancer.
Diphenyl difluoroketone (EF24), a molecule with structural similarity curcumin, is reported to significantly inhibit proliferation of various cancer cells.8 Thomas et al.9 find that treatment of MDA‐MB231 breast and PC3 prostate cancer cells with EF24 can lead to inhibition of HIF‐1α transcriptional activity. In addition, previous reports show that the oncogenic factor HIF‐1α is implicated in regulating aerobic glycolysis and regulating the expression of Glut1.10 Together, these results support targeting Glut1 as a site for chemotherapeutic intervention using EF24.
Materials and Methods
Cell culture
A2780 cells were obtained from the European Collection of Cell Cultures (ECACC). SKOV‐3 and OVCAR‐3 cells were obtained from the American Type Culture Collection (ATCC; LGC Promochem, Manassas, VA, USA). All cell lines were cultured in DMEM supplemented with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin.
Reagents
EF24 was purchased from Sigma (St. Louis, MO, USA). DMSO was added to culture at 0.1% (v/v) as a solvent control. Monoclonal anti‐GLUT1 and anti‐β‐actin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Measurement of glucose uptake and the rate of glycolysis
Glucose uptake and the rate of glycolysis were measured by determining the uptake of 2‐[3H] deoxyglucose by cells and the conversion of 5‐[3H] glucose to 3H2O, respectively, as described previously.11, 12, 13, 14
Measurement of lactate production
Lactate levels in the culture media of cells were determined by using a Lactate Assay Kit (Biovision, Mountain View, CA, USA). Cells (1 × 105) were seeded onto 12‐well plates for 12 h and then replenished with fresh phenol red‐free medium (Sigma). Cells were then incubated for 16–24 h and the culture medium was collected for measurement of lactate concentrations. Lactate levels were determined using the Lactate Assay Kit and normalized with cell number.
Migration and invasion assays
Cell migration assays were performed in the BD Falcon 24‐multiwell insert system (BD Biosciences, San Jose, CA, USA), as previously described.15 For the Matrigel invasion assay, filters were precoated with 30 μL Matrigel (BD Biosciences) for 3 h. Culture medium containing 10% FBS was added to lower chambers and aliquots of 1–3 × 104 cells in 300 μL of FBS‐free medium were seeded into upper chambers. Cells on the lower surface of the membrane were fixed with ice‐cold methanol and stained with crystal violet. Cell numbers were counted under an optical microscope (×100). Each experiment was repeated at least three times.
Subcutaneous xenograph experiments
All mice were obtained from the laboratory animal center of the Chinese academy of sciences, Shanghai. The experimental protocol was reviewed and approved by the Committee on the Use of Live Animals in Teaching and Research of the Harbin Medical University, Harbin, China (SYSK 2010‐012). Tumor size was measured twice weekly using a digital caliper. The tumor volume was calculated using the formula: length × (width)2 × 0.52 and subsequently transformed into relative values (V; V = V t/V 0, where V 0 is the tumor volume at initiation of treatment, whereas V t is the tumor volume at any given day during entire treatment period).
Immunofluorescence assay
SKOV‐3 cells were washed and fixed with 3% paraformaldehyde or 100% methanol and permeabilized in 0.1% Triton X‐100. Incubation with monoclonal mouse anti‐Glut1 antibody (Santa Cruz Biotechnology) for 30 min was followed by incubation with FITC‐labeled anti‐mouse IgG secondary antibody (Santa Cruz Biotechnology). Immunostained cells were photographed under an inverted fluorescence microscope (×400).
Intraperitoneally metastasis assay
Five‐week‐old female nude (BALB/c) mice were injected i.p. with SKOV‐3 control shRNA or SKOV‐3 Glut1 shRNA cells for examination of their peritoneal metastatic potential.16 In brief, the cells (5 × 106) were isolated and suspended in Hanks balanced salt solution for injection in a volume of 0.1 mL with a 30‐gauge needle. The mice (n = 6/group) were injected i.p. with vehicles or EF24 (10 mg/kg/day) treatment five times per week beginning on the day of implantation. The number and extent of overt metastases were then quantified 35 days after cell injection.
Lung metastasis assay
SKOV‐3 shControl cells and SKOV‐3 shGlut1 cells (1 × 106/0.2 mL) were injected into nude mice via tail veins to imitate tumor metastasis. Experimental animals (n = 6/group) received vehicles (normal saline containing 1% DMSO) or EF24 (10 mg/kg/day) i.p. injection five times per week beginning on the day of implantation. The mice were killed 4 weeks after the inoculation and lungs were removed and fixed in formaldehyde.
Cell viability assays
SKOV‐3, A2780 and OVCAR‐3 cells were cultured in 96‐well plates, and EF24 was administrated with increasing doses of 0, 0.5, 1, 2, 4 and 8 μM for 48 and 72 h. DMSO was added as a solvent control with increasing concentrations of 0, 0.017, 0.033, 0.067, 0.133 and 0.267% (v/v). Cell viability was examined by MTT assay, as described previously.17
Real‐time PCR, western blot analysis
TaqMan real‐time PCR was performed as described previously.18, 19
Statistical analysis
Groups were compared using a two‐tailed Student's t‐test (Prism4; GraphPad Software, La Jolla, CA, USA) and P < 0.05 was considered significant.
Results
EF24 treatment attenuates ovarian cancer cell proliferation and metastasis
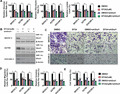
Our data for the MTT assay presented in Figure 1(a) indicate that EF24 significantly suppressed proliferation of SKOV‐3, A2780 and OVCAR‐3 cells within a 48‐h period, which continues to 72 h. To determine the effect of EF24 on migration of ovarian cancer cells, SKOV‐3, A2780 and OVCAR‐3 cells were treated with vehicle (1% DMSO) or 3 μM EF24 for 48 h. Then the treated cells were wounded by scratching and maintained for an additional 24 h. As shown in Figure S1(a,b), strong flattening and spreading of SKOV‐3, A2780 and OVCAR‐3 cells were observed in the group of vehicle treatment, whereas the treatment of EF24 attenuated the flattening and spreading of SKOV‐3, A2780 and OVCAR‐3 cells. Migration and invasion were examined next, using Transwell assays. Again, the migration ability of SKOV‐3, A2780 and OVCAR‐3 cells was hindered by treatment of 3 μM EF24 (Fig. 1b,c). Furthermore, the Matrigel invasion assay showed that 3 μM EF24 decreased the number of cells penetrating through Matrigel to approximately 45% compared with the control group (Fig. S1c). To evaluate the change in oxidative phosphorylation when glucose uptake was restricted, total ATP level were determined after 3 μM EF24 treatment for 48 h. As shown in Figure S3(a), decreased ATP level was observed after 3 μM EF24 treatment. However, the extent of decrease was much less than the reduction of glycolysis. Moreover, alteration of energy metabolism often links to change of oxidative stress and the decrease of cell viability might be affected by oxidative stress. Accordingly, the ROS levels were determined. As shown in Figure S3(b), significantly elevated levels of ROS were observed after 3 μM EF24 treatment for 48 h. These results are consistent with Brain K. Adams's finding that EF24 can increase the ROS level and induce apoptosis in cancer cells through a redox‐dependent mechanism.20
Figure 1.
EF24 treatment attenuates ovarian cancer cell proliferation, metastasis and glucose metabolism. (a) SKOV‐3, A2780 and OVCAR‐3 cells were incubated with EF24 in various concentrations. *P < 0.05, compared with cells treated with DMSO for 48 h; #P < 0.05, compared with cells treated with DMSO for 72 h. (b) Representative images of the migration assay in SKOV‐3, A2780 and OVCAR‐3 cells after treatment of 3 μM EF24. (c) Relative changes of migration cell number in SKOV‐3, A2780 and OVCAR‐3 cells after 3 μM EF24 treatment. (d) Relative changes of glucose uptake, glycolysis and lactate production in SKOV‐3, A2780 and OVCAR‐3 cells after treatment of 3 μM EF24. *P < 0.01.
EF24 treatment blocked glucose metabolism of ovarian cancer cells in vitro
Using SKOV‐3, A2780 and OVCAR‐3 cells, we analyzed the regulation effect of EF24 on the Warburg effect, which is characterized by higher glucose uptake, a higher rate of glycolysis, and higher lactate production in cancer cells than in normal cells.13 As shown in Figure 1(c), 3 μM EF24 treatment significantly decreased glucose uptake, the rate of glycolysis and lactate production compared with the vehicle treatment in SKOV‐3, A2780 and OVCAR‐3 cells. These results indicate that EF24 is an effective inhibitor against aerobic glycolysis.
EF24 treatment attenuates glucose transporter 1 expression in ovarian cancer cells
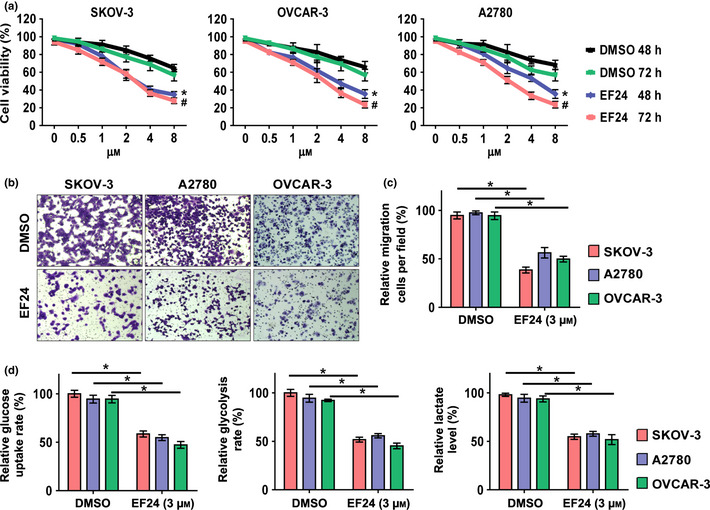
Considering the important role of Glut1 in regulating glucose metabolism,16, 20 it is useful to examine whether EF24 targets Glut1 for its anti‐metabolism effect. As shown in Figure 2(a), EF24 treatment significantly downregulates the basal protein level of Glut1 in a dose‐dependent manner in SKOV‐3, A2780 and OVCAR‐3 cells. Hypoxia condition of 1% O2 was induced, which is reported to induce the expression of HIF‐1α and its target gene Glut1.10 As shown in Figure 2(b), hypoxia condition markedly increased the protein level of Glut1 in cells treated with the vehicle. However, no significant induction of Glut1 expression was observed in EF24 (3 μM) treatment cells. Immunofluoresence staining was also performed. Notably, EF24 (3 μM) treatment could attenuate the expression of Glut1 in both basal and induction levels (Fig. 2c). Then, when the mRNA level of Glut1 was evaluated, no significant change was observed, which indicated that EF24 performs the regulation of Glut1 in a post‐transcriptional manner (Fig. 2d).
Figure 2.
EF24 treatment attenuates Glut1 expression in ovarian cancer cells. (a) The basal level of Glut1 protein expression could be downregulated in SKOV‐3, A2780 and OVCAR‐3 cells after EF24 treatment. (b) The induction of Glut1 expression under hypoxia condition could be blocked by EF24 treatment. (c) The basal and induction level of Glut1 could be inhibited in immunofluoresence staining assay. (d) The mRNA level of Glut1 was examined by real‐time PCR.
Glucose transporter 1 is responsible for EF24‐mediated inhibition of glucose metabolism, metastasis and proliferation in ovarian cancer cells
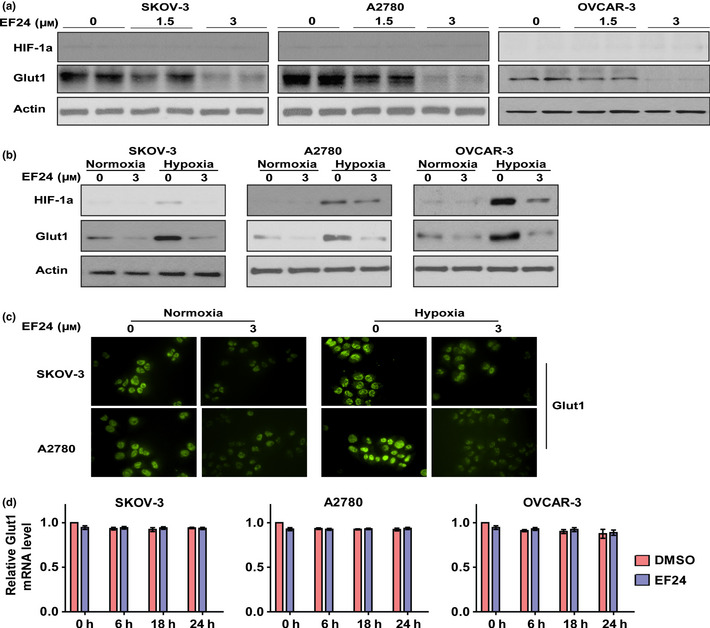
To determine whether Glut1 contributes to the function of EF24 in regulating glucose metabolism, metastasis and proliferation, the expression of Glut1 was knocked down using shRNA. As shown in Figure 3(a), Glut1 knockdown significantly impaired glucose uptake, the rate of glycolysis and lactate production in SKOV‐3, A2780 and OVCAR‐3 cells. Knockdown of Glut1 24 h prior to EF24 (3 μM) treatment resulted in lower glucose uptake, rate of glycolysis and lactate production compared with individual treatment; however, the effects were less than additive. These results indicate that Glut1 is an important mediator of EF24 in regulating glucose metabolism. To evaluate the role of Glut1 in regulating ovarian cancer metastasis, Glut1 was overexpressed or knocked down in cells (Figs 3b,S2c). As shown in Figure S2(a,b), Glut1 overexpression increased SKOV‐3, A2780 and OVCAR‐3 cell migration; however, Glut1 knockdown significantly reduced migration in SKOV‐3, A2780 and OVCAR‐3 cells (Fig. 3c). Treatment using 3 μM EF24 after Glut1 knockdown resulted in higher inhibition of cell proliferation and migration than individual treatment (Fig. 3d,e), but the effect is less than additive. These results suggest that Glut1 is an important mediator for EF24 in regulating cell proliferation and migration.
Figure 3.
Glut 1 is responsible for EF24‐mediated induction of glucose metabolism, metastasis and proliferation in ovarian cancer cells. (a) Relative changes of glucose uptake, glycolysis and lactate production after the treatment of EF24, Glut1 knockdown and combination in SKOV‐3, A2780 and OVCAR‐3 cells. (b) The protein level of Glut1 after the treatment of EF24, Glut1 knockdown and combination. (c) Representative images of the migration assay in SKOV‐3, A2780 and OVCAR‐3 cells after treatment of EF24, Glut1 knockdown and combination. (d) Relative changes of migration cell number in SKOV‐3, A2780 and OVCAR‐3 cells after treatment of EF24, Glut1 knockdown and combination. (e) Relative cell proliferation rate in SKOV‐3, A2780 and OVCAR‐3 cells after treatment of EF24, Glut1 knockdown and combination. *P < 0.01.
Glucose transporter 1 contributes to the role of EF24 in inhibiting xenograph tumor growth and metastasis
To examine whether Glut1 mediates the effect of EF24 in an in vivo xenograph tumor model, nude mice were established using SKOV‐3 cells transfected with control shRNA or Glut1 shRNA. As shown in Figure 4(a,b), the tumors of SKOV‐3 shControl cells were significantly much smaller after the treatment of EF24 than the treatment of vehicle. EF24 treatment could also decrease the size of tumors of SKOV‐3 shGlut1 cells compared with the vehicle treatment. However, the inhibition effect was much smaller. The Glut1 protein levels are shown in Figure 4(c). These results indicate that Glut1 is a possible mediator for the role of EF24 in inhibiting xenograph tumor growth. In addition, we further examined the therapeutic effect of EF24 against metastasis in vivo and the mediate role of Glut1. Peritoneal metastasis model was introduced.16 SKOV‐3 shControl and shGlut1 cells were injected i.p. into nude mice, which were then treated with EF24 or vehicle. For the SKOV‐3 shControl tumors, vehicle treatment mice manifested several disseminated and enlarged metastatic nodules throughout the peritoneal cavity, including the diaphragm, intestine and mesentery. However, the number of metastatic nodules was significantly reduced in mice treated with EF24. In contrast, a smaller number of metastatic nodules were found in the mice injected with SKOV‐3 shGlut1 cells, and EF24 treatment caused attenuated therapeutic effect compared with the SKOV‐3 shControl group (Fig. 4d,e). We also established peripheral intravascular implanted metastatic models by injecting SKOV‐3 shControl or SKOV‐3 shGlut1cells into nude mice through the tail vein. The same result was observed with Glut1 knockdown: EF24 treatment caused an attenuated therapeutic effect by blocking lung metastasis of SKOV‐3 cells compared with cells without Glut1 knockdown (Fig. 4f). These results thus suggest that EF24 would be a potent reagent against ovarian cancer tumor metastasis and Glut1 is a possible mediator of EF24.
Figure 4.
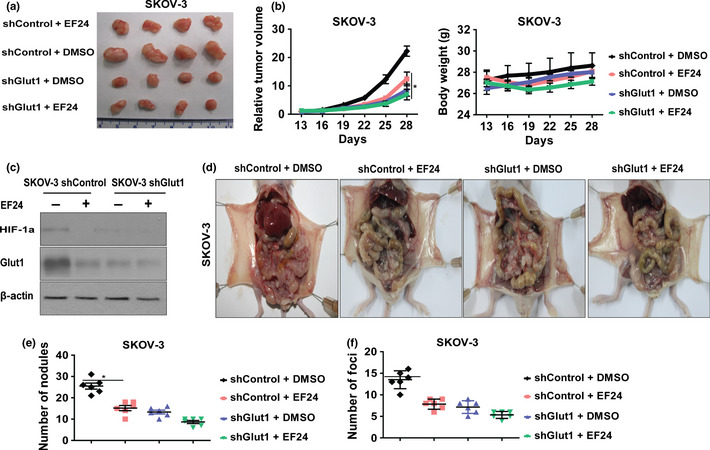
Glut1 contributes to the role of EF24 in inhibiting xenograft tumor growth and metastasis. (a) Representative SKOV‐3 shControl and SKOV‐3 shGlut1 xenograph tumor images at the end of the treatment of vehicle and EF24 (10 mg/kg/day). (b) Volume of xenograph tumors and the body weight were determined at different time points. (c) The protein level of Glut1 in each group of xenograph tumors. (d) Representative SKOV‐3 shControl and SKOV‐3 shGlut1 intraperitoneal metastasis images after the treatment of EF24. (e) The average number of intraperitoneal metastasis foci per mouse was calculated. (f) The average number of lung metastasis foci per mouse was calculated. *P < 0.01.
Discussion
The Warburg effect is defined by an increased utilization of glucose via glycolysis as a cellular resource, and is a common phenotype of cancerous cells. In addition to the dependency on glycolysis, cancer cells exhibit other metabolic characteristics such as glutamine metabolism and fumarate respiration. Apart from glucose, glutamine levels are found to be high in all tumor cells. Glutaminolysis is an energy‐producing pathway. Tumor cells are known to overexpress mitochondrial phosphate‐dependent and phosphate‐independent glutaminase and NAD(P)‐dependent malate decarboxylase, which leads to degradation of glutamine to glutamate and ammonia, which triggers autophagy and provides extra energy to survive in glucose deprived conditions. Glutamate is further oxidized to α‐ketoglutarate, which can enter the citric acid cycle to produce ATP. In addition to glutamine metabolism, Soga et al. provide evidence that the energy generation pathway of cancer, especially under hypoxic and nutrient‐deprived conditions, relies on fumarate respiration. Fumarate respiration confers upon cells the ability to generate ATP by converting fumarate to succinate, instead of oxygen to water, as the final electron transport step in the reverse reaction of succinate dehydrogenase. Succinate, the end‐product of fumarate respiration, was found to be markedly higher, as were fumarate and malate levels, in colon, lung and prostate tumor tissues than in their corresponding normal tissues. Therefore, high concentrations of these metabolites in tumors might be attributed to upregulation of fumarate respiration, which would facilitate tumor growth and proliferation, even under hypovascular microenvironment. Renewed interest in cancer metabolism has generated hope that a new class of therapeutic agents may finally appear on the stage for cancer therapy.
Previous research has shown that EF24, a molecule with structural similarity to curcumin, can inhibit proliferation of a variety of cancer cells with 10‐fold greater potency than curcumin in cell death induction.9 In the present research, our results demonstrated that EF24 could inhibit not only the proliferation but also the invasion and migration of ovarian cancer cells.
More important, our results provided evidence to indicate that EF24 treatment could reverse the Warburg effect in ovarian cancer cells. As in ovarian cancer cells, increased metabolic activity and glucose concentration has been linked to aggressiveness of cancer.22 Thus, targeting the glycolytic pathway may kill the malignant cells and likely have broad therapeutic implications. In the present research, significantly decreased glucose uptake, the rate of glycolysis, and lactate production were observed after 3 μM EF24 treatment. These results might contribute to the observation that EF24 could inhibit the proliferation, invasion and metastasis of ovarian cancer cells. The reason may be that the more aggressive tumors, which are likely to have a greater demand for metabolic energy and, hence, for glucose, frequently exhibit this metabolic alteration and reveal an increasing dependency on the glycolytic pathway. The increased glycolysis results in acute and chronic acidification of the local environment through the conversion of pyruvate to lactic acid. This microenvironmental acidosis leads to invasion and metastasis through inhibition of gap‐junction conductance, and, probably, activation of metalloproteinases promoting the degration of the extracellular matrix and basement membrane.23
In addition, numerous studies have demonstrated that Glut1 is the rate‐limiting factor for anaerobic glycolysis in various cancers, including ovarian cancer.24 The results of one study on 94 patients suffering from malignant or benign ovarian tumors showed that the expression of Glut1 was positive in invasive ovarian carcinomas, moderate in borderline tumors and weak to negative in all benign ovarian neoplasms, indicating Glut1 as a diagnostic tool and therapeutic target in ovarian cancer.25 In our in vitro and xenograph tumor research, we found that as an important mediator of glucose metabolism, Glut1 contributes to the function of EF24 in both energy metabolism and metastasis. However, it is important to realize that each cell may have different basal levels of Glut1 and respond differently to EF24 treatment. In our experiment, A2780 and SKOV‐3 cells had higher Glut1 levels than OVCAR‐3 cells and 1.5 µM EF24 treatment led to a significant decrease of Glut1 expression, which was not so clear in SKOV‐3 and OVCAR‐3 cells. However, when 3 µM EF24 was introduced, the expression of Glut1 was reduced so significantly that it was barely detectable in all three ovarian cancer cells, indicating that 3 µM EF24 was able to block almost all Glut1 expression.
In conclusion, we reported in this study that the inhibitory effects of EF24 on the energy metabolism in ovarian cancer cells through downregulation of Glut1 expression. This effect may further lead to the inhibition of cell migration, invasion and tumor metastasis. Therefore, EF24 is a possible candidate for anti‐metastasis therapeutic applications for ovarian cancer.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. EF24 treatment blocked ovarian cancers motility and invasion.
Fig. S2. Glut1 over‐expression increase ovarian cancer cells metastasis.
Fig. S3. Decreased ATP levels and increased ROS levels in ovarian cancer cells were observed after EF24 treatment.
Data S1. Glut1 mediated the function of EF24 in inhibiting ovarian cancer cell metastasis.
Acknowledgments
We acknowledge the excellent technical assistance of Dr Yingjian Liang in in vitro studies. This work was supported by the Heilongjiang Province Youth Fund (QC08C79). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
(Cancer Sci 2013; 104: 1690–1696)
References
- 1. Lowe KA, Chia VM, Taylor A et al An international assessment of ovarian cancer incidence and mortality. Gynecol Oncol 2013; 130: 107–14. [DOI] [PubMed] [Google Scholar]
- 2. Warburg O. On the origin of cancer cells. Science 1956; 123: 309–14. [DOI] [PubMed] [Google Scholar]
- 3. Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene 2006; 25: 4633–46. [DOI] [PubMed] [Google Scholar]
- 4. Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther 2009; 121: 29–40. [DOI] [PubMed] [Google Scholar]
- 5. Osthus RC, Shim H, Kim S et al Deregulation of glucose transporter 1 and glycolytic gene expression by c‐Myc. J Biol Chem 2000; 275: 21797–800. [DOI] [PubMed] [Google Scholar]
- 6. Kato M, Yamamoto S, Takano M, Matsubara O, Furuya K. Aberrant expression of the mammalian target of rapamycin, hypoxia‐inducible factor‐1alpha, and glucose transporter 1 in the development of ovarian clear‐cell adenocarcinoma. Int J Gynecol Pathol 2012; 31: 254–63. [DOI] [PubMed] [Google Scholar]
- 7. Amann T, Hellerbrand C. GLUT1 as a therapeutic target in hepatocellular carcinoma. Expert Opin Ther Targets 2009; 13: 1411–27. [DOI] [PubMed] [Google Scholar]
- 8. Sun A, Shoji M, Lu YJ, Liotta DC, Snyder JP. Synthesis of EF24‐tripeptide chloromethyl ketone: a novel curcumin‐related anticancer drug delivery system. J Med Chem 2006; 49: 3153–8. [DOI] [PubMed] [Google Scholar]
- 9. Thomas SL, Zhong D, Zhou W et al EF24, a novel curcumin analog, disrupts the microtubule cytoskeleton and inhibits HIF‐1. Cell Cycle 2008; 7: 2409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Airley RE, Mobasheri A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics. Chemotherapy 2007; 53: 233–56. [DOI] [PubMed] [Google Scholar]
- 11. Koivisto UM, Martinez‐Valdez H, Bilan PJ, Burdett E, Ramlal T, Klip A. Differential regulation of the GLUT‐1 and GLUT‐4 glucose transport systems by glucose and insulin in L6 muscle cells in culture. J Biol Chem 1991; 266: 2615–21. [PubMed] [Google Scholar]
- 12. Christofk HR, Vander Heiden MG, Harris MH et al The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008; 452: 230–3. [DOI] [PubMed] [Google Scholar]
- 13. Bensaad K, Tsuruta A, Selak MA et al TIGAR, a p53‐inducible regulator of glycolysis and apoptosis. Cell 2006; 126: 107–20. [DOI] [PubMed] [Google Scholar]
- 14. Gravells P, Hoh L, Canovas D, Rennie IG, Sisley K, Bryant HE. Resistance of uveal melanoma to the interstrand cross‐linking agent mitomycin C is associated with reduced expression of CYP450R. Br J Cancer 2011; 104: 1098–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Reilly N, Gallagher C, Reddy Katikireddy K, Clynes M, O'Sullivan F, Kavanagh K. Demodex‐associated Bacillus proteins induce an aberrant wound healing response in a corneal epithelial cell line: possible implications for corneal ulcer formation in ocular rosacea. Invest Ophthalmol Vis Sci 2012; 53: 3250–9. [DOI] [PubMed] [Google Scholar]
- 16. Ponnusamy MP, Lakshmanan I, Jain M et al MUC4 mucin‐induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells. Oncogene 2010; 29: 5741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lai JP, Chien JR, Moser DR et al hSulf1 Sulfatase promotes apoptosis of hepatocellular cancer cells by decreasing heparin‐binding growth factor signaling. Gastroenterology 2004; 126: 231–48. [DOI] [PubMed] [Google Scholar]
- 18. Alpini G, Glaser SS, Zhang JP et al Regulation of placenta growth factor by microRNA‐125b in hepatocellular cancer. J Hepatol 2011; 55: 1339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Nat Acad Sci U S A 2010; 107: 7455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adams BK, Cai J, Armstrong J et al EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox‐dependent mechanism. Anticancer Drugs. 2005; 16: 263–75. [DOI] [PubMed] [Google Scholar]
- 21. Martin HJ, Kornmann F, Fuhrmann GF. The inhibitory effects of flavonoids and antiestrogens on the Glut1 glucose transporter in human erythrocytes. Chem Biol Interact 2003; 146: 225–35. [DOI] [PubMed] [Google Scholar]
- 22. Fabian C, Koetz L, Favaro E, Indraccolo S, Mueller‐Klieser W, Sattler UG. Protein profiles in human ovarian cancer cell lines correspond to their metabolic activity and to metabolic profiles of respective tumor xenografts. FEBS J 2012; 279: 882–91. [DOI] [PubMed] [Google Scholar]
- 23. Stern R, Shuster S, Neudecker BA, Formby B. Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp Cell Res 2002; 276: 24–31. [DOI] [PubMed] [Google Scholar]
- 24. Amann T, Maegdefrau U, Hartmann A et al GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol 2009; 174: 1544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rudlowski C, Moser M, Becker AJ et al GLUT1 mRNA and protein expression in ovarian borderline tumors and cancer. Oncology 2004; 66: 404–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. EF24 treatment blocked ovarian cancers motility and invasion.
Fig. S2. Glut1 over‐expression increase ovarian cancer cells metastasis.
Fig. S3. Decreased ATP levels and increased ROS levels in ovarian cancer cells were observed after EF24 treatment.
Data S1. Glut1 mediated the function of EF24 in inhibiting ovarian cancer cell metastasis.


