Abstract
The ubiquitous Ca2+ release-activated Ca2+ (CRAC) channel is crucial to many physiological functions. Both gain and loss of CRAC function is linked to disease. While ORAI1 is a crucial subunit of CRAC channels, recent evidence suggests that ORAI2 and ORAI3 heteromerize with ORAI1 to form native CRAC channels. Furthermore, ORAI2 and ORAI3 can form CRAC channels independently of ORAI1, suggesting diverse native CRAC stoichiometries. Yet, most available CRAC modifiers are presumed to target ORAI1 with little knowledge of their effects on ORAI2/3 or heteromers of ORAIs. Here, we used ORAI1/2/3 triple-null cells to express individual ORAI1, ORAI2, ORAI3 or ORAI1/2/3 concatemers. We reveal that GSK-7975A and BTP2 essentially abrogate ORAI1 and ORAI2 activity while causing only a partial inhibition of ORAI3. Interestingly, Synta66 abrogated ORAI1 channel function, while potentiating ORAI2 with no effect on ORAI3. CRAC channel activities mediated by concatenated ORAI1–1, ORAI1–2 and ORAI1–3 dimers were inhibited by Synta66, while ORAI2–3 dimers were unaffected. The CRAC enhancer IA65 significantly potentiated ORAI1 and ORAI1–1 activity with marginal effects on other ORAIs. Further, we characterized the profiles of individual ORAI isoforms in the presence of Gd3+ (5μM), 2-APB (5μM and 50μM), as well as changes in intracellular and extracellular pH. Our data reveal unique pharmacological features of ORAI isoforms expressed in an ORAI-null background and provide new insights into ORAI isoform selectivity of widely used CRAC pharmacological compounds.
Keywords: Calcium signaling, CRAC channels, ORAI1/2/3, pharmacological compounds, pH
Graphical Abstract
Introduction
The store-operated Ca2+ entry (SOCE) pathway mediated by the Ca2+ release-activated Ca2+ (CRAC) channel is a ubiquitous receptor-activated Ca2+ entry route across the plasma membrane that is critical to many cellular functions in mammalian organisms[1–8]. CRAC channel function depends on two classes of membrane proteins: 1) The Ca2+ sensing stromal interaction molecule (STIM) located in the membrane of the endoplasmic reticulum (ER); and 2) the plasma membrane channel proteins, ORAIs[1–4]. Agonists acting on membrane receptors that couple to the phosphoinositide-specific phospholipase C (PLC) cause production of inositol-1,4,5-trisphosphate (IP3) from the breakdown of phosphatidylinositol(4,5)bisphosphate (PIP2)[9]. It is clearly established that the depletion of ER Ca2+ by the action of IP3 on IP3 receptors cause STIM1 proteins to translocate to ER-plasma membrane junctions where they trap ORAI1 channel protein to activate CRAC channels and induce SOCE[1, 2, 4, 10]. Both loss or gain of function mutations in STIM1 and ORAI1 have been linked to many diseases, including severe combined immune-deficiency (SCID), autoimmunity, ectodermal dysplasia, muscle weakness, tubular aggregate myopathy, and Miosis[6, 11]. Further, remodeling of STIM and ORAI protein expression contributes to an increasing number of cardiovascular and respiratory diseases and cancers[12, 13], highlighting the potential therapeutic use of specific CRAC channel modifiers during disease [14].
Mammals express two STIM proteins (STIM1/2) and three ORAI proteins (ORAI1–3), which are encoded by five independent genes. There is strong evidence that mammalian CRAC channels are hexamers of ORAI proteins [15–17] and that genuine and optimal CRAC channel activation requires that each ORAI subunit within hexameric CRAC is bound to one STIM protein[18, 19]. While STIM1 and ORAI1 are the bona fide components of CRAC channels, increasing evidence suggest that ORAI2 and ORAI3 proteins heteromerize with ORAI1 under native conditions to fine tune CRAC channel activity[20–22]. STIM2, long considered as a mediator of homeostatic SOCE under unstimulated basal conditions, has recently been shown to recruit STIM1 to ORAI1 clusters to amplify CRAC channel activity under relatively weak agonist stimulation[23–25]. Furthermore, in some cell types such as neurons, monocytes and certain cancer cells, STIM2, ORAI2 and ORAI3 mediate a sizeable portion of native SOCE[26–32], suggesting that CRAC channels encoded by ORAI2 or ORAI3 independently of ORAI1 likely exist at least in some cell types, especially when ORAI1 expression is low or absent.
While there are currently no inhibitors or activators of CRAC channels that are in use in the clinic, several CRAC channel inhibitors have undergone clinical trials in recent years[14]. Current CRAC channel modifiers commercially available have been presumed to act on ORAI1 in native cell systems where other STIM/ORAI isoforms are present[33–42]. Furthermore, overexpression studies have focused on STIM1/ORAI1-mediated CRAC channel pharmacology in wildtype cells where these CRAC currents might be contaminated by endogenous STIM/ORAI isoforms[36–38, 40, 42]. Here, we used our ORAI1/2/3 triple knockout HEK293 (ORAI-TKO) cells developed recently[22], which provides an ORAI-null background, to co-express STIM1 with individual ORAI isoforms or combinations of ORAI concatenated dimers. We used whole cell patch clamp electrophysiology to determine the unique pharmacological features of CRAC currents (ICRAC) mediated by different ORAIs. In addition to the established CRAC channel inhibitors 2-Aminoethoxydiphenyl borate (2-APB)[36, 38, 41, 42] and the trivalent ion Gd3+[43, 44], we evaluated other CRAC channels inhibitors, the pyrazole derivatives BTP2 and GSK-7975A, Synta66[38, 39] as well as a new CRAC enhancer IA65 characterized by our group[26]. Previous studies suggested that Ca2+ signaling in immune cells is remodeled within the inflammatory environment with upregulation of ORAI3. Unlike ORAI1, ORAI3 appears resistant to inhibition by the oxidant-rich inflammatory environment[32, 45]. We also studied the effects of intracellular and extracellular oxidizing and reducing environments on the activity of CRAC channels [46–49], while considering each ORAI isoform independently expressed in an ORAI-null background. Our results reveal unique pharmacological features of different ORAI isoforms and concatenated ORAI combinations and provide a comprehensive pharmacological guide for the identification of the molecular nature of CRAC channels in various native cell types and tissues with distinct patterns of ORAI isoform expression.
Materials and Methods
Reagents
2-aminoethoxydiphenyl borate (2-APB) (Catalog number: 100065–100MG), GSK-7975A (Catalog number: 534351), and Synta66 (Catalog number: SML1949–5MG) were purchased from Millipore-Sigma. BAPTA, Tetracesium Salt (Catalog number: B1212) was purchased from Invitrogen. BTP2 (catalog number: 3939) was purchased from TOCRIS. GdCl3 was from Acros Organics. IA65 was recently described [26]. All other reagents used in this study were purchased from Thermo Fisher. The transfection kit for HEK293 cells (Catalog number: VCA-1003) was from Lonza.
Cell culture and transfections
HEK293 cells (ATCC) were transfected with 4.0 μg EYFP-STIM1 along with 1.0 μg of either CFP-ORAI1, CFP-ORAI2, CFP-ORAI3, or CFP-tagged concatenated ORAI dimers[15, 22] and seeded on 30 mm round glass coverslips in 6-well plates one day before patch-clamp recordings. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 1% l-glutamine, 1% sodium pyruvate, 10% fetal bovine serum (FBS), and 1% Antibiotic-Antimycotic (100X) (Thermo Fisher, catalog number: 15240062), at 37°C in a humidified atmosphere containing 5% CO2. Detailed methods on the generation of ORAI1/2/3 triple knockout HEK293 cells using CRISPR/Cas9 from ORAI1 single knockout cells were previously described [22, 26, 27, 50, 51].
Patch clamp electrophysiology
Whole-cell patch clamp electrophysiological recordings were performed with an Axopatch 200B and Digidata 1440A (Molecular Devices). Patch pipettes were pulled from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL) with a P-1000 Flaming/Brown micropipette puller (Sutter Instrument) and polished with DMF1000 (World Precision Instruments). Resistances of filled patch pipettes were 2 to 4 MΩ. Under whole-cell configuration, only cells with small series resistances (< 8 MΩ) and tight seals (>16 GΩ) were chosen to perform recordings. A programed voltage ramps from −140 mV to +100 mV (from a holding potential of +30 mV) was applied every 3 seconds. 8 mM MgCl2 was included in the pipette solution to inhibit TRPM7 currents. Clampfit 11.1 software (Molecular Devices) was used to analyze data. Bath solution: 115 mM Na-methanesulfonate, 10 mM CsCl, 1.2 mM MgSO4, 10 mM Hepes, 20 mM CaCl2, and 10 mM glucose (pH adjusted to 7.4 with NaOH). Pipette solution: 115 mM Cs-methanesulfonate, 20 mM Cs-BAPTA, 8 mM MgCl2, and 10 mM Hepes (pH adjusted to 7.2 with CsOH). For extracellular pH effects on ICRAC: Bath solution: 115 mM Na-methanesulfonate, 10 mM CsCl, 1.2 mM MgSO4, 10 mM Hepes, 20 mM CaCl2, and 10 mM glucose (pH adjusted to 5.3, 7.4 and 9.5 with either HCl or NaOH). For intracellular pH effects on ICRAC: Pipette solution: 115 mM Cs-methanesulfonate, 20 mM Cs-BAPTA, 8 mM MgCl2, and 10 mM Hepes (pH adjusted to 5.0, 7.2 and 9.5 with either HCl or CsOH). For ORAI1- and ORAI2-mediated current recordings, we always subtract the first sweep immediately after break-in to correct for any contribution from leak currents. Because ORAI3 tend to generate pre-activated currents, we subtract the last sweep after block with 5μ M Gd3+.
Statistical analysis
All values are presented as boxplots with the 25th to 75th percentile range, mean and median for each condition. Paired Sample t-Test or one-way ANOVA were used for two or three groups comparisons, respectively. Data were plotted using Origin software, with p < 0.05 considered significant.
Results and Discussion
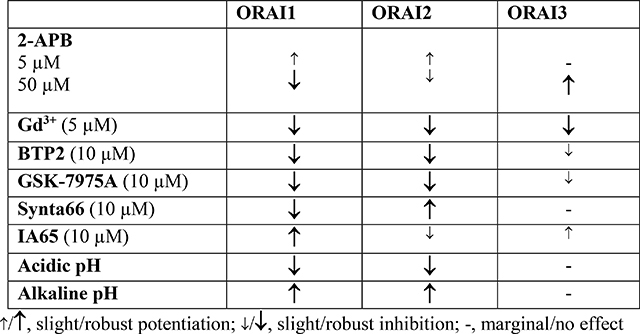
To rule out confounding effects from endogenous ORAI isoforms, we used ORAI1/2/3 triple knockout HEK293 (ORAI-TKO) cells, in which we co-expressed STIM1 with various ORAI variants and recorded steady-state CRAC currents mediated by these ORAI variants followed by addition of CRAC channel inhibitors and potentiators. To minimize contributions from CRAC channel slow Ca2+-dependent inactivation (slow CDI), we depleted the ER stores by inclusion of 20 mM of the fast Ca2+ chelator BAPTA in the patch pipette. Previous studies have showed that native CRAC channels are inhibited by low concentrations of trivalent lanthanides (1–5 μM Gd3+)[26, 28, 29, 33, 35, 43, 44, 52]. While the trivalent lanthanides Gd3+ and La3+ are broad inhibitors of many ion channels and transporters, low concentrations of these trivalent ions (<10 μM) selectively block highly Ca2+ selective channels such as CRAC channels and L-type voltage-gated Cav1.2 Ca2+ channels by interfering with the selectivity filter within the channel pore[53–56], suggesting that Ca2+ selectivity and inhibition by low μM lanthanides go hand in hand. We show here that 5 μM Gd3+ equally and significantly blocks CRAC currents mediated by ORAI1, ORAI2 and ORAI3 (Fig. S1). These results are consistent with the fact that all ORAI isoforms mediate Ca2+ selective CRAC conductances and that the pore regions of all ORAIs are highly homologous[1].
2-APB is an extensively characterized pharmacological modulator of CRAC channels, which was shown to exhibit both a potentiating and inhibitory effect on native CRAC channel activity depending on the concentration used. Low concentration (1–10 μM) of 2-APB potentiated CRAC currents (ICRAC), while high concentration (20–50 μM) of 2-APB inhibited ICRAC [36, 40–42]. In studies of ORAI isoforms ectopically expressed in wildtype HEK293 cells, high concentrations of 2-APB potentiated ORAI3 and altered its ion selectivity [36, 38, 40, 42]. Here we show that 5 μM 2-APB potentiates ICRAC mediated by ORAI1 and ORAI2 but has no effect on ORAI3 ICRAC (Fig. S2). However, 50 μM 2-APB completely abrogates ORAI1, but only slightly inhibits ORAI2. Further, 50 μM 2-APB significantly potentiated ORAI3 and altered its CRAC current/voltage (I/V) relationship revealing outward rectification (Fig. S3). These results are consistent with previous findings showing that 2-APB causes a dilation of ORAI3 pore rendering the channel non-selective[57]. Our findings on ORAI isoform expression in ORAI-TKO cells show that ORAI1 is more sensitive to inhibition by 2-APB than ORAI2.
Most ion channels are regulated by changes of internal and external pH. Native CRAC channels and those mediated by ORAI isoforms expressed in wildtype HEK293 cells have been shown to be regulated by acidic or alkaline pH[46–49]. Intracellular (Fig. S4) or extracellular acidification (Fig. S5; pH=5.0–5.3) inhibited CRAC currents mediated by ORAI1 and ORAI2, but had no effect on ORAI3 ICRAC, when compared to physiological pH (pH=7.2). Conversely, intracellular (Fig. S4) and extracellular alkalization (Fig. S5; pH=9.5) potentiated ORAI1 and ORAI2, with marginal effect on ORAI3. Early studies, most of which predate the discovery of STIM and ORAI proteins have reported the regulation of native ICRAC by both extracellular and intracellular pH in Jurkat T-cells, macrophages and neutrophils[58–61]. These reports showed that extracellular and intracellular acidification inhibits while alkalization enhances ICRAC. ICRAC generated through ectopic expression of ORAI1 (with STIM1) showed similar sensitivities to both internal and external low and high pH albeit with some subtle discrepancies, including those related to the pKa values of acidic pH block [46, 49]. These discrepancies are likely due to the notion that CRAC channels in native cell systems are contributed by homomeric and/or heteromeric assemblies of several ORAI isoforms[62]. Previous studies showed that several residues mediate the sensitivity of ORAI1 to external pH and these include D110, D112, E106 and E190[46, 48, 49]. Tsujikawa et al showed that H155 located in the TM2-TM3 intracellular loop mediates ORAI1 sensitivity to internal pH[49]. Interestingly, all these residues including H155 are conserved in ORAI3, suggesting that additional residues or pathways are mediating the resistance of ORAI3 to changes in pH. Oxidation of C195 of ORAI1 was shown to mediate its H2O2-dependent inhibition [35, 45]. ORAI3 is resistant to H2O2-dependent inhibition and the equivalent residue of C195 in ORAI3 is G170[45]. It is interesting to note that reactive oxygen species such as H2O2 are typically high in the tumor microenvironment which is also acidic[63]. The ability of ORAI3 to maintain activity in the tumor microenvironment or in inflammatory environments is likely important and deserves further attention [64]. Our results show that ORAI1 and ORAI2 are significantly inhibited by both intracellular and extracellular acidification and potentiated by alkalization, while ORAI3 is only marginally affected. The molecular determinants of these differences in pH sensitivity between ORAI3 and ORAI1/2 and whether G170 contributes to ORAI3 relative resistance to pH require further investigations. Beck et al. showed that ectopically expressed ORAI3 is more significantly affected by low and high pH by comparison to our results[46]. The reasons for this apparent discrepancy are unknown but the presence of native ORAI isoforms during the recordings of Beck et al. could contribute to the pH sensitivity of ectopically expressed ORAI isoforms.
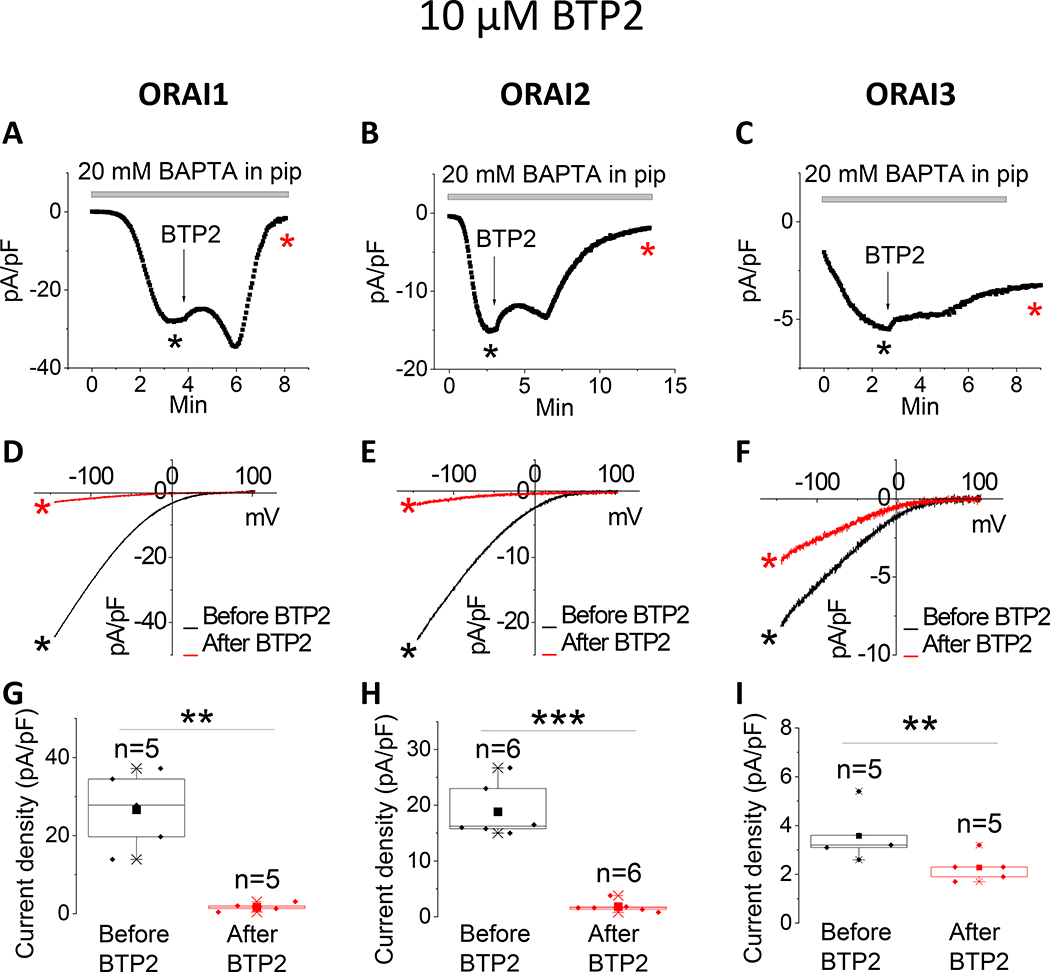
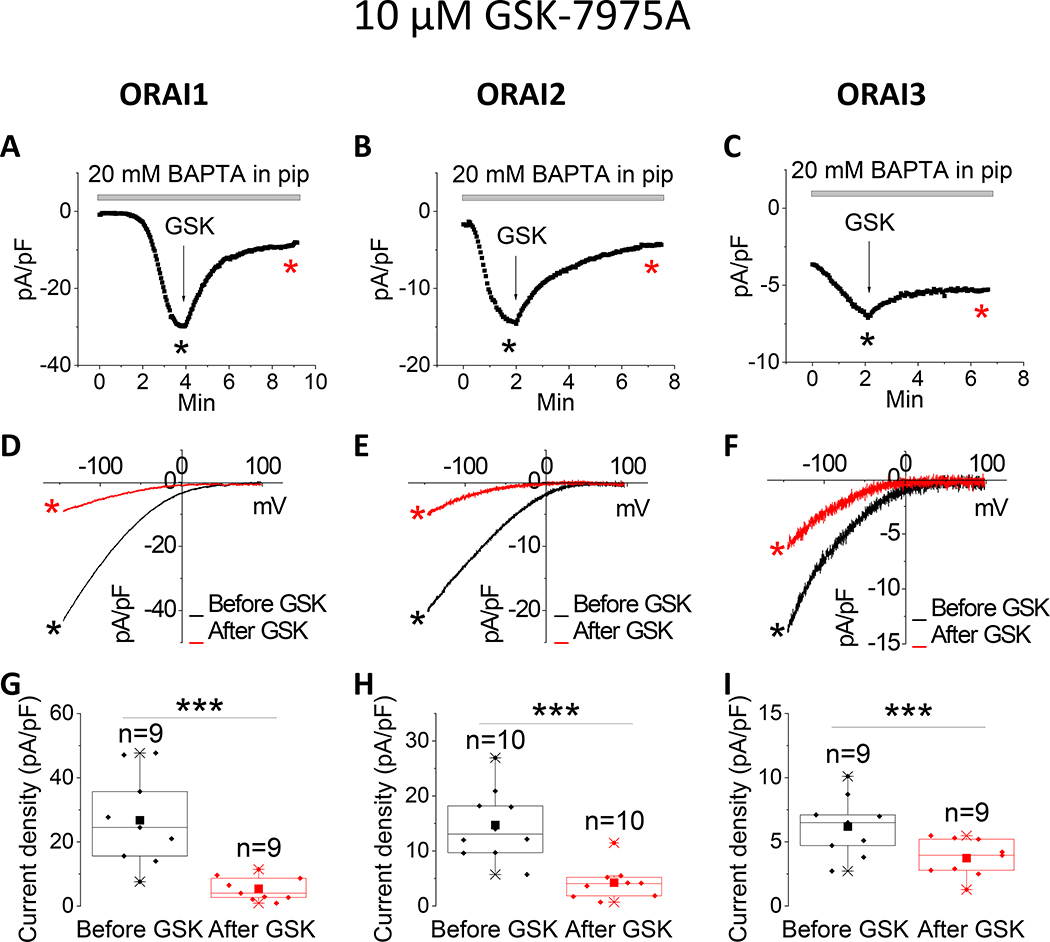
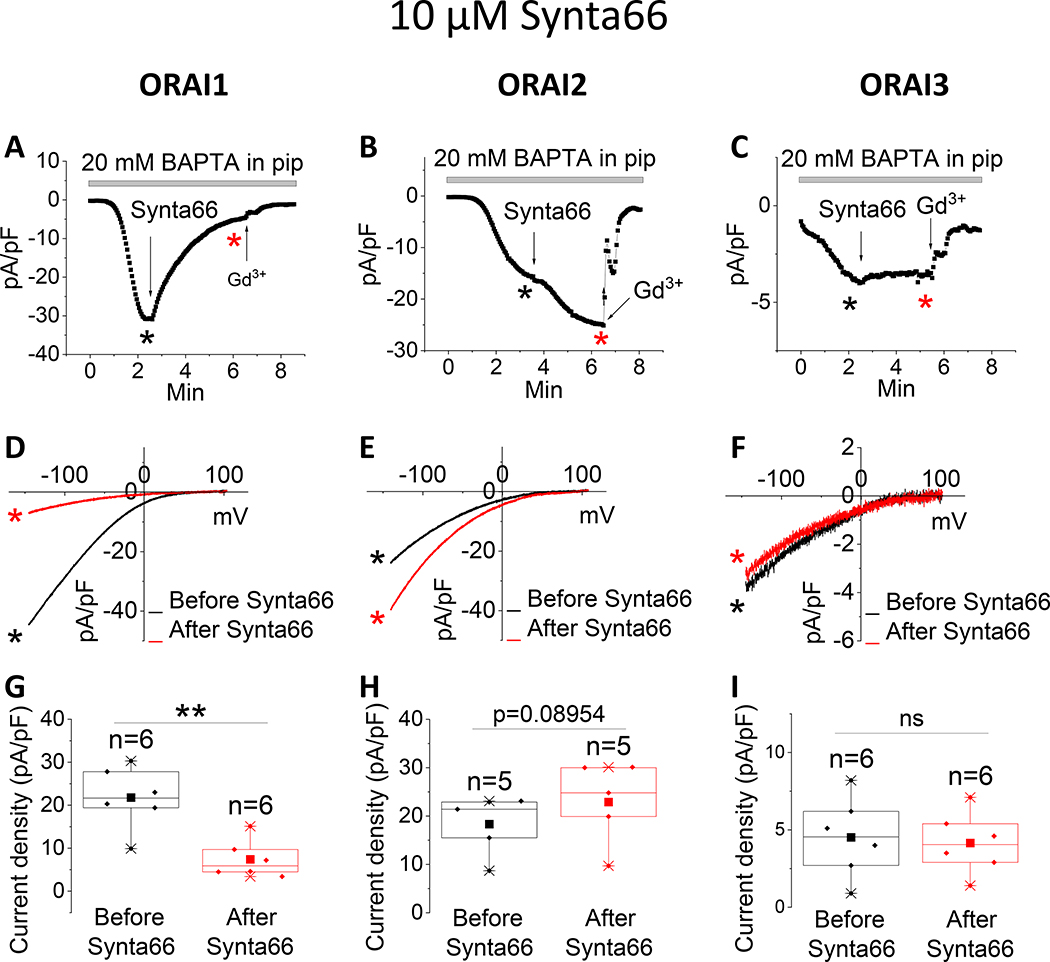
The pyrazole derivatives BTP2 and GSK-7975A have been extensively used as pharmacological blockers of CRAC channels. Derler et al. reported that GSK-7975A inhibited CRAC currents mediated by ectopically expressed ORAI1 and ORAI3 in wildtype HEK293 cells [37]; the effects of GSK-7975A on CRAC currents mediated by ORAI2 was not tested. We show that 10 μM of either BTP2 (Fig. 1) or GSK-7975A (Fig. 2) significantly inhibits ORAI1 and ORAI2, while causing only a partial inhibition of ORAI3. Interestingly, the Synta66 compound, which gained significant attention recently as a CRAC channel blocker[34, 37, 39], caused a significant inhibition of ORAI1 while potentiating ORAI2 and having no effect on ORAI3 when used at 10 μM (Fig. 3).
Figure 1. Differential effects of BTP2 on ORAI isoforms.
(A-C) Representative time course of ICRAC mediated by either ORAI1, ORAI2 or ORAI3 (at −100 mV) after whole-cell mode with a pipette solution containing 20 mM BAPTA. 10 μM BTP2 was applied to the bath when current reached steady state. (D-F) Representative I/V relationships were taken from (A-C) where indicated by the color-coded asterisks. (G-I) Data from several recordings representing steady state ICRAC current density (at −100 mV) before and after addition of BTP2 represented as boxplots with the 25th to 75th percentile range, mean and median for each condition. Data were statistically analyzed using Pair-Sample t-Test (**p<0.01 and ***p<0.001).
Figure 2. Differential effects of GSK-7975A on ORAI isoforms.
(A-C) Representative time course of ICRAC mediated by either ORAI1, ORAI2 or ORAI3 (at −100 mV) after whole-cell mode with a pipette solution containing 20 mM BAPTA. 10 μM GSK-7975A was applied to the bath when current reached steady state. (D-F) Representative I/V relationships were taken from (A-C) where indicated by the color-coded asterisks. (G-I) Data from several recordings representing steady state ICRAC current density (at −100 mV) before and after addition of GSK-7975A represented as boxplots with the 25th to 75th percentile range, mean and median for each condition. Data were statistically analyzed using Pair-Sample t-Test (***p<0.001).
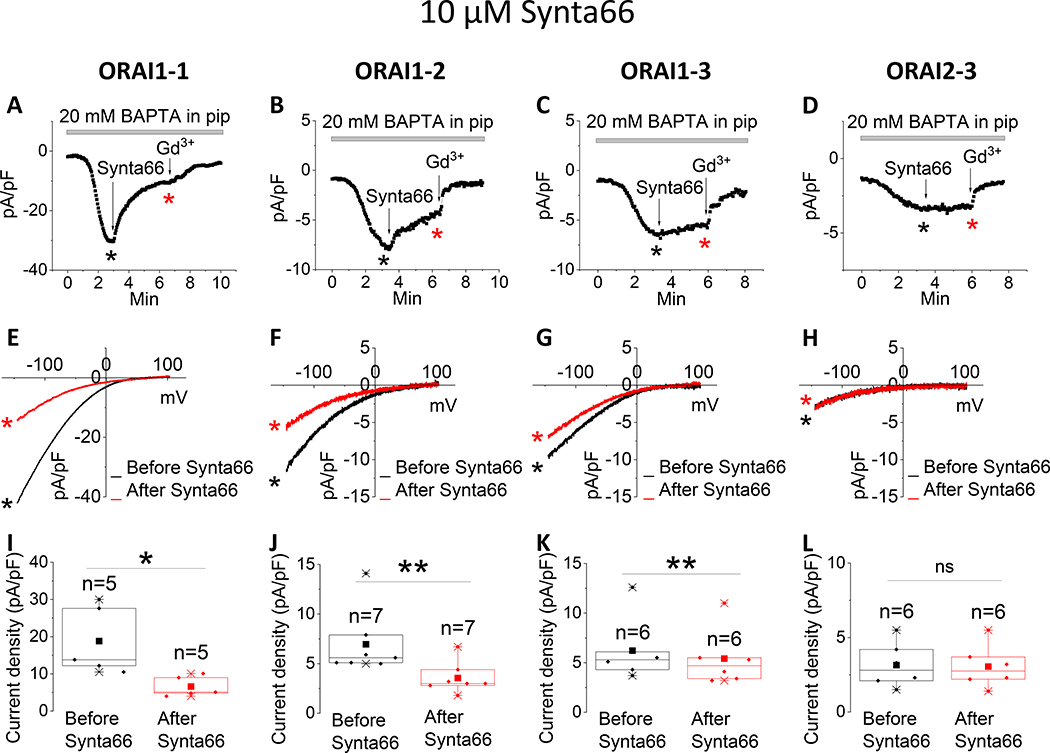
Figure 3. Differential effects of Synta66 on ORAI isoforms.
(A-C) Representative time course of ICRAC mediated by either ORAI1, ORAI2 or ORAI3 (at −100 mV) after whole-cell mode with a pipette solution containing 20 mM BAPTA. 10 μM Synta66 was applied to the bath when current reached steady state. At the end of recording, 5 μM Gd3+ was subsequently applied to the bath. (D-F) Representative I/V relationships were taken from (A-C) where indicated by the color-coded asterisks. (G-I) Data from several recordings representing steady state ICRAC current density (at −100 mV) before and after addition of Synta66 represented as boxplots with the 25th to 75th percentile range, mean and median for each condition. Data were statistically analyzed using Pair-Sample t-Test (**p<0.01 and ns, not significant).
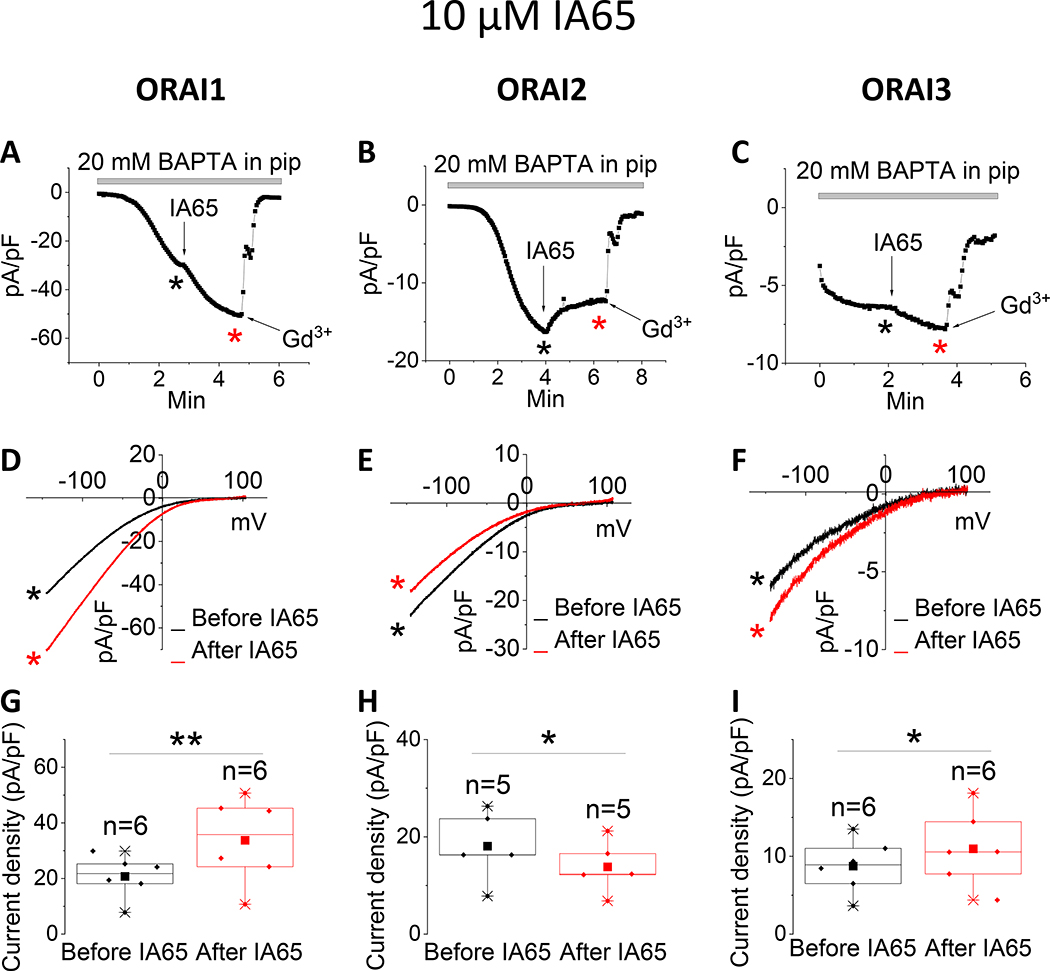
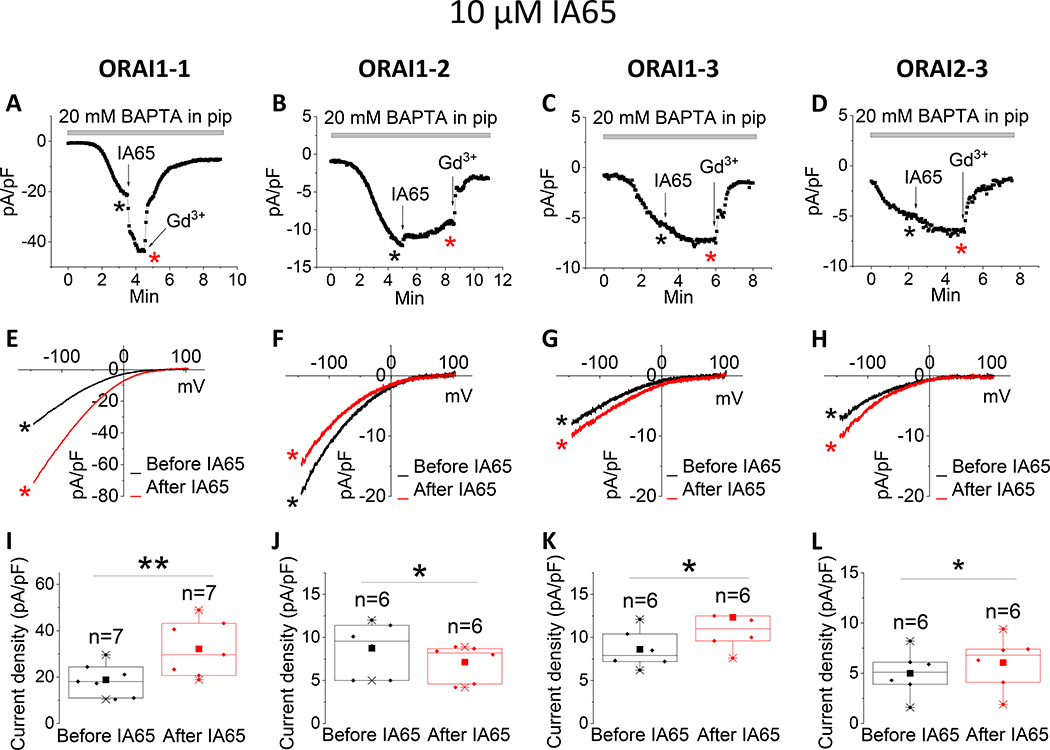
Recently, we discovered that the compound IA65 (3-bromo-1,1,1-trifluoro-3-phenylpropan-2 -one), is a native CRAC channel enhancer in various cell types, including MCF7 breast cancer cells, primary smooth muscle cells and skeletal muscle fibers [26]. We used CRISPR/Cas9 in MCF7 cells in which SOCE is mediated by a mixture of ORAI1 and ORAI3 to demonstrate that IA65 is an ORAI1 enhancer, having only marginal potentiating effects on ORAI3. Here, we tested side by side the effects of IA65 (10μM) on all three ORAI isoforms. We confirmed our previous findings that IA65 significantly potentiated ORAI1 while only mildly potentiating ORAI3-mediated ICRAC. Surprisingly, IA65 slightly inhibited ICRAC mediated by ORAI2 (Fig. 4).
Figure 4. Differential effects of IA65 on ORAI isoforms.
(A-C) Representative time course of ICRAC mediated by either ORAI1, ORAI2 or ORAI3 (at −100 mV) after whole-cell mode with a pipette solution containing 20 mM BAPTA. 10 μM IA65 was applied to the bath when current reached steady state. At the end of recording, 5 μM Gd3+ was subsequently applied to the bath. (D-F) Representative I/V relationships were taken from (A-C) where indicated by the color-coded asterisks. (G-I) Data from several recordings representing steady state ICRAC current density (at −100 mV) before and after addition of IA65 represented as boxplots with the 25th to 75th percentile range, mean and median for each condition. Data were statistically analyzed using Pair-Sample t-Test (*p<0.05 and **p<0.01).
Because Synta66 and IA65 had both inhibitory and potentiating effects on ICRAC mediated by different ORAI isoforms, we chose these two compounds to perform further studies testing their effects on CRAC currents mediated by ORAI concatenated dimers, namely ORAI1–1, ORAI1–2, ORAI1–3 and ORAI2–3. Synta66 significantly inhibited ICRAC mediated by ORAI1–1 concatenated homodimers to a similar extent to that of ORAI1. However, Synta66 only partially inhibited ICRAC mediated by ORAI1–2 and ORAI1–3 concatenated heterodimers, while having no effect on ORAI2–3 (Fig. 5), suggesting that within an ORAI heteromer the Synta66 pharmacological profiles of ORAI1 and ORAI3 dominate over that of ORAI2. IA65 significantly potentiated ICRAC mediated by ORAI1–1 concatenated homodimers to a similar extent to that of ORAI1. However, IA65 slightly inhibited ICRAC mediated by ORAI1–2 while slightly potentiating ORAI1–3 and ORAI2–3 concatenated heterodimers (Fig. 6), suggesting that within an ORAI heteromer IA65 pharmacological profile of ORAI2 dominates over that of ORAI1. The stoichiometry of native CRAC channels is likely more complex, involving variable numbers of ORAI isoform subunits within each hexamer. Assuming the position of each ORAI isoform within a hexameric CRAC channel is biologically relevant, native CRAC channels count 92 different combinations[22]. Nevertheless, Synta66 and IA65 could prove useful in understanding the molecular composition of SOCE in neuronal cell types where ORAI2 appears to be the dominant ORAI[30]. Since STIM proteins associate closely with ORAI and become an integral part of the CRAC channel during its activation, the potential role of the compounds studied herein in altering STIM/ORAI interactions and/or their ability to differentially affect STIM1 versus STIM2 requires future FRET and patch clamp investigations.
Figure 5. Effects of Synta66 on ORAI concatenated dimers.
(A-D) Representative time course of ICRAC mediated by either ORAI1–1, ORAI1–2, ORAI1–3 or ORAI2–3 concatenated dimers (at −100 mV) after whole-cell mode with a pipette solution containing 20 mM BAPTA. 10 μM Synta66 was applied to the bath when current reached steady state. At the end of recording, 5 μM Gd3+ was subsequently applied to the bath. (E-H) Representative I/V relationships were taken from (A-D) where indicated by the color-coded asterisks. (I-L) Data from several recordings representing steady state ICRAC current density (at −100 mV) before and after addition of Synta66 represented as boxplots with the 25th to 75th percentile range, mean and median for each condition. Data were statistically analyzed using Pair-Sample t-Test (*p<0.05, **p<0.01, ns not significant).
Figure 6. Effects of IA65 on ORAI concatenated dimers.
(A-D) Representative time course of ICRAC mediated by either ORAI1–1, ORAI1–2, ORAI1–3 or ORAI2–3 concatenated dimers (at −100 mV) after whole-cell mode with a pipette solution containing 20 mM BAPTA. 10 μM IA65 was applied to the bath when current reached steady state. At the end of recording, 5 μM Gd3+ was subsequently applied to the bath. (E-H) Representative I/V relationships were taken from (A-D) where indicated by the color-coded asterisks. (I-L) Data from several recordings representing steady state ICRAC current density (at −100 mV) before and after addition of IA65 represented as boxplots with the 25th to 75th percentile range, mean and median for each condition. Data were statistically analyzed using Pair-Sample t-Test (*p<0.05, **p<0.01).
In summary, our findings define unique pharmacological features of each ORAI isoform and how heteromeric ORAI assemblies alter this pharmacology. Use of these differentially selective pharmacological activators and inhibitors of CRAC could help determine the molecular composition and the relative contribution of each ORAI isoform to native CRAC channels in various cell types and primary tissues.
Supplementary Material
Highlights.
ORAI channel isoforms are distinctly affected by pH and various pharmacological inhibitors and activators
Synta66 inhibits ORAI1 and potentiates ORAI2 whereas IA65 potentiates ORAI1 and inhibits ORAI2
ORAI heteromerization through concatenated constructs shapes the pharmacological profiles of CRAC channels
Distinct ORAI isoform pharmacology could be useful for determining the relative contribution of each ORAI isoform to native CRAC channels in various primary cells.
Acknowledgments
Research in the Trebak laboratory is supported by the National Heart, Lung, and Blood Institute (R35-HL150778 to M.T.)
Footnotes
Competing interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Prakriya M, Lewis RS, Store-Operated Calcium Channels, Physiol Rev, 95 (2015) 1383–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Soboloff J, Rothberg BS, Madesh M, Gill DL, STIM proteins: dynamic calcium signal transducers, Nat Rev Mol Cell Biol, 13 (2012) 549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Trebak M, Kinet JP, Calcium signalling in T cells, Nat Rev Immunol, 19 (2019) 154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Trebak M, Putney JW Jr., ORAI Calcium Channels, Physiology (Bethesda), 32 (2017) 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Trebak M, Zhang W, Ruhle B, Henkel MM, Gonzalez-Cobos JC, Motiani RK, Stolwijk JA, Newton RL, Zhang X, What role for store-operated Ca(2)(+) entry in muscle?, Microcirculation, 20 (2013) 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Feske S, CRAC channels and disease - From human CRAC channelopathies and animal models to novel drugs, Cell Calcium, 80 (2019) 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gudlur A, Zeraik AE, Hirve N, Hogan PG, STIM calcium sensing and conformational change, J Physiol, 598 (2020) 1695–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yeung PS, Yamashita M, Prakriya M, Molecular basis of allosteric Orai1 channel activation by STIM1, J Physiol, 598 (2020) 1707–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Berridge MJ, The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease, Physiol Rev, 96 (2016) 1261–1296. [DOI] [PubMed] [Google Scholar]
- [10].Lunz V, Romanin C, Frischauf I, STIM1 activation of Orai1, Cell Calcium, 77 (2019) 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lacruz RS, Feske S, Diseases caused by mutations in ORAI1 and STIM1, Ann N Y Acad Sci, 1356 (2015) 45–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Johnson M, Trebak M, ORAI channels in cellular remodeling of cardiorespiratory disease, Cell Calcium, 79 (2019) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vashisht A, Trebak M, Motiani RK, STIM and Orai proteins as novel targets for cancer therapy. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis, Am J Physiol Cell Physiol, 309 (2015) C457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stauderman KA, CRAC channels as targets for drug discovery and development, Cell Calcium, 74 (2018) 147–159. [DOI] [PubMed] [Google Scholar]
- [15].Cai X, Zhou Y, Nwokonko RM, Loktionova NA, Wang X, Xin P, Trebak M, Wang Y, Gill DL, The Orai1 Store-operated Calcium Channel Functions as a Hexamer, J Biol Chem, 291 (2016) 25764–25775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hou X, Pedi L, Diver MM, Long SB, Crystal structure of the calcium release-activated calcium channel Orai, Science, 338 (2012) 1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yen M, Lokteva LA, Lewis RS, Functional Analysis of Orai1 Concatemers Supports a Hexameric Stoichiometry for the CRAC Channel, Biophys J, 111 (2016) 1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yen M, Lewis RS, Physiological CRAC channel activation and pore properties require STIM1 binding to all six Orai1 subunits, J Gen Physiol, 150 (2018) 1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yen M, Lewis RS, Numbers count: How STIM and Orai stoichiometry affect store-operated calcium entry, Cell Calcium, 79 (2019) 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eckstein M, Vaeth M, Aulestia FJ, Costiniti V, Kassam SN, Bromage TG, Pedersen P, Issekutz T, Idaghdour Y, Moursi AM, Feske S, Lacruz RS, Differential regulation of Ca(2+) influx by ORAI channels mediates enamel mineralization, Sci Signal, 12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vaeth M, Yang J, Yamashita M, Zee I, Eckstein M, Knosp C, Kaufmann U, Karoly Jani P, Lacruz RS, Flockerzi V, Kacskovics I, Prakriya M, Feske S, ORAI2 modulates store-operated calcium entry and T cell-mediated immunity, Nat Commun, 8 (2017) 14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yoast RE, Emrich SM, Zhang X, Xin P, Johnson MT, Fike AJ, Walter V, Hempel N, Yule DI, Sneyd J, Gill DL, Trebak M, The native ORAI channel trio underlies the diversity of Ca(2+) signaling events, Nat Commun, 11 (2020) 2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ong HL, de Souza LB, Zheng C, Cheng KT, Liu X, Goldsmith CM, Feske S, Ambudkar IS, STIM2 enhances receptor-stimulated Ca(2)(+) signaling by promoting recruitment of STIM1 to the endoplasmic reticulum-plasma membrane junctions, Sci Signal, 8 (2015) ra3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Son GY, Subedi KP, Ong HL, Noyer L, Saadi H, Zheng C, Bhardwaj R, Feske S, Ambudkar IS, STIM2 targets Orai1/STIM1 to the AKAP79 signaling complex and confers coupling of Ca(2+) entry with NFAT1 activation, Proc Natl Acad Sci U S A, 117 (2020) 16638–16648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Subedi KP, Ong HL, Son GY, Liu X, Ambudkar IS, STIM2 Induces Activated Conformation of STIM1 to Control Orai1 Function in ER-PM Junctions, Cell Rep, 23 (2018) 522–534. [DOI] [PubMed] [Google Scholar]
- [26].Azimi I, Stevenson RJ, Zhang X, Meizoso-Huesca A, Xin P, Johnson M, Flanagan JU, Chalmers SB, Yoast RE, Kapure JS, Ross BP, Vetter I, Ashton MR, Launikonis BS, Denny WA, Trebak M, Monteith GR, A new selective pharmacological enhancer of the Orai1 Ca(2+) channel reveals roles for Orai1 in smooth and skeletal muscle functions, ACS Pharmacol Transl Sci, 3 (2020) 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Emrich SM, Yoast RE, Xin P, Zhang X, Pathak T, Nwokonko R, Gueguinou MF, Subedi KP, Zhou Y, Ambudkar IS, Hempel N, Machaca K, Gill DL, Trebak M, Cross-talk between N-terminal and C-terminal domains in stromal interaction molecule 2 (STIM2) determines enhanced STIM2 sensitivity, J Biol Chem, 294 (2019) 6318–6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Motiani RK, Abdullaev IF, Trebak M, A novel native store-operated calcium channel encoded by Orai3: selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells, J Biol Chem, 285 (2010) 19173–19183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA, Trebak M, Orai3 is an estrogen receptor alpha-regulated Ca(2)(+) channel that promotes tumorigenesis, FASEB J, 27 (2013) 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stegner D, Hofmann S, Schuhmann MK, Kraft P, Herrmann AM, Popp S, Hohn M, Popp M, Klaus V, Post A, Kleinschnitz C, Braun A, Meuth SG, Lesch KP, Stoll G, Kraft R, Nieswandt B, Loss of Orai2-Mediated Capacitative Ca(2+) Entry Is Neuroprotective in Acute Ischemic Stroke, Stroke, 50 (2019) 3238–3245. [DOI] [PubMed] [Google Scholar]
- [31].Berna-Erro A, Braun A, Kraft R, Kleinschnitz C, Schuhmann MK, Stegner D, Wultsch T, Eilers J, Meuth SG, Stoll G, Nieswandt B, STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death, Sci Signal, 2 (2009) ra67. [DOI] [PubMed] [Google Scholar]
- [32].Saul S, Gibhardt CS, Schmidt B, Lis A, Pasieka B, Conrad D, Jung P, Gaupp R, Wonnenberg B, Diler E, Stanisz H, Vogt T, Schwarz EC, Bischoff M, Herrmann M, Tschernig T, Kappl R, Rieger H, Niemeyer BA, Bogeski I, A calcium-redox feedback loop controls human monocyte immune responses: The role of ORAI Ca2+ channels, Sci Signal, 9 (2016) ra26. [DOI] [PubMed] [Google Scholar]
- [33].Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M, Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation, Circ Res, 103 (2008) 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Azimi I, Bong AH, Poo GXH, Armitage K, Lok D, Roberts-Thomson SJ, Monteith GR, Pharmacological inhibition of store-operated calcium entry in MDA-MB-468 basal A breast cancer cells: consequences on calcium signalling, cell migration and proliferation, Cell Mol Life Sci, 75 (2018) 4525–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ben-Kasus Nissim T, Zhang X, Elazar A, Roy S, Stolwijk JA, Zhou Y, Motiani RK, Gueguinou M, Hempel N, Hershfinkel M, Gill DL, Trebak M, Sekler I, Mitochondria control store-operated Ca(2+) entry through Na(+) and redox signals, EMBO J, 36 (2017) 797–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].DeHaven WI, Smyth JT, Boyles RR, Bird GS, Putney JW Jr., Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry, J Biol Chem, 283 (2008) 19265–19273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Derler I, Schindl R, Fritsch R, Heftberger P, Riedl MC, Begg M, House D, Romanin C, The action of selective CRAC channel blockers is affected by the Orai pore geometry, Cell Calcium, 53 (2013) 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jairaman A, Prakriya M, Molecular pharmacology of store-operated CRAC channels, Channels (Austin), 7 (2013) 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li J, McKeown L, Ojelabi O, Stacey M, Foster R, O’Regan D, Porter KE, Beech DJ, Nanomolar potency and selectivity of a Ca(2)(+) release-activated Ca(2)(+) channel inhibitor against store-operated Ca(2)(+) entry and migration of vascular smooth muscle cells, Br J Pharmacol, 164 (2011) 382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R, CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties, Curr Biol, 17 (2007) 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Prakriya M, Lewis RS, Potentiation and inhibition of Ca(2+) release-activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors, J Physiol, 536 (2001) 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang SL, Kozak JA, Jiang W, Yeromin AV, Chen J, Yu Y, Penna A, Shen W, Chi V, Cahalan MD, Store-dependent and -independent modes regulating Ca2+ release-activated Ca2+ channel activity of human Orai1 and Orai3, J Biol Chem, 283 (2008) 17662–17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Trebak M, Bird GS, McKay RR, Putney JW Jr., Comparison of human TRPC3 channels in receptor-activated and store-operated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition, J Biol Chem, 277 (2002) 21617–21623. [DOI] [PubMed] [Google Scholar]
- [44].Trebak M, St JBG, McKay RR, Birnbaumer L, Putney JW Jr., Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels, J Biol Chem, 278 (2003) 16244–16252. [DOI] [PubMed] [Google Scholar]
- [45].Bogeski I, Kummerow C, Al-Ansary D, Schwarz EC, Koehler R, Kozai D, Takahashi N, Peinelt C, Griesemer D, Bozem M, Mori Y, Hoth M, Niemeyer BA, Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling, Sci Signal, 3 (2010) ra24. [DOI] [PubMed] [Google Scholar]
- [46].Beck A, Fleig A, Penner R, Peinelt C, Regulation of endogenous and heterologous Ca(2)(+) release-activated Ca(2)(+) currents by pH, Cell Calcium, 56 (2014) 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gavriliouk D, Scrimgeour NR, Grigoryev S, Ma L, Zhou FH, Barritt GJ, Rychkov GY, Regulation of Orai1/STIM1 mediated ICRAC by intracellular pH, Sci Rep, 7 (2017) 9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Scrimgeour NR, Wilson DP, Rychkov GY, Glu(1)(0)(6) in the Orai1 pore contributes to fast Ca(2)(+)-dependent inactivation and pH dependence of Ca(2)(+) release-activated Ca(2)(+) (CRAC) current, Biochem J, 441 (2012) 743–753. [DOI] [PubMed] [Google Scholar]
- [49].Tsujikawa H, Yu AS, Xie J, Yue Z, Yang W, He Y, Yue L, Identification of key amino acid residues responsible for internal and external pH sensitivity of Orai1/STIM1 channels, Sci Rep, 5 (2015) 16747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Johnson MT, Gudlur A, Zhang X, Xin P, Emrich SM, Yoast RE, Courjaret R, Nwokonko RM, Li W, Hempel N, Machaca K, Gill DL, Hogan PG, Trebak M, L-type Ca(2+) channel blockers promote vascular remodeling through activation of STIM proteins, Proc Natl Acad Sci U S A, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang X, Pathak T, Yoast R, Emrich S, Xin P, Nwokonko RM, Johnson M, Wu S, Delierneux C, Gueguinou M, Hempel N, Putney JW Jr., Gill DL, Trebak M, A calcium/cAMP signaling loop at the ORAI1 mouth drives channel inactivation to shape NFAT induction, Nat Commun, 10 (2019) 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M, Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration, FASEB J, 23 (2009) 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Babich O, Reeves J, Shirokov R, Block of CaV1.2 channels by Gd3+ reveals preopening transitions in the selectivity filter, J Gen Physiol, 129 (2007) 461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Beedle AM, Hamid J, Zamponi GW, Inhibition of transiently expressed low- and high-voltage-activated calcium channels by trivalent metal cations, J Membr Biol, 187 (2002) 225–238. [DOI] [PubMed] [Google Scholar]
- [55].Lansman JB, Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit, J Gen Physiol, 95 (1990) 679–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Malasics A, Boda D, Valisko M, Henderson D, Gillespie D, Simulations of calcium channel block by trivalent cations: Gd(3+) competes with permeant ions for the selectivity filter, Biochim Biophys Acta, 1798 (2010) 2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Schindl R, Bergsmann J, Frischauf I, Derler I, Fahrner M, Muik M, Fritsch R, Groschner K, Romanin C, 2-aminoethoxydiphenyl borate alters selectivity of Orai3 channels by increasing their pore size, J Biol Chem, 283 (2008) 20261–20267. [DOI] [PubMed] [Google Scholar]
- [58].Guse AH, Roth E, Emmrich F, Ca2+ release and Ca2+ entry induced by rapid cytosolic alkalinization in Jurkat T-lymphocytes, Biochem J, 301 (Pt 1) (1994) 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kerschbaum HH, Cahalan MD, Monovalent permeability, rectification, and ionic block of store-operated calcium channels in Jurkat T lymphocytes, J Gen Physiol, 111 (1998) 521–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Malayev A, Nelson DJ, Extracellular pH modulates the Ca2+ current activated by depletion of intracellular Ca2+ stores in human macrophages, J Membr Biol, 146 (1995) 101–111. [DOI] [PubMed] [Google Scholar]
- [61].Sandoval AJ, Riquelme JP, Carretta MD, Hancke JL, Hidalgo MA, Burgos RA, Store-operated calcium entry mediates intracellular alkalinization, ERK1/2, and Akt/PKB phosphorylation in bovine neutrophils, J Leukoc Biol, 82 (2007) 1266–1277. [DOI] [PubMed] [Google Scholar]
- [62].Yoast RE, Emrich SM, Trebak M, The Anatomy of CRAC channel(s), Curr Opin Physiology, 10.1016/j.cophys.2020.07.012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ji K, Mayernik L, Moin K, Sloane BF, Acidosis and proteolysis in the tumor microenvironment, Cancer Metastasis Rev, 38 (2019) 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Frisch J, Angenendt A, Hoth M, Prates Roma L, Lis A, STIM-Orai Channels and Reactive Oxygen Species in the Tumor Microenvironment, Cancers (Basel), 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.