Abstract
OBJECTIVES:
Sepsis has been called a “disease of the elderly,” and as in-hospital mortality has decreased, more sepsis survivors are progressing into poorly characterized long-term outcomes. The purpose of this study was to describe the current epidemiology of sepsis in older adults compared with middle-aged and young adults.
DESIGN:
Prospective longitudinal study with young (≤45 years), middle-aged (46-64 years), and older (≥65 years) patient groups.
SETTING:
University tertiary medical center.
PARTICIPANTS:
A total of 328 adult surgical intensive care unit (ICU) sepsis patients.
MEASUREMENTS:
Patients were characterized by (1) baseline demographics and predisposition, (2) septic event, (3) hospital outcomes and discharge disposition, (4) 12-month mortality, and (5) Zubrod Performance Status, physical function (Short Physical Performance Battery and handgrip strength), and cognitive function (Hopkins Verbal Learning Test, Controlled Oral Word Association, and Mini-Mental Status Examination) at 3-, 6-, and 12-month follow-up. Loss to follow-up was due to death (in 68), consent withdrawal (in 32), and illness and scheduling difficulties: month 3 (in 51), month 6 (in 29), and month 12 (in 20).
RESULTS:
Compared with young and middle-aged patients, older patients had (1) significantly more comorbidities at presentation (eg, chronic renal disease 6% vs 12% vs 21%), intra-abdominal infections (14% vs 25% vs 37%), septic shock (12% vs 25% vs 36%), and organ dysfunctions; (2) higher 30-day mortality (6% vs 4% vs 17%) and fewer ICU-free days (median = 25 vs 23 vs 20); (3) more progression into chronic critical illness (22% vs 34% vs 42%) with higher poor disposition discharge to non-home destinations (19% vs 40% vs 62%); (4) worse 12-month mortality (11% vs 14% vs 33%); and (5) poorer Zubrod Performance Status and objectively measured physical and cognitive functions with only slight improvement over 12-month follow-up.
CONCLUSION:
Compared with younger patients, older sepsis survivors suffer both a higher persistent disability burden and 12-month mortality.
Keywords: sepsis, older adults, health outcomes, cognitive function, physical function
Despite decades of extensive research, sepsis remains a common, costly, and debilitating syndrome.1 The Surviving Sepsis Campaign was initiated in 2004 as an international effort to develop and implement evidence-based care guidelines.2 As a result of effective implementation, in-hospital mortality of sepsis has decreased substantially.3,4 Many intensive care unit (ICU) patients who previously succumbed to early refractory shock and multiple organ failure (MOF) now survive their hospitalization. However, a disturbing number of these sepsis survivors develop a clinical trajectory of chronic critical illness (CCI) with a prolonged ICU course and high resource utilization. They are commonly discharged to skilled nursing facilities (SNFs) and long-term acute care facilities (LTACs), experience sepsis recidivism, ongoing physical and cognitive disabilities, and up to 40% are dead at 1 year.5
Sepsis has been long recognized as the “quintessential disease of the elderly.”6 The incidence of sepsis and in-hospital mortality increases exponentially beyond the age of 65 years.7,8 Although older adults constitute only one-fifth of the US population, they account for nearly two-thirds of the patients admitted to hospitals with sepsis. From 1996 to 2008, hospitalizations for sepsis in US Medicare recipients rose from roughly 400,000 cases to more than 1 million.9 Consistent with improved outcomes associated with implementation of the Surviving Sepsis Campaign guidelines, in-hospital mortality of older patients decreased substantially from 28% to 16%.3,4 Unfortunately, at 3-year follow-up, their mortality exceeded 70% and has not changed over the time period.3,4 Their recovery from sepsis is hampered by baseline comorbidities and frailty as well as physical and cognitive disabilities.10 Additionally, “septic autocannibalism” (well described in surgical ICUs) causes loss of lean body mass despite early nutritional support.11 In older patients, this can accelerate age-related muscle loss and anabolic resistance,1,12,13 thus limiting rehabilitation potential. Acute delirium is also commonly seen in older septic patients. It was once thought to be reversible, but studies showed that sepsis causes inflammation in the brain and microinfarctions that can cause long-term impairment of global cognition with an increased risk of dementia.14–16 Other retrospective studies showed that older sepsis survivors have poor quality of life, frequently develop cognitive and functional disability, and require substantial ongoing acute and long-term care 17–19
Although age was shown to be an independent predictor of mortality in critically ill septic patients, the other important epidemiological aspects of sepsis across age groups have not been described objectively.20 Therefore, the purpose of this study is to characterize (1) predisposing factors, (2) the severity of the septic insult, (3) organ dysfunction resolution and clinical trajectories, (4) traditional ICU outcomes, and (5) detailed postdischarge 1-year outcomes across age groups. These study results are to inform geriatric medicine providers that older sepsis survivors are more likely to progress into poor long-term outcomes with nonrecovery from severe disabilities and significant mortality.
METHODS
Study Design and Population
This is a prospective longitudinal observational cohort study that enrolled surgical ICU patients with new-onset sepsis over 4 years ending December 31, 2018. The University of Florida (UF) Health Shands Hospital in Gainesville is a level 1 trauma and tertiary care center with two trauma/surgical ICUs totaling 48 beds that served as the recruitment base for this cohort study. Together, the Acute Care Surgery and ICU teams manage more than 2,600 critically ill patients annually. Each ICU has a dedicated surgical critical care team including an attending intensivist, critical care fellows, surgical and anesthesia residents, advanced practice providers, and 24/7 coverage by attending acute care surgeons.21 The study was approved by the UF institutional review board and registered with clinicaltrials.gov (NCT02276417).
The patient or legally authorized representative provided informed consent within 96 hours after the patient qualified for study inclusion. If not obtained within 96 hours, all patient data and biological samples were destroyed. Details of the study design with inclusion and exclusion criteria as well as the clinical and laboratory standard operating procedure utilized were published.21 In brief, inclusion criteria included (1) age 18 years or older, (2) clinical diagnosis of sepsis defined by 2001 consensus guidelines, and (3) entrance into an electronic medical record sepsis screening and evidence-based management protocols. Exclusion criteria eliminated patients whose baseline immunosuppression, end-stage comorbidities, or severe functional injuries would be a primary determinant of their long-term outcomes and thus confound outcome assessment.
The initial septic events were adjudicated at weekly Sepsis and Critical Illness Research Center (SCIRC) meetings to ensure the appropriate diagnosis of sepsis, its severity, and site of infection. Clinical data were collected by research nurses into an established sepsis database including baseline demographics, comorbidities, body mass index (BMI), morbid obesity (BMI >40), admission diagnosis, reason for admission, infection diagnosis, and sepsis severity defined by 2001 consensus guidelines. Predicted mortality was assessed by the Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores at 24 hours.22 Infections were defined using Centers for Disease Control and Intervention definitions, and sepsis was classified as “present on admission” if diagnosed within 48 hours and “hospital acquired” if diagnosed 48 hours after hospital admission. Timing and need for invasive vs noninvasive source control procedure were recorded. Hospital outcomes included need for mechanical ventilation as well as ventilation, ICU, and hospital-free days (these were calculated by subtracting the number of days after sepsis protocol onset from 30 days).
Secondary infections were defined as any probable or microbiologically confirmed bacterial, yeast, fungal, or viral infection requiring treatment and occurring at least 48 hours after sepsis protocol onset during the index hospitalization. Infections within 48 hours of sepsis onset were considered coexisting and therefore excluded. Secondary infections were presented as mean per patient and secondary infections per 100 hospital person-days (to adjust for the time at risk).
Organ dysfunction progression was assessed by serial SOFA scores. MOF was defined by the Denver Score, and acute kidney injury was defined by the Kidney Disease: Improving Global Outcomes score. Patients were classified by three inpatient clinical trajectories: early death, rapid recovery, and CCI. Early death was defined as death within 14 days of sepsis onset. CCI was defined as an ICU stay greater than or equal to 14 days with evidence of persistent organ dysfunction by SOFA. Rapid recovery patients were those not meeting criteria for early death or CCI.
Discharge disposition was classified based on known associations with long-term outcomes as either “good” (home with or without health care services or rehabilitation facility) or “poor” non-home destinations (LTACs, SNFs, another acute care hospital, hospice, or inpatient death). Mortality information was acquired from the Social Security death index, and the causes of death were adjudicated from the medical records. For this analysis, the study patients were divided into three groups by age: young patients (≤45 years), middle-aged patients (46-64 years), and older patients (≥65 years).
Long-Term Outcomes
Performance status was measured by Eastern Cooperative Oncology Group/World Health Organization/Zubrod Performance Status that ranges from 0 to 5, with increasing score reflecting worse performance status: (0) asymptomatic (fully active); (1) Symptomatic but completely ambulatory (restricted in physically strenuous activity); (2) symptomatic, less than 50% of time in bed during the day (ambulatory and capable of all self-care but unable to perform any work activities); (3) symptomatic, more than 50% of time in bed but not bedbound (capable of only limited self-care); (4) bedbound (completely disabled, incapable of any self-care); and (5) death.21 Baseline (ie, prehospitalization) performance status was based on patient/proxy-reported 4-week recall assessment as soon as possible after sepsis onset.23
Objective level of physical function was assessed by two tests: (1) Short Physical Performance Battery (SPPB) that evaluates lower extremity function based on a timed short-distance walk, repeated chair stands, and balance test,21 and 2) handgrip strength measured in the dominant hand using an adjustable hydraulic dynamometer (Jamar Hydraulic Hand Dynamometer, Model No. BK-7498; Fred Sammons, Burr Ridge, IL, USA). The better of two trials was recorded.21
Cognitive function was assessed by three tests: (1) Hopkins Verbal Learning Test (HVLT) by reading aloud a list of 12 words to participants after which they are asked to recall as many of the words as possible; delayed word recall occurs after a 20-minute delay,24 (2) Controlled Oral Word Association (COWA) requires the individual to name as many words as possible that begin with a given letter,25 and (3) Mini-Mental Status Examination (MMSE) consists of two sections that contain 11 tasks of cognition. Scores range from 0 to 30. Scores of 24 and lower indicate cognitive impairment.26
Assessments at follow-up visits were performed in-person by clinical research coordinators at our institution or participants’ home (or by phone if unable to schedule a visit): Zubrod Performance Status, HVLT, and COWA.
Study Enrollment and Retention
A total of 328 enrolled subjects had completed the 12-month follow-up by May 31, 2019. There were 27 inpatient deaths, 23 died between discharge and 3 months, and 25 withdrew consent before 3 months, resulting in 253 survivors eligible for 3-month follow-up testing. Another 10 patients who died between 3 and 6 months, with an additional 4 patients withdrawing consent, resulted in 239 patients eligible for 6-month follow-up. Between the 6- and 12-month follow-up, 8 patients died and 3 withdrew consent, leaving 228 eligible for testing at 12 months. Supplementary Figure S1 visualizes study enrollment and 12-month follow-up. Most common reasons for missed follow-up evaluations at 3, 6, and 12 months were hospitalization/rehab (7%, 1%, and <1%), unable to schedule (6%, 9%, and 7%), too sick (2%, 1%, and <1%), and refused/uninterested in study participation (2%, <1%, and <1%).
Statistical Analysis
Data are presented as frequency and percentage, mean and standard deviation/standard error, or median and quartiles. The Fisher exact test and the Kruskal-Wallis test were used for comparison of categorical and continuous variables, respectively. SOFA scores between groups were compared using the Kruskal-Wallis test at 1, 4, 7, and 14 days after sepsis onset. SOFA score was imputed for living patients discharged before day 14. For patients with a poor discharge disposition, the last available in-hospital component scores were carried forward. Similarly, for patients with a good disposition, the last available in-hospital component scores were used for hepatic, coagulation, and renal component scores; respiratory and central nervous system components were assumed to be 0. Inverse probability weighting based on concurrent adjudicated Zubrod score was used to account for missing follow-up data, as well as absence due to death. All significance tests were two-sided, with P ≤ .05 considered statistically significant. Statistical analyses were performed with SAS software (SAS Institute, Cary, NC). We further divided the older group into 65 to 74 vs 75 years and older, but given the lack of differences between these subgroups, we included these results in the supplementary material (Supplementary Tables S1–S5 and Supplementary Figures S2 and S3).
RESULTS
There were 328 study patients of which 176 (53%) were male; mean age was 62 years. They were predominantly non-Hispanic whites with a median BMI of 29.2, and 58 (18%) were morbidly obese. Most had multiple comorbidities; the most common were hypertension, coronary artery disease, and chronic lung disease. Roughly 60% had sepsis present on admission. One-quarter of the patients presented in septic shock, and the most common sites of infections were intra-abdominal, surgical site infection, and pneumonia. Two-thirds required a source control procedure of which 70% were invasive and 30% were noninvasive.
Table 1 lists the baseline demographics, characteristics of the septic event, and predisposition by age groups. Compared with the young and middle-aged patients, the older patients had a lower BMI (with less morbid obesity) and, as expected, substantially more comorbidities (60% had three or more). Of note, the incidence of liver disease, substance abuse, and active cancer were not different between the age groups. Compared with the young and middle aged, the older patients had more intra-abdominal infections, more septic shock, and higher APACHE II scores.
Table 1.
Baseline Demographics, Characteristics of the Inciting Septic Event, and Predisposition by Age Groups
| Age groups n (%) | Young 65 (20) | Middle aged 131 (40) | Older 132 (40) |
|---|---|---|---|
| Male, n (%) | 34 (52) | 68 (52) | 74 (56) |
| Age, y, median (IQR 25th, 75th) | 35 (28, 42)a,b | 58 (53, 62)c | 72 (69, 77) |
| Race, n (%) | |||
| White | 59 (91) | 113 (86) | 121 (92) |
| African American | 5 (8) | 16 (12) | 9 (7) |
| American Indian | 1 (2) | 0 (0) | 0 (0) |
| Asian | 0 (0) | 0 (0) | 1 (1) |
| Other | 0 (0) | 1 (1) | 0 (0) |
| Unknown | 0 (0) | 1 (1) | 1 (1) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 3 (5) | 4 (3) | 1 (1) |
| Not Hispanic or Latino | 62 (95) | 127 (97) | 131 (99) |
| BMI, median (25th, 75th) | 30.3 (25.8, 40.4)b | 29.8 (25.1,39.1)c | 28.2 (24.4, 33.8) |
| Morbid obesity n (%) | 17 (26)b | 28 (21)c | 13 (9.8) |
| Comorbidities, n (%) | |||
| Hypertension | 18 (28)a,b | 80 (61)c | 105 (80) |
| Coronary artery disease | 4 (6)b | 21 (16)c | 58 (44) |
| Diabetes | 11 (17)a,b | 51 (39) | 52 (39) |
| Chronic lung disease | 1 (2)a,b | 28 (21) | 37 (28) |
| Atrial fibrillation | 0 (0)a,b | 9 (7) | 29 (22) |
| Chronic renal disease | 4 (6)b | 15 (12) | 27 (21) |
| Heart failure | 2 (3)a,b | 12 (9)c | 28 (21) |
| Peripheral artery disease | 3 (5)a,b | 8 (6)c | 28 (21) |
| Active cancer | 5 (8) | 24 (18) | 20 (15) |
| Prior stroke | 1 (2) | 12 (9) | 12 (9) |
| Substance abuse | 4 (6) | 12 (9) | 8 (6) |
| Dementia | 0 (0) | 1 (1) | 6 (5) |
| Liver disease | 1 (2) | 4 (3) | 2 (2) |
| ESRD | 1 (2) | 4 (3) | 3 (2) |
| No. of comorbidities (adjudicated), n (%) | a,b | c | |
| 0 | 29 (45) | 18 (14) | 5 (4) |
| 1 | 16 (25) | 31 (24) | 20 (15) |
| 2 | 11 (17) | 29 (22) | 28 (21) |
| ≥3 | 9 (14) | 53 (41) | 79 (60) |
| Reason for hospital admission, n (%) | |||
| Planned surgery | 11 (17) | 27 (21) | 28 (21) |
| Trauma | 10 (15) | 13 (10) | 10 (8) |
| Active infection | 41 (63) | 78 (60) | 79 (60) |
| Noninfectious/Chronic problems | 3 (5) | 13 (10) | 15 (11) |
| Emergency surgery (within 24 h), n (%) | 39 (60) | 71 (54) | 60 (46) |
| Sepsis present on admission (≤48 h), n (%) | 45 (69) | 82 (63) | 74 (56) |
| Hospital-acquired sepsis (>48 h), n (%) | 20 (31) | 49 (37) | 58 (44) |
| Type of infection, n (%) | |||
| Intra- abdominal | 9 (14)b | 33 (25)c | 49 (37) |
| Surgical site infection | 21 (32)b | 31 (24) | 23 (17) |
| Pneumonia | 11 (17) | 23 (18) | 23 (17) |
| Necrotizing soft tissue infection | 10 (15)b | 22 (17)c | 7 (5) |
| Urosepsis | 7 (11) | 13 (10) | 19 (14) |
| Other | 6 (9) | 7 (5) | 9 (7) |
| Catheter-related bloodstream infection | 1 (2) | 2 (2) | 2 (2) |
| Sepsis severity, n (%) | |||
| Sepsis | 31 (48)a,b | 36 (28) | 31 (23) |
| Severe sepsis | 26 (40) | 62 (47) | 54 (41) |
| Septic shock | 8 (12)a,b | 33 (25) | 47 (36) |
| APACHE II score (24 h), median (IQR 25th, 75th) | 12 (7, 18)a,b | 16 (11,22)c | 20 (15.5, 26) |
| SOFA score (24 h), median (IQR 25th, 75th) | 6 (3, 8)a,b | 7 (4, 10)c | 8 (6, 10) |
| Culture negative | 18 (28) | 49 (37) | 56 (42) |
| Culture positive | 47 (72) | 82 (63) | 76 (58) |
| Bacterial: gram positive | 9 (19) | 18 (22) | 22 (29) |
| Bacterial: gram negative | 17 (37) | 36 (44) | 30 (40) |
| Fungal | 2 (4) | 5 (6) | 3 (4) |
| Polymicrobial | 19 (40)b | 23 (28) | 21 (27) |
| Sepsis source control procedure, n (%) | 46 (71) | 95 (73) | 83 (63) |
| Invasive procedures | 27 (59) | 67 (71) | 64 (77) |
| Noninvasive procedures | 19 (41) | 28 (29)c | 19 (23) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation; BMI, body mass index; ESRD, end-stage renal disease; IQR, interquartile range; SOFA = Sequential Organ Failure Assessment.
Statistical differences were labeled as follows:
Young vs middle-aged.
Young vs older adults.
Middle-aged vs older adults, with statistical significance set at P < .05.
Figure 1 depicts serial SOFA scores over 14 days by age group. It is noteworthy that SOFA scores were increased in the older patients upon presentation, resolving more slowly and remaining higher than in the young and middle-aged patients.
Figure 1.
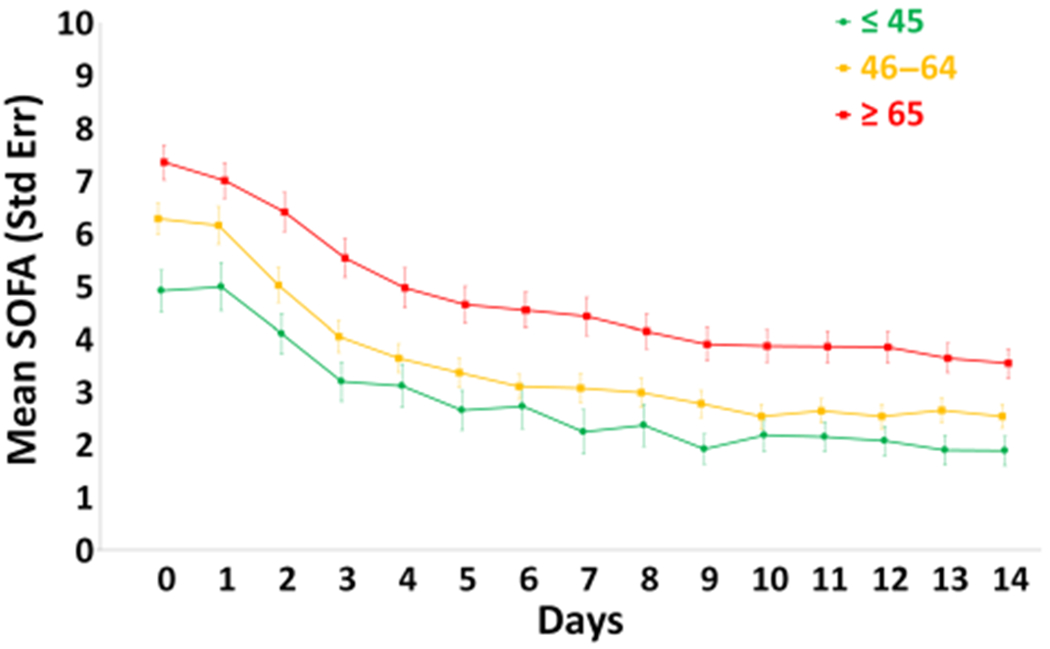
Sequental Organ Failure Assessment (SOFA) scores over 14 days by age groups. Data are presented as mean standard ± error with statistical significance set at P < .05. [Color figure can be viewed at wileyonlinelibrary.com]
Table 2 lists hospital and postdischarge outcomes by age groups. Compared with the young and middle aged, the older patients had substantially worse hospital outcomes with an increased need for mechanical ventilation with fewer ventilation-, ICU-, and hospital-free days. The 30-day mortality was greater in older patients with more CCI and a notably common (62%) poor discharge disposition to non-home destinations.
Table 2.
Characteristics of the Hospital and Postdischarge Outcomes by Age Group
| Age groups n (%) | Young 65 (20%) | Middle age 131 (40%) | Older 132 (40%) |
|---|---|---|---|
| Need for mechanical ventilation, n (%) | 37 (57)b | 81 (62)c | 99 (75) |
| Ventilator-free days, 30 d, median (IQR 25th, 75th) | 28 (26, 30)b | 28 (23, 30)c | 27 (17.5, 30) |
| ICU-free days, 30 d, median (IQR 25th, 75th) | 25 (19, 27)b | 23 (12, 26)c | 10 (6, 25) |
| Hospital-free days, 30 d, median (IQR 25th, 75th) | 17 (6, 23)b | 15 (1,21) | 10 (0, 20) |
| Secondary infections/Patient, mean (SD) | .4 (.8)b | .5 (.9) | .6 (.8) |
| Secondary infections/100 hospital days, mean (SD) | 2.3 (7)b | 1.8 (3.3) | 2.7 (4.6) |
| AKI, n (%) | 33 (51) | 73 (56) | 86 (65.2) |
| KDIGO stage 1 | 15 (46) | 33 (45) | 34 (40) |
| KDIGO stage 2 | 10 (30) | 27 (37) | 28 (33) |
| KDIGO stage 3 | 8 (24) | 13 (18) | 24 (27) |
| MOF frequency, n (%) by Denver Score | 4 (6)b | 17 (13) | 29 (22) |
| 30-d mortality, n (%) | 4 (6)b | 5 (4)a | 23 (17) |
| Clinical trajectory, n (%) | |||
| Early death | 2 (3) | 3 (2) | 9 (7) |
| CCI | 14 (22)b | 45 (34) | 55 (42) |
| Rapid recovery | 49 (75)b | 83 (63) | 68 (52) |
| Discharge disposition, n (%) | |||
| “Good” disposition | 53 (82)a,b | 79 (60)c | 50 (38) |
| Home | 22 (42)b | 28 (35)c | 13 (26) |
| Home care | 31 (58)b | 43 (55)c | 24 (48) |
| Rehab | 0 (0)b | 8 (10) | 13 (26) |
| “Poor” disposition, n (%) | 12 (19)a,b | 52 (40)c | 82 (62) |
| Long-term care hospital | 5 (42)b | 18 (35) | 28 (34) |
| Skilled nursing | 1 (8)a,b | 21 (40) | 28 (34) |
| Another hospital | 3 (25) | 6 (12) | 1 (1) |
| Hospice | 0 (0) | 2 (3) | 6 (7) |
| Death | 3 (25) | 5 (10)c | 19 (24) |
| No. of readmissions, mean (SD) | 1.1 (2) | 1.2 (2) | .9 (1) |
Abbreviations: AKI, acute kidney injury; CCI, chronic critical illness; IQR, interquartile range; KDIGO = Kidney Disease: Improving Global Outcomes; MOF, multiorgan failure; SD, standard deviation.
Statistical difference was labeled as follows:
Young vs middle aged.
Young vs older adults.
Middle-aged vs older adults, with statistical significance set at P < .05.
Figure 2A depicts 12-month survival estimates by age group. Compared with the younger and middle aged, the older patients had increased 3-month (9% vs 8% vs 25%), 6-month (11% vs 11% vs 30%), and 12-month (11% vs 14% vs 32%) mortality. Supplementary Table S1 lists the causes of 30 days and more than 30 days mortality by age group. Figure 2B depicts Zubrod Performance Status by age groups at baseline and at 3-, 6-, and 12-month follow-up. Baseline scores showed that pre-sepsis, the three age groups had similar good functional status (young = 1.1 ± .17; middle aged = 1.6 ± .12; older = 1.4 ± .11). Post-sepsis, the older patient group’s performance status worsened notably to a moderate disability range at 3, 6, and 12 months (3.1 ± .2, 3.0 ± .2, 2.9 ± .2). These scores are worse than the mild disability scores of the young (1.9 ± .2, 1.5 ± .2, 1.4 ± .2) and middle-aged (2.3 ± .1, 2.0 ± .1 5, 2.0 ± .2) patients. Of note, performance status did not improve over the follow-up in the older patients, but they did return close to baseline in the young patients.
Figure 2.
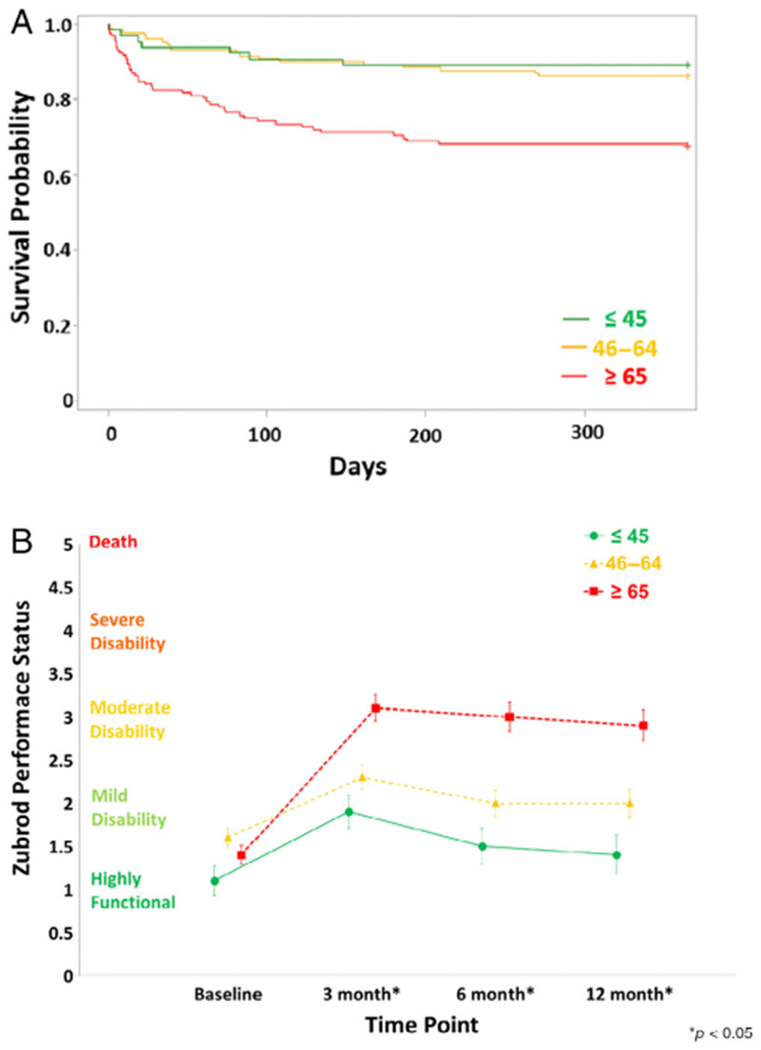
(A) The 12-month survival estimates and (B) Zubrod Performance Status at baseline and at 3-, 6-, and 12-month follow-up by age groups. Data for Zubrod Performance Status are presented as mean standard ± error with statistical significance set at P < .05.
Table 3 depicts the long-term follow-up of physical function and cognitive status by age groups. For lower extremity gait, balance, and chair standing assessed by SPPB, older patients were worse at 3 months compared with the young and middle-aged patients. At 6 months, SPPB improved somewhat in the older patients and remained worse than the younger patients. At 12 months, there was further improvement, but a statistical trend (P = .08) in the difference between the young and old remained. Handgrip strength was better for young adults than in middle-aged and older adults at 3 months. Compared with the young, it remained at similarly low levels in the middle-aged and older patients. At 12 months, the older patients remained lower than the young and middle-aged patients.
Table 3.
Long-Term Follow-Up of Physical Function and Cognitive Status for Overall and by Age Group
| Age groups n (%) | Young 65 (20%) | Middle aged 131 (40%) | Older 132 (40%) |
|---|---|---|---|
| Physical function | |||
| Total SPPB, mean (SE) | |||
| 3 mo | 7.6 (1.0)b | 5.5 (.7)c | 3.5 (.7) |
| 6 mo | 8.2 (1.1)b | 5.9 (.7) | 4 (.8) |
| 12 mo | 7.9 (1.6) | 5.1 (.9) | 4.9 (1.1) |
| Handgrip strength | |||
| 3 mo | 30.5 (2.7)a,b | 23.2 (1.5) | 22.5 (2.6) |
| 6 mo | 48.5 (5.9)a,b | 25.2 (1.3) | 22.9 (1.1) |
| 12 mo | 36.7 (2.5)a,b | 27.8 (1.6)c | 18.8 (2.2) |
| Cognitive function | |||
| Hopkins Verbal Learning Test, mean (SE) | |||
| 3 mo | |||
| Total recall | 26.3 (1.0)a,b | 20.8 (1.1) | 18.8 (1.0) |
| Delayed recall | 9.7 (.5)a,b | 6.5 (.6) | 5.4 (.7) |
| Retention | 91.7 (2.7)a,b | 72.8 (5.9) | 65.1 (6.8) |
| 6 mo | |||
| Total recall | 26.4 (1.0)a,b | 23.5 (.7)c | 19.1 (1.8) |
| Delayed recall | 9.3 (.4)a,b | 8.1 (.3)c | 5.4 (.9) |
| Retention | 92.4 (1.5)b | 86.3 (3.0)c | 60.7 (8.9) |
| 12 mo | |||
| Total recall | 28 (.9)a,b | 22 (1.9) | 20.8 (1.3) |
| Delayed recall | 9.7 (.4)a,b | 6.5 (1.1) | 6.9 (.5) |
| Retention | 91.3 (2.4)a | 67.3 (11.2) | 81 (5.1) |
| COWA, mean (SE) | |||
| 3 mo | 40.8 (2.0)a,b | 28.8 (2.4) | 28.5 (1.8) |
| 6 mo | 38.5 (1.5)a,b | 32.8 (1.4) | 26.5 (1.8) |
| 12 mo | 37.3 (2.1)b | 31.3 (5.9) | 27 (2.5) |
| MMSE, mean (SE) | |||
| 3 mo | 90.2 (2.6)b | 84.3 (2.9) | 81.9 (3.2) |
| 6 mo | 95.2 (1.6)a,b | 89.9 (1.3)c | 78.6 (2.8) |
| 12 mo | 92.4 (2.9)b | 86.8 (4.4) | 85.1 (2.2) |
Abbreviations: COWA, Controlled Oral Word Association; MMSE, Mini-Mental State Examination; SE, standard error; SPPB, Short Physical Performance Battery.
Statistical difference was labeled as follows:
Young vs middle aged.
Young vs older adults.
Middle-aged vs older adults, with statistical significance set at P < .05.
Cognitive function assessed by HVLT was poorer at 3 months in older and middle-aged compared with young adults. Compared with the older patients, there was an improvement in the delayed recall and retention in the middle aged at 6 months, but these scores worsened to similarly low levels as seen in the older patients at 12 months and were lower than the younger patients. None of the results of cognitive function were different between the middle-aged and older adults. HVLT delayed recall improved in older adults but did not change in middle-aged and young adults. HVLT word retention improved in older adults, did not change in young adults, and trended toward a decrease in middle-aged adults over 12 months.
Similar findings were found with COWA and MMSE. The middle-aged and older patients had significantly worse scores at 3, 6, and 12 months than the younger patients, with the exception of an increase in MMSE in middle-aged patients at 6 months.
DISCUSSION
The major findings of this study were that when compared with the young and middle aged, older patients at presentation had more comorbidities (such as chronic renal disease, coronary artery disease, and chronic lung disease). These increase the risk of sepsis and likely hampered recovery. Interestingly, older patients had more intra-abdominal infections. They were more likely to present in septic shock with worse initial SOFA scores and slower resolution of organ dysfunctions. They had a notably increased 30-day mortality with fewer ICU-free days and more progression into CCI. Older patients were much more likely to have a poor disposition discharge to non-home destinations and substantially worse 12-month mortality. Zubrod Performance Status and functional tests were worse with only slight improvement at 12-month follow-up.
The findings in this article are not unexpected, but to our knowledge, this is the first prospective comprehensive characterization of the in-hospital and postdischarge long-term outcomes in sepsis survivors across age groups including objectively measured physical and cognitive function outcomes and self-reported functional status. Our results are consistent with a recently published multicenter study by Yende et al27 of 483 patients who survived hospitalization for sepsis and were followed prospectively for 12 months. Average age was greater than 60 years, and most (78%) had at least one chronic disease. Intra-abdominal was the third most common site of infection, and APACHE II score was similar to our cohort. In contrast with our ICU population, their patients had a much lower SOFA score at enrollment, and most were discharged to home.27
The explanation how aging effects the underlying pathobiology of sepsis resulting in these poor long-term outcomes is multifactorial. At baseline, advanced age increases the risk for sepsis and affects the severity of sepsis. Many potential factors including comorbidities (such as chronic lung disease and renal insufficiency), exposure to procedures, malnutrition, increased aspiration risk (from altered mental status and decreased gag/cough reflex), and immobility predispose older patients to develop sepsis. Additionally, the diagnosis of sepsis is commonly delayed in older patients because of a blunted systemic inflammatory response and the presence of comorbidities that can cause confounding symptoms.16,28 As a result, older patients are more likely to present later in the process and progress into septic shock (due to limited cardiac reserve).29 Baseline pre-sepsis sarcopenia, frailty, and cognitive disabilities all adversely affect recovery. However, the septic systemic inflammatory response can cause loss of vital muscle mass (worsening physical function) and increase microglial activation, oxidative damage, mitochondrial dysfunction, and altered synaptic plasticity in the brain (worsening cognitive function).15
ICU care inherent to sepsis (eg, bed rest, mechanical ventilation, sedatives) also has long-term adverse effects on physical and cognitive function.18 Older sarcopenic patients have anabolic resistance that makes them nonresponsive to nutritional interventions.30 Moreover, the dysregulated systemic immune response is clearly affected by aging.15,31 At baseline, older patients have alterations in adaptive immunity (decreased lymphocyte number and function) that makes them more susceptible to infectious challenges.16 Whereas younger patients are capable of returning to a balanced state of innate and adaptive immunity, older patients have difficulty returning it to homeostasis.32 Although other mechanisms are likely involved in this dyshomeostasis, ongoing UF SCIRC animal and human studies indicate that persistent emergency myelopoiesis contributes to the simultaneous low-grade inflammation (promoting catabolism and anabolic resistance) and immunosuppression (increasing the risk of secondary infections) of post-intensive care syndrome in our CCI patients.33
This persistent inflammation and immunosuppression host response is consistent with the previously cited study by Yende et al. They showed that two-thirds of their cohort exhibited this host response endotype, and these patients had more hospital readmissions, higher mortality attributable to cardiovascular disease, and higher all-cause 1-year mortality compared with the remaining patients who returned to a normal host response.27
Strengths
The present study has a number of strengths. First, the study cohort was managed by evidence-based protocols, thus reducing the confounding effect of variable care. Second, an established sepsis database designed to characterize the epidemiology of MOF was used. Third, this prospective longitudinal design included multiple objective measures to assess physical and cognitive function with in-person and phone follow-up. Fourth, the high retention rates at follow-up visits provided new information about long-term outcomes after sepsis among different age groups.
Limitations
First, this was an observational study, and thus it is difficult to differentiate causation from correlation. Logistic regression analyses by age group could provide some additional insight by adjusting for comorbidities at baseline, but the subgroups were too small to draw reliable conclusions on the effect of age.
Second, this study was performed within two surgical ICUs at a single tertiary care regional medical center. This confounds the generalizability of the observations. Third, comorbidities play an important role in the predisposition and outcomes of sepsis in aging patients. We obtained comorbidity data by concurrent chart review, but a more in-depth interview with the patient/family plus objective biomarkers (such as hemoglobin A1C for diabetes) would have allowed quantitation of poor control or severity. Fourth, we did not have information about the physical and cognitive function of patients before an admission to the ICU. Additionally, we did not have information about trauma to upper and lower limbs at admission and eventual readmission for an orthopedic procedure during follow-up that might have influenced the functional testing at follow-up. However, only a small fraction (9%) of the cohort suffered trauma.
In conclusion, as overall early mortality after sepsis has decreased, a substantial portion (approximately 20%) of older patients still die within 30 days. However, for geriatric medicine providers, it is noteworthy that older ICU sepsis survivors are likely to be discharged to non-home destination with severe non-recovering disabilities and an ongoing 1-year mortality more than 30%.
Supplementary Material
Additional Supporting Information may be found in the online version of this article.
Supplementary Figure S1: Consolidated Standards of Reporting Trials (CONSORT) diagram and retention rates of 12-month follow-up.
Supplementary Figure S2: (A) The 12-month survival estimates and (B) Zubrod Performance Status at baseline and at 3-, 6-, and 12-month follow-up (B) by age groups including a breakdown of the older group into 65-74 and ≥75 years.
Supplementary Figure S3: SOFA scores over 14 days by age groups including a breakdown of the older group into 65-74 and ≥75 years.
Supplementary Table S1: Post-sepsis Causes of Death by All Age Groups.
Supplementary Table S2: Baseline Demographics, Characteristics of the Inciting Septic Event, and Predisposition by Older Age Groups.
Supplementary Table S3: Admission Service and Types of Sepsis Source Control by all Age Groups.
Supplementary Table S4: Characteristics of the Hospital and Post-discharge Outcomes by Older Age Groups.
Supplementary Table S5: Long-term Follow-up of Physical Function and Cognitive Status for Overall and by Older Age Groups.
ACKNOWLEDGMENTS
We would like to acknowledge the invaluable contributions of the University of Florida Sepsis and Critical Illness Research Center staff including Jennifer Lanz, Ruth Davis, Jillianne Brakenridge, Ashley McCray, Bridget Baisden, Ricky Ungaro, Dina Nacionales, Marvin Dirain, Tabitha Johns, and Ada Malcolm.
Financial Disclosure: This work was supported by the National Institute of General Medical Sciences (NIGMS) grants: R01 GM-113945 (Philip A. Efron) and P50 GM-111152 (to all authors) awarded by the NIGMS, AHA Career Development Award 18CDA34080001 (Robert T. Mankowski). Support was also provided by National Institute on Aging grants R03 AG056444 (Scott C. Brakenridge) and P30 AG028740 (Robert T. Mankowski, Stephen D. Anton, and Christiaan Leeuwenburgh).
Sponsor’s Role: The funding bodies had no role in the design of the study, collection, analysis and interpretation of data, and writing the manuscript.
Footnotes
Conflict of Interest: The authors have declared no conflicts of interest for this article.
REFERENCES
- 1.Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319(1):62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–873. [DOI] [PubMed] [Google Scholar]
- 3.McKinley BA, Moore LJ, Sucher JF, et al. Computer protocol facilitates evidence-based care of sepsis in the surgical intensive care unit. J Trauma. 2011;70(5):1153–1166; discussion 1166-1167. [DOI] [PubMed] [Google Scholar]
- 4.El Solh AA, Akinnusi ME, Alsawalha LN, Pineda LA. Outcome of septic shock in older adults after implementation of the sepsis “bundle.” J Am Geriatr Soc. 2008;56(2):272–278. [DOI] [PubMed] [Google Scholar]
- 5.Brakenridge SC, Efron PA, Cox MC, et al. Current epidemiology of surgical sepsis: discordance between inpatient mortality and 1-year outcomes. Ann Surg. 2019;270(3):502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milbrandt EB, Eldadah B, Nayfield S, Hadley E, Angus DC. Toward an integrated research agenda for critical illness in aging. Am J Respir Crit Care Med. 2010;182(8):995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. [DOI] [PubMed] [Google Scholar]
- 8.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. [DOI] [PubMed] [Google Scholar]
- 9.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anton SD, Woods AJ, Ashizawa T, et al. Successful aging: advancing the science of physical independence in older adults. Ageing Res Rev. 2015; 24(Pt B):304–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore FA, Moore EE, Billiar TR, Vodovotz Y, Banerjee A, Moldawer LL. The role of NIGMS P50 sponsored team science in our understanding of multiple organ failure. J Trauma Acute Care Surg. 2017;83(3):520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji Y, Cheng B, Xu Z, et al. Impact of sarcopenic obesity on 30-day mortality in critically ill patients with intra-abdominal sepsis. J Crit Care. 2018;46:50–54. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015; 14(1):58–74. [DOI] [PubMed] [Google Scholar]
- 14.Ehlenbach WJ, Sonnen JA, Montine TJ, Larson EB. Association between sepsis and microvascular brain injury. Crit Care Med. 2019;47(11):1531–1538. [DOI] [PubMed] [Google Scholar]
- 15.Barter J, Kumar A, Stortz JA, et al. Age and sex influence the hippocampal response and recovery following sepsis. Mol Neurobiol. 2019;56(12):8557–8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowe TA, McKoy JM. Sepsis in older adults. Infect Dis Clin North Am. 2017;31(4):731–742. [DOI] [PubMed] [Google Scholar]
- 17.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–1283. [DOI] [PubMed] [Google Scholar]
- 18.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson S, Ruokonen E, Varpula T, Ala-Kokko TI, Pettila V, Finnsepsis SG. Long-term outcome and quality-adjusted life years after severe sepsis. Crit Care Med. 2009;37(4):1268–1274. [DOI] [PubMed] [Google Scholar]
- 20.Gardner AK, Ghita GL, Wang Z, et al. The development of chronic critical illness determines physical function, quality of life, and long-term survival among early survivors of sepsis in surgical ICUs. Crit Care Med. 2019;47(4):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loftus TJ, Mira JC, Ozrazgat-Baslanti T, et al. Sepsis and Critical Illness Research Center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017;7(7):e015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 23.West HJ, Jin JO. JAMA oncology patient page. Performance status in patients with cancer. JAMA Oncol. 2015;1(7):998. [DOI] [PubMed] [Google Scholar]
- 24.Woods SP, Scott JC, Dawson MS, et al. Construct validity of Hopkins Verbal Learning Test-revised component process measures in an HIV-1 sample. Arch Clin Neuropsychol. 2005;20(8):1061–1071. [DOI] [PubMed] [Google Scholar]
- 25.Ross TP, Calhoun E, Cox T, Wenner C, Kono W, Pleasant M. The reliability and validity of qualitative scores for the Controlled Oral Word Association Test. Arch Clin Neuropsychol. 2007;22(4):475–488. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 27.Yende S, Kellum JA, Talisa VB, et al. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open. 2019;2(8):e198686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kale SS, Yende S. Effects of aging on inflammation and hemostasis through the continuum of critical illness. Aging Dis. 2011;2(6):501–511. [PMC free article] [PubMed] [Google Scholar]
- 29.Fleg JL, Strait J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail Rev. 2012;17(4-5):545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haran PH, Rivas DA, Fielding RA. Role and potential mechanisms of anabolic resistance in sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3(3):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stortz JA, Hollen MK, Nacionales DC, et al. Old mice demonstrate organ dysfunction as well as prolonged inflammation, immunosuppression, and weight loss in a modified surgical sepsis model. Crit Care Med. 2019;47(11):e919–e929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76(1):21–29; discussion 29-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horiguchi H, Loftus TJ, Hawkins RB, et al. Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front Immunol. 2018;9:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supplementary Figure S1: Consolidated Standards of Reporting Trials (CONSORT) diagram and retention rates of 12-month follow-up.
Supplementary Figure S2: (A) The 12-month survival estimates and (B) Zubrod Performance Status at baseline and at 3-, 6-, and 12-month follow-up (B) by age groups including a breakdown of the older group into 65-74 and ≥75 years.
Supplementary Figure S3: SOFA scores over 14 days by age groups including a breakdown of the older group into 65-74 and ≥75 years.
Supplementary Table S1: Post-sepsis Causes of Death by All Age Groups.
Supplementary Table S2: Baseline Demographics, Characteristics of the Inciting Septic Event, and Predisposition by Older Age Groups.
Supplementary Table S3: Admission Service and Types of Sepsis Source Control by all Age Groups.
Supplementary Table S4: Characteristics of the Hospital and Post-discharge Outcomes by Older Age Groups.
Supplementary Table S5: Long-term Follow-up of Physical Function and Cognitive Status for Overall and by Older Age Groups.


