Abstract
Purpose
Emphysema and chronic obstructive lung disease were previously identified as major risk factors for severe disease progression in COVID-19. Computed tomography (CT)-based lung-density analysis offers a fast, reliable, and quantitative assessment of lung density. Therefore, we aimed to assess the benefit of CT-based lung density measurements to predict possible severe disease progression in COVID-19.
Material and methods
Thirty COVID-19-positive patients were included in this retrospective study. Lung density was quantified based on routinely acquired chest CTs. Presence of COVID-19 was confirmed by reverse transcription polymerase chain reaction (RT-PCR). Wilcoxon test was used to compare two groups of patients. A multivariate regression analysis, adjusted for age and sex, was employed to model the relative increase of risk for severe disease, depending on the measured densities.
Results
Intensive care unit (ICU) patients or patients requiring mechanical ventilation showed a lower proportion of medium- and low-density lung volume compared to patients on the normal ward, but a significantly larger volume of high-density lung volume (12.26 dl IQR 4.65 dl vs. 7.51 dl vs. IQR 5.39 dl, p = 0.039). In multivariate regression analysis, high-density lung volume was identified as a significant predictor of severe disease.
Conclusions
The amount of high-density lung tissue showed a significant association with severe COVID-19, with odds ratios of 1.42 (95% CI: 1.09-2.00) and 1.37 (95% CI: 1.03-2.11) for requiring intensive care and mechanical ventilation, respectively. Acknowledging our small sample size as an important limitation; our study might thus suggest that high-density lung tissue could serve as a possible predictor of severe COVID-19.
Keywords: CT scan, spiral CT, SARS Coronavirus, SARS-CoV
Introduction
The recent pandemic outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) poses an increasing challenge to healthcare systems worldwide. Apart from an early detection of the resulting coronavirus disease 2019 (COVID-19), efficient and fast identification of patients at risk of severe disease progression is crucial for triage and successful patient treatment. In many hospitals, computed tomography (CT) is used to quickly identify patients with suspected COVID-19 [1,2]. Although CT is not as accurate as reverse transcription polymerase chain reaction (RT-PCR) of throat or deep nasal swaps, it may enable faster results than RT-PCR and might thus help to detect affected patients earlier, isolate them in time, and initiate further treatment. Furthermore, CT is also important for disease follow-up [3]. In addition to the detection of pulmonary infiltrates, CT also allows for an evaluation of the unaffected lung parenchyma and identification of additional comorbidities such as emphysema or fibrosis.
Several studies have recently been conducted to identify risk factors for severe progression of COVID-19 with treatment in the intensive care unit (ICU) and the potential need for invasive ventilation and/or even extracorporeal membrane oxygenation [4-7]. Among those risk factors, emphysema, and chronic obstructive lung disease were described as particularly relevant [4,8,9].
The use of CT for lung density analysis is already established and superior to subjective visual assessment [10,11]. Early quantification of reduced lung density as a correlate of emphysema based on routinely acquired chest CT images could therefore be useful in identifying patients at risk of severe disease progression. Therefore, this study aims to investigate the association between CT-based measurements of lung density and COVID-19 severity.
Material and methods
Data for this retrospective study were collected from non-contrast low-dose chest CTs, routinely acquired between March 2020 and April 2020. We included patients who presented at the emergency department of our institution and in whom SARS-CoV-2 was confirmed by RT-PCR. This study was approved by the institutional review board (EA 4/140/17).
Computed tomography imaging protocol
Patients were positioned in supine position and asked to hold their breath in deep inspiration during image acquisition. Two different types of scanners were used at our institution, an 80-slice scanner (Aquilion Prime, Canon Medical Systems Cooperation, Otawara, Japan) and a 64-slice scanner (Lightspeed VCT, General Electric, Boston, Massachusetts, United States), to perform a low-dose examination of the chest in patients with suspected SARS-CoV-2 infection. The imaging protocol for the Canon Aquilion Prime was as follows: 0.27 s rotation time, 100 kV tube voltage, automatic modulation of tube current between 10 and 100 mA, a noise index of 27, pitch factor of 1.388, and a reconstructed slice thickness of 0.5 mm. For the GE Lightspeed VCT, rotation time was 0.35 s, 100 kV tube voltage, automatic tube current modulation between 10 and 100 mA, noise index of 30, pitch factor of 1.375, and a reconstructed slice thickness of 0.625 mm. Image reconstruction was performed using a lung- and soft-tissue kernel (Canon Aquilion Prime: Fc01 and Fc85, GE Lightspeed VCT “standard” and “lung”) and moderate iterative reconstruction.
Data acquisition
Lung density measurements were performed using Vital’s Vitrea™ Advanced Visualisation applications (Canon Medical Systems Cooperation, Otawara, Japan). The whole lung was quantified based on predefined thresholds: Lung tissue with densities between –1024 and –920 Hounsfield units (HU) was defined as low density, between –920 and –720 HU as medium density, and lung tissue between –720 and 0 HU as high density. Data were then exported as structured text files for further analysis.
Follow-up chest radiographs were used to obtain clinical outcome data. By identifying the referring ward in our radiology information system, we identified patients requiring ICU treatment; the need of invasive mechanical ventilation was assessed through interpretation of chest radiographs. Further patient characteristics such as weight or height, sex, or age were stored in tabular form as comma- separated values.
Statistical analysis
Statistical analysis was performed with the “R” statistical programming language and the “tidyverse” library [12,13]. Due to the small sample size, all variables were expressed as median and interquartile range (IQR). The Wilcoxon rank sum test was used to compare two groups of variables. A multivariate linear regression analysis, corrected for age and sex, was conducted to calculate odds ratios for lung density measurements with mechanical ventilation and ICU treatment as outcome parameters. A p-value of < 0.05 was considered statistically significant.
Results
In this retrospective study, 30 chest CT images of patients with COVID-19, confirmed by RT-PCR, were analysed. The average age was 67.85 IQR: 14.73 years. 57% of the patients (n = 17) were male (average age 67.2 IQR: 15.6 years) and 43% (n = 13) were female (70.6 IQR: 15.5 years). During treatment at our site, 43% (n = 13) of patients had to be transferred to the ICU. 23% of all patients (n = 7) also required mechanical ventilation at some point during their stay in the hospital. None of the patients was mechanically ventilated at the time of the CT scan. Table 1 gives an overview of the patient characteristics.
Table 1.
Distribution of patient characteristics
| Parameter | All patients | Male | Female |
|---|---|---|---|
| Age | 67.85 IQR: 14.73 | 67.2 IQR: 15.6 | 70.6 IQR: 15.5 |
| n | 30 | 17 | 13 |
| Intensive care treatment | 13 | 9 | 4 |
| Mechanical ventilation | 7 | 6 | 1 |
| Overall lung volume (dl) | 27.10 IQR: 12.54 | 26.87 IQR: 21.18 | 27.34 IQR: 6.46 |
| Low-density volume (dl) | 4.13 IQR: 5.36 | 4.39 IQR: 5.58 | 4.09 IQR: 4.01 |
| Low-density percentage | 0.16 IQR: 0.13 | 0.16 IQR: 0.14 | 0.16 IQR: 0.12 |
| Medium-density volume (dl) | 22.65 IQR: 12.91 | 22.58 IQR: 15.38 | 22.72 IQR: 9.72 |
| High-density volume (dl) | 10.23 IQR: 5.39 | 10.84 IQR: 4.99 | 6.77 IQR: 6.02 |
| Low-density index | 0.16 IQR: 0.13 | 0.16 IQR: 0.14 | 0.16 IQR: 0.12 |
| PD15 (g/l) | 77.0 IQR: 35.5 | 77 IQR: 34 | 76 IQR: 35 |
PD15 – 15th percentile of lung density
Lung density and requirement of intensive care treatment
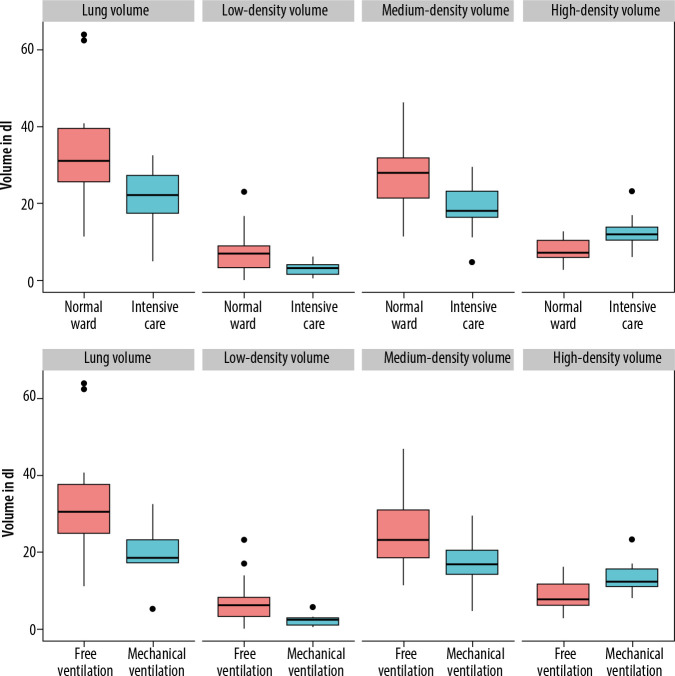
Patients requiring treatment on the ICU had significantly lower overall lung volume compared to patients on the normal ward (22.22 IQR: 13.98 dl vs. 31.16 IQR: 9.99 dl) (p = 0.003). Surprisingly, patients on the ICU showed a lower proportion of medium- and low-density lung volume compared to patients on the normal ward (medium-density volume: 18.13 IQR: 6.76 dl vs. 27.92 IQR: 10.4 dl, p = 0.012; low-density volume: 3.14 IQR: 5.73 dl vs. 6.92 IQR: 2.61 dl, p = 0.014). Regarding high-density lung volume, patients on the ICU showed a significantly higher proportion of high-density lung volume compared to patients on the normal ward (11.96 IQR: 3.62 dl vs. 7.21 IQR: 4.4 dl, p = 0.007). There was no sex-specific difference between low-, medium-, or high-density volume (p-values 0.45 and 0.89). The photodiode (PD) 15 (15th percentile of lung density) was lower for normal ward patients (62 IQR: 52 g/l) compared to ICU patients (93 IQR: 22 g/l), indicating an overall lower lung density of patients on the normal ward. Figure 1 and Table 2 give an overview of lung density and requirement of ICU treatment.
Figure 1.
Lung density distributions in patients
Table 2.
Distribution of lung density measurements in normal ward and intensive care unit
| Parameter | Normal ward | Intensive care unit | p |
|---|---|---|---|
| Overall lung volume (dl) | 31.16 IQR: 9.99 | 22.22 IQR: 13.98 | 0.003* |
| Low-density volume (dl) | 6.92 IQR: 2.61 | 3.14 IQR: 5.73 | 0.014* |
| Low-density percentage | 0.22 IQR: 0.16 | 0.10 IQR: 0.08 | 0.076 |
| Medium-density volume (dl) | 27.92 IQR: 10.40 | 18.13 IQR: 6.76 | 0.012* |
| High-density volume (dl) | 7.21 IQR: 4.40 | 11.96 IQR: 3.62 | 0.007* |
| Low-density index | 0.22 IQR: 0.16 | 0.10 IQR: 0.08 | 0.076 |
| PD15 (g/l) | 62 IQR: 52 | 93 IQR: 22 | 0.301 |
Significant p-values are indicated by asterisks. PD15 – 15th percentile of lung density
Lung density and requirement of mechanical ventilation
Out of 11 patients requiring invasive ventilation during treatment of COVID-19, only one patient was female. Therefore, no subgroup analysis was performed to compare male and female patients. Patients who later required mechanical ventilation had a lower initial total lung volume of 18.42 IQR: 12.6 dl compared to patients breathing freely (30.4 IQR: 5.96 dl, p = 0.012). There was also a significant difference in low-density volume between the two groups (5.98 IQR: 1.7 dl vs. 2.22 IQR: 5.01 dl, p = 0.019); however, the percentage of low-density volume in relation to overall lung volume was not significantly different (19% vs. 12%, p = 0.13). The high-density volume was significantly larger in patients who required invasive ventilation during hospitalisation (7.51 IQR: 5.39 dl vs. 12.26 IQR: 4.65 dl, p = 0.039). PD15 was lower in freely breathing patients (71 IQR: 33.5 g/l) than in mechanically ventilated patients (96 IQR: 20.5 g/l), but this difference did not reach the predefined significance level (p = 0.41). Figure 1 and Table 3 give an overview on lung-density and the requirement of ICU treatment.
Table 3.
Distribution of lung volumes in freely breathing and mechanically ventilated patients
| Parameter | Free breathing | Mechanical ventilation | p | |
|---|---|---|---|---|
| All patients | ||||
| Overall lung volume (dl) | 30.4 IQR: 5.96 | 18.42 IQR: 12.60 | 0.012* | |
| Low-density volume (dl) | 5.98 IQR: 1.70 | 2.22 IQR: 5.01 | 0.019* | |
| Medium-density volume (dl) | 23.17 IQR: 12.52 | 16.76 IQR: 6.40 | 0.046* | |
| High-density volume (dl) | 7.51 IQR: 5.39 | 12.26 IQR: 4.65 | 0.039* | |
| Low-density index | 0.18 IQR: 0.14 | 0.08 IQR: 0.05 | 0.130 | |
| PD15 (g/l) | 71 IQR: 33.50 | 96 IQR: 20.50 | 0.405 | |
| Male | ||||
| Overall lung volume (dl) | 32.73 IQR: 15.37 | 19.16 IQR: 7.05 | 0.040* | |
| Low-density volume (dl) | 6.38 IQR: 1.25 | 2.22 IQR: 4.65 | 0.062* | |
| Medium-density volume (dl) | 0.2 IQR: 0.12 | 0.08 IQR: 0.07 | 0.234 | |
| High-density volume (dl) | 29.59 IQR: 14.03 | 16.85 IQR: 5.76 | 0.094 | |
| Low-density index | 10.39 IQR: 4.82 | 13.11 IQR: 5.43 | 0.121 | |
| PD15 (g/l) | 0.2 IQR: 0.12 | 0.08 IQR: 0.07 | 0.234 | |
Significant p-values are indicated by asterisks. PD15 – 15th percentile of lung density
Influence of lung-density on requiring intensive care treatment or invasive ventilation
In a multivariate regression analysis, adjusted for age and sex, different measurements for lung density were analysed to determine how they affect the probability of a patient requiring treatment in the ICU and/or mechanical ventilation. Because patients on the ICU showed a lower overall lung volume, an increase of lung volume from 20 dl to 30 dl would be associated with a 26% lower risk for requiring intensive care treatment. On the other hand, an increase in high-density volume from 10 dl to 20 dl led to a 48% increased risk for treatment on the ICU. However, the 95% CI for all measurements except high-density volume showed odds below and above 1; hence, no clear positive or negative influence of the measured values could be determined with certainty. Table 4 gives an overview of odds ratios derived from logistic regression analysis.
Table 4.
Overview of the calculated odds ratios
| Parameter | Odds ratio | 95% CI | |
|---|---|---|---|
| Requiring intensive care | |||
| Overall lung volume (dl) | 0.866 | 0.74-0.95 | |
| Low-density volume (dl) | 0.699 | 0.47-0.91 | |
| Medium-density volume (dl) | 0.002 | 0-2.97 | |
| High-density volume (dl) | 0.878 | 0.77-0.97 | |
| Low-density index | 1.416 | 1.09-2.00 | |
| PD15 (g/l) | 1.009 | 0.99-1.03 | |
| Mechanical ventilation | |||
| Overall lung volume (dl) | 0.882 | 0.76-0.97 | |
| Low-density volume (dl) | 0.649 | 0.35–0.93 | |
| Medium-density volume (dl) | 0.002 | 0-19.92 | |
| High-density volume (dl) | 0.880 | 0.75-0.98 | |
| Low-density index | 1.366 | 1.03-2.11 | |
| PD15 (g/l) | 1.007 | 0.98-1.04 | |
PD15 – 15th percentile of lung density
Discussion
In this study, we investigated whether quantification of lung density based on CT images would correlate with the severity of COVID-19. Our hypothesis was that patients with emphysema and subsequently reduced lung density were at increased risk of developing a more serious infection and would therefore require treatment on the ICU and/or invasive ventilation. However, this hypothesis could not be confirmed. Instead, only higher lung density was associated with severe COVID-19, probably resulting from an increased proportion of pulmonary infiltrates.
It is likely that patients with chronic obstructive pulmonary disease (COPD) might suffer from more severe disease progression. Leung et al. stated that this might be due to poor lung reserves in the case of infection and an overexpression of the ACE-2 receptor, which plays an important role as an entry receptor for the fusion of SARS-CoV-2 and airway epithelia [14,15]. A recent meta-analysis by Zhao et al. found a fourfold higher risk of developing severe COVID-19 in COPD patients, and an even more elevated risk for COPD patients remaining active smokers [16]. In addition, earlier studies have shown that acute exacerbation comes with an increased risk of cardiovascular events, further deteriorating the patients’ prognosis, which might be another important risk factor in specific COPD phenotypes with frequent exacerbations [17]. However, further research regarding the association between COVID-19 progression and COPD-phenotypes (e.g. with predominant emphysema or asthma overlap) are needed because the currently available literature is sparse.
Previous studies aimed to identify patients with emphysema and COPD, at risk for severe COVID, using CT. Lyu et al. previously investigated the performance of chest CT for the identification of critical cases [18]. They observed 51 patients and found both a higher lung density (defined by > 779 HU) and a reduced volume of the medium-density lung in critically ill patients (n = 24) [18]. While we also observed a higher lung density in critically ill patients (defined by 720–0 HU), we found an increased volume of medium-density lung in severely affected patients. Colombi et al. demonstrated that a decrease in well aired lung tissue corresponded to a higher likelihood of transmission to the ICU or death [19]. Yu et al. analysed the distribution, size, and density of ground glass opacities in patients affected with COVID-19 [20]. They were able to show that an increase in the size and density of infiltrations corresponded to a worse clinical outcome [20]. This is also consistent with our observations that larger areas of opacification lead to an overall increase in the volume of high-density lung tissue. Ground class opacities are a typical CT sign of viral infection, including COVID-19 [21]. The pathophysiological correlation with ground glass opacities is either interstitial oedema and/or incomplete filling of the alveolar space with fluid [22]. As a result, the overall density of the lung tissue increases.
Compared to lung-density values reported in literature for lung-healthy patients, the population of this study showed a normal distribution of low-density lung percentage and no higher prevalence of emphysema compared to the normal population. Cheng et al. analysed 2351 lung-healthy patients for lung density on CT and found a low-density percentage of 17–19% [23], comparable to the values reported in Table 1. It is therefore possible that in a patient population with lung disease and a significantly higher proportion of emphysema, CT-based quantification and analysis of low-density lung-volume could still be useful to model the severity of COVID-19. Another confounder and thus limitation of the present study results from the fact that patients severely affected by COVID-19 were unable to hold their breath properly and/or breathe in deeply during the CT examination, possibly causing the lower overall lung volume of patients in the ICU and/or with mechanical ventilation. It is also possible that a larger lung volume allowed patients to tolerate larger areas of pulmonary infiltrates, before the lung volume required for gas exchange became too small. Furthermore, other factors, such as increased intra-abdominal pressure in obese patients, might also have contributed to the reduction in lung volume.
If the lung parenchyma expands during deep inspiration, the overall lung density decreases [24,25]. This could also explain why, contrary to our previous assumptions, the lung density as well as the percentage of low-density lung in less severely ill patients tended to be lower. In line with this, PD15 did not change between both groups, suggesting different depth of inspiration.
Furthermore, it is possible, that patients with severe emphysema, but also with severe infection and large pulmonary infiltrates, may have shown falsely high lung density values. The inclusion of confirmatory spirometry for assessing lung volume would have been desirable for validation of the measurements obtained from CT. However, having all patients with COVID-19 undergo spirometry was not possible because of hygiene regulations and the often poor condition of the patients.
Additional limitations of the present work include the retrospective design and the small sample size. Finally, because many patients were still hospitalised at the time of analysis, further endpoints such as the duration of ICU treatment or intubation could not be evaluated. It should also be emphasised that lung density is not the only risk factor for severe COVID-19, but rather one of many risk factors, some of which have been identified as particularly important. One of these risk factors is obesity; it was shown that an increased body mass index or increased areas of visceral fatty tissue was associated with more severe COVID-19 [26,27]. Another important risk factor is ethnicity; it was observed that especially Black and Indigenous Americans were at higher risk for COVID-19 infection, severe disease progression, and death [28]. This underlines that more attention needs to be paid to associations between COVID-19 progression and ethnicity [29]. However, because the population in the present study was predominantly white and the sample size was small, it was not possible to take ethnicity into account.
Conclusions
Contrary to our expectations and to previous research, larger proportions of medium- and low-density lung tissue showed no association with severe course of COVID-19. Only high-density lung was identified as a possible predictor of severe COVID-19, probably due to the higher proportion of pulmonary infiltrates in critically ill patients.
Acknowledgments
LCA is grateful for her participation in the BIH Charité – Junior Clinician and Clinician Scientist Program funded by the Charité – Universitaetsmedizin Berlin and the Berlin Institute of Health.
Conflict of interest
B. Hamm has received research grants for the Department of Radiology, Charité-Universitätsmedizin Berlin from the following companies and institutions: (1) Abbott, (2) Actelion Pharmaceuticals, (3) Bayer Schering Pharma, (4) Bayer Vital, (5) BRACCO Group, (6) Bristol-Myers Squibb, (7) Charite Research Organisation GmbH, (8) Deutsche Krebshilfe, (9) Dt. Stiftung für Herzforschung, (10) Essex Pharma, (11) EU Programmes, (12) FibrexMedical Inc, (13) Focused Ultrasound Surgery Foundation, (14) Fraunhofer Gesellschaft, (15) Guerbet, (16) INC Research, (17) lnSightec Ud, (18) IPSEN Pharma, (19) Kendlel MorphoSys AG, (20) Lilly GmbH, (21) Lundbeck GmbH, (22) MeVis Medical Solutions AG, (23) Nexus Oncology, (24) Novartis, (25) Parexel Clinical Research Organisation Service, (26) Perceptive, (27) Pfizer GmbH, (28) Philipps, (29) Sanofis-Aventis S.A., (30) Siemens, (31) Spectranetics GmbH, (32) Terumo Medical Corporation, (33) TNS Healthcare GMbH, (34) Toshiba, (35) UCB Pharma, (36) Wyeth Pharma, (37) Zukunftsfond Berlin (TSB), (38) Amgen, (39) AO Foundation, (40) BARD, (41) BBraun, (42) Boehring Ingelheimer, (43) Brainsgate, (44) PPD (Clinical Research Organisation), (45) CELLACT Pharma, (46) Celgene, (47) CeloNova Bio-Sciences, (48) Covance, (49) DC Deviees, Ine. USA, (50) Ganymed, (51) Gilead Sciences, (52) GlaxoSmithKline, (53) ICON (Clinical Research Organisation), (54) Jansen, (55) LUX Bioseienees, (56) MedPass, (57) Merek, (58) Mologen, (59) Nuvisan, (60) Pluristem, (61) Quintiles, (62) Roehe, (63) SehumaeherGmbH (Sponsoring eines Workshops), (64) Seattle Geneties, 65) Symphogen, (66) TauRx Therapeuties Ud, (67) Accovion, (68) AIO: Arbeitsgemeinschaft Internistische Onkologie, (69) ASR Advanced sleep research, (70) Astellas, (71) Theradex, (72) Galena Biopharma, (73) Chiltern, (74) PRAint, (75) lnspiremd, (76) Medronic, (77) Respicardia, (78) Silena Therapeutics, (79) Spectrum Pharmaceuticals, (80) St Jude, (81) TEVA, (82) Theorem, (83) Abbvie, (84) Aesculap, (85) Biotronik, (86) Inventivhealth, (87) ISATherapeutics, (88) LYSARC, (89) MSD, (90) Novocure, (91) Ockham Oncology, (92) Premier-Research, (93) Psi-cro, (94) Tetec-ag, (95) Winicker-Norimed, (96) Achaogen Inc, (97) ADIR, (98) Astra Zeneca AB, (99) Demira Inc, (100) Euroscreen S.A., (101) Galmed Research and Development Ltd, (102) GETNE, (103) Guidant Europe NV, (104) Holaira Inc, (105) Immunomedics Inc, (106) Innate Pharma, (107) Isis Pharmaceuticals Inc, (108) Kantar Health GmbH, (109) MedImmune Inc, (110) Medpace Germany GmbH (CRO), (111) Merrimack Pharmaceuticals Inc, (112) Millenium Pharmaceuticals Inc, (113) Orion Corporation Orion Pharma, (114) Pharmacyclics Inc, (115) PIQUR Therapeutics Ltd, (116) Pulmonx International Sárl, (117) Servier (CRO), (118) SGS Life Science Services (CRO), and (119) Treshold Pharmaceuticals Inc. S.M. Niehues has received funding from Bayer Vital, Bracco, Guerbet, Canon Medical Systems and Teleflex medical. The funding had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The remaining authors declare that they have no conflicts of interest and did not receive any funds. There are no patents, products in development, or marketed products to declare.
References
- 1.Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol 2020; 214: 1280-1286. [DOI] [PubMed] [Google Scholar]
- 2.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; 296: 200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology 2020; 295: 200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain V, Yuan JM. Systematic review and meta-analysis of predictive symptoms and comorbidities for severe COVID-19 infection. medRxiv 2020. 10.1101/2020.03.15.20035360. [DOI] [PMC free article] [PubMed]
- 5.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan M, Yin W, Tao Z, et al. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One 2020; 15: e0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, He Y, Yang H, et al. Development and validation a nomogram for predicting the risk of severe COVID-19: a multi-center study in Sichuan, China. PLoS One 2020; 15: e0233328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis 2020; 94: 91-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One 2020; 15: e0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bankier AA, Maertelaer VD, Keyzer C, et al. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology 1999; 211: 851-858. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka S, Yamashiro T, Washko GR, et al. Quantitative CT assessment of chronic obstructive pulmonary disease. RadioGraphics 2010; 30: 55-66. [DOI] [PubMed] [Google Scholar]
- 12.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing 2013. [Google Scholar]
- 13.Wickham H. Tidyverse: easily install and load the ‘tidyverse’. R package version 2017; 1: 2017. [Google Scholar]
- 14.Leung JM, Niikura M, Yang CWT, et al. COVID-19 and COPD. Eur Respir J 2020; 56: 2002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J 2020; 55: 2000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol 2020; 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunisaki KM, Dransfield MT, Anderson JA, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med 2018; 198: 51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyu P, Liu X, Zhang R, et al. The performance of chest CT in evaluating the clinical severity of COVID-19 pneumonia: identifying critical cases based on CT characteristics. Invest Radiol 2020: 55: 412-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colombi D, Bodini FC, Petrini M, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology 2020; 296: E86-E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Q, Wang Y, Huang S, et al. Multicenter cohort study demonstrates more consolidation in upper lungs on initial CT increases the risk of adverse clinical outcome in COVID-19 patients. Theranostics 2020; 10: 5641-5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, Pan J, Teng D, et al. Interpretation of CT signs of 2019 novel coronavirus (COVID-19) pneumonia. Eur Radiol 2020; 30: 5455-5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diederich S. High resolution computed tomography of the lungs: ground glass opacity and its differential diagnosis. Radiologe 2010; 50: 1141-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng T, Li Y, Pang S, et al. Normal lung attenuation distribution and lung volume on computed tomography in a Chinese population. Int J Chron Obstruct Pulmon Dis 2019; 14: 1657-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert HB, Robert AW, Kirk G, et al. Lung density changes with growth and inflation. Chest 2015; 148: 995-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas M, Hamm B, Niehues SM. Automated lung volumetry from routine thoracic CT scans: how reliable is the result? Acad Radiol 2014; 21: 633-638. [DOI] [PubMed] [Google Scholar]
- 26.Petersen A, Bressem K, Albrecht J, et al. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism 2020; 110: 154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng KI, Gao F, Wang X-B, et al. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism 2020; 108: 154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yancy CW. COVID-19 and African Americans. JAMA 2020; 323: 1891-1892. [DOI] [PubMed] [Google Scholar]
- 29.Pareek M, Bangash MN, Pareek N, et al. Ethnicity and COVID-19: an urgent public health research priority. Lancet 2020; 395: 1421-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]