Dear Editor
As the world struggles to cope with coronavirus disease 2019 (COVID-19), deciphering the immunological protection is imperative to develop an efficient vaccine, convalescent plasma-based therapies and revise mitigation measures. Once severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) enters the host via contact, droplet or aerosol-mediated transmission [1], the non-specific innate immune response is followed by antigen-specific adaptive immunity mediated by B cells (humoral immunity) producing neutralizing antibodies and T cells (cellular immunity) including CD8+, CD4+ and regulatory T cells.
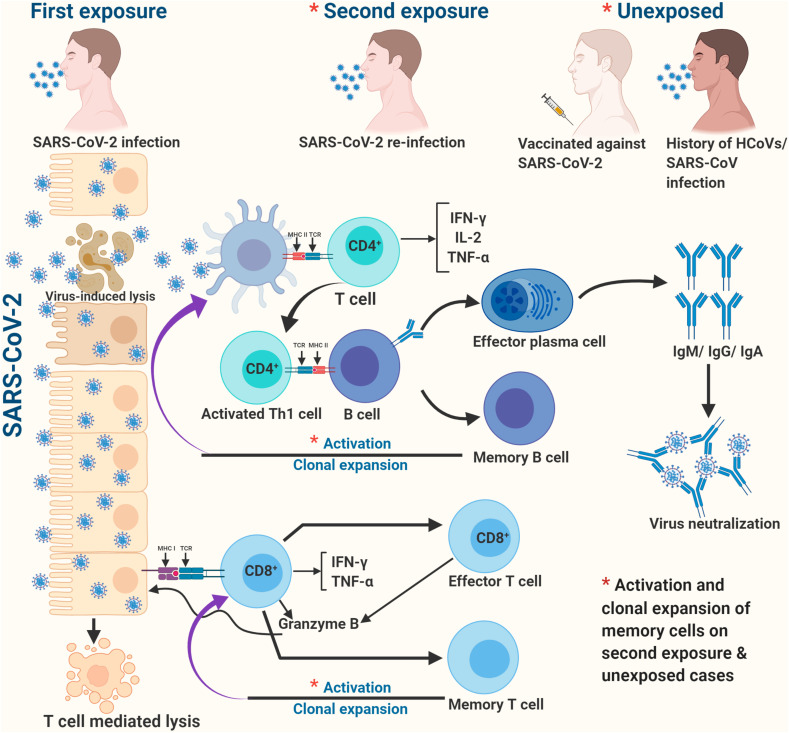
After the first encounter with SARS-CoV-2, B and T cells retain the immunological memory, which enables quicker and stronger response (protective immunity) on the subsequent encounter with same or closely-related (cross-reaction) pathogen and might contribute to herd immunity (Figure 1 ). However, it's unknown whether antibodies or T cells confer protective immunity to SARS-CoV-2, and if so, it's strength and durability. Efforts are underway to identify the correlates of immunological protection through studies on animal models, variable disease outcomes and closely-related coronaviruses.
Fig. 1.
A hypothetical illustration of protective immunity against COVID-19.
The individual variations in protective immunity can occur due to genetic differences. The human leukocyte antigen (HLA) molecules of a haplotype having more binding specificities to SARS-CoV-2 peptides on antigen-presenting cells could cause genetic advantage [2], and these loci need to be identified as biomarkers of immunological protection.
With respect to humoral immunity, neutralizing IgG antibodies may be a correlate of protective immunity. In an outbreak with 85% attack rate, the neutralizing antibodies from previous SARS-CoV-2 infection were significantly associated with protection against re-infection (also observed in animal models like rhesus macaques) [3]. IgG against the receptor-binding domain of spike protein have high specificity and sensitivity and sustain in patients up to 75 days [3]. Another study deciphered that neutralizing antibodies decline within 2–3 months in COVID-19 recovered patients. One mathematical model also suggested shortly durable immunity [4].
The durability of neutralizing antibodies in other human coronaviruses may be relevant for comparison. Among the seven pathogenic coronaviruses of human beings, HCoV-229E, HCoV-NL63, HCoV-OC43 and HCoV-HKU1 cause mild disease (common cold); whereas, SARS-CoV, MERS-CoV and SARS-CoV-2 are highly pathogenic. Antibody titers lack longevity and wane substantially one year post-infection in common cold coronaviruses; three years in SARS-CoV; and persist for 2 years after recovery from severe MERS-CoV infection [5]. As SARS-CoV-2 infection has mostly asymptomatic or mild clinical presentation, like common cold coronaviruses, rapidly waning antibody responses following primary infection or immunization (compared to severe cases) may allow susceptibility to re-infection. The secretory IgA as protective neutralizing antibodies against SARS-CoV-2 should also be explored as mucosal immunity provides protection by intranasal immunization against closely-related SARS-CoV and MERS-CoV [5].
The evidences are emerging about the role of cellular responses in protective immunity against COVID-19. A recent study showed robust and highly functional T cell-mediated response (even in antibodies-seronegative individuals) elicited by SARS-CoV-2, which accord long-term protection [3]. Even if the infection is not severe enough to result in measurable antibodies, memory T cell (MTC) may offer long-lasting protective immunity. In closely-related human coronaviruses like SARS-CoV where CD4+ and CD8+ T cell responses have been identified 4 and 6 years post-infection, respectively, with no identifiable memory B cell (MBC) responses in blood [5]. Similarly, MTC responses have been detected in MERS-survivors 2 years post-infection.
Another study confirmed the sustained protective immunity against COVID-19, where potent antiviral MBCs and MTCs increased numerically over 3 months after symptom-onset with MBCs expressing neutralizing antibody receptors and MTCs expanding and producing IFN-γ on antigen encounter [3].
The genetically similar fragments of SARS-CoV-2 and common cold coronaviruses exhibit cross-reactivity via MTCs. This might cause milder clinical outcomes in some people. The CD4+ T cells reactive to SARS-CoV-2 detected in 40–60% of unexposed individuals suggest T cell-mediated recognition due to cross-reactive common cold coronaviruses [3]. Similarly, MTCs displaying cross-reactivity to nucleocapsid protein of SARS-CoV-2 have been found in individuals recovered from SARS-CoV outbreak 17 years back [3].
The validity of protective immunity is under scanner by first documentation of COVID-19 re-infection (milder illness in first-episode and asymptomatic on re-infection after 142 days) using comparative genomic analysis to differentiate viral shedding [6]. The residual low antibody titer and T cell immunity might have ameliorated severity during re-infection. The re-infection reported in COVID-19 and seasonal common cold coronaviruses implicate that vaccines may not provide life-long protection against COVID-19 and may require booster doses.
The evidences from human coronaviruses-based studies and preliminary data on SARS-CoV-2 indicate that both humoral and cellular adaptive immunity mediate the immunological protection, and the vaccine should induce both of them robustly. Further, documented COVID-19 re-infection shouldn't be discouraging as receding severity on subsequent episodes indicates priming of adaptive immunity. Realistically, herd immunity to SARS-CoV-2 can be achieved through vaccination instead of natural infection. The present-day caveat is waiving off of immunity passports and compliance of mitigation measures by recovered patients as diligently as the unexposed population at risk.
Ethical approval
This article does not require any human/animal subjects to acquire such approval.
Funding
There is no funding source used for this paper.
CRediT authorship contribution statement
Priyanka: Conceptualization, Data curation, Visualization, Writing - original draft, Writing - review & editing. Om Prakash Choudhary: Conceptualization, Supervision, Writing - original draft, Writing - review & editing. Indraj Singh: Writing - review & editing.
Declaration of competing interest
All authors report no conflicts of interest relevant to this article.
Acknowledgements
The figure was created with BioRender (https://biorender.com/).
References
- 1.Priyanka, Choudhary O.P., Singh I., Patra G. Aerosol transmission of SARS-CoV-2: the unresolved paradox. Travel Med Infect Dis. 2020;37:101869. doi: 10.1016/j.tmaid.2020.101869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chitguppi R. Humans develop robust immune response & memory that may provide lasting protection against Covid-19. Dental Tribune. 2020;37 doi: 10.13140/RG.2.2.14791.93606. [DOI] [Google Scholar]
- 4.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., Hu J.L., Xu W., Zhang Y., Lv F.J., Su K., Zhang F., Gong J., Wu B., Liu X.M., Li J.J., Qiu J.F., Chen J., Huang A.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 5.Sariol A., Perlman S. Lessons for COVID-19 immunity from other coronavirus infections. Immunity. 2020;53(2):248–263. doi: 10.1016/j.immuni.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To K.K.W., Hung I.F.N., Ip J.P., Chu A.W.H., Chan W.M., Tam A.R. Coronavirus disease 2019 (COVID-19) re-infection by a phylogenetically distinct severe acute respiratory syndrome coronavirus 2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020:ciaa1275. doi: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]