Abstract
Reports of the incidence of acute kidney injury in patients with coronavirus disease 2019 (COVID-19) have varied greatly from 0.5% to as high as 39%, with onset generally within 7 days from time of admission. The nature of the kidney insult is acute tubular necrosis, immune cell infiltration, or rhabdomyolysis, as demonstrated in autopsy reports. Moreover, infection with COVID-19 has been associated with coagulation abnormalities, as well as complement-mediated generalized thrombotic microvascular injury. These patients have been found to have high D-dimer, fibrin degradation product, and fibrinogen values, an elevated international normalized ratio, normal partial thromboplastin time, and normal platelet count values. Renal artery thrombosis is a rare condition, the most common cause of which is atrial fibrillation. However, bilateral completely occlusive renal artery thrombosis is even rarer. We present a case of a patient with COVID-19 on systemic anticoagulation therapy who presented with a serum creatinine level of 6.04 mg/dL requiring the initiation of kidney replacement therapy and was found to have bilateral renal artery thrombosis.
Index Words: COVID-19, coronavirus 2019, renal artery thrombosis, acute kidney injury
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has presented the medical community and society at large with unprecedented challenges. The virus has had devastating effects on multiple organ systems. Hypercoagulability and thrombotic disease have also been observed at alarmingly high levels. The incidence of thrombotic disease in patients with COVID-19 has been reportedly as high as 31%.1 Zuo et al2 even found that 52% of hospitalized patients with COVID-19 had antiphospholipid antibodies. A rare condition seen in patients in a prothrombotic state is renal artery thrombosis. A large case series of emergency department patients revealed a prevalence of 0.02/1,000,3 whereas a series of 14,000 autopsies revealed a prevalence of 14/1,000.4 The most common cause of renal artery thrombosis is atrial fibrillation, followed by cholesterol-based emboli.
The development of moderate to severe acute kidney injury in COVID-19–positive patients has been associated with a significant mortality risk.5,6 Those patients’ 180-day mortality rate was 58% compared with their counterparts without acute kidney injury at 28%.6 Furthermore, the angiotensin-converting enzyme 2 receptor, located on the surface of renal tubular cells among other cells, has been identified as the receptor that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses to facilitate viral entry into target cells.7 Furthermore, kidney damage has been reported in 80% of COVID-19–associated pneumonia cases.8
The resultant increase in demand for kidney replacement therapy has presented a challenge to medical institutions all around the world, with as many as 5% of intensive care unit patients requiring dialysis during the second week of infection.9
Case Report
We present a case of a woman in her 60s with a medical history of paroxysmal atrial fibrillation receiving apixaban, hypertension, heart failure with preserved ejection fraction, and gastroesophageal reflux who presented to the Mount Sinai Hospital with a 2-day history of shortness of breath and productive cough. She was found to be in atrial fibrillation with a rapid ventricular rate that resolved with intravenous diltiazem administration, as well as hypoxic respiratory failure requiring bilevel positive airway pressure. COVID-19 was diagnosed and she was started on treatment with hydroxychloroquine, azithromycin, and methylprednisolone. Her respiratory status improved and she was placed on oxygen nasal cannula as needed.
Admission laboratory blood work was significant for a white blood cell count of 36.8 ×103/μL, serum creatinine level of 6.04 mg/dL (last known creatinine was 0.64 mg/dL on July 6, 2019), serum blood urea nitrogen level of 53 mg/dL, serum lactate dehydrogenase level of 2,600 U/L, and a brain natriuretic peptide level of 1,126 pg/mL. Urinalysis was significant for 4 to 10 red blood cells, 5 to 10 white blood cells, and protein excretion ≥ 500 mg/dL. The patient’s last known urinalysis was from July 2019, and results were unremarkable and negative for blood and protein.
A kidney ultrasound demonstrated echogenic kidneys bilaterally with no hydronephrosis, mass, or kidney stone visualized. The patient was started on intermittent hemodialysis through a femoral central venous catheter before being transitioned to peritoneal dialysis. Both the central venous catheter and peritoneal dialysis catheter exit sites were complicated by consistent oozing requiring multiple daily dressing changes.
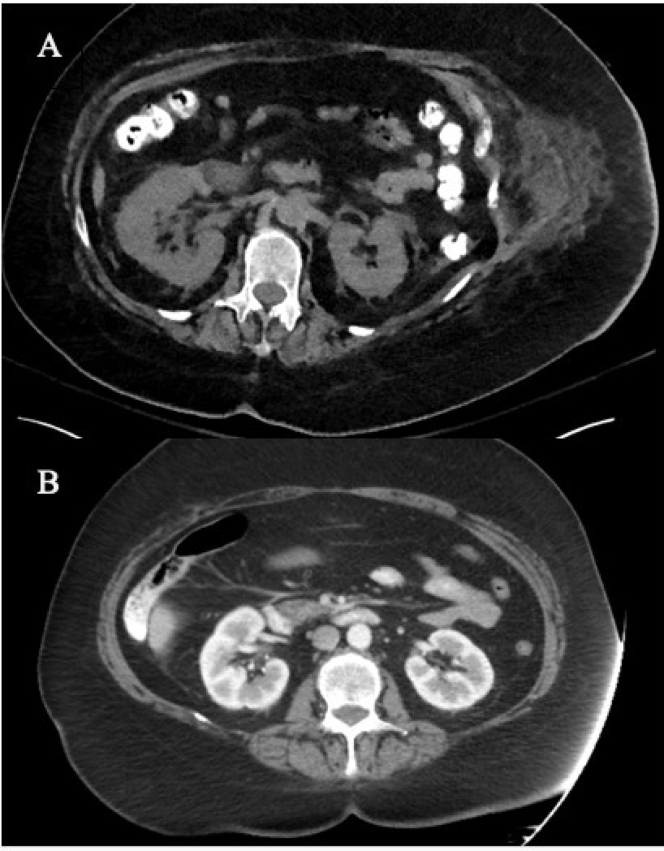
The patient’s peritoneal fluid was blood tinged for the first 2 days, and her hemoglobin level remained stable at 13.6 g/dL. Her hemoglobin level then decreased to 5.5 g/dL over the following 43 hours. Multiphase contrast-enhanced computed tomography angiography of the abdomen and pelvis was performed. Incidentally, bilateral renal artery thrombosis (Fig 1A) and thrombosis of the proximal celiac artery with distal reconstitution were identified, with no evidence of acute arterial extravasation.
Figure 1.
(A) Multiphase contrast-enhanced computed tomography angiogram of the abdomen and pelvis shows bilateral renal artery thrombosis. (B) Computed tomography with intravenous contrast of the abdomen and pelvis from July 1, 2019, shows no renal artery thrombosis.
Although the kidneys appeared atrophic and lobulated in contour with perinephric fat stranding, they were of similar size to the patient’s last known computed tomography results with contrast of the abdomen and pelvis (Fig 1B).
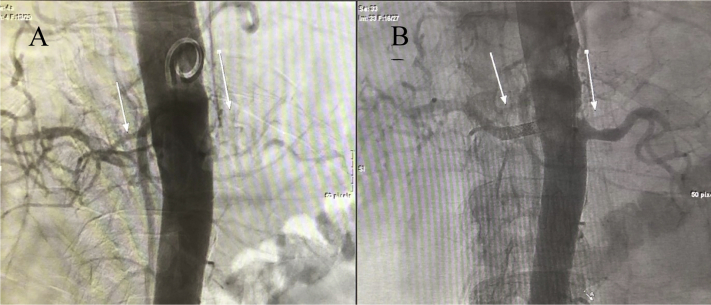
On hospital day 8, the day after the angiogram, the patient underwent elective bilateral renal artery angiography, confirming complete occlusion of the bilateral renal arteries. Successful percutaneous bilateral renal artery aspiration thrombectomy and thrombolysis were then performed, with stent placement in the right renal artery, with widely patent renal arteries on the completion angiogram and restoration of blood flow (Fig 2). Further imaging revealed a pulmonary embolism and thrombotic occlusion of the left subclavian artery.
Figure 2.
(A) Complete occlusion of bilateral renal arteries. (B) Successful bilateral renal artery aspiration percutaneous thrombectomy and thrombolysis. Arrows indicate the renal arteries.
A hypercoagulable workup was undertaken (summarized in Table 1) that revealed an elevated prothrombin time, activated partial thromboplastin time (aPTT), and international normalized ratio, all measured while the patient was still receiving apixaban, 5 mg, twice daily. The anti-cardiolipin antibody immunoglobulin G (IgG) and IgM levels were checked before heparin initiation postthrombectomy and both were within normal limits. The remaining hypercoagulable markers were checked while the patient was receiving a heparin drip and revealed an elevated D-dimer, elevated fibrin degradation dimer, normal fibrinogen, low antithrombin III antigen, and low protein C and S levels. Lupus anticoagulant panel was checked while the patient was receiving apixaban and the result interpretation for lupus anticoagulant was officially negative. However, there remained a possibility of low-level lupus anticoagulant.
Table 1.
Hypercoagulable Workup Test Results
| Laboratory Test | Result | Reference Range |
|---|---|---|
| Prothrombin time, s | 19.6 | 12.3-14.9 |
| aPTT, s | 35.2 | 25.4-34.9 |
| INR | 1.7 | |
| D-Dimer, μg/mL | 13.54 | 0.00-0.50 |
| Fibrin degradation dimer, μg/mL | 13.56 | 0.00-0.50 |
| Fibrinogen, mg/dL | 350 | 175-450 |
| Antithrombin III antigen | 47% | 77%-124% |
| Protein C activity | 50% | 70%-130% |
| Protein S activity | 29% | 55%-123% |
| Anti-cardiolipin antibody IgG, GPL | 22.7 | 0.0-22.9 |
| Anti-cardiolipin antibody IgM, GPL | 3.5 | 0.0-10.9 |
| Lupus anticoagulant panel | ||
| aPTT, s | 38.2 | 25.4-34.9 |
| aPTT PNP mix immediate, s | 30.8 | 25.4-34.9 |
| aPTT PNP immediate, s | 28.5 | 25.4-34.9 |
| aPTT actin factor sensitive, s | 26.1 | 23.2-27.0 |
| DRVVT screen, s | 52.2 | ≤41 |
Abbreviations: aPTT, activated partial thromboplastin; DRVVT, dilute Russell’s viper venom time; GPL, 1 μg of immunoglobulin G antibody; IgG, immunoglobulin G; INR, international normalized ratio; PNP, pooled normal plasma.
The patient’s hospital course thereafter was complicated by hemodynamic instability due to the mentioned blood loss, requiring intravenous pressors and blood transfusions. Four days postreperfusion, her kidney function remained poor, with serum creatinine level of 9.42 mg/dL, and she remained oliguric.
Discussion
As we continue to learn more about the COVID-19 virus and study clinical outcomes of infected patients, our knowledge of its complications and preparedness for their onset will subsequently improve. To date, there have been reported thrombotic complications seen in COVID-19–infected patients, including pulmonary emboli, deep vein thromboses, and catheter-related thromboses, as well as arterial ischemic strokes.10
Possible causes of our patient’s thrombophilia are many; perhaps the most obvious is atrial fibrillation. Other causes to consider include deficiencies of coagulation pathway factors, protein C, protein S, and antithrombin III. Moreover, given the reported thrombotic complications of COVID-19, our differential includes COVID-19 as the possible cause.
The hypercoagulable workup revealed lower than normal antithrombin III antigen levels, as well as low activity of both protein C and protein S. Decreased antithrombin III antigen levels can be seen in various forms of nephropathy as a result of severe proteinuria11 (urinalysis from the day of admission demonstrated protein excretion ≥ 500 mg/dL). Decreased protein C and S activity can be seen with kidney injury, with some studies even indicating significant elevations in anti-protein C and S antibodies.
In line with a previous report by Tang et al,12 our patient had an elevated fibrin degradation dimer level. However, contrary to Zhang et al’s13 findings in which there were 3 cases of thrombosis associated with increased anticardiolipin immunoglobulin levels, our patient had normal anticardiolipin immunoglobulin levels.
Bowles et al14 found that 91% of their patients who were SARS-CoV-2–positive and with a prolonged aPTT were also positive for lupus anticoagulant. In our patient, the lupus anticoagulant panel revealed an aPTT that was more prolonged than the screening aPTT performed at the same time (38.2 vs 35.2 seconds); this is an expected variation given that the lupus anticoagulant panel plasma sample is frozen before testing. Although we did not test for individual coagulation pathway factor levels, the normal aPTT actin factor sensitive indicates normal coagulation pathway factor levels.
We acknowledge that to perform an accurate reliable thrombophilia workup the patient in question should be off all forms of anticoagulation therapy. However, given the clinical context, it would not be ethical to withhold the anticoagulation therapy in a patient with known atrial fibrillation who developed bilateral renal artery thromboses while receiving systemic anticoagulation.
Renal artery thrombosis is a rare condition. Among patients with atrial fibrillation with peripheral arterial thromboembolic events, renal arteries are the least affected at only 2% of cases (as opposed to the extremities; 61%).15 Moreover, complete infarction is the most rare, typically seen after trauma or interventions involving the aorta, neither of which was the case with our patient who was also receiving systemic anticoagulation both before and during her presentation. For these reasons, we believe that it is unlikely that atrial fibrillation was the underlying cause in this case.
It was anticipated that the renal artery thrombectomy could have potentially salvaged the patient’s kidney function. However, the repeated administration of iodinated contrast and subsequent hemodynamic instability due to continued bleeding (the source of which is yet to be identified) may have hampered recovery from severe prolonged kidney ischemia.
To our knowledge, this is the first reported case of bilateral renal artery thrombosis in a COVID-19–infected patient receiving systemic anticoagulation. Assessing kidney perfusion in oliguric patients with COVID-19 with acute kidney injury may be of importance in guiding the clinical workup and medical decision making. If caught at an early phase, there may be a greater chance of salvaging kidney function.
Article Information
Authors’ Full Names and Academic Degrees
Osama El Shamy, MD, Nitzy Munoz-Casablanca, MD, Steven Coca, DO, Shuchita Sharma, MD, Robert Lookstein, MD, and Jaime Uribarri, MD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Consent
The authors declare that they have obtained consent from the patient discussed in the report.
Peer Review
Received May 15, 2020. Evaluated by 1 external peer reviewer, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form July 27, 2020.
Footnotes
Complete author and article information provided before references.
References
- 1.Rico-Mesa J.S., Rosas D., Ahmadian-Tehrani A., White A., Anderson A.S., Chilton R. The role of anticoagulation in COVID-19-induced hypercoagulability. Curr Cardiol Rep. 2020;22(7):53. doi: 10.1007/s11886-020-01328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuo Y., Estes S.K., Gandhi A.A. Prothrombotic antiphospholipid antibodies in COVID-19. medRxiv [Preprint] 2020 doi: 10.1101/2020.06.15.20131607. [DOI] [Google Scholar]
- 3.Hoxie H., Coggin C. Renal infarction. Arch Intern Med. 1940;65:587–594. [Google Scholar]
- 4.Domanovits H., Paulis M., Nikfardjam M., Meron G., Kurkciyan I., BankierAA Acute renal infarction. Clinical characteristics of 17 patients. Medicine (Baltimore) 1999;78:386–394. doi: 10.1097/00005792-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 5.McNicholas B.A., Rezoagli E., Pham T. Impact of early acute kidney injury on management and outcome in patients with acute respiratory distress syndrome: a secondary analysis of a multicenter observational study. Crit Care Med J. 2020;47(9):1216–1225. doi: 10.1097/CCM.0000000000003832. [DOI] [PubMed] [Google Scholar]
- 6.Liu K., Glidden D., Eisner M.D. Predictive and pathogenic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007;35(12):2755–2761. [PMC free article] [PubMed] [Google Scholar]
- 7.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pei G., Zhang Z., Peng J. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(5):1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.G., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klok F.A., Kruip M., van der Meer N. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Z., Wang F., Liang M. SerpinC1/antithrombin III in kidney-related disease. Clin Sci. 2017;131:823–831. doi: 10.1042/CS20160669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowles L., Platton S., Yatrey N. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. 2020;383:288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost L., Engholm G., Johnsen S., Moller H., Henneberg E.W., Husted S. Incident thromboembolism in the aorta and the renal, mesenteric, pelvic, and extremity arteries after discharge from the hospital with a diagnosis of atrial fibrillation. Arch Intern Med. 2001;161:272–276. doi: 10.1001/archinte.161.2.272. [DOI] [PubMed] [Google Scholar]