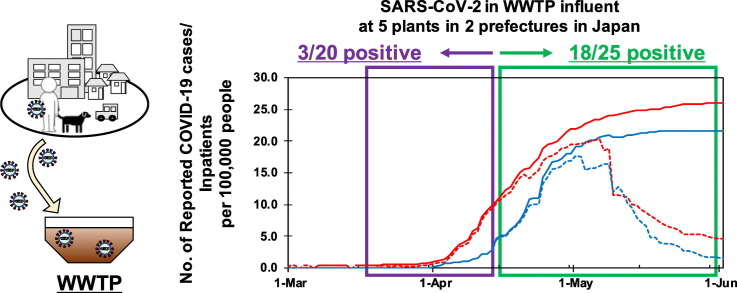
Abstract
The presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in wastewater samples has been documented in several countries. Wastewater-based epidemiology (WBE) is potentially effective for early warning of a COVID-19 outbreak. In this study, presence of SARS-CoV-2 RNA in wastewater samples was investigated and was compared with the number of the confirmed COVID-19 cases in the study area during COVID-19 outbreak in Japan. In total, 45 influent wastewater samples were collected from five wastewater treatment plants in Ishikawa and Toyama prefectures in Japan. During the study period, the numbers of confirmed COVID-19 cases in these prefectures increased from 0.3 and 0 to >20 per 100,000 people. SARS-CoV-2 ribonucleic acid (RNA) in the samples was detected using several PCR-based assays. Of the 45 samples, 21 were positive for SARS-CoV-2 according to at least one of the three quantitative RT-PCR assays. The detection frequency increased when the number of total confirmed SARS-CoV-2 cases in 100,000 people exceeded 10 in each prefecture; however, SARS-CoV-2 could also be detected at a low frequency even when the number was below 1.0. SARS-CoV-2 in wastewater could be detected in the early stage of the epidemic, even if the number of confirmed cases potentially underestimates the actual numbers of cases. This suggests that WBE approach can potentially act as an early warning of COVID-19 outbreaks in Japan.
Keywords: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Coronavirus infectious disease 2019 (COVID-19), Wastewater-based epidemiology (WBE), qRT-PCR, PEG precipitation
Graphical abstract
1. Introduction
The recent novel coronavirus disease (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has led to more than 40 million confirmed cases and more than one million deaths worldwide, as of October 23, 2020 (WHO, 2020). In some countries, COVID-19 cases are still increasing after occasional lifting of lockdowns and reopening of businesses Wastewater-based epidemiology (WBE) is surveillance of epidemiological information of people in a sewer catchment by monitoring the pathogen in wastewater. WBE is regarded as an effective approach for providing a snapshot of the epidemic situation in an entire catchment area and has been applied to enteric viruses such as norovirus and enteroviruses (Choi et al., 2018; Yang et al., 2015). WBE could provide an early warning of possible re-outbreaks and seasonal outbreaks in the future.
Recently, detection of SARS-CoV-2 in wastewater was reported in the Netherlands, Australia, the United States (US), China, Italy, Spain, India, and Japan (Ahmed et al., 2020a; Haramoto et al., 2020; Kumar et al., 2020; La Rosa et al., 2020; Medema et al., 2020; Nemudryi et al., 2020; Randazzo et al., 2020; Rimoldi et al., 2020; Sherchan et al., 2020). SARS-CoV-2 was present in wastewater, because it is shed, not only in respiratory secretions, but in the feces of patients (Tang et al., 2020; Wölfel et al., 2020; Zhang et al., 2020a; Zhang et al., 2020b). Importantly, pre-symptomatic and asymptomatic patients, as well as symptomatic patients, were shown to have a viral load in feces (Tang et al., 2020; Wölfel et al., 2020; Zhang et al., 2020b). The proportion of asymptomatic infection was reportedly as 18–50% (Mizumoto et al., 2020; Nishiura et al., 2020; Buitrago-Garcia et al., 2020; Lavezzo et al., 2020; White et al., 2020). These people with mild or no symptoms may not be included in clinical surveillance, because most of them do not visit clinics or hospitals. Much larger population of asymptomatic and mildly symptomatic infections have been implied by a surveillance of antibodies to SARS-CoV-2 in 10 sites in the United States, in which the estimated number of infections were 6 to 24 times greater than that of confirmed cases (Havers et al., 2020). In addition, the number of reported COVID-19 cases may be biased by the condition of medical services in each country (e.g. access to medical services, capacity to carry out PCR tests, stay-at-home policies for mildly symptomatic patients, responses to medical collapse, and similar). Transmission by such undiagnosed patients can adversely affect the initial containment of COVID-19. Some studies have argued that the number of cases is underestimated in Japan, because of its low capacity to conduct PCR tests (Omori et al., 2020). SARS-CoV-2 excreted from asymptomatic and pre-symptomatic individuals can be present in wastewater and therefore, WBE is potentially predict the overall epidemic status, without the risk of such biases. This implies that WBE could be a more sensitive early epidemic predictor than clinical surveillance and effective in verifying the coverage of clinical surveillance in various medical situations. To assess the application of WBE as an early warning tool for COVID-19 outbreaks, it is necessary to verify correlations between the occurrence of SARS-CoV-2 in wastewater and the number of COVID-19 cases in each country.
Some studies reported the presence of SARS-CoV-2 RNA in wastewater even before the identification of the first COVID-19 case in the study area (Medema et al., 2020; Randazzo et al., 2020). The sensitivity of WBE depends on the viral load in the feces of infected people. Detection of SARS-CoV-2 in wastewater could be less sensitive than that of norovirus, because the viral load of SARS-CoV-2 in feces is reportedly 1–2 log10 lower than that of norovirus (Hata and Honda, 2020). In the early stage of the epidemic, SARS-CoV-2 in wastewater should be, if present, highly diluted by wastes from the vast majority of uninfected individuals. SARS-CoV-2 is an enveloped virus, while typical viral pathogens in water, such as noroviruses, are non-enveloped viruses. Commonly used methods for concentrating viruses in water are developed for non-enveloped viruses and might not be effective for SARS-CoV-2 (Kitajima et al., 2020). Several RT-PCR assays for SARS-CoV-2 are now available, but their applicability to wastewater samples has not been properly determined. These factors indicate that the detection of SARS-CoV-2 in wastewater is more challenging than that of norovirus and other enteric viruses. At this time, verifying the effectiveness of concentration methods and downstream RT-PCR assays is necessary to ensure WBE's reliability.
The objective of this study was to verify the WBE approach for COVID-19 by comparing the detected concentration of SARS-CoV-2 in wastewater with the COVID-19 cases reported by the clinical surveillance. The detected concentrations of SARS-CoV-2 RNA in wastewater would reflect true prevalence of COVID-19 infection in the sewer catchment including clinically undiagnosed patients, while the number of clinically reported cases covers only diagnosed patients and also depends on the number of PCR diagnosis. In this study, wastewater samples were taken in two prefectures, Ishikawa and Toyama in Japan, which had the highest number of reported COVID-19 cases per population, with the exception of the Tokyo Metropolitan district. Multiple qRT-PCR assays for detection of SARS-CoV-2 RNA were comparatively applied. At the beginning of the study period, the numbers of confirmed COVID-19 cases in Ishikawa and Toyama prefectures were only 0.3 and 0 per 100,000 people (4 and no confirmed cases), respectively. Since the number of PCR diagnosis was limited in the early stage of outbreak in Japan, SARS-CoV-2 RNA could be detectable in wastewater earlier than clinical reports. Moreover, the viral RNA concentration possibly increases along with the increase of clinically reported COVID-19 cases. This enabled us to examine the sensitivity of WBE for COVID-19 relative to the reported COVID-19 cases per population in the target areas.
2. Material and methods
2.1. Sample collection
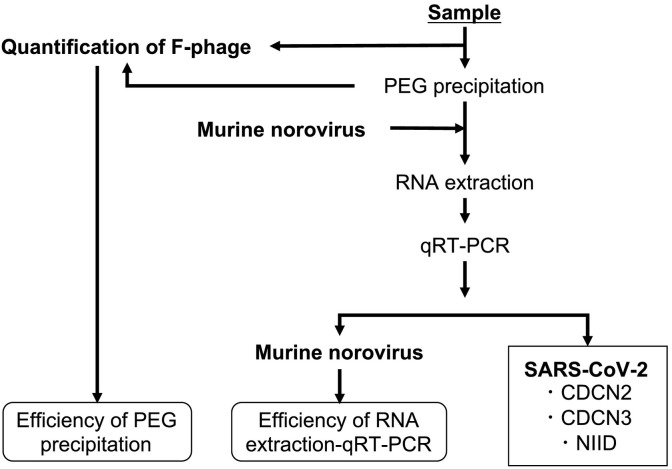
Influent wastewater samples were collected at five wastewater treatment plants (WWTPs): WWTPs A,B, and C in Ishikawa prefecture, and D and E in Toyama prefecture. These WWTPs were built to treat maximum volumes of 12,800; 53,300; 156,000; 82,500; and 143,500 m3/day of wastewater in total and serve populations of 31,501; 112,396; 150,223; 169,400; and 233,480, respectively (see Table S1 in the Supplementary Material). Physico-chemical characteristics (water temperature, pH, SS, and COD, measured by each WWTP) of the influent wastewater in each WWTP during the study period are summarized in Table S2 in the Supplementary Material. At each WWTP, 100 mL grab samples were collected in the morning (between 9:00 and 10:00), weekly or biweekly from March 5 to May 29, 2020. At WWTP C, the sample collection was conducted until April 21. At WWTP E, the sample collection started on May 15. Consequently, a total of 45 samples were obtained. Among them, four samples collected during March 6 and 27 were kept frozen at −80 °C, while the other sample were kept on ice until the analysis, which took place within three days of the collection. Sample storage in frozen form and on ice are reported to be similarly effective for a couple of days (Olson et al., 2004). Each sample was split into 80 mL and 20 mL subsamples, which were then subjected to a virus concentration process and quantification of F-phages to estimate the efficiency of the virus concentration process, respectively (see Fig. 1 ).
Fig. 1.
Flow diagram of the sample processing for confirming the presence of SARS-CoV-2 in wastewater using qRT-PCR assays and for assessing the virus detection efficiency with process controls.
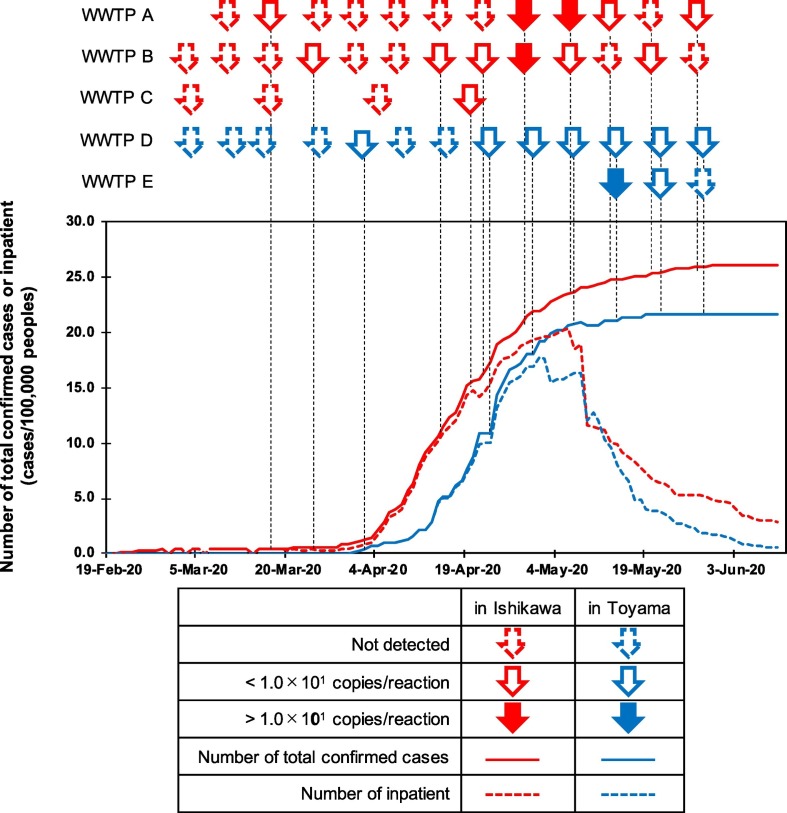
At the beginning of the sampling period, there were only four and zero COVID-19 confirmed cases in Ishikawa and Toyama prefectures, respectively. The reported cases increased during the study period to reach 21.9 and 18.1 cases per 100,000 people in Ishikawa and Toyama prefectures, respectively, by the end of April. The numbers of reported cases per population in Ishikawa and Toyama prefectures were ranked as the second and third in Japan, respectively, following the Tokyo Metropolitan area (see Fig. 2 ). The reported cases per 100,000 people in Toyama reached 21.6 on May 19 and did not increase thereafter, while the number in Ishikawa gradually increased to 26.0 by the end of the study period on May 29.
Fig. 2.
Temporal relationships between the number of SARS-CoV-2 infection cases and the presence of SARS-CoV-2 in wastewater samples in Ishikawa and Toyama prefectures. Arrows indicate the date and WWTP of the sample collection and occurrence of SARS-CoV-2 in the sample: arrows surrounded by a dashed line indicate that SARS-CoV-2 was not detected in the sample; white arrows indicate SARS-CoV-2 was detected but was not quantifiable (1.0 × 100–1.0 × 101 copies/reaction); colored arrows indicate SARS-CoV-2 was detected with quantifiable levels (1.2 × 104–3.5 × 104 copies/L). For samples deemed positive by multiple qRT-PCR assays, the assay showing the highest detected concentration was selected. Solid line and dashed lines indicate the number of confirmed cases and inpatients, respectively, in each prefecture.
2.2. Virus concentration by polyethylene glycol precipitation (PEG precipitation)
Polyethylene glycol precipitation described by Jones and Johns (2009) was applied for concentrating viruses in the wastewater samples with some modifications. PEG precipitation has been suggested to be effective for enveloped viruses in wastewater and surface water samples (Ahmed et al., 2020b; Deboosere et al., 2011; Honjo et al., 2010; Torii et al., 2020). Prior to the virus concentration, the 80 mL subsamples were centrifuged at 3000 ×g for 5 min. The supernatant was recovered and mixed with 8.0 g of PEG8000 and 4.7 g of sodium chloride to final concentrations of 10% and 1 M, respectively. The mixture was incubated overnight on a shaker at 4 °C. Subsequently, the mixture was centrifuged at 10,000 ×g for 30 min. The supernatant was discarded and the resulting pellets were resuspended in 500 μL of phosphate buffer as a virus concentrate.
2.3. RNA extraction
Murine norovirus was used as a molecular process control to monitor the efficiencies of the RNA extraction-RT-qPCR processes. Briefly, 140 μL of the virus concentrate was spiked with 1.7 × 104 copies (2 μL of a viral stock) of MNV and subjected to RNA extraction using a QIAamp® Viral RNA mini kit (Qiagen Company, Hilden, Germany) to obtain 60 μL of RNA extract, in accordance with the manufacturer's instructions. The RNA extract was then subjected to qRT-PCR assays for detection of SARS-CoV-2 and the spiked MNV.
2.4. qRT-PCR assays
TaqMan-based qRT-PCR assays for the quantitative detection of SARS-CoV-2 and spiked MNV were performed with a qTOWER3 (Analytik Jena AG, Jena, Germany). For quantification of the MNV RNA, a primer and TaqMan probe set described by Kitajima et al. (2008) was utilized (Table 1 ). For the quantification of SARS-CoV-2, two and one primer and TaqMan probe sets, described by Centers for Disease Control and Prevention (CDC) in the USA (Centers for Disease Control and Prevention, 2020) (CDCN2 and CDCN3 assays, which were originally denoted as 2019-nCoV_N2 and 2019-nCoV_N3, respectively) and Shirato et al. (2020) (NIID assay, which was originally denoted as NIID_2019-nCoV_N), respectively, were used separately (see Table 1). Shirato et al. (2020) described two reverse primers for NIID assay. In this study, one of them (NIID_2019-nCoV_N_R2ver3, denoted as NIID-R in Table 1) was used, because it does not mismatch with reference strains (for example, Wuhan-Hu-1 strain, GenBank accession # MN908947.3). A reaction mixture (20 μL) was prepared using a One Step PrimeScript™ RT-PCR Kit (Perfect Real Time) (TaKaRa). Briefly, 20 μL of a reaction mixture was prepared by mixing 5 μL of RNA extract with 10 μL of a 2 × OneStep RT-PCR Buffer III, 0.4 μL of a TaKaRa Ex Taq HS, 0.4 μL of a PrimeScript™ RT enzyme Mix II, 400 nM each of forward- and reverse primers, 100 nM of a TaqMan probe, and nuclease-free water. qRT-PCR was performed under the following thermal cycling conditions: RT reaction at 42 °C for 5 min, initial denaturation and inactivation of the RT enzyme at 95 °C for 10 s, followed by 50 cycles of amplification with denaturation at 95 °C for 5 s, and annealing and extension at specific temperatures for each assay (see Table 1) for 30 s.
Table 1.
Primer and TaqMan probe sequences used for the detection of SARS-CoV-2 in this study.
| Assay | Name | Function | Sequence (5′ → 3′)a, b | Location | Temperature for annealing/extension | Reference |
|---|---|---|---|---|---|---|
| 2019-nCoV_N2 | CDCN2-F | Forward primer | TTACAAACATTGGCCGCAAA | 29,164–29,183 | 55 °C | CDC (2020) |
| CDCN2-R | Reverse primer | GCGCGACATTCCGAAGAA | 29,213–29,230 | |||
| CDCN2-P | TaqMan Probe | ACAATTTGCCCCCAGCGCTTCAG- | 29,188–29,210 | |||
| 2019-nCoV_N3 | CDCN3-F | Forward primer | GGGAGCCTTGAATACACCAAAA | 28,681–28,702 | 55 °C | CDC (2020) |
| CDCN3-R | Reverse primer | TGTAGCACGATTGCAGCATTG | 28,732–28,752 | |||
| CDCN3-P | TaqMan Probe | AYCACATTGGCACCCGCAATCCTG- | 28,704–28,727 | |||
| NIID_2019-nCoV_N | NIID-F | Forward primer | AAATTTTGGGGACCAGGAAC | 29,125–29,144 | 60 °C | Shirato et al. (2020) |
| NIID-R | Reverse primer | TGGCACCTGTGTAGGTCAAC | 29,263–29,282 | |||
| NIID-P | TaqMan Probe | ATGTCGCGCATTGGCATGGA | 29,222–29,241 | |||
| MNV | MKMNVF | Forward primer | CGGTGAAGTGCTTCTGAGGTT | 6330–6350 | 56 °C | Kitajima et al. (2008) |
| MKMNVR | Reverse primer | GCAGCGTCAGTGCTGTCAA | 6371–6389 | |||
| MKMNVP | TaqMan Probe | CGAACCTACATGCGTCAG | 6352–6369 |
All TaqMan probes were labeled with 5′-FAM and 3′-TAMRA.
The corresponding nucleotide positions of SARS-CoV-2 Wuhan-Hu-1 strain and MNV strain S7-PP3 (GenBank acc. No. MN908947.3 and AB435515.1, respectively).
To obtain a calibration curve, a 10-fold serial dilution (concentrations of 1.0 × 100 to 1.0 × 106 copies/reaction) of a standard DNA (plasmid DNA or oligo DNA) containing the target sequence was amplified. If the resultant Ct value from a sample corresponded to >1.0 copy/reaction, the sample was determined to be positive for SARS-CoV-2. Considering the sample volume reduction during the concentration and RNA extraction processes, 1.0 copy/reaction (5 μL RNA extract) is equivalent to approximately 1.0 × 103 copies/L in the original sample. SARS-CoV-2 in samples detected at <1.0 × 101 copy/reaction was not considered to be quantifiable. Absence of the positive signal in the no-template control was confirmed in every qRT-PCR run to exclude the potential contamination of the template into the reagents.
2.5. Amplicon sequencing
Positive qRT-PCR products obtained from the samples collected until April 24 (9 products from 7 samples) were subjected to amplicon sequencing. The products were separated by electrophoresis in a 1.5% agarose gel. PCR products of the expected size (67 bp, 72 bp, and 158 bp for CDCN2, CDCN3, and NIID assays, respectively) were excised from the gel and purified with a NucleoSpin® Gel and PCR Clean-up (TaKaRa). Both strands of the purified products were sequenced by the conventional Sanger sequencing.
2.6. Determining the efficiencies of the virus concentrations and RNA extraction-qRT-PCR
F-phages in the samples, before and after the concentration process, were quantified using a plate counting assay employing Salmonella typhimurium WG49 as a host strain, as described previously (Mooijman et al., 2002). The efficiency of the virus concentration process was estimated by comparing the F-phage concentration in each sample before the concentration process with that after the concentration process.
The efficiency of the RNA extraction qRT-PCR was estimated by comparing the observed gene concentration of the spiked MNV in nuclease-free water with that in the concentrate.
Of note, F-phages and MNV are not enveloped viruses, while SARS-CoV-2 is an enveloped virus. Therefore, efficiencies determined by these controls are not necessarily correlate with those of SARS-CoV-2. These controls were used for the purpose to check a manipulation failure, which possibly causes substantial loss of SARS-CoV-2.
2.7. Collection of data on clinical surveillance
The reported numbers of COVID-19 cases in Ishikawa and Toyama prefectures were derived from a database maintained by the Ministry of Health, Labor and Welfare in Japan (https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00086.html). The numbers of inpatients in each prefecture were calculated by subtracting the numbers of discharged and dead from the number of total confirmed cases.
2.8. Statistical analysis
The correlations between the determined SARS-CoV-2 concentrations in the wastewater samples and the reported numbers of COVID-19 cases were determined by Spearman rank correlation test using R software version 3.6.1.
3. Results
3.1. Efficiency of the virus detection
The recovery efficiency for F-phages during the concentration process was 45% geometric mean with 2.1 geometric standard deviation and always exceeded 10% (n = 45). In this study, 4 samples were kept frozen, while other 41 samples were kept on ice until the analysis. All the 4 frozen samples were collected during March. The geometric mean F-phages concentration in the frozen samples was 2.1 × 102 PFU/mL with 1.1 geometric standard deviation (n = 4), which was not substantially different from those in other samples collected in March (3.6 × 102 PFU/mL with 1.4 geometric standard deviation, n = 9). This indicates that the difference in the sample storage did not substantially affect virus quantification in this study. The RNA extraction-qRT-PCR efficiency, determined with the spiked MNV, was 83% geometric mean with 2.0 geometric standard deviation and always exceeded 10% (n = 45). Some samples resulted in >100% the efficiency in the concentration and RNA extraction-qRT-PCR. This might be attributed by low plaque counts, even though they were always >10 PFU/plate, and relatively low extraction efficiency for the positive control, respectively. Presumptive virus detection efficiency, which could be estimated by multiplying these efficiencies, was 38% geometric mean with 2.9 geometric standard deviation and below 10% for 6 out of 45 samples (3.7% was the lowest).
3.2. Detection of SARS-CoV-2 using qRT-PCR assays
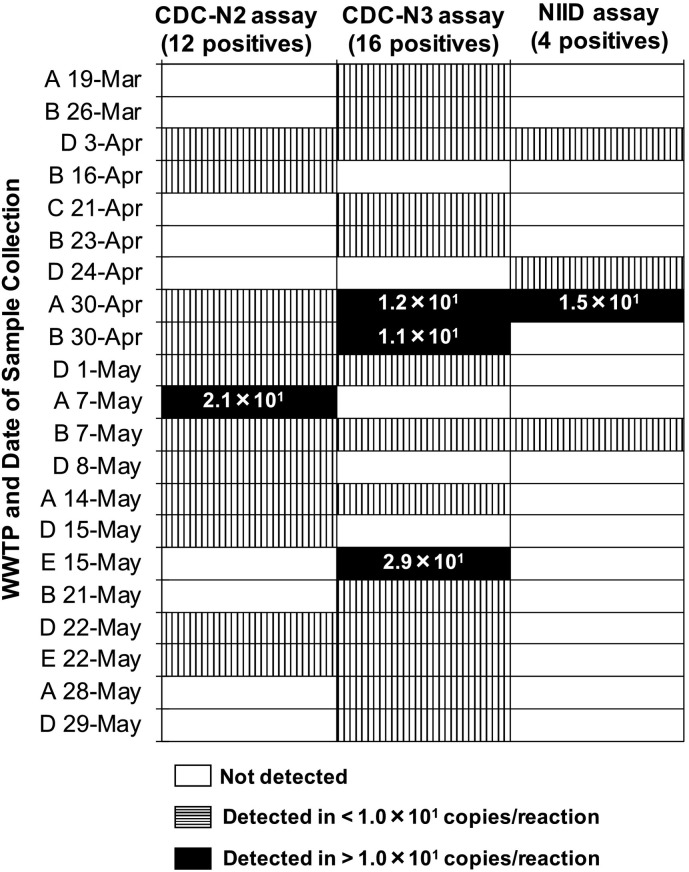
In this study, three qRT-PCR assays (CDCN2, CDCN3, and NIID assays) were applied separately. Of the 45 tested samples, 21 were positive according to at least one of the assays (see Table 2 and Fig. 3 ). Among them, 8 were positive for more than one assay. The CDCN3 assay showed the highest detection frequency (16 positive out of 45 samples), which was followed by CDCN2 assay (12 positive out of 45 samples). The NIID assay resulted in a lower detection frequency (4 positive out of 45 samples). N3 assay also tended to show higher detected concentration. However, even with N3 assay, only 3 samples, which were collected during April 30 to May 15 at WWTP A, B, and E, exceeded the detected concentration of 1.0 × 101 copies/reaction. Additionally, a sample collected on May 7 at WWTP A exceeded the detected concentration of 1.0 × 101 copies/reaction by N2 assay. Concentrations of SARS-CoV-2 in these four samples could be calculated as 1.2 × 104–3.5 × 104 copies/L. The amplicons were verified with their size by gel electrophoresis and Sanger direct sequencing. The sequence from the amplicon by CDCN3 assay for WWTP D on April 3 and by NIID assay for WWTP D on April 24 (Table 2 and Fig. 3) were partially identical to a reference sequence of SARS-CoV-2 Wuhan-Hu-1 strain (GenBank accession number: MN908947) (Fig. S1). Other sequence reads had partial match with primer sequences but no matches with GenBank database.
Table 2.
Summary of samples deemed positive for SARS-CoV-2 by qRT-PCR assays.
| Date | WWTPa | Total confirmed cases per 100,000 people |
Efficiency of the detection process |
qRT-PCR |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Ishikawa | Toyama | Concentration |
RNA extraction qRT-PCR |
Total |
CDCN2 |
CDCN3 | NIID | ||
| % | % | % | copies/Lb | ||||||
| March 19 | A | 0.4 | 0.0 | 260 | 72 | 190 | Neg. | Pos. | Neg. |
| March 27 | B | 0.5 | 0.0 | 76 | 50 | 38 | Neg. | Pos. | Neg. |
| April 3 | D | 1.4 | 0.8 | 130 | 100 | 130 | Pos. | Pos. | Pos. |
| April 16 | B | 12.2 | 5.1 | 10 | 49 | 4.9 | Pos. | Neg. | Neg. |
| April 21 | C | 15.7 | 10.9 | 88 | 41 | 36 | Neg. | Pos. | Neg. |
| April 23 | B | 17.4 | 11.0 | 36 | 50 | 18 | Neg. | Pos. | Neg. |
| April 24 | D | 18.9 | 14.3 | 52 | 31 | 16 | Neg. | Neg. | Pos. |
| April 30 | A | 21.9 | 18.1 | 41 | 190 | 76 | Pos. | 1.2 × 104 | 1.3 × 104 |
| April 30 | B | 21.9 | 18.1 | 43 | 190 | 82 | Pos. | 1.2 × 104 | Neg. |
| May 1 | D | 22.0 | 19.2 | 49 | 120 | 60 | Pos. | Pos. | Neg. |
| May 7 | A | 23.7 | 20.9 | 45 | 120 | 52 | 1.8 × 104 | Neg. | Neg. |
| May 7 | B | 23.7 | 20.9 | 110 | 110 | 120 | Pos. | Pos. | Pos. |
| May 8 | D | 24.1 | 21.0 | 170 | 190 | 310 | Pos. | Neg. | Neg. |
| May 14 | A | 24.8 | 21.1 | 17 | 130 | 22 | Pos. | Pos. | Neg. |
| May 15 | D | 24.8 | 21.3 | 40 | 170 | 67 | Pos. | Neg. | Neg. |
| May 15 | E | 24.8 | 21.3 | 42 | 130 | 57 | Neg. | 3.5 × 104 | Neg. |
| May 21 | B | 25.4 | 21.6 | 31 | 130 | 40 | Neg. | Pos. | Neg. |
| May 22 | D | 25.5 | 21.6 | 25 | 170 | 42 | Pos. | Pos. | Neg. |
| May 22 | E | 25.5 | 21.6 | 33 | 130 | 44 | Pos. | Pos. | Neg. |
| May 28 | A | 25.9 | 21.6 | 27 | 120 | 31 | Neg. | Pos. | Neg. |
| May 29 | D | 26.0 | 21.6 | 26 | 56 | 14 | Neg. | Pos. | Neg. |
WWTPs A, B, and C were located in Ishikawa prefecture, while D and E were located in Toyama prefecture.
Quantification was done only if the detected SARS-CoV-2 RNA concentration was >1.0 × 101 copies/reaction. Pos.: Positive with a detected concentration of 1.0 × 100–1.0 × 101 copies/reaction; Neg.: Negative.
Fig. 3.
Comparison of the qRT-PCR assays applied for detection of SARS-CoV-2 in the wastewater samples. Each column indicates the sample, which was determined to be positive by at least one of the assays, and the assay. White, vertically striped, and black columns indicate that SARS-CoV-2 was not detected, was detected in <1.0 × 101 copies/reaction, and was detected in >1.0 × 101 copies/reaction, respectively. Numbers shown on black columns indicate SARS-CoV-2 concentrations determined by the assay (copies/reaction).
The detection frequency seemed to be higher when the number of total confirmed SARS-CoV-2 cases per 100,000 people exceeded 10 in each prefecture (after April 14 and 21 in Ishikawa and Toyama prefectures, respectively) (see Fig. 2 and Table 2). The SARS-CoV-2 detection frequency was 15% (3 positive out of 20 samples) before the number became >10, whereas it increased to 75% (18 positive out of 25 samples) after the number became >10. Until April 24, the samples tended to be positive according to any one of the qRT-PCR assays, but thereafter, each sample was positive according to multiple qRT-PCR assays (mostly the CDCN2 and CDCN3 assays). Concurrently, the observed concentration tended to be higher and sporadically exceeded 1.0 × 101 copies/reaction, as mentioned above. Correlations between the number of confirmed cases and determined SARS-CoV-2 concentration in the samples were further evaluated by Spearman rank correlation test (Table 3 ). In Ishikawa prefecture, the concentration determined by CDCN2 assay revealed a weak correlation (ρ = 0.39, p<0.05), which was improved to be a moderate correlation (ρ = 0.45, p<0.01) by applying the highest concentration in the three assays. The data obtained in Toyama prefecture did not show any significant relationship. When the data in Ishikawa and Toyama prefectures were mixed, CDCN2 and CDCN3 assays revealed weak correlations (ρ = 0.38 and 0.39, respectively, p<0.05). Again, the correlations were improved by applying the highest concentration in the three methods (ρ = 0.51, p<0.01).
Table 3.
Spearman rank correlations between detected SARS-CoV-2 in the wastewater samples and the numbers of reported COVID-19 cases in Ishikawa and Toyama prefectures. Only significant correlations at the p<0.05 and p<0.01 are shown.
| Prefecture | qRT-PCR assay |
|||
|---|---|---|---|---|
| N2 | N3 | NIID | All assaysa | |
| Ishikawa | 0.39⁎ | – | – | 0.45⁎ |
| Toyama | – | – | – | – |
| Both | 0.38⁎⁎ | 0.39⁎⁎ | – | 0.51⁎⁎ |
: The highest detected concentration among the three assays was selected for the analysis.
: 0.01<p<0.05.
: p<0.01.
4. Discussion
4.1. Efficacy of PEG precipitation for detecting SARS-CoV-2 in wastewater samples
Most of previous studies investigated pathogenic and indicator viruses in water, targeting non-enveloped viruses (Haramoto et al., 2018). Consequently, retrospective virus concentration methods were optimized for non-enveloped viruses and may be less effective for enveloped viruses like SARS-CoV-2; for instance, adsorption-elution methods, using an electropositive or electronegative microfilter and glass wool, were definitely effective for non-enveloped viruses (Cashdollar and Wymer, 2013), but resulted in inefficient recovery (around 10% or lower) for at least some enveloped viruses (Blanco et al., 2019; Deboosere et al., 2011; Francy et al., 2013; Haramoto et al., 2009; Honjo et al., 2010). Notably, recent studies compared the efficiencies of virus concentration methods using spiked murine hepatitis virus (MHV), which is regarded as a human CoV surrogate, and indicated that some methods using an electronegative microfilter can efficiently recover SARS-CoV-2 (Ahmed et al., 2020b). A size-exclusive method using ultrafiltration is also widely applied for concentrating viruses, including enveloped viruses like influenza-A virus and SARS-CoV-2 (Ahmed et al., 2020a; Heijnen and Medema, 2011; Medema et al., 2020; Nemudryi et al., 2020). One of the drawbacks of the ultrafiltration-based method is the cost of the ultrafiltration unit; for example, Centricon® Plus-70, which can process up to 70 mL samples, costs approximately 50 USD per unit and may not be suitable for frequent use. Additionally, a recent study suggested that Centricon® Plus-70 is not effective for recovering SARS-CoV-2 (Ahmed et al., 2020b). By contrast, PEG precipitation is one of the most cost-effective methods for concentrating viruses at low water sample volumes (around 100 mL; Lewis and Metcalf, 1988). The technique is effective for variety of proteins (Atha and Ingham, 1981); therefore, it might be effective for detecting SARS-CoV-2, even though only a limited number of studies have applied the technique to enveloped viruses in wastewater and surface water (Ahmed et al., 2020b; Deboosere et al., 2011; Honjo et al., 2010). Of these, Ahmed et al. (2020b) revealed that the recovery efficiency of MHV spiked in wastewater was 44.0 ± 27.7%. Other two studies (Deboosere et al., 2011; Honjo et al., 2010) successfully detected enveloped viruses (Influenza A virus and Cyprinid Herpesvirus 3) from lake water samples by adsorption-elution methods and following PEG precipitation, even though their recovery efficiencies by PEG precipitation were not clearly shown. For PEG precipitation, several conditions (i.e., concentrations of PEG and NaCl) have been applied (Jones and Johns, 2009). Of these, we selected relatively high concentrations of PEG and NaCl (10% and 1.0 M), in accordance with Jones and Johns (2009). As expected, the recovery efficiency of F-phages in this study, 45% geometric mean and always above 10%, can be considered to be sufficiently high and stable (Haramoto et al., 2018). In this study, F-phages were selected because they are abundantly present in wastewater and are easily enumerated by a simple plate counting assay. F-phages are non-enveloped viruses and their recovery efficiency is not necessarily correlate with SARS-CoV-2. As mentioned above, previous studies have suggested that PEG precipitation is effective for enveloped viruses in wastewater samples (Ahmed et al., 2020b; Deboosere et al., 2011; Honjo et al., 2010). The stably sufficient recovery efficiency of F-phages observed in this study can indicate that a substantial loss of SARS-CoV-2 during the PEG precipitation did not occur. However, an efficient method for recovering SARS-CoV-2 in wastewater, and an appropriate recovery control to ascertain the efficient recovery of SARS-CoV-2, should be identified in the future.
Wastewater samples after virus concentration processes can result in inefficient nucleic acid extraction and RT-PCR (Haramoto et al., 2018). In this study, to see the efficiency of the extraction and RT-PCR processes, MNV, a non-enveloped virus, was used. No notable loss of RNA or inhibitory effect, which reduce the detection efficiency of MNV to less than 10%, was observed in this study. The structural difference between MNV and SARS-CoV-2 might affect the efficiency of RNA extraction process, by which envelope and capsid are denatured. Our results, the sufficiently high detection efficiencies for the spiked MNV, does not necessarily indicate those for SARS-CoV-2. Enveloped viruses such as MHV and phi 6 phage may work better than MNV as the control. However, even with MNV, a manipulation failure, which also affects the SARS-CoV-2 detection, can be identified.
4.2. Comparison of the qRT-PCR assays for detection of SARS-CoV-2 RNA
Several molecular assays have been designed for detection of SARS-CoV-2 (CDC, 2020; Chu et al., 2020; Corman et al., 2020; Shirato et al., 2020). Although some studies evaluated the effectiveness of these assays comparatively (Jung et al., 2020; Medema et al., 2020; Nalla et al., 2020; Nemudryi et al., 2020; Randazzo et al., 2020; Vogels et al., 2020), a gold standard method has not yet been determined. In this study, three qRT-PCR assays were comparatively applied. Across the 45 samples tested, the CDCN3 assay resulted in the highest detection frequency and tended to show higher detected concentrations; the NIID assay resulted in a far lower detection frequency and seemed to be inefficient. Some samples that were negative according to the CDCN3 assay were positive according to the CDCN2 assay. These inconsistent results among the assays may be because some assays failed to amplify genes of specific SARS-CoV-2 strains. Similarly, inconsistent results among with different target genes have been observed in previous studies (Medema et al., 2020; Randazzo et al., 2020). Considering that most of the SARS-CoV-2 positive samples resulted in low detected concentrations (<1.0 × 101 copies/reaction), it is also possible that the target gene was occasionally absent in the reaction mixture. Spearman rank correlation test revealed that the detected SARS-CoV-2 concentration was better correlated to the number of reported cases when all the assays were taken into account. This implies the assays complement each other. The possibility of false positives also needs to be considered. In this study, no positive signal was observed from the negative control, which was included in all the qRT-PCR runs. Gel electrophoresis and amplicon sequencing can also be applied to exclude the possibility of false positives (Ahmed et al., 2020a). In this study, sequences of two out of nine amplicons were partially identical to a reference SARS-CoV-2 sequence. However, others had only partial matches with primer sequences. This is probably because Sanger sequencing is not reliable for short amplicons (<100 bp). Application of cloning-sequencing or next generation sequencing would be better to confirm positive results by qRT-PCR.
4.3. Relationship between the occurrence of SARS-CoV-2 in wastewater and the number of COVID-19 cases
The detection frequency of SARS-CoV-2 in investigated wastewater samples seemed to accord with the numbers of confirmed cases and with those of inpatients in both prefectures, even though the number of confirmed cases probably underestimates the actual number. The detection frequency appeared to be higher when the number of confirmed cases exceeded 10 per 100,000 people (Fig. 2 and Table 2); moreover, no positive results were obtained for the samples collected in Toyama prefecture during March, when no confirmed SARS-CoV-2 infection cases were reported there. Notably, SARS-CoV-2 in wastewater samples could be detected even when the number of cases were supposed to be low (<1.0 confirmed cases per 100,000 people), although the detection frequency decreased (see Fig. 2 and Table 2). In this study, grab samples were collected. Compared with a 24-hours composite sample, SARS-CoV-2 concentration in a grab sample is expected to be variable. Especially when the number of infected individuals is low and the contamination of SARS-CoV-2 in wastewater occurs intermittently at a level close to the limit of detection, a grab sample can provide more chances for detecting SARS-CoV-2. On the contrary, the concentration of SARS-CoV-2 in a composite sample is supposed to be averaged and more representative. Therefore, to see a correlation between the number of COVID-19 cases and the concentration of SARS-CoV-2 in wastewater, employing composite samples might be better. For better characterization of grab sample and composite sample, comparing these two sampling approaches would be necessary. One SARS-CoV-2 positive sample collected during March was collected at WWTP A. This positive result might also be attributable to the relatively small population served by the WWTP (31,501 people), since small WWTPs have a limited dilution effect and may be more sensitive to the presence of infected individuals. The estimated number of inpatients in Ishikawa and Toyama prefectures began to decrease on May 7 and 9, respectively; furthermore, in Toyama prefecture, the number of reported cases stopped increasing on May 19. Thereafter, the concentration and/or detection frequency of SARS-CoV-2 in the samples was expected to decrease, but such a tendency was not clearly observed. In some cases, SARS-CoV-2 has been reported to be shed from stools, even after it has become undetectable in the respiratory tract (Wu et al., 2020; Wölfel et al., 2020), for which SARS-CoV-2 shed from discharged individuals may be an explanation. Presence of undiagnosed or asymptomatic infection may also be an explanation. For better application of WBE, further studies are necessary regarding the presence of SARS-CoV-2 in wastewater when its prevalence is decreasing. Additionally, stability of SARS-CoV-2 and its RNA in wastewater also needs to be studied further. A recent study revealed that the temperature is one of the most important factors on the decay of SARS-CoV-2 RNA (Ahmed et al., 2020c). As have been pointed, effects of other factors such as level of organic matter, pH, and light availability also needs to be evaluated (Wartecki and Rzymski, 2020).
4.4. Implications for WBE
Previous studies have suggested that the concentration of SARS-CoV-2 in wastewater is affected by the number of infected people in the catchment area (Ahmed et al., 2020a; Medema et al., 2020); however, there seems to be a discrepancy in the observed relationships between the concentrations in wastewater and the numbers of infected people. Ahmed et al. (2020a) could not identify SARS-CoV-2 in a wastewater sample in Queensland until the number of confirmed cases reached around 100 cases per 100,000 people, and the observed concentration was below the limit of quantification at that time. By contrast, Medema et al. (2020) and Randazzo et al. (2020) obtained positive results before the first confirmed case in the study areas. This is, at least partly, because the number of the confirmed cases did not necessarily reflect the actual prevalence of the infection at a given time point, which might be better correlated to the concentration of SARS-CoV-2 in the wastewater. In Japan, it took a few days to confirm a case by clinical surveillance after the symptoms first appear or a person becomes infected; there may also be unconfirmed asymptomatic cases. Another possible reason is that sewer catchment areas cover only sections of prefectures. Since COVID-19 cases were reported on a prefecture basis in Japan, the sampled area included the number of cases reported outside the sewer catchment of the target WWTP. Even if it was detected once due to the presence of patients in the catchment area, it would no longer be detected if the patients moved outside that area for hospitalization. The difference in the methods applied also needs to be concerned: the collection of composite samples or grab samples, as discussed above, and the methods of sample concentration, nucleic acid extraction, and qRT-PCR. In the context of WBE, further data regarding the relationship between the numbers of cases and the concentrations of SARS-CoV-2 in wastewater needs to be accumulated.
5. Conclusions
The presence of SARS-CoV-2 in wastewater samples collected from five WWTPs in two prefectures in Japan was investigated. SARS-CoV-2 is more likely to be detected when COVID-19 is prevalent in the catchment area, although SARS-CoV-2 was detectable even before the number of cases reaches <1.0 per 100,000 people. The detection frequency remained high even after increase in the number of cases stopped, presumably due to shedding from discharged or undiagnosed individuals. Applying multiple qRT-PCR assays is recommendable because they may work complementary. Grab samples, which seem to be less representative than composite samples, were collected in this study. Testing composite sample can potentially show better correlation between SARS-CoV-2 in the sample and the number of cases. The observed SARS-CoV-2 concentrations in most of the samples were low, <1.0 × 101 copies/reaction. Controls used in this study were non-enveloped viruses, but it would be better to use enveloped viruses to assess the assay reliability. To improve the applicability of WBE, validation of the way of sample collection, improvement of the assay sensitivity, and identification of suitable controls are necessary.
CRediT authorship contribution statement
Akihiko Hata: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Visualization. Hiroe Hara-Yamamura: Investigation, Writing - review & editing. Yuno Meuchi: Investigation. Shota Imai: Investigation. Ryo Honda: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare no conflicts of interest associated with this manuscript.
Acknowledgements
This work was supported by JST MIRAI Program (Grant No. JPMJMI18DC) and Hiramoto-Gumi Inc., a civil engineering company in Japan.
Editor: Ewa Korzeniewska
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.143578.
Appendix A. Supplementary data
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., Mcminn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;379:139960. doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddell S., Sherchan S.P., Simpson S.L., Sirikanchana K., Symonds E.M., Verhagen R., Vasan S.S., Kitajima M., Bivins A. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atha D.H., Ingham K.C. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J. Biol. Chem. 1981;256:12108–12117. [PubMed] [Google Scholar]
- Blanco A., Abid I., Al-Otaibi N., Pérez-Rodríguez F.J., Fuentes C., Guix S., Pintó R.M., Bosch A. Glass wool concentration optimization for the detection of enveloped and non-enveloped waterborne viruses. Food Environ. Virol. 2019;11:184–192. doi: 10.1007/s12560-019-09378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitrago-Garcia, D., Egli-Gany, D., Counotte, M.J., Hossmann, S., Imeri, H., Ipekci, A.M., Salanti, G., Low, N., 2020. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 17, e1003346. [DOI] [PMC free article] [PubMed]
- Cashdollar J.L., Wymer L. Methods for primary concentration of viruses from water samples: a review and meta-analysis of recent studies. J. Appl. Microbiol. 2013;115:1–11. doi: 10.1111/jam.12143. [DOI] [PubMed] [Google Scholar]
- . Centers for Disease Control and Prevention, Respiratory Viruses Branch, Division of Viral Diseases Instructions. 2020. Real-Time RT-PCR Panel for Detection 2019-Novel Coronavirus Centers for Disease Control and Prevention, Respiratory Viruses Branch, Division of Viral Diseases, Accessed February 28, 2020.
- Choi P.M., Tscharke B.J., Donner E., O’Brien J.W., Grant S.C., Kaserzon S.L., Mackie R., O’Malley E., Crosbie N.D., Thomas K.V., Mueller J.F. Wastewater-based epidemiology biomarkers: past, present and future. TrAC - Trends Anal. Chem. 2018;105:453–469. [Google Scholar]
- Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y., Ng D.Y.M., Wan C.K.C., Yang P., Wang Q., Peiris M., Poon L.L.M. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020;66(4):549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Luisa Schmidt M., GJC Mulders D., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C., Victor C.M., Olfert L., Marco K., Richard M., Adam M., Daniel C.K., Tobias B., Sebastian B., Julia S., Marie Luisa S., Daphne G.J.C, .M., Bart H.L., der Veer Bas V., den Brink Sharon V., Lisa W., Gabriel G., Jean-Louis R., Joanna E., Maria Z., Malik P., Herman G., Chantal R. Detection of 2019-nCoV by RT-PCR. Euro. Surveill. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. (pii=2000045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboosere N., Horm S.V., Pinon A., Gachet J., Coldefy C., Buchy P., Vialette M. Development and validation of a concentration method for the detection of influenza a viruses from large volumes of surface water. Appl. Environ. Microbiol. 2011;77:3802–3808. doi: 10.1128/AEM.02484-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francy D.S., Stelzer E.A., Brady A.M.G., Huitger C., Bushon R.N., Ip H.S., Ware M.W., Villegas E.N., Gallardo V., Lindquist H.D.A. Comparison of filters for concentrating microbial indicators and pathogens in lake water samples. Appl. Environ. Microbiol. 2013;79:1342–1352. doi: 10.1128/AEM.03117-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Katayama H., Ito T., Ohgaki S. Development of virus concentration methods for detection of koi herpesvirus in water. J. Fish Dis. 2009;32:297–300. doi: 10.1111/j.1365-2761.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D., Katayama H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Honda R. Potential sensitivity of wastewater monitoring for SARS-CoV-2: comparison with norovirus cases. Environ. Sci. Technol. 2020;54(11):6451–6452. doi: 10.1021/acs.est.0c02271. [DOI] [PubMed] [Google Scholar]
- Havers F.P., Reed C., Lim T., Montgomery J.M., Klena J.D., Hall A.J., Fry A.M., Cannon D.L., Chiang C.F., Gibbons A., Krapiunaya I., Morales-Betoulle M., Roguski K., Rasheed M.A.U., Freeman B., Lester S., Mills L., Carroll D.S., Owen S.M., Johnson J.A., Semenova V., Blackmore C., Blog D., Chai S.J., Dunn A., Hand J., Jain S., Lindquist S., Lynfield R., Pritchard S., Sokol T., Sosa L., Turabelidze G., Watkins S.M., Wiesman J., Williams R.W., Yendell S., Schiffer J., Thornburg N.J. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, march 23-may 12, 2020. JAMA Intern. Med. 2020;30329:1–11. doi: 10.1001/jamainternmed.2020.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen L., Medema G. Surveillance of influenza a and the pandemic influenza A (H1N1) 2009 in sewage and surface water in the Netherlands. J. Water Health. 2011;9:434–442. doi: 10.2166/wh.2011.019. [DOI] [PubMed] [Google Scholar]
- Honjo M.N., Minamoto T., Matsui K., Uchii K., Yamanaka H., Suzuki A. a, Kohmatsu Y., Iida T., Kawabata Z. Quantification of cyprinid herpesvirus 3 in environmental water by using an external standard virus. Appl. Environ. Microbiol. 2010;76:161–168. doi: 10.1128/AEM.02011-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.H., Johns M.W. Improved detection of F-specific RNA coliphages in fecal material by extraction and polyethylene glycol precipitation. Appl. Environ. Microbiol. 2009;75:6142–6146. doi: 10.1128/AEM.00436-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., Park G.S., Moon J.H., Ku K., Beak S.H., Lee C.S., Kim Seil, Park E.C., Park D., Lee J.H., Byeon C.W., Lee J.J., Maeng J.S., Kim S.J., Kim Seung Il, Kim B.T., Lee M.J., Kim H.G. Comparative analysis of primer-probe sets for RT-qPCR of COVID-19 causative virus (SARS-CoV-2) ACS Infect. Dis. 2020;6:2513–2523. doi: 10.1021/acsinfecdis.0c00464. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Tohya Y., Matsubara K., Haramoto E., Utagawa E., Katayama H., Ohgaki S. Use of murine norovirus as a novel surrogate to evaluate resistance of human norovirus to free chlorine disinfection in drinking water supply system. Environ. Eng. Res. 2008;45:361–370. (in Japanese) [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. The first proof of the capability of wastewater surveillance for COVID-19 in India through the detection of the genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746(141326) doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Ferraro G.B., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzo E., Franchin E., Ciavarella C., Cuomo-Dannenburg G., Barzon L., Del Vecchio C., Rossi L., Manganelli R., Loregian A., Navarin N., Abate D., Sciro M., Merigliano S., De Canale E., Vanuzzo M.C., Besutti V., Saluzzo F., Onelia F., Pacenti M., Parisi S.G., Carretta G., Donato D., Flor L., Cocchio S., Masi G., Sperduti A., Cattarino L., Salvador R., Nicoletti M., Caldart F., Castelli G., Nieddu E., Labella B., Fava L., Drigo M., Gaythorpe K.A.M., Brazzale A.R., Toppo S., Trevisan M., Baldo V., Donnelly C.A., Ferguson N.M., Dorigatti I., Crisanti A. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo. Nature. 2020;584:425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G.D., Metcalf T.G. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl. Environ. Microbiol. 1988;54:1983–1988. doi: 10.1128/aem.54.8.1983-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ Sci Technol Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooijman K. a, Bahar M., Muniesa M., Havelaar A.H. Optimisation of ISO 10705-1 on enumeration of F-specific bacteriophages. J. Virol. Methods. 2002;103:129–136. doi: 10.1016/s0166-0934(02)00004-6. [DOI] [PubMed] [Google Scholar]
- Nalla, A.K., Casto, A.M., Huang, M.L.W., Perchetti, G.A., Sampoleo, R., Shrestha, L., Wei, Y., Zhu, H., Jerome, K.R., Greninger, A.L., 2020. Comparative performance of SARS-CoV-2 detection assays using seven different primer-probe sets and one assay kit. J. Clin. Microbiol. 58(6), e00557-20. [DOI] [PMC free article] [PubMed]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Reports Med. 2020;1:100098. doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Kobayashi T., Suzuki A., Jung S.-M., Hayashi K., Kinoshita R., Yang Y., Yuan B., Akhmetzhanov A.R., Linton N.M., Miyama T. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int. J. Infect. Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M.R., Axler R.P., Hicks R.E. Effects of freezing and storage temperature on MS2 viability. J. Virol. Methods. 2004;122:147–152. doi: 10.1016/j.jviromet.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Omori R., Mizumoto K., Chowell G. Changes in testing rates could mask the novel coronavirus disease (COVID-19) growth rate. Int. J. Infect. Dis. 2020;94:116–118. doi: 10.1016/j.ijid.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a Low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto1 D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K., Nao N., Katano H., Takayama I., Saito S., Kato F., Katoh H., Sakata M., Nakatsu Y., Mori Y., Kageyama T., Matsuyama S., Takeda M. Development of genetic diagnostic methods for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn. J. Infect. Dis. 2020;73:304–307. doi: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- Tang A., Tong Z., Wang H., Dai Y., Li K., Liu J., Wu W., Yuan C., Yu M., Li P., Yan J. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis. 2020;26:1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S., Furumai H., Katayama H. Applicability of polyethylene glycol precipitation followed by acid guanidinium thiocyanate-phenol-chloroform extraction for the detection of SARS-CoV-2 RNA from municipal wastewater. Sci. Total Environ. 2020:143067. doi: 10.1016/j.scitotenv.2020.143067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels, C.B.F., Brito, A.F., Wyllie, A.L., Fauver, J.R., Ott, I.M., Kalinich, C.C., Petrone, M.E., Casanovas-Massana, A., Catherine Muenker, M., Moore, A.J., Klein, J., Lu, P., Lu-Culligan, A., Jiang, X., Kim, D.J., Kudo, E., Mao, T., Moriyama, M., Oh, J.E., Park, A., Silva, J., Song, E., Takahashi, T., Taura, M., Tokuyama, M., Venkataraman, A., Weizman, O. El, Wong, P., Yang, Y., Cheemarla, N.R., White, E.B., Lapidus, S., Earnest, R., Geng, B., Vijayakumar, P., Odio, C., Fournier, J., Bermejo, S., Farhadian, S., Dela Cruz, C.S., Iwasaki, A., Ko, A.I., Landry, M.L., Foxman, E.F., Grubaugh, N.D., 2020. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT–qPCR primer–probe sets. Nat. Microbiol. In press. [DOI] [PMC free article] [PubMed]
- Wartecki A., Rzymski P. On the coronaviruses and their associations with the aquatic environment and wastewater. Water. 2020;12:1–27. [Google Scholar]
- White E.M., Santostefano C.M., Feifer R.A., Kosar C.M., Blackman C., Gravenstein S., Mor V. Asymptomatic and Presymptomatic severe acute respiratory syndrome coronavirus 2 infection rates in a multistate sample of skilled nursing facilities. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.5664. (Accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization Coronavirus disease (COVID-2019) situation reports [WWW document] 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports URL.
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Kasprzyk-Hordern B., Frost C.G., Estrela P., Thomas K.V. Community sewage sensors for monitoring public health. Environ. Sci. Technol. 2015;49:5845–5846. doi: 10.1021/acs.est.5b01434. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J. Med. Virol. 2020 doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen C., Zhu S., Shu C., Wang D., Song J. Notes from the field isolation of 2019-nCoV from a stool specimen of a laboratory- confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Wkly. 2020;2(8):123–124. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.