Abstract
Background
Switching antiretroviral regimens when human immunodeficiency virus (HIV) viremia is controlled for a new regimen is challenging when there is the potential for prior nucleoside reverse-transcriptase inhibitor (NRTI) resistance. The objective was to study virologic outcomes after switching to dolutegravir compared with remaining on a boosted protease inhibitor (protease inhibitor/ritonavir [PI/r]) regimen in people with HIV (PWH) with prior documented virologic failure and/or exposure to mono/dual NRTIs.
Methods
We used the Quebec HIV Cohort including 10 219 PWH whose data were collected at 4 sites in Montreal, Canada. We included all PWH with documented virologic failure or exposure to mono/dual NRTI therapy who were virologically suppressed on a PI/r-based regimen for at least 6 months on or after January 1, 2014 (n = 532). A marginal structural Cox model analysis was used to estimate the effect of the switch to dolutegravir on virologic outcome compared with remaining on PI/r. The outcome was defined as 2 consecutive viral loads (VLs) >50 copies/mL or 1 VL >50 copies/mL if it occurred at the last VL available.
Results
Among 532 eligible participants, 216 (40.6%) had their regimen switched to dolutegravir with 2 NRTIs, whereas 316 (59.4%) remained on the PI/r with 2 NRTIs. The weighted hazard ratio for the effect of dolutegravir switch on virologic failure compared with patients whose regimen remained on PI/r was 0.57 (95% confidence interval, 0.21–1.52).
Conclusions
We did not find evidence of an increased risk for virologic failure after switching to dolutegravir from PI/r among patients with previous virologic failure or prior exposure to mono/dual NRTI.
Keywords: antiretroviral regimen (ART), dolutegravir switch, protease inhibitor/ritonavir (PI/r), previously documented virologic failure and prior exposure to mono/dual NRTI combination antiretroviral therapy
Therapeutic switches to dolutegravir with 2 NRTIs provide a safe option for patients with a viremia suppressive antiretroviral regimen in the presence of prior documented virologic failure and prior exposure to mono/dual NRTI therapy.
It remains unknown whether people with human immunodeficiency virus (PWH) who are virologically suppressed on a boosted protease inhibitor (protease inhibitor/ritonavir [PI/r]) regimen but who are at risk for prior nucleoside reverse-transcriptase inhibitors (NRTIs) resistance can be safely switched to dolutegravir. The European AIDS Clinical Society [1] and US Department of Health and Human Services [2] recommend, when contemplating a regimen switch in these situations, to maintain a suppressive regimen or to replace it only if the other 2 NRTIs in the antiretroviral therapy (ART) combination are effective in maintaining virologic suppression [1] or to consult a human immunodeficiency virus (HIV) specialist [2].
Previous studies with the first-generation of integrase strand transfer inhibitors (INSTIs) have shown that the replacement of an ART among patients with previously documented virologic failures, or proven or suspected resistance mutations, lead to more virologic failures than the maintenance of the boosted protease inhibitors (PIs) regimen. For example, the SWITCHMRK study showed that replacing a lopinavir/ritonavir regimen with raltegravir led to more virologic failures in a population with prior treatment failures [3]. After these results, switch studies have excluded patients with prior virologic failures or resistance mutations or those who were exposed to suboptimal mono/dual NRTI therapies [4–7].
However, even if these strategies have never been tested, there are indirect indications that the second generation of INSTIs could be an effective therapeutic choice for switch strategies in experienced patients with documented virologic failure or resistance mutation. Unlike raltegravir and elvitegravir, INSTIs such as dolutegravir or bictegravir are recognized to have a high genetic barrier with fewer side effects and lower risk for resistance mutations at failure [8–11]. The efficacy of dolutegravir is well recognized for the replacement of ART among patients without documented virologic failure or resistance mutations [4, 8, 12]. Some studies have also shown that dolutegravir was superior or noninferior to the first generation of INSTIs or to PI/r in experienced and naive patients [13–16]. Moreover, some treatment failure studies have shown the superiority of a dolutegravir-based regimen. The DAWNING study [17] showed the superiority of dolutegravir with 2 NRTIs when comparing dolutegravir to PI/r among patients with current virologic failure to a first-line non-NRTI (NNRTI)-based regimen. The SAILING study [18] showed that given with an optimized background regimen, dolutegravir was superior to raltegravir in experienced patients failing their current regimen. These data support the high genetic barrier to resistance of dolutegravir and its potential role in switching therapy in case of prior presumed or documented resistance.
The objective of our study was to analyze virologic outcomes after switching to dolutegravir compared with remaining on a boosted PI (PI/r) regimen in PWH with prior documented virologic failure and/or exposure to mono/dual NRTIs. A weakness of many observational studies is that they ignore the longitudinal nature of exposure and confounding. The aim of our study was also to avoid these pitfalls by applying an established causal inference method to provide strong evidence with “real-world” data.
METHODS
The Quebec HIV Cohort is an observational cohort of PWH followed in 4 centers that specialized in HIV care in Montreal. Sites included 2 community clinics, “Clinique Médicale l’Actuel (CMA)” and “Clinique de Médecine Urbaine du Quartier Latin (CMUQL)”, and 2 hospital clinics, “Centre Hospitalier de l’Université de Montréal (CHUM)” and “McGill University Health Center (MUHC).” Patient charts were used to prospectively collect data at every clinical visit that usually takes place every 3 to 6 months. Data collection was done independently in each center. It began in 1985 at CMA, in 1997 at CMUQL, and in 1989 at both CHUM and MUHC. Data were integrated into individual databases using ACCESS and were merged into a central database using encryption while considering visits belonging to the same patient followed across several sites. After the validation step (data completeness and correction), 10 219 PWH were included in the cohort, 5844 of whom were currently being followed in clinics as of August 31, 2017. The following variables were assessed: date of HIV diagnosis, sociodemographic variables (age, sex, centers), and risk factors for HIV acquisition. We also used time-varying variables documented or potentially updated at every patient’s visit including ART received, resistance mutations, and biochemical laboratory results (CD4 count, viral load [VL], and testing for antihepatitis C virus antibodies and hepatitis B surface antigen). The mean (standard deviation [SD]) and median (interquartile range) age at entry in the Quebec HIV Cohort were 37.4 years (10.3) and 36.4 years (30.1–43.8), respectively, males constituted 84.1% of the cohort, and patients belonged to different risk groups (men who have sex with men, injection drug users, those who were infected by vertical transmission, and those from endemic countries).
We included all PWH in the cohort who, on January 1, 2014 or later, had documented virologic failure and/or who had been exposed to mono/dual NRTI therapy, and who were virologically supressed on a PI/r-based ART for at least 6 months. The PIs considered in the study included lopinavir, darunavir, or atazanavir. The inclusion of PWH with previously documented virologic failure in the cohort was based on the definition of virologic failure recommended by the Guidelines for adults living with HIV used by health professionals in Quebec [19], which is defined as follows: a documented VL >1000 copies/mL after 16 weeks of therapy, or VL >400 copies/mL after 24 weeks, or 2 consecutive VL >50 copies/mL after 48 weeks or after having reached suppressive viremia (<50 copies/mL), or VL >50 copies/mL at discontinuation treatment. Prior exposure to mono/dual NRTI therapy was defined as any previous exposure to a single NRTI or 2 NRTIs for at least 1 month. The mono-NRTI therapy included zidovudine or didanosine used until 1997. Dual NRTI therapy included the following combinations: lamivudine/zidovudine, lamivudine/stavudine, didanosine/lamivudine, zalcitabine/zidovudine, didanosine/zidovudine, zalcitabine/lamivudine, and didanosine/stavudine that were used between 1996 and 2005.
There were 532 PWH who, on January 1, 2014 or later, had documented virologic failure and/or who had been exposed to mono/dual NRTI therapy based on these definitions, and who were all on a virologically suppressed PI/r-based ART for at least 6 months. This included 15 new users of PIs who fulfilled these criteria after January 1, 2014.
Patient Consent Statement
This study was approved by the Research Ethics Boards of the Sainte Justine University Hospital Center, MUHC, and CHUM with a waiver of consent.
Statistical Analysis
A survival analysis was conducted to analyze the risk of virologic failure of patients initially on a virologically suppressed boosted PI (PI/r) regimen where some eventually switched to dolutegravir. The index date or time zero was defined as January 1, 2014 or the earliest subsequent date where the inclusion criteria were met. An exploratory Kaplan-Meier plot with an unadjusted Peto-Prentice test were used to estimate and compare the cumulative incidence of virologic outcome in the group that maintained their original regimen versus the patients who eventually switched (PI/r + 2 NRTIs maintenance versus dolutegravir + 2NRTIs switch). Following the Target Trials approach [20], for the main analysis treatment switch was defined as the first change in regimen from PI/r to dolutegravir, and switched patients were considered exposed to dolutegravir thereafter. The follow-up time was considered from time 0 until a virologic failure occurred or, for censored observations, the last visit for which a VL measurement was available. In the case of therapy interruption outside of the treatment switch of interest, patients were censored on the date of interruption. The outcome of postindex date virologic failure was defined as 2 consecutive VL >50 copies/mL or 1 VL >50 copies/mL if the last VL available was VL >50 copies/mL.
We fit a marginal structural Cox model to estimate the effect of time-dependent exposure to dolutegravir on the hazard of virologic outcome using the approach described by Fewell et al [21, 22]. The database was discretized into 5-month intervals. We applied stabilized inverse probability of treatment and censoring weights (IPW) using logistic regression models for the probability of therapy switch to dolutegravir and for the probability of not being censored at every 5-month time point (t). These models were adjusted for past CD4 counts (continuous) as a time-dependent variable and baseline covariates (measured at time zero), namely, ART duration (continuous), HIV infection duration (continuous), and age (continuous). Missing CD4 values were replaced by the most recent documented CD4 value. A 5% truncation was used for the censoring weights due to large values. To approximate the hazard ratio (HR) for the effect of exposure at time t to dolutegravir with 2 NRTIs on virologic outcome in the marginal structural Cox model, we used our IPW in a pooled logistic regression conditional on switch status at time t [23]. All statistical analyses were performed using STATA version 14 software with the pscore package (StataCorp, College Station, TX).
RESULTS
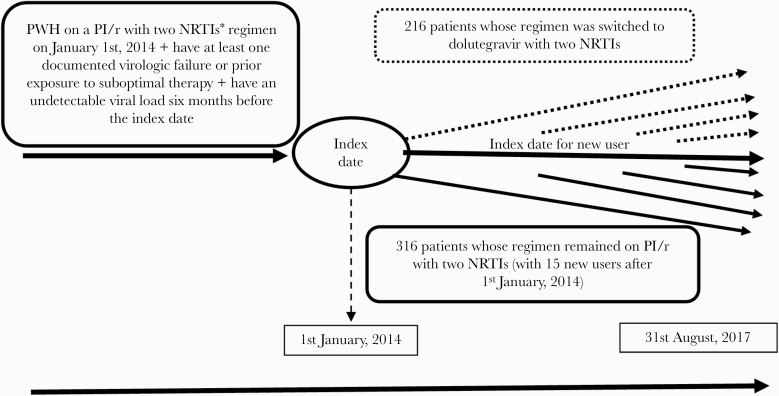
Among 532 eligible participants, 216 (40.6%) had their regimen switched to dolutegravir with 2 NRTIs, whereas 316 (59.4%) remained on their PI/r with 2 NRTIs regimen throughout follow-up. Our definition of previously documented virologic failure included virtually no patient (only 2 per group) with a failure based on a VL 50–100. Most patients continued the same 2 NRTIs when switching to dolutegravir with 17.6% (38 of 216) who also have had a switch to 2 new NRTIs. Figure 1 shows the details regarding the selection of PWH in the study. Table 1 shows the characteristics of patients at index date according to exposure status; mean age (SD) was 50.8 years (9.5) and 52.6 years (8.6) for patients whose regimen was switched to dolutegravir and for those who remained on PI/r, respectively. The NRTI backbones used with dolutegravir in the switch group were abacavir/lamivudine (73.6%) or tenofovir disoproxil/emtricitabine (26.4%). In the PI/r maintenance group, 39.2% used abacavir/lamivudine, 58.9% used tenofovir disoproxil/emtricitabine, and 1.9% used tenofovir disoproxil/lamivudine, and the PI used was lopinavir in 26.6% (84 of 316), darunavir in 39.6% (125 of 316), and atazanavir in 33.8% (107 of 316). There were 199 PWH tested for mutations before time 0, from which 84 cases documented M184 V/I mutations. Among the subjects tested in the dolutegravir switch group, 32.5% (25 of 77) had the M184 V/I mutation, whereas 48.4% (59 of 122) of those tested in the PI/r maintenance group had that mutation. Among the 25 PWH with M184V whose regimen was switched to dolutegravir, 60% (15 of 25) included the backbone abacavir/lamivudine and 40% (10 of 25) included tenofovir disoproxil fumarate/emtricitabine. There was no virologic failure in this subpopulation. Among the subjects tested for genotyping before time 0, other NRTIs resistance mutations (in mutation sites: M41, K65, D67, T69, K70, L74, Y115, Q151, L210, and T215) have been found in 37.7% (29 of 77) patients of the dolutegravir switch group and in 46.7% (57 of 122) of the PI/r group. One PI mutation was documented in a patient who switched to dolutegravir compared with 0 patients in the PI/r group, whereas INSTI resistance mutations (all mutation site E138) were documented among 4 patients in the dolutegravir group compared with 5 in the PI/r group.
Figure 1.
Patients from the Quebec HIV Cohort who were eligible for the study. CI, confidence interval; PHW, people with human immunodeficiency virus; PI/r, protease inhibitor/ritonavir; NRTIs, nucleoside reverse-transcriptase inhibitors. *NRTIs = abacavir + lamivudine or tenofovir disoproxil + emtricitabine or tenofovir disoproxil + lamivudine.
Table 1.
Baseline Characteristics of Patients (n = 532) With Prior Virologic Failure or Exposure to Mono/Dual NRTI Therapy According to ART Exposure Group
| Patient Characteristics Measured at Baseline (Index Date) | PWH Whose Regimen Was Switched to Dolutegravir With 2 NRTIs (n = 216) | PWH Whose Regimen Remained on PI/r With 2 NRTIs (n = 316) | |
|---|---|---|---|
| Age in years | Mean (SD) | 50.8 (9.5) | 52.6 (8.6) |
| Median (IQR) | 51.2 (44.9–56.9) | 52.4 (47.5–57.8) | |
| Sex, N (%) | Male | 190 (87.9%) | 272 (86.1%) |
| Female | 26 (12.1%) | 44 (13.9%) | |
| Risk factor for HIV acquisition, N (%) | MSM | 153 (70.8%) | 222 (70.2%) |
| Bisexual | 7 (3.2%) | 10 (3.2%) | |
| Heterosexual | 46 (21.3%) | 59 (18.7%) | |
| From endemic | 20 (9.3%) | 33 (10.4%) | |
| Vertical transmission | 2 (0.9%) | 1 (0.3%) | |
| Backbones, N (%) | Abacavir/lamivudine | 159 (73.6%) | 124 (39.2%) |
| Tenofovir disoproxil/ emtricitabine | 57 (26.4%) | 186 (58.9%) | |
| Tenofovir disoproxil/ lamivudine | 0 (0%) | 6 (1.9%) | |
| CD4 count (cells/mm3) | Mean (SD) | 675.9 (287.9) | 618.9 (288.9) |
| Median (IQR) | 621.5 (480.0–851.0) | 590.0 (430.0–748.0) | |
| HIV Infection duration (in year) | Mean (SD) | 15.3 (6.1) | 16.8 (4.9) |
| Median (IQR) | 15.9 (11.0–19.0) | 17.5 (15.5–19.3) | |
| ART duration (in years) | Mean (SD) | 13.6 (5.0) | 16.8 (4.9) |
| Median (IQR) | 15.2 (9.9–17.0) | 17.5 (15.5–19.3) | |
| Mutations 184V/I, N (%) | Yes | 25 (32.5%) | 59 (48.4%) |
| No | 52 (67.5%) | 63 (51.6%) | |
| Not tested | 139 | 194 | |
| Other NRTI mutationsa | Yes | 29 (37.7%) | 57 (46.7%) |
| No | 48 (62.3%) | 65 (53.3%) | |
| Not tested | 139 | 194 | |
| Previously documented virologic failure | Yes | 204 (94.4%) | 152 (48.1%) |
| No | 12 (5.6%) | 164 (51.9%) | |
| Previous exposure to mono/ dual NRTI therapyb | Yes | 64 (29.6%) | 217 (68.7%) |
| No | 152 (70.4%) | 99 (31.3%) | |
| Hepatitis B history, N (%) | Positive for HBsAg | 8 (3.7%) | 39 (12.3%) |
| Hepatitis C history, N (%) | Positive for anti-HCV | 28 (12.9%) | 46 (14.6%) |
Abbreviations: ART, antiretroviral therapy; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range (25%–75%); MSM, men who have sex with men; NRTI, nucleoside reverse-transcriptase inhibitor; PI/r, protease inhibitor/ritonavir; PWH, people with HIV; SD, standard deviation.
aOthers NRTIs mutations in mutation sites: M41, K65, D67, T69, K70, L74, Y115, Q151, L210, and T215.
bMonotherapy or dual therapy with 1 or 2 NRTIs for at least 1 month before baseline.
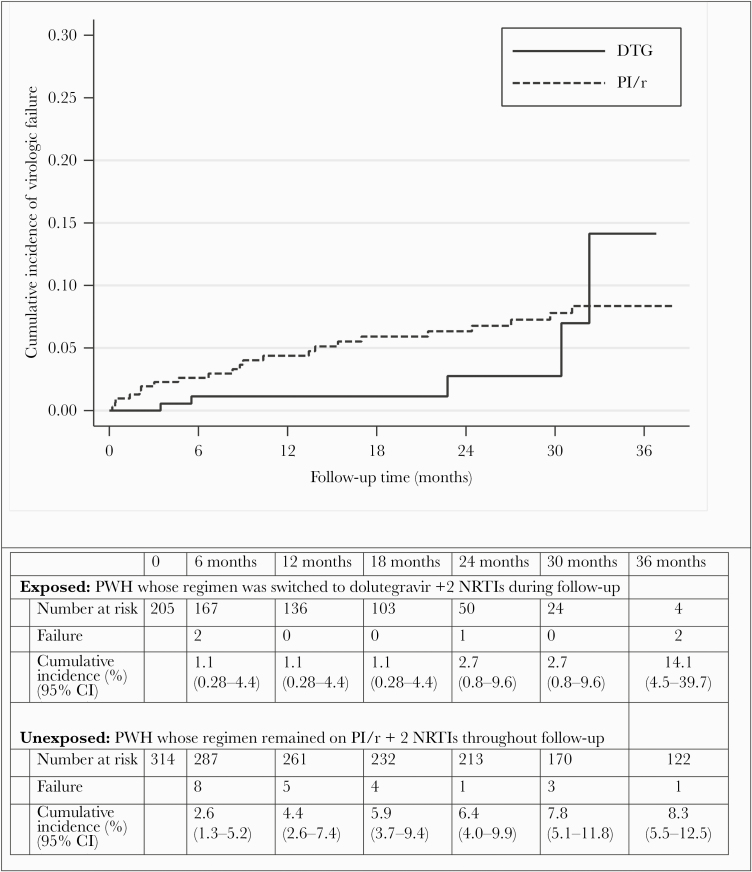
Figure 2 shows the crude cumulative incidence of patients whose regimen was switched to dolutegravir and those whose regimen was maintained on PI/r. A total of 28 postindex virologic failures occurred in the cohort (5 in the dolutegravir switch group and 23 in the PI/r maintenance group). The crude cumulative incidence of virologic failure at 2 years was 1.1% (95% confidence interval [CI], 0.2–4.4) for patients whose regimen was switched to dolutegravir and 6.3% (95% CI, 4.0–9.8) for those who remained on PI/r (Peto-Prentice test P = .15). Virologic failure outcome was defined using the last available VL >50 copies/mL for 80% (4 of 5) and 30.4% (7 of 23) of the patients who switch to dolutegravir and those who remain on PI/r, respectively. Among patients who switch to dolutegravir (n = 216) and among those who remained on PI/r (n = 316), 20 and 86 were censored because of treatment discontinuation, respectively. Table 2 presents the HR estimated following the IPW marginal structural model. The point estimate suggests that dolutegravir switch is associated with a better outcome, but the wide CIs for the HR do not allows us to conclude that a difference exists (weighted HR, 0.57; 95% CI, 0.21–1.52). If we restricted the analysis to only patients with documented M184 mutation (n = 84, total follow-up time of 152.9 person-years), the crude cumulative incidence of virologic failure at 24 months for patients whose regimen was switched to dolutegravir and for those whose regimen remained on PI/r were 0% (0–0) and 4.3% (1.7%–16.3%), respectively (P value/log-rank test = .42) (data not shown).
Figure 2.
Cumulative incidence of postindex virologic failure for people with human immunodeficiency virus (PWH) whose regimen was maintained on protease inhibitor/ritonavir (PI/r) relative to those whose regimen was switched to dolutegravir (DTG). Peto-Prentice P = .15. CI, confidence interval; NRTIs, nucleoside reverse-transcriptase inhibitors.
Table 2.
Marginal Structural Cox Model Estimates for the Effect of Treatment Regimen on Postindex Virologic Failure (n = 532)
| Exposure | Person-Years | Number of Virologic Failure | Crude HR (95% CI) | IPTW×IPCW-Weighted Marginal Structural Model HR (95% CI) |
|---|---|---|---|---|
| Exposure to PI/r with 2 NRTIs | 723.56 | 23 | 1 (reference) | 1 (reference) |
| Exposure to dolutegravir with 2 NRTIs | 291.54 | 5 | 0.54 (0.23–1.24) | 0.57 (0.21–1.52) |
Abbreviations: CI, confidence interval; HR, hazard ratio; IPCW, inverse probability of censoring weights; IPTW, inverse probability of treatment weights; NRTIs, nucleoside reverse-transcriptase inhibitors; PI/r, protease inhibitor/ritonavir.
DISCUSSION
There is only indirect evidence that a 2-NRTI + PI/r virologically suppressed regimen could be replaced by a 2-NRTI + dolutegravir regimen, and these switches are not openly recommended for patients with previously documented virologic failure or prior exposure to suboptimal mono/dual NRTI [1, 2]. Our study, undertaken among patients with previously documented virologic failure or prior exposure to suboptimal therapy, brings new support to this strategy. In addition, it suggests that virologic outcomes may be better after dolutegravir switch compared with PI/r maintenance.
Previous studies (4 randomized controlled trials [RCTs] and 1 observational study) have clearly shown the virologic efficacy of dolutegravir in both naive and experienced patients [14, 15, 24–26]. Dolutegravir may also offer some advantages including a high genetic barrier and a reduced risk of lipid disorders [4, 27]. Integrase strand transfer inhibitors are also recognized to not alter the size of virus reservoirs or immune activation [28]. However, there is uncertainty regarding the safety of dolutegravir switch among PWH who have previously documented virologic failure, prior exposure to suboptimal therapy, or presence of resistance mutations. Some studies (RCTs, meta-analyzes, and observational studies) have clearly shown the superiority of dolutegravir over other first-generation INSTIs [29–32]. Some studies have also shown the superiority or noninferiority of dolutegravir compared with PI/r regimen in patients without documented virologic failure or resistance mutations. For example, the NEAT022 study [4], an RCT, showed no statistically significant difference between patients whose regimen was switched to dolutegravir and those who remained on PI/r: 92.2% of switched patients (n = 205) and 87.0% of maintained patients (n = 210) had a VL <50 copies/mL at 96 weeks of follow-up for a mean difference of 5.2% (95% CI, −0.6 to 11). This study concluded that dolutegravir was noninferior but excluded patients with documented prior virologic failure or resistance mutations. In the context of the treatment of a current virologic failure, DAWNING [17], an RCT among patients failing a first-line NNRTI-based regimen, showed a superiority of dolutegravir with 2 NRTIs compared with PI/r with 2 NRTIs after 48 weeks of follow-up with an adjusted mean difference of 13.8% (95% CI, 7.3–20.3). Chen et al [33], in an observational study of patients on a virologically suppressed regimen, did not show a difference in the virologic outcome (VL >50 copies/mL) at 48 weeks between PWH whose regimen was switched to dolutegravir (1.1%) compared with those who remained PI/r (3.8%), for a mean difference of −2.7% (95% CI, −5.5 to 0.5). In this study, 44.4% and 19.5% of patients had previous documented virologic failures in the group on dolutegravir and on PI/r, respectively. It is interesting to note that 97.1% (34 of 35) and 96.2% (25 of 26) of switched and unswitched patients with documented M184 mutations had a VL <50 copies/mL at 48 weeks, respectively. These results were corroborated by the study by Sörstedt et al [34] who compared patients with previous NRTI mutations (122 whose regimen was switched to dolutegravir and 122 whose regimen was maintained on PI/r) and found that the proportions of virologic success were 96.7% and 97.5%, respectively, after a median of follow-up of 78 weeks. In this study, M184 V/I and NRTI mutations were the most common mutations and were documented in 36.5% and 27.7% of patients in the dolutegravir and PI/r groups, respectively. It is interesting to note that there was an absence of new NRTI and INSTI mutations in patients who switched on dolutegravir. The study 4030 [35] also showed the virologic efficacy of dolutegravir in patients with pre-existing NRTI resistance. In this study, at 48 weeks, 89.4% (42 of 47) and 94.1% (32 of 34) had a VL <50 copies/mL for patients with M184V/I on bictegravir and dolutegravir, respectively. Finally, the prospective cohort study of 5 European cohorts studying patients on abacavir/lamivudine/dolutegravir did not conclude a difference in virologic outcomes between the groups of patients with M184 mutation [36]. In this study with a median follow-up of 289 days, the virologic failure incidence in patients with and without M184V/I mutation was not statistically significant different (29.8 per 1000 person-years [95% CI, 11.2–79.4] vs 13.6 [95% CI, 8.4–21.8], respectively). Our study, undertaken among patients with previously documented virologic failure and/or prior exposure to mono/dual NRTI combination ART, did not demonstrate a difference in the virologic outcomes of patients whose regimen was switched to dolutegravir in comparison with those whose regimen was maintained on PI/r.
Our study also has significant methodological strengths despite certain weaknesses including the potential for unmeasured confounding bias, inherent to all observational design. We used marginal structural models to control for time-dependent confounding by CD4 count that also has the potential to be influenced by prior exposures and other variables. Marginal structural models allow us to define causal effects between the time-dependent exposure and the outcome, although estimation depends on several factors including the above-mentioned, no-unmeasured-confounders assumption and a well defined exposure (comparison of treatment options after a virologic failure or after switch). The analysis was based on an established causal inference method conceived to provide strong evidence using observational and real-world data. Valid causal inference relies on underlying assumptions that include positivity, consistency, noninterference, and absence of unmeasured confounders [37]. Positivity was empirically validated by verifying the size of the weights. Compliance with the consistency criterion is credible because we followed the Target Trials approach [20], and the observed exposure corresponds to an intervention that we could hypothetically assign. Compliance with the noninterference criterion is also credible because it does not seem plausible that the exposure of one person affects the counterfactual result of another. The absence of unmeasured confounding factors cannot be guaranteed, although subject matter knowledge leads us to believe that the likely confounding variables have been measured and considered in the model. Our study also has a high potential for generalizability with a nonnegligible sample size of patients followed across 4 sites with appreciable follow-up time. However, it is important to mention that a very small number of virologic failure occurred during follow-up, which limits the power of the study for the comparison of treatment groups. It would also have been interesting to have genotyping data from patients who experienced virologic failures after time 0, but this was not done systematically in our observational study.
CONCLUSIONS
Our study adds to the body of evidence suggesting that switching to dolutegravir is likely a safe option for patients controlled on PI/r who have prior resistance, although larger studies are still needed to have more precise estimates of virologic failure among such patients. Dolutegravir, recognized as offering some advantages including a high genetic mutation barrier, reduced risk of lipid disorders, and no impact on the virus size reservoirs or immune activation [4, 27, 28], may offer a safe and desirable option for ART replacement for patients on a PI/r regimen with antecedent of virologic failure or prior exposure to mono/dual NRTI.
Acknowledgments
We are grateful to Anne-Fanny Vassal, Maliheh Vaziri, and Mariève Beauchamp (Clinique Médicale l’Actuel [CMA]), Meryem Merabi (Clinique de Médecine Urbaine du Quartier Latin [CMUQL]), Catherine Deshaies and Joseph Niyibizi (Sainte-Justine Hospital), Tudor Luncean (Centre Hospitalier de l’Université de Montréal), Jessica Lumia and Leo Wong (McGill University Health Center), and all other contributing research staff personnel for help in collecting and managing data in all sites.
Authors’ contributions. All authors of this research paper have directly contributed to the conception and design (H. T., M. N. S., J.-G. B., A. d. P., M. E. S., R. T., M. K., C. T., M. D., M. R., S. F. G.), or acquisition of data (M. N. S., J. G. B., R. T., M. K., C. T., L. L., Z. R. G., C. P., N. M., M. D., M. R., I. H., H. T.), or data management, analysis and interpretation (M. N. S., H. T., M. C., M. E. S., S. F. G., J.-G. B., A. d. P., C. L., N. M., M. D., A. T.) of the study. M. N. S., H. T., J.-G. B., and A. d. P. wrote the first draft of the manuscript. All authors have subsequently read, revised, and approved the version that is being submitted.
Financial support. This work was funded by an unrestricted Grant from ViiV Healthcare and the Réseau sida et maladies infectieuses (SIDA-MI) du Fonds de recherche du Québec – Santé (FRQS). M. N. S. was supported by a doctoral award from Islamic Development Bank, Merit scholarship Program for High Technology (2013–2016) and a doctoral award from Université de Montréal (additional tuition fees waiver scholarships for international student and scholarships for end of PhD studies). H. T. holds a salary award (Research Scholar) from the FRQS and a New investigator salary award from Canadian Institutes of Health Research (CIHR). A. d. P. holds a salary award (Research Scholar) from the FRQS. M. K. is supported by a Tier I Canada Research Chair. M. E. S. holds a New Investigator Salary Award from CIHR.
Potential conflicts of interest. H. T. has received unrestricted grants from ViiV Healthcare, Merck, and Gilead Sciences. J.-G. B. has received honoraria for consulting for ViiV Healthcare, Merck, and Gilead Sciences and for participation as a speaker at conferences from Merck and Gilead Sciences. His institution (CMUQL) has received research grants from GlaxoSmithKline, Merck, and Gilead Sciences. A. d. P.’s institution participates in several pharmaceutical clinical trials for human immunodeficiency virus antiretrovirals and hepatitis C virus treatments in which she is the site coinvestigator or principal investigator (ViiV Healthcare, Janssen, Merck, Gilead Sciences) and has received honoraria for consulting in advisory board meetings for ViiV Healthcare and Merck. R. T. has received honoraria for consulting for ViiV Healthcare, Merck, and Gilead Sciences and for participation as a speaker at conferences from Merck and Gilead Sciences. His institution (CMA) has received research grants from GlaxoSmithKline, Gilead Sciences, Merck, and Janssen. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. EACS Guidelines version 10.0 Available at: https://www.eacsociety.org/files/guidelines-10.0_final_2_2.pdf. Accessed April 15, 2020.
- 2. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Departements of Health and Human Services; Available at: https://files.aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 6 April 2020. [Google Scholar]
- 3. Eron JJ, Young B, Cooper DA, et al. ; SWITCHMRK 1 and 2 investigators Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet 2010; 375:396–407. [DOI] [PubMed] [Google Scholar]
- 4. Gatell JM, Assoumou L, Moyle G, et al. Immediate versus deferred switching from a boosted protease inhibitor-based regimen to a dolutegravir-based regimen in virologically suppressed patients with high cardiovascular risk or age >/=50 years: final 96-week results of the NEAT022 Study. Clin Infect Dis 2019; 68:597–606. [DOI] [PubMed] [Google Scholar]
- 5. Arribas JR, Pialoux G, Gathe J, et al. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis 2014; 14:581–9. [DOI] [PubMed] [Google Scholar]
- 6. Trottier B, Lake J, Logue K, et al. Switching to abacavir/dolutegravir/lamivudine fixed dose combination (ABC/DTG/3TC FDC) from a PI, INI or NNRTI based regimen maintains HIV suppression. 55th Interscience Conference on Antimicrobial Agents and Chemotherapy (San Diego, CA). 17-21 September, 2015. [Google Scholar]
- 7. Pozniak A, Markowitz M, Mills A, et al. Switching to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of non-nucleoside reverse transcriptase inhibitor with emtricitabine and tenofovir in virologically suppressed adults with HIV (STRATEGY-NNRTI): 48 week results of a randomised, open-label, phase 3b non-inferiority trial. Lancet Infect Dis 2014; 14:590–9. [DOI] [PubMed] [Google Scholar]
- 8. Aboud M, Orkin C, Podzamczer D, et al. Efficacy and safety of dolutegravir-rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the randomised, open-label, phase 3 SWORD-1 and SWORD-2 studies. Lancet HIV 2019; 6:e576–87. [DOI] [PubMed] [Google Scholar]
- 9. Navarro J, Santos JR, Silva A, et al. Effectiveness of once/day dolutegravir plus boosted darunavir as a switch strategy in heavily treated patients with human immunodeficiency virus. Pharmacotherapy 2019; 39:501–7. [DOI] [PubMed] [Google Scholar]
- 10. Nyaku AN, Zheng L, Gulick RM, et al. ; ACTG A5353 Study Team Dolutegravir plus lamivudine for initial treatment of HIV-1-infected participants with HIV-1 RNA <500 000 copies/mL: week 48 outcomes from ACTG 5353. J Antimicrob Chemother 2019; 74:1376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joly V, Burdet C, Landman R, et al. ; LAMIDOL Study Group Dolutegravir and lamivudine maintenance therapy in HIV-1 virologically suppressed patients: results of the ANRS 167 trial (LAMIDOL). J Antimicrob Chemother 2019; 74:739–45. [DOI] [PubMed] [Google Scholar]
- 12. Trottier B, Lake JE, Logue K, et al. Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, Phase IIIb study. Antivir Ther 2017; 22:295–305. [DOI] [PubMed] [Google Scholar]
- 13. Clotet B, Feinberg J, van Lunzen J, et al. ; ING114915 Study Team Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–31. [DOI] [PubMed] [Google Scholar]
- 14. Raffi F, Rachlis A, Stellbrink HJ, et al. ; SPRING-2 Study Group Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013; 381:735–43. [DOI] [PubMed] [Google Scholar]
- 15. Baldin G, Ciccullo A, Capetti A, et al. Efficacy and safety of switching to dolutegravir plus emtricitabine/tenofovir disoproxil fumarate (TDF) or elvitegravir/cobicistat/emtricitabine/TDF in virologically suppressed HIV-infected patients in clinical practice: results from a multicentre, observational study. HIV Med 2019; 20:164–8. [DOI] [PubMed] [Google Scholar]
- 16. Orrell C, Hagins DP, Belonosova E, et al. ; ARIA study team Fixed-dose combination dolutegravir, abacavir, and lamivudine versus ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate and emtricitabine in previously untreated women with HIV-1 infection (ARIA): week 48 results from a randomised, open-label, non-inferiority, phase 3b study. Lancet HIV 2017; 4:e536–46. [DOI] [PubMed] [Google Scholar]
- 17. Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis 2019; 19:253–64. [DOI] [PubMed] [Google Scholar]
- 18. Cahn P, Pozniak AL, Mingrone H, et al. ; extended SAILING Study Team Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013; 382:700–8. [DOI] [PubMed] [Google Scholar]
- 19. [La thérapie antirétrovirale pour les adultes infectés par le VIH - Guide pour les professionnels de la santé du Québec ] Available at: https://publications.msss.gouv.qc.ca/msss/document-000733/?&date=DESC&sujet=vih-sida&critere=sujet. Accessed January 10, 2019.
- 20. Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 2016; 183:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fewell Z, Hernan MA, Wolfe F, et al. Controlling for time-dependent confounding using marginal structural models. Stata J 2004; 4:402–20. [Google Scholar]
- 22. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000; 11:561–70. [DOI] [PubMed] [Google Scholar]
- 23. Xiao Y, Abrahamowicz M, Moodie EE. Accuracy of conventional and marginal structural Cox model estimators: a simulation study. Int J Biostat 2010; 6:Article 13. [DOI] [PubMed] [Google Scholar]
- 24. Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet 2017; 390:2063–72. [DOI] [PubMed] [Google Scholar]
- 25. Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017; 390:2073–82. [DOI] [PubMed] [Google Scholar]
- 26. Raffi F, Jaeger H, Quiros-Roldan E, et al. ; extended SPRING-2 Study Group Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2013; 13:927–35. [DOI] [PubMed] [Google Scholar]
- 27. Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moron-Lopez S, Navarro J, Jimenez M, et al. Switching from a protease inhibitor-based regimen to a dolutegravir-based regimen: a randomized clinical trial to determine the effect on peripheral blood and ileum biopsies from antiretroviral therapy-suppressed human immunodeficiency virus-infected individuals. Clin Infect Dis 2019; 69:1320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eron JJ, Clotet B, Durant J, et al. ; VIKING Study Group Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis 2013; 207:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castagna A, Maggiolo F, Penco G, et al. ; VIKING-3 Study Group Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis 2014; 210:354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rusconi S, Adorni F, Tau P, et al. ; ARCA (Antiviral Response Cohort Analysis) Dolutegravir (DTG)-containing regimens after receiving raltegravir (RAL) or elvitegravir (EVG): durability and virological response in a large Italian HIV drug resistance network (ARCA). J Clin Virol 2018; 105:112–7. [DOI] [PubMed] [Google Scholar]
- 32. Jiang J, Xu X, Guo W, et al. Dolutegravir(DTG, S/GSK1349572) combined with other ARTs is superior to RAL- or EFV-based regimens for treatment of HIV-1 infection: a meta-analysis of randomized controlled trials. AIDS Res Ther 2016; 13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen GJ, Sun HY, Chang SY, et al. Effectiveness of switching from protease inhibitors to dolutegravir in combination with nucleoside reverse transcriptase inhibitors as maintenance antiretroviral therapy among HIV-positive patients. Int J Antimicrob Agents 2019; 54:35–42. [DOI] [PubMed] [Google Scholar]
- 34. Sörstedt E, Carlander C, Flamholc L, et al. Effect of dolutegravir in combination with nucleoside reverse transcriptase inhibitors (NRTIs) on people living with HIV who have pre-existing NRTI mutations. Int J Antimicrob Agents 2018; 51:733–8. [DOI] [PubMed] [Google Scholar]
- 35. Sax PE, Rockstroh JK, Luetkemeyer AF, et al. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Olearo F, Nguyen H, Bonnet F, et al. The impact of M184V/I mutation on the efficacy of abacavir/lamivudine/dolutegravir regimens prescribed in treatment-experienced patients. J Int AIDS Soc 2018; 21(Supplement 8):10–11. [Google Scholar]
- 37. Hernan MA, Robins JM. Causal Inference: What If. Boca Raton: Chapman & Hall/CRC; 2020. [Google Scholar]