Summary
Background
The possibility of 2019 novel coronavirus disease (COVID-19) transmission to neonates through breast milk remains unverified.
Methods
This paper presents the interim results of a longitudinal study being carried out in Hubei province. As of 1 April 2020, 24 mothers confirmed with COVID-19, 19 mothers suspected with COVID-19 but Polymerase chain reaction negative, and 21 mothers without COVID-19 and their neonates have been recruited. Telephone follow-up was conducted to collect information on breastfeeding practices. Forty-four breast milk samples were collected from 16 of the 24 mothers with confirmed COVID-19 for the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) ribonucleic acid (RNA) and antibodies (IgM and IgG) testing.
Findings
The average mother-child separation time was 36•7 ± 21•1 days among mothers confirmed with COVID-19, significantly longer than that of the suspected group (16•6 ± 13•1 days) and control group (10•5 ± 8•2 days). Both the COVID-19 confirmed (58•3%) and suspected (52•6%) groups presented significantly lower rates of breastfeeding as compared with the control group (95•2%). All 44 breast milk samples tested negative for the SARS-CoV-2 nucleic acid. Thirty-eight breast milk samples underwent antibody testing and all tested negative for IgG. Twenty-one breast milk samples from 8 women tested positive for IgM, while the remaining samples from 11 women tested negative.
Interpretation
Considering the lack of evidence for SARS-CoV-2 transmission through breast milk, breastfeeding counselling along with appropriate hand hygiene precautions and facemasks should be provided to all pregnant women.
Funding
The study was funded by the Hong Kong Committee for UNICEF.
Keywords: COVID-19, Neonate, Breastfeeding, Breast milk, Antibody
Research in context.
Evidence before this study: We searched PubMed, Elsevier ScienceDirect, Wiley Online Library, SpringerLink and the China National Knowledge Infrastructure database for articles published up to 18th September 2020, using the keywords “COVID-19″, “coronavirus”, “SARS-CoV-2″, “2019-nCoV”, AND “pregnancy”, “vertical transmission”, “breast milk”, “antibody” AND “neonate”, “case report”. Sixteen studies have published the testing of SARS-CoV-2 RNA in breast milk, half of which reported with negative results from the intermittently collected samples. However, a few studies reported positive SARS-CoV-2 ribonucleic acid (RNA) in breast milk on 1–13 days post-delivery, or 1–5 days after the onset of symptoms of COVID-19. The positive rate of RNA was nearly 14•29% (20/140 samples from 9/49 patients). Even though one study reported positive results of one neonate's nasopharyngeal swab, stool, and blood samples after breastfeeding by the positive mother, neither the mother nor infant exhibited any symptoms. The largest cohort study published thus far found no evidence of mother-to-child transmission when neonates roomed-in with mothers and breastfed, but the possibility of COVID-19 transmission to neonates through breast milk has been postulated. The SARS-CoV-2 antibodies of IgA, IgM and IgG in breast milk had also been reported, but the number of samples was limited and the effects of prolonged mother-child separation on breastfeeding practices due to quarantine control measures have not been reported.
Added value of this study: The possibility of SAR-CoV-2 transmission through breast milk and its antibodies in breast milk of mothers confirmed with COVID-19, as well as the impacts of prolonged mother-child separation on breastfeeding practices due to quarantine control measures were investigated in this study. There was no evidence of SARS-CoV-2 in any collected samples of the breast milk form 16 mothers confirmed with COVID-19 between 3–79 days from the onset of COVID-19 symptoms. The IgM antibody was detected in the breast milk of some mothers confirmed with COVID-19 between 3–68 days after the onset of symptoms. IgG antibody for SARS-CoV-2 was not detected in the breast milk samples in our study even several weeks post infection. Breastfeeding was less common among neonates born to both confirmed mothers and suspected/PCR negative mothers (58•3% and 52•6%, respectively) compared to the control group (95•2%). Mothers with confirmed COVID-19 delayed initiation of breastfeeding or feeding of expressed breast milk to infants until three weeks after delivery primarily due to strict isolation and quarantine measures.
Implications of all the available evidence: Breastfeeding practices were severely impacted during the COVID-19 epidemic, as the Chinese national guidelines called for suspension of breastfeeding and the separation among both confirmed and suspected/PCR negative mothers and their infants. Considering the lack of evidence for transmission of SARS-CoV-2 through breast milk, health care workers should provide adequate breastfeeding counseling along with appropriate hand hygiene precautions and facemasks to all pregnant women to minimize the risk of transmission, and the decision of breastfeeding should be made together by the families and doctors. The separation of mothers and infants should be avoided wherever possible. Additionally, a longer follow-up period is likely needed to detect IgG antibody.
Alt-text: Unlabelled box
1. Introduction
The Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) is highly contagious, and was first identified in Wuhan, China in December 2019 [1]. The outbreak has spread to 188 countries and regions around the world, with more than 31•6 million confirmed cases as of 23 September 2020 [2].
Hundreds of confirmed maternal cases of the 2019 novel coronavirus disease (COVID-19) have been reported globally [3,4]. Most research studies thus far have not found evidence of vertical transmission of COVID-19 from mother to child [5], [6], [7], [8], [9]. However, some studies found that a few neonates born to mothers with confirmed COVID-19 presented positive SARS-CoV-2 Polymerase Chain Reaction (PCR) results, including one neonate with a nasopharyngeal swab positive for SARS-CoV-2 PCR within 16 h after birth [10], and three of 33 neonates with nasopharyngeal and anal swabs positive for SARS-CoV-2 PCR within two days after birth [11]. There have been no confirmed cases of vertical transmission of SARS-CoV-2 from mother to child through breastfeeding, but the possibility of COVID-19 transmission to neonates through breast milk has been postulated. Until September 18, 2020, sixteen studies have published the testing of SARS-CoV-2 ribonucleic acid (RNA) in breast milk, half of which reported with negative results from the intermittently collected samples [7,[12], [13], [14], [15], [16], [17]]. However, a few studies reported positive SARS-CoV-2 RNA in breast milk on 1–13 days post-delivery [18], [19], [20], [21], [22], [23], or 1–5 days after the onset of symptoms of COVID-19 [24,25], and one study did not include the testing time [26]. The positive rate of RNA was nearly 14•29% (20/140 samples from 9/49 patients). Even though Bastug and colleagues reported positive results of one neonate's nasopharyngeal swab, stool, and blood samples after breastfeeding by the positive mother, neither the mother nor infant exhibited any symptoms [21]. However, the largest cohort study published thus far found no evidence of mother-to-child transmission when neonates roomed-in with mothers and breastfed [27]. In the past few months, the SARS-CoV-2 antibodies of IgA, IgG and IgM in breast milk have also been reported [13,28,29], but the number of samples was limited and the effects of prolonged mother-child separation on breastfeeding practices due to quarantine control measures have not been reported. The risk of transmission through breast milk and impact of COVID-19 infection on neonates need to be carefully balanced with the early- and longer-term negative health consequences of not breastfeeding.
A multi-center longitudinal research study is being carried out in Wuhan, the epicenter of the COVID-19 outbreak in China. The parent study began on 10 February 2020. This study presents interim results of the multi-center research focused on breastfeeding practices in the first 56 days after delivery and the presence of SARS-CoV-2 nucleic acid and its antibodies (IgM and IgG) in the breast milk of mothers confirmed with COVID-19 post-delivery. We present the following paper in accordance with the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) statement and protocols.
2. Methods
2.1. Participants
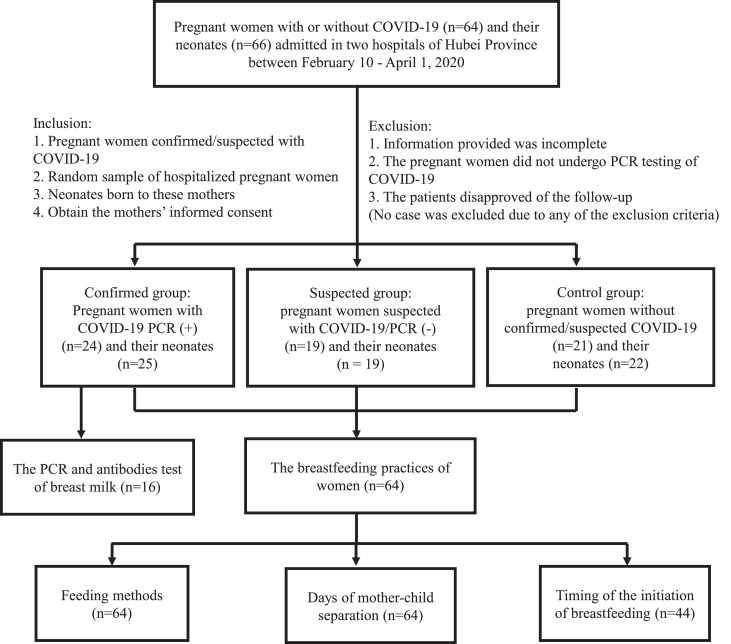
Participants were recruited from Hubei Province for a multicenter longitudinal study on the transmission of COVID-19 and its impact on health and developmental outcomes among neonates born to mothers with confirmed/suspected COVID-19. The present study aims to explore the short- and medium-term health and developmental outcomes of neonates born to mothers with confirmed/suspected COVID-19. While recruitment of participants is ongoing, as of 1 April 2020, 24 pairs of pregnant women confirmed with COVID-19 and their neonates (Confirmed Group, including one pair of twins), 19 pairs of pregnant women suspected of COVID-19 but found to be PCR negative and their neonates (Suspected Group), and 21 pairs of pregnant women without confirmed/suspected COVID-19 and their neonates (Control Group, including one pair of twins) have been recruited and matched by gestational age.
The diagnostic criteria by the National Health Commission of the People's Republic of China was used for the mothers confirmed or suspected with COVID-19 [30]. All mothers diagnosed with COVID-19 were enrolled in this study. Each mother had a positive SARS-CoV-2 nucleic acid test on a throat swab or serological antibodies, with at least one clinical manifestation (e.g., fever, respiratory tract symptoms, chest computerized tomography (CT) of pneumonia, or abnormal blood routine) concurrently. All the mothers with suspected COVID-19 who subsequently had a negative SARS-CoV-2 nucleic acid test of a throat swab and a negative serological test were recruited into the suspected group. Mothers who were free from COVID-19 related diseases but admitted for birth or pregnancy complications (e.g., prematurity, hyperbilirubinemia, neonatal amniotic fluid/meconium aspiration syndrome, neonatal bacterial pneumonia), in the same hospitals as the confirmed and suspected groups were recruited for the control group; additionally, women in the control group had normal chest CT and had a negative SARS-CoV-2 nucleic acid testing.
2.2. Data collection and methods
Telephone based follow-ups were conducted at the 3rd, 7th, 14th, 28th, 42rd, and 56th day after delivery to collect information on breastfeeding practices, including the feeding method (exclusive breastfeeding or feeding expressed breast milk, breastfeeding or feeding expressed breast milk mixed with formula feeding, and only formula feeding), the days of mother-child separation, and the timing of initiation of breastfeeding or feeding with expressed breast milk, if applicable.
Breast milk samples were collected from 16 of the 24 COVID-19 confirmed mothers for SARS-CoV-2 nucleic acid and antibody (IgM and IgG) testing. Among the remaining eight mothers in the confirmed group, one refused breast milk testing, two stopped expressing breast milk due to mastitis since the third day after delivery, and the remaining five failed to collect samples of breast milk due to isolation and strict transportation limitations for the researchers. Breast milk samples were attempted to be collected at 10 different time points, including the day of delivery, as well as the 3rd, 7th, 14th, 21st, 28th, 35th, 42nd, 56th and 70th day post-delivery. Mothers were requested to wear masks and wash hands before collecting breast milk samples. The breast milk was expressed by electric breast pump and utensils were disinfected by boiling water prior to each use. All samples were immediately transferred to RNase-free sample tubes and stored at -20 °C until testing.
The testing was conducted by Wuhan Institution of Virology under the Chinese Academy of Sciences by methods reported previously [31,32]. Considering the potential exposure risk of infection during testing, all samples were incubated at 56 °C for 30–60 min before testing to inactivate the virus, according to kit instructions used in this study and the World Health Organization (WHO)’s laboratory technical guidelines [33,34]. FineMag viral DNA/RNA extraction kits with rapid magnetic bead method (Genfine Biotech Co LTD, Beijing, FMY502T5-TR) were applied to extract the RNA of specimens. Automated Nucleic Acid Extractor (Hubei Xinzongke Viral Disease Control Bio-Tech LTD, Wuhan, NZKEX-32) was used for RNA extraction.
2019-nCoV Nucleic Acid Detection Kits (Hubei Xinzongke Viral Disease Control Bio-Tech LTD, Wuhan, NZK-H20008) and RT-PCR were used to detect the RdRp gene and N gene of SARS-CoV-2, and the human Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as an internal control [31,35]. All RT-PCRs ran on a Bio-Rad CFX96 Real-time Detection System. The reaction mixture consisted of 14 μL reaction buffer, 1 μL enzyme solution, 5 μL RNA template, and 5 μL RNase-free pure water. The RT-PCR reactions were set to 42 °C for 5 min, incubated at 95 °C for 1 min, denatured at 95 °C for 45 cycles for 10 s, and fluorescence signal was acquired at 60 °C for 30 s. After the reaction, RdRp gene, N gene and GAPDH gene were detected by the fluorescent channels FAM, HEX/VIC, and ROX, respectively. The diagnostic criteria are based on the recommendations of the National Institute for Viral Disease Control and Prevention of China. Procedures to prevent sample contamination were in accordance with standard laboratory practices.
Diagnostic Kits for IgM/IgG Antibodies to SARS-CoV-2 (Enzyme linked immunosorbent assay, Livzon Pharmaceutical Group Inc., Zhuhai, 2,020,020,308) were used to detect IgM and IgG in breast milk [32]. After inactivation, samples were diluted in dilution buffer (1:100 for IgM and 1:20 for IgG) and added to the plates coated with anti-human IgM μ chain for IgM and SARS-CoV-2 nucleocapsid (N) protein for IgG, respectively. The absorbance value indicating the relative antibody level in the sample—was tested under 450 nm/630 nm wavelength. Antibody levels equal to or higher than the cut off values of 0•10 for IgM and 0•13 for IgG were considered positive.
2.3. Statistical analysis
IBM SPSS Statistics 22•0 software was used for statistical analysis. Categorical variables are presented with number and proportion (%) and continuous variables are presented with mean and standard deviation. One-way ANOVA was used to compare the data of three groups that conformed to normality and homogeneity of variance. Kruskal-Wallis test was used to compare data of the three groups that did not conform to normality or homogeneity of variance. Chi-square test was used to compare proportions of the three groups. A value of P <0•05 was considered statistically significant.
2.4. Ethical approval
This study has been registered in the Chinese Clinical Trial Register (ChiCTR2000033960). All procedures performed in the study involving the collection of human samples were in accordance with the ethical standards approved by the Ethical Committee of Hubei Provincial Maternal and Child Health Hospital (NO. 2020–IEC–XM010). All participants gave their written permission for both their own and their children's participation in the study.
2.5. Role of funding source
The study was funded by the Hong Kong Committee for UNICEF, who was fully independent to the research design, data collection, analysis, interpretation, writing or decision to submit the paper for publication. This study group hasn't been paid to write this article by any pharmaceutical company or other agency. Prof Shiwen Xia has full access to all the data in the study and has final responsibility for the decision to submit for publication.
3. Results
3.1. Basic information of mothers and their neonates
Among the 64 mothers across the three groups, one confirmed mother was enrolled in the study 49 days before delivery; 25 mothers were enrolled on the day of delivery (6 confirmed, 2 suspected and 17 control); and the remaining were enrolled between 1–31 days after delivery (17 confirmed, 17 suspected and 4 control; Table 1 and Fig. 1).
Table 1.
Basic information of the mothers and their neonates in the three groups.
| Items | Confirmed group | Suspected group | Control group | F/χ2 value | P value |
|---|---|---|---|---|---|
| Maternal Information | |||||
| Number of mothers | n = 24 | n = 19 | n = 21 | ||
| Enrolled before delivery | 1 | 0 | 0 | ||
| Enrolled at the day of delivery | 6 | 2 | 17 | ||
| Enrolled after delivery | 17 | 17 | 4 | ||
| Age | 29•8 ± 3•7 | 32•5 ± 5•1 | 30•9 ± 3•2 | 2•81# | 0•25 |
| High school education or below | 11 (45•8%) | 9 (47•4%) | 12 (57•1%) | 0•65 | 0•72 |
| Gestational hypertension | 5 (20•8%) | 3 (15•8%) | 1 (4•8%) | 2•8 | 0•25 |
| GDM& | 4 (16•7%) | 3 (15•8%) | 5 (23•8%) | 0•52 | 0•77 |
| Gestational thyroid disease | 1 (4•2%) | 4 (21•1%) | 2 (9•5%) | 3•11 | 0•21 |
| Fetal distress | 4 (16•7%) | 4 (21•1%) | 1 (4•8%) | 2•76 | 0•25 |
| Intrauterine infection | 5 (20•8%) | 5 (26•3%) | 3 (14•3%) | 0•91 | 0•63 |
| PROM& >2 h | 5 (20•8%) | 6 (31•6%) | 6 (28•6%) | 0•69 | 0•71 |
| Cesarean delivery | 19 (79•2%) | 12 (63•2%) | 11 (52•4%) | 3•64 | 0•16 |
| Abnormal amniotic fluid | 1 (4•2%) | 3 (15•8%) | 5 (23•8%) | 4•04 | 0•13 |
| Abnormal umbilical cord | 3 (12•5%) | 5 (26•3%) | 5 (23•8%) | 1•56 | 0•46 |
| Abnormal placenta | 2 (8•3%) | 2 (10•5%) | 7 (33•3%) | 5•44 | 0•07 |
| Delivery complications | 1 (4•2%) | 0 (0•0%) | 2 (9•5%) | 2•70 | 0•26 |
| Maternal length of stay (d) | 16•5 ± 10•5 | 4•7 ± 1•0 | 4•8 ± 1•6 | 19•46# | < 0•001⁎⁎ |
| Neonatal information | |||||
| Number of neonates | n = 25 | n = 19 | n = 22 | ||
| Twins | 2 (8•0%) | 0 (0•0%) | 2 (9•1%) | 2•84 | 0•24 |
| Male infants | 15 (60•0%) | 8 (42•1%) | 13 (59•1%) | 1•67 | 0•43 |
| GA& (w) | 38•2 ± 2•1 | 37•1 ± 3•2 | 37•7 ± 2•5 | 1•01# | 0•61 |
| Birth weight (kg) | 3•0 ± 0•5 | 2•8 ± 0•7 | 3•0 ± 0•5 | 1•15 | 0•32 |
| SGA& | 3 (12•0%) | 4 (21•1%) | 3 (13•6%) | 0•72 | 0•70 |
| Preterm infants | 7 (28•0%) | 5 (26•3%) | 8 (36•4%) | 0•59 | 0•75 |
| 1 min Apgar score | 9•2 ± 0•8 | 8•8 ± 1•7 | 9•6 ± 1•0 | 6•63# | 0•04* |
| 5 min Apgar score | 9•8 ± 0•4 | 9•6 ± 1•3 | 9•8 ± 0•9 | 2•19# | 0•34 |
| Birth asphyxia | 0 (0•0%) | 3 (15•8%) | 1 (4•5%) | 5•47 | 0•07 |
| PCIS& <90 | 3 (12•0%) | 6 (31•6%) | 4 (18•2%) | 2•59 | 0•27 |
| Mortality | 0 (0•0%) | 0 (0•0%) | 0 (0•0%) | N/A | N/A |
| Neonatal length of stay (d) | 6•6 ± 6•5 | 11•2 ± 10•4 | 8•5 ± 6•4 | 2•81# | 0•25 |
GDM, Gestational diabetes mellitus; PROM, Premature rupture of membrane; GA, Gestational age; SGA, Small for gestational age; PCIS, Pediatric Critical Illness Score
Kruskal-Wallis test
P < 0•05
P < 0•01
Fig. 1.
Research protocol and process of this study. The participants were enrolled between February 10 - April 1, 2020, and the follow-up data was collected by April 30, 2020.
Among the 24 mothers confirmed with COVID-19, their average age was 29•8 years old. The average days to delivery from the onset of COVID-19 symptoms was nine days. Two mothers had a positive PCR test result prior to delivery, and 22 mothers had positive results postnatally. All mothers confirmed with COVID-19 had abnormal chest imaging, although none of them had severe COVID-19 symptoms. Fifteen had fever or respiratory tract symptoms, while the remaining cases did not have typical COVID-19 symptoms (1 had decreased amniotic fluid, 2 had premature rupture of membrane, and 6 were asymptomatic). Nine were transferred from the delivery hospitals to the designated hospital for COVID-19 treatment once confirmed, and the remaining were isolated in the delivery hospitals for treatment until discharge. Among them, 20•8% had underlying pregnancy complications (e.g., hypertension, diabetes, thyroid disease, fetal distress, or intrauterine infection during pregnancy). cesarian section was performed on 79•2% of the mothers. The average number of days from the onset of symptoms until recovery with negative PCR testing was 16•4 ± 15•6 days (range 3–62 days).
We enrolled 19 mothers in the suspected group and 21 mothers in the control group, with average ages of 32•5 and 30•9 years, respectively. Among these two groups, the percentages of pregnancy complications were 31•6% and 28•6%, and cesarian section was performed on 63•2% and 52•4% of the mothers, respectively. There were no statistical differences between the three groups in maternal age, educational level, presence of underlying pregnancy complications, delivery mode, or incidence of birth complications and abnormalities (e.g., amniotic fluid, umbilical cord and placenta abnormalities, and fetal distress) (Table 1). The average length of maternal hospitalization was 16•5 ± 10•5 days for the confirmed group, which was significantly longer than that of the suspected group (4•7 ± 1•0 days) and the control group (4•8 ± 1•6 days) (P < 0•001).
There was a total of 66 neonates born to the 64 mothers (one pair of twins in the confirmed and control groups, respectively). Among the 25 neonates born to confirmed mothers, seven were premature, three were small for gestational age (SGA), and none experienced birth asphyxia. SARS-CoV-2 nucleic acid testing was also performed on neonates in the confirmed and suspected groups in this study, and no positive results was found (a detailed study on neonates will be published in another paper). The average length of hospitalization was 6•6 ± 6•5 days, 11•2 ± 10•4 days, and 8•5 ± 6•4 days for neonates born to confirmed, suspected, and control group mothers, respectively. There were no significant differences between the three groups of neonates in the length of hospitalization, gender, gestational age, birth weight, as well as the incidence of small for gestational age (SGA), prematurity, birth asphyxia, and critical illness (Table 1).
3.2. Breastfeeding practices
As of 30 April 2020, telephone-based follow-ups were conducted with all enrolled mothers across three groups between 30–80 days. In the confirmed, suspected and control groups, the average duration (days) of mother-child separation immediately after birth was 36•7 ± 21•1, 16•6 ± 13•1, and 10•5 ± 8•2, respectively (Table 2). The rates of any kind of breastfeeding (including exclusive breast milk feeding and breast milk combined with formula feeding) in the confirmed, suspected and control group were 58•3%, 52•6% and 95•2% within 56 days after birth, respectively. The rates of exclusive breastfeeding (including bottle feeding expressed breast milk and direct breastfeeding by mothers) were 4•2%, 15•8% and 47•6%, respectively (Table 2). Both confirmed and suspected groups presented significantly lower rates of breastfeeding as compared with the control group (P = 0•006).
Table 2.
Breastfeeding practices among the confirmed, suspected and control groups.
| Items | Confirmed group | Suspected group | Control group | F/χ2 value | P value |
|---|---|---|---|---|---|
| Feeding method | |||||
| Exclusive breastfeeding | 1 (4•2%) | 3 (15•8%) | 10 (47•6%) | 12•96 | 0•002⁎⁎ |
| Breastfeeding combined with formula | 13 (54•2%) | 7 (36•8%) | 10 (47•6%) | 1•29 | 0•53 |
| Formula feeding | 10 (41•6%) | 9 (47•4%) | 1 (4•8%) | 10•37 | 0•006⁎⁎ |
| Any breastfeeding | 14 (58•3%) | 10 (52•6%) | 20 (95•2%) | 10•37 | 0•006⁎⁎ |
| Days of mother-child separation (d) | 36•7 ± 21•1 | 16•6 ± 13•1 | 10•5 ± 8•2 | 20•63# | < 0•001⁎⁎ |
| Timing of the initiation of breastfeeding& | |||||
| Initiated Breastfeeding ≤ 7 days | 2 (14•3%) | 0 (0•0%) | 13 (65•0%) | 16•12 | < 0•001⁎⁎ |
| Initiated Breastfeeding at 8–14 days | 1 (7•1%) | 3 (30•0%) | 5 (25•0%) | 8•79 | 0•012* |
| Initiated Breastfeeding at 15–21 days | 1 (7•1%) | 4 (40•0%) | 0 (0•0%) | 3•01 | 0•22 |
| Initiated Breastfeeding at 22–28 days | 4 (28•6%) | 2 (20•0%) | 2 (10•0%) | 3•28 | 0•19 |
| Initiated Breastfeeding ≥ 29 days | 6 (42•9%) | 1 (10•0%) | 0 (0•0%) | 11•27 | 0•004⁎⁎ |
P < 0•01
P < 0•05
Kruskal-Wallis test
The initiation of breastfeeding included the initiation of breastfeeding or initiation of feeding expressed breast milk to neonates.
Expressing breast milk was the preferred feeding method adopted by 44 of the 64 mothers recruited in the study. Ten out of 14 confirmed mothers who fed their babies with expressed breast milk began expressing breast milk after delivery, but did not initiate feeding breast milk to their baby until 22–29 days after delivery, primarily due to strict isolation and quarantine measures (Table 2). Only two mothers in the confirmed group initiated feeding breast milk to their baby within seven days after delivery, one mother initiated between 8–14 days after delivery, and one mother initiated between 15–21 days after delivery (Table 2). In the suspected group, none of the 10 mothers who fed their babies with expressed breast milk initiated feeding breast milk to their baby within seven days after delivery, while one mother initiated later than 29 days after birth (Table 2). In the control group, 20 out of the 21 mothers breastfed their babies, with 13 out of the 20 mothers initiating breastfeeding within seven days after delivery (Table 2).
3.3. Testing of SARS-CoV-2 nucleic acid and antibodies in breast milk among mothers confirmed with COVID-19
We anticipated collecting 160 samples of breast milk, however, due to challenges faced during the COVID-19 epidemic, only 44 samples were successfully collected from 16 of the 24 mothers confirmed with COVID-19. Among those, one sample was collected on the day of delivery, seven samples on day 3, seven samples on day 7, eight samples on day 14, two samples on day 21, nine samples on day 28, three samples on day 35, four samples on day 42, two samples on day 56 and one sample on day 70 post-delivery.
Out of the 44 breast milk samples, six samples from two mothers completed SARS-CoV-2 nucleic acid testing without the antibody results because the antibodies testing guidance and kits were not available until 9 March 2020. The remaining 38 breast milk samples from 15 mothers completed both the SARS-CoV-2 nucleic acid testing and the IgM & IgG antibody testing. Additionally, among the 16 mothers who had their breast milk tested, 10 had symptom onset 1–65 days prior to delivery, while six developed symptoms between the day of delivery and 4 days post-delivery.
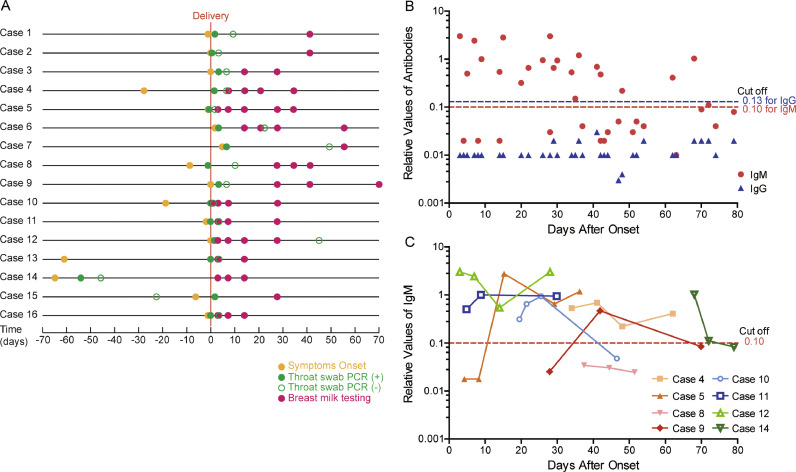
All 44 breast milk samples tested negative for SARS-CoV-2 (Table 3). Samples were collected from mothers between 3–62 days from the onset of COVID-19 symptoms (Fig. 2A). Thirty-eight breast milk samples underwent antibody testing and all tested negative for the IgG antibody. Twenty-one breast milk samples from eight mothers tested positive for IgM, while the remaining 17 samples from 11 mothers tested negative for IgM (Fig. 2A, 2B). The timing of initial IgM positive results among the eight mothers was between days of 3–79 post onset of COVID-19 symptoms (Fig. 2B). Three of eight mothers had an eventual IgM negative result following the initial positive result 47–72 days post symptom onset (Fig. 2B).
Table 3.
IgM, IgG and PCR testing of SARS-CoV-2 in breast milk of mothers confirmed with COVID-19.
| NO. | Items | 1 d | 3 d | 7 d | 14 d | 21 d | 28 d | 35 d | 42 d | 56 d | 70 d |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | IgM | 0•02 | |||||||||
| Case 2 | 0•02 | ||||||||||
| Case 3 | 0•02 | 0•03 | |||||||||
| Case 4 | 0•53 | 0•69 | 0•22 | 0•41 | |||||||
| Case 5 | 0•02 | 0•02 | 2•81 | 0•66 | 1•20 | ||||||
| Case 6 | 0•04 | ||||||||||
| Case 7 | 0•05 | ||||||||||
| Case 8 | 0•04 | 0•03 | 0•03 | ||||||||
| Case 9 | 0•03 | 0•48 | 0•09 | ||||||||
| Case 10 | 0•32 | 0•66 | 0•95 | 0•05 | |||||||
| Case 11 | 0•50 | 1•00 | 0•94 | ||||||||
| Case 12 | 3•03 | 2•43 | 0•54 | 3•01 | |||||||
| Case 13 | 0•01 | 0•04 | |||||||||
| Case 14 | 1•03 | 0•11 | 0•08 | ||||||||
| Case 15 | 0•15 | ||||||||||
| Case 16 | |||||||||||
| Case 1 | IgG | 0•01 | |||||||||
| Case 2 | 0•01 | ||||||||||
| Case 3 | 0•01 | 0•01 | |||||||||
| Case 4 | 0•01 | 0•03 | 0•003 | 0•01 | |||||||
| Case 5 | 0•01 | 0•01 | 0•01 | 0•02 | 0•02 | ||||||
| Case 6 | 0•02 | ||||||||||
| Case 7 | 0•01 | ||||||||||
| Case 8 | 0•01 | 0•01 | 0•01 | ||||||||
| Case 9 | 0•01 | 0•01 | 0•02 | ||||||||
| Case 10 | 0•01 | 0•01 | 0•01 | 0•003 | |||||||
| Case 11 | 0•01 | 0•01 | 0•01 | ||||||||
| Case 12 | 0•01 | 0•01 | 0•01 | 0•01 | |||||||
| Case 13 | 0•01 | 0•01 | |||||||||
| Case 14 | 0•02 | 0•02 | 0•02 | ||||||||
| Case 15 | 0•01 | ||||||||||
| Case 16 | |||||||||||
| Case 1 | PCR | (-) | |||||||||
| Case 2 | (-) | ||||||||||
| Case 3 | (-) | (-) | |||||||||
| Case 4 | (-) | (-) | (-) | (-) | |||||||
| Case 5 | (-) | (-) | (-) | (-) | (-) | ||||||
| Case 6 | (-) | (-) | (-) | (-) | |||||||
| Case 7 | (-) | ||||||||||
| Case 8 | (-) | (-) | (-) | ||||||||
| Case 9 | (-) | (-) | (-) | ||||||||
| Case 10 | (-) | (-) | (-) | (-) | |||||||
| Case 11 | (-) | (-) | (-) | ||||||||
| Case 12 | (-) | (-) | (-) | (-) | |||||||
| Case 13 | (-) | (-) | |||||||||
| Case 14 | (-) | (-) | (-) | ||||||||
| Case 15 | (-) | ||||||||||
| Case 16 | (-) | (-) | (-) |
The cut off value was 0•10 for IgM and 0•13 for IgG; if the antibody level was equal to or higher than the cut off value, it was considered as positive result.
Fig. 2.
The IgM, IgG and PCR testing results of SARS-CoV-2 in breast milk of mothers with COVID-19. (A) The time points of the onset of symptoms and breast milk sample collection among mothers confirmed with COVID-19 who underwent breast milk testing. (B) Seventeen breast milk samples tested negative both for the IgG and IgM antibodies, and 21 samples from eight women tested positive for IgM but negative for IgG. The IgM positive samples were collected 3–68 days from the onset of symptoms. (C) The changes of IgM values among eight mothers who had three or more breast milk samples collected for antibody testing.
Additionally, the IgM positive samples were collected on average 30•8 ± 19•4 days from the onset of COVID-19 symptoms, while IgM negative samples were collected on average 43•4 ± 21•3 days after the onset of symptoms (Fig. 2B). However, the difference of the sample collection time between the IgM positive and negative groups did not reach statistical significance, likely due to the small sample size (Mann-Whitney U test, P = 0•052).
4. Discussion
4.1. The breastfeeding practices among mothers with or without COVID-19
The benefits of breastfeeding (e.g., reducing mortality, sepsis; facilitating the development of the neonatal immune system and maternal bonding and attachment) have been fully reported and emphasized in current studies and guidelines [36], [37], [38]. Our study found that mothers with confirmed and suspected COVID-19 delayed initiation of breastfeeding or feeding of expressed breast milk to infants until three weeks, and one and half weeks after delivery, respectively, as compared with the control group who initiated breastfeeding in the first week after delivery. While all the confirmed and suspected mothers were guided on expressing breast milk after delivery, they were unable to initiate feeding breast milk to their baby primarily due to strict isolation and quarantine measures, especially during the first three months of the COVID-19 outbreak. For the control group, the neonates and their mothers were admitted to the same hospital as the confirmed group due to non-COVID-19 related complications of pregnancy and delivery, which contributed to mother-child separation and late onset of breastfeeding.
We found that breastfeeding was less common among neonates born to both confirmed mothers and suspected/PCR negative mothers (58•3% and 52•6%, respectively) compared to the control group (95•2%). The low breastfeeding rates among the confirmed and suspected groups may be due primarily to the recommendation of the Chinese national guidelines that breastfeeding should be suspended for women who give birth and are COVID-19 suspected or confirmed cases [30]. Current Chinese guidelines continue to recommend that breastfeeding should be suspended until the mother's nucleic acid test is negative [39]. Women with COVID-19 in our study also reported their concerns about the safety of breastfeeding.
Another important factor that likely contributed to lower rates of breastfeeding was the longer period of mother-child separation (36•7 ± 21•1 days postpartum) among the confirmed group. Mother-child separation has been considered harmful for the early development of infants and may increase maternal physical and psychological problems [40], [41], [42]. Chinese national guidelines for COVID-19 suggest an additional 14-days of isolation for confirmed/suspected patients after they are discharged from the hospital, even for suspected mothers with negative test results [30]. The United States Centers for Disease Control and Prevention also considers the separation of the newborn from a mother with suspected or confirmed COVID-19 to reduce the risk of spreading the virus to the newborn [43,44]. These measures likely resulted in prolonged mother-child separation, which likely contributed to delayed breastfeeding and reduced milk production.
However, delayed breastfeeding initiation is associated with neonatal morbidity [44]. WHO advises that mothers should not be separated from their infants unless the mother is too sick to care for her baby [45]. We recommend that healthcare workers should strengthen breastfeeding guidance and health education to mothers with confirmed and suspected COVID-19 to improve the rate of breastfeeding during hospitalization and once they are discharged. The risks and benefits of temporary mother-child separation should be discussed with the mother by her healthcare team. Furthermore, when the mother's milk is not available, milk banking and donor milk are recommended to ensure breastfeeding [46,47].
4.2. The possibility of SARS-CoV-2 transmission through breast milk
Our study results indicate that the breast milk of mothers confirmed with COVID-19 was negative for SARS-CoV-2 nucleic acid at least in the first 10 weeks post-delivery. Even among five mothers with nine breast milk samples collected prior to the mothers’ recovery from COVID-19 infection, SARS-CoV-2 nucleic acid testing was negative. This finding supports prior studies that SARS-CoV-2 nucleic acid has not been detected in breast milk [7,[12], [13], [14], [15], [16], [17]], even when the mother was still positive for SARS-CoV-2 [13]. Although recent studies reported a 14•29% (20/140) positive rate of SARS-CoV-2 RNA in breast milk, these studies only identified viral RNA by RT-PCR test rather than the infectious virus [18], [19], [20], [21], [22], [23], [24], [25], [26]. Two cases of neonatal infection have been reported due to unprotected contact with undiagnosed mothers, while there were ten neonates who did not become infected by mothers who directly breastfed while wearing a mask [28]. This suggests that respiratory droplets are more likely to spread the virus from mother to newborn than through breast milk. It supports our findings that mothers who wore masks and washed hands before collecting breast milk samples all had negative SARS-CoV-2 RNA in their breast milk.
According to the over 800 neonates reported in the literature, the incidence of vertical transmission has proven to be low (nearly 4•2%) [28]. Furthermore, the majority of studies reported no respiratory or other illness in neonates born to mothers with confirmed/suspected SARS-CoV-2 infection [28,33]. According to the current evidences, the WHO recommends that the neonates born to mothers with suspected or confirmed COVID-19 should be breastfed within 1 hour of birth with maternal masks and handwashing [45,48]. Moreover, the American Academy of Pediatrics—which initially supported the separation of newborns and advised against direct breastfeeding—has changed its recommendation to discourage separation and encourage breastfeeding based on current evidences [49]. Although the benefits of breastfeeding may outweigh the risks [50], considering the positive results of one neonate's nasopharyngeal swab, stool, and blood samples after breastfeeding by the positive mother (although the infant did not exhibit any symptoms), the breastfeeding decision should be made together by the families and doctors [21]. Additionally, pasteurization (62•5 °C for 30 min) of breast milk has been reported to eliminate the replication-competent SARS-CoV-2 or viral RNA, which could also be used to might benefit the safety of breastfeeding [24].
4.3. Antibodies of SARS-CoV-2 in breast milk of mothers confirmed with COVID-19
In our study, 21 of the 38 samples collected from eight confirmed mothers were positive for IgM between 3–68 days after the onset of COVID-19 symptoms. We also found that most of the samples with IgM positive were collected within the first 40 days from the onset of COVID-19 symptoms as compared with the IgM-negative samples. Previous studies tested IgG and IgM in blood samples which showed that the median seroconversion time for IgM and IgG were day-12 and day-14 after the onset of disease, respectively [51]. Furthermore, a study reported positive results of secretory-IgA (sIgA), IgM and IgG in breast milk samples which were obtained 14–30 days after maternal symptoms had abated [29]. However, the antibody concentration might differ between serum and breast milk which needs further investigation. Breast milk of mothers infected with influenza has been shown to contain IgM, IgA and IgG antibodies, which has been hypothesized to neutralize viruses in breast milk or in the gastrointestinal or respiratory tracts [52]. Given that antibodies may help to neutralize SARS-CoV-2 [53], it is theoretically possible that breast milk secreted by lactating mothers with positive IgM may have a positive impact on infant health, although more data is needed to study.
However, we did not detect IgG for SARS-CoV-2 in the breast milk samples even several weeks post infection; this was inconsistent with previous studies and needs further investigation [13,28,29]. IgG antibodies are primarily expressed after recovery from an infectious disease and last for months or years [54]. It has been reported that the positive rate of SARS-CoV-2 IgG in serum is only 11•8% at seven days after infection and increased gradually to 100% at three months post infection [54]. In our study, most of the samples (31 of 38) for antibody testing were collected within two months after the onset of symptoms, and none were collected after three months following symptoms onset. A longer follow-up period is likely needed to confirm whether IgG (comprises only ~2% of breast milk Ig) [29] of SARS-CoV-2 is expressed in breast milk. In addition, a previous study demonstrated that IgG concentration in breast milk was reduced by 34% after pasteurization (62•5 °C for 30 min, IgM was not detected), which may be a contributing factor for our negative IgG results [55]. Additionally, sIgA is another common antibody that maybe expressed in breast milk, which had been identified as a dominant antibody in breast milk following recovery from COVID-19 [29]. However, we failed to test the sIgA of SARS-CoV-2 in breast milk because the kits were not available during the study period and the milk samples were not available anymore to redo the testing.
Four major limitations should be taken into consideration when interpreting the findings. Firstly, while our study had a larger sample than in some prior studies, the sample sizes for each group remained relatively small with limited power to detect differences by group. Secondly, we had incomplete breast milk sampling from each mother due to challenging field conditions, resulting in low numbers of breast milk samples for antibody testing. Thirdly, we also failed to test the sIgA of SARS-CoV-2 in breast milk because of unavailable testing kits. Lastly, the national protocols for isolation limited support for expressing milk among the mothers, and the inactivation process (incubated at 56 °C for 30–60 min before testing) may have reduced the detection of RNA and antibodies across the samples [24].
In summary, breastfeeding practices were severely impacted during the COVID-19 epidemic among both confirmed and suspected/PCR negative mothers particularly due to local recommendations to suspend breastfeeding for COVID-19 confirmed/suspected mothers. Our study also highlighted the late initiation of breastfeeding even among mothers without confirmed or suspected COVID-19. There was no evidence of the SARS-CoV-2 virus in breast milk. The presence of IgM in some samples suggests the possibility that breast milk might have a protective effect on newborns. Considering the lack of evidence of SARS-CoV-2 transmission through breast milk, health education and counseling on breastfeeding support should be strengthened to all pregnant women and their family members. The separation of mothers and infants should be avoided wherever possible. Nevertheless, in view of the possibility of respiratory, contact or breastfeeding transmission of the SARS-CoV-2 during breastfeeding, the decision should be made together by the families and doctors, and appropriate hand hygiene precautions and facemasks are recommended to minimize the risk of transmission.
Contributors
SX designed and led the study. SP, HZ, LY, LC, YC, PZ, H. Liu and H. Li contributed to the collection of medical records, breast milk samples and the follow-up. SP analyzed the original data and wrote the manuscript. XH, MD, AN contributed to the study design, results interpretation as well as the reviewing and revision of the manuscript. SP and SX made substantial revision of the manuscript. JX supported samples testing.
Declaration of Competing Interest
The authors of this paper have no conflict of interest.
The opinions expressed in this paper are solely those of the authors and do not necessarily represent the official position of UNICEF.
Acknowledgments
Acknowledgments
The study was funded by the Hong Kong Committee for UNICEF, who was fully independent to the research design, data collection, analysis, interpretation, writing or decision to submit the paper for publication. This study group hasn't been paid to write this article by any pharmaceutical company or other agency.
Prof Shiwen Xia has full access to all the data in the study and has final responsibility for the decision to submit for publication. Dr. France Begin (UNICEF Headquarter) provided valuable technical inputs to this manuscript.
Data sharing statements
No new data were created or analyzed in this study. The de-identified data supporting the conclusions of this article can be made available from the corresponding author upon reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2020.100045.
Appendix. Supplementary materials
References
- 1.Wu F., Zhao S., Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2020. https://coronavirus.jhu.edu/map.html (accessed Sep 23, 2020).
- 3.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99(7):823–829. doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan J., Guo J., Fan C. Coronavirus disease 2019 (COVID-19) in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;S0002-9378(20):30462. doi: 10.1016/j.ajog.2020.04.014. –2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karimi-Zarchi M., Neamatzadeh H., Dastgheib S.A. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr Pathol. 2020;39(3):246–250. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu H., Wang L., Fang C. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):56–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu D., Sang L., Du S., Li T., Chang Y., Yang X.A. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J Med Virol. 2020 doi: 10.1002/jmv.25927. ; published online Apr 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng Z., Wang J., Mo Y. Unlikely SARS-CoV-2 vertical transmission from mother to child: A case report. J Infect Public Health. 2020;13(5) doi: 10.1016/j.jiph.2020.04.004. 818–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Valdez L.M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37(8):861–865. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingkong Z., Shiwen X., Wenhao Y. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. ; published online Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perrone S., Giordano M., Meoli A. Lack of viral transmission to preterm newborn from a COVID-19 positive breastfeeding mother at 11 days postpartum. J Med Virol. 2020 doi: 10.1002/jmv.26037. ; published online Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Y., Chi X., Hai H. Antibodies in the breast milk of a maternal woman with COVID-19. Emerg Microbes Infect. 2020;9(1):1467–1469. doi: 10.1080/22221751.2020.1780952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S., Guo L., Chen L. A case report of neonatal 2019 coronavirus disease in China. Clin Infect Dis. 2020;71(15):853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvatori G., De Rose D.U., Concato C. Managing COVID-19-positive maternal-infant dyads: an Italian experience. Breastfeed Med. 2020;15(5):347–348. doi: 10.1089/bfm.2020.0095. [DOI] [PubMed] [Google Scholar]
- 16.Fan C., Lei D., Fang C. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa226. ; published online Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong L., Tian J., He S. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groß R., Conzelmann C., Müller J.A. Detection of SARS-CoV-2 in human breastmilk. Lancet. 2020;S0140-6736(20):31181–31188. doi: 10.1016/S0140-6736(20)31181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu C., Liu W., Su H. Breastfeeding risk from detectable severe acute respiratory syndrome coronavirus 2 in breastmilk. J Infect. 2020;81(3):452–482. doi: 10.1016/j.jinf.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa S., Posteraro B., Marchetti S. Excretion of SARS-CoV-2 in human breast milk. Clin Microbiol Infect. 2020;26(10):1430–1432. doi: 10.1016/j.cmi.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastug A., Hanifehnezhad A., Tayman C. Virolactia in an asymptomatic mother with COVID-19. Breastfeed Med. 2020;15(8):488–491. doi: 10.1089/bfm.2020.0161. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y., Liu C., Dong L. Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. BJOG. 2020;127(9):1109–1115. doi: 10.1111/1471-0528.16276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buonsenso D., Costa S., Sanguinetti M. Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am J Perinatol. 2020;37(8):869–872. doi: 10.1055/s-0040-1710541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers C., Krogstad P., Bertrand K. Evaluation for SARS-CoV-2 in breast milk from 18 infected women. JAMA. 2020 doi: 10.1001/jama.2020.15580. published online Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tam P.C.K., Ly K.M., Kernich M.L. Detectable severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human breast milk of a mildly symptomatic patient with coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa673. ; published online May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirtsman M., Diambomba Y., Poutanen S.M. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ. 2020;192(24):E647–EE50. doi: 10.1503/cmaj.200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvatore C.M., Han J.Y., Acker K.P. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health. 2020;S2352-4642(20):30235. doi: 10.1016/S2352-4642(20)30235-2. –2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyle M.H., Glassman M.E., Khan A. A review of newborn outcomes during the COVID-19 pandemic. Semin Perinatol. 2020 doi: 10.1016/j.semperi.2020.151286. ; published online Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox A., Marino J., Amanat F. Evidence of a significant secretory-IgA-dominant SARS-CoV-2 immune response in human milk following recovery from COVID-19. medRxiv. 2020 doi: 10.1101/2020.05.04.20089995. published online May 8. [DOI] [Google Scholar]
- 30.National Health Commission of the People's Republic of China. The diagnosis and treatment scheme of novel coronavirus pneumonia (trial seventh edition). Mar 4, 2020. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml (accessed Apr 30, 2020).
- 31.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu W., Liu L., Kou G. Evaluation of Nucleocapsid and Spike Protein-based ELISAs for detecting antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58(6):e00461. doi: 10.1128/JCM.00461-20. –20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Manual for the laboratory diagnosis and virological surveillance of influenza. 2011. https://www.who.int/influenza/gisrs_laboratory/manual_diagnosis_surveillance_influenza/en/ (accessed May 27, 2020).
- 34.World Health Organization. Laboratory testing strategy recommendations for COVID-19: interim guidance. Jan 17, 2020. https://www.who.int/publications-detail/laboratory-testing-strategy-recommendations-for-covid-19-interim-guidance (accessed May 27, 2020).
- 35.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.UNICEF. From the first hour of life: Making the case for improved infant and young child feeding everywhere. Oct, 2016. https://data.unicef.org/resources/first-hour-life-new-report-breastfeeding-practices/ (accessed Sep 4, 2020).
- 37.Ochoa T.J., Mendoza K., Carcamo C. Is mother's own milk lactoferrin intake associated with reduced neonatal sepsis, necrotizing enterocolitis, and death? Neonatology. 2020;117(2):167–174. doi: 10.1159/000505663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verhasselt V. Breastfeeding, a personalized medicine with influence on short- and long-term immune health. Nestle Nutr Inst Workshop Ser. 2020;94:48–58. doi: 10.1159/000505578. [DOI] [PubMed] [Google Scholar]
- 39.Pu J., Liu X.X. Systematic perinatal management of the pregnant women and neonates during the epidemic of COVID-19. Zhonghua Fu Chan Ke Za Zhi. 2020;55(3):153–156. doi: 10.3760/cma.j.cn112141-20200221-00123. [DOI] [PubMed] [Google Scholar]
- 40.Bystrova K., Ivanova V., Edhborg M. Early contact versus separation: effects on mother-infant interaction one year later. Birth. 2009;36(2):97–109. doi: 10.1111/j.1523-536X.2009.00307.x. [DOI] [PubMed] [Google Scholar]
- 41.Tomori C., Gribble K., Palmquist A.E.L., Ververs M.-T., Gross M.S. When separation is not the answer: Breastfeeding mothers and infants affected by COVID-19. Matern Child Nutr. 2020 doi: 10.1111/mcn.13033. ; published online May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmquist A.E.L., Holdren S.M., Fair C.D. "It was all taken away": lactation, embodiment, and resistance among mothers caring for their very-low-birth-weight infants in the neonatal intensive care unit. Soc Sci Med. 2020;244 doi: 10.1016/j.socscimed.2019.112648. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. If you are pregnant, breastfeeding, or caring for young children. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnancy-breastfeeding.html (accessed June 25, 2020).
- 44.Smith E.R., Locks L.M., Manji K.P. Delayed breastfeeding initiation is associated with infant morbidity. J Pediatr. 2017;191:57–62. doi: 10.1016/j.jpeds.2017.08.069. .e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. Clinical management of COVID-19. May 27, 2020. https://www.who.int/publications-detail/clinical-management-of-covid-19 (accessed May 27, 2020).
- 46.Marinelli K.A. International perspectives concerning donor milk banking during the SARS-CoV-2 (COVID-19) pandemic. J Hum Lact. 2020;36(3):492–497. doi: 10.1177/0890334420917661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Association EMB. COVID-19: EMBA position statement. Feb 25, 2020. https://europeanmilkbanking.com/covid-19-emba-position-statement/ (accessed May 27, 2020).
- 48.World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions: scientific brief, 09 July 2020. July 9, 2020. https://apps.who.int/iris/handle/10665/333114 (accessed Sep 7, 2020).
- 49.American Academy of Pediatrics. FAQs: management of infants born to mothers with COVID-19. July 15, 2020. https://services.aap.org/en/pages/2019-novel-coronaviruscovid-19-infections/clinical-guidance/faqs-management-of-infantsborn-to-covid-19-mothers/ (accessed Sep 7, 2020).
- 50.Williams J., Namazova-Baranova L., Weber M. The importance of continuing breastfeeding during coronavirus disease-2019: in support of the world health organization statement on breastfeeding during the pandemic. J Pediatr. 2020;223:234–236. doi: 10.1016/j.jpeds.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J., Yuan Q., Wang H. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. ; published online Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demers-Mathieu V., Huston R.K., Markell A.M., McCulley E.A., Martin R.L., Dallas D.C. Antenatal influenza a-specific IgA, IgM, and IgG antibodies in mother's own breast milk and donor breast milk, and gastric contents and stools from preterm infants. Nutrients. 2019;11(7):1567. doi: 10.3390/nu11071567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Catalan-Dibene J. Human antibodies can neutralize SARS-CoV-2. Nat Rev Immunol. 2020;20(6):350. doi: 10.1038/s41577-020-0313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin Q., Zhu L., Ni Z., Meng H., You L. Duration of serum neutralizing antibodies for SARS-CoV-2: Lessons from SARS-CoV infection. J Microbiol Immunol Infect. 2020;S1684-1182(20):30075. doi: 10.1016/j.jmii.2020.03.015. –X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evans T.J., Ryley H.C., Neale L.M., Dodge J.A., Lewarne V.M. Effect of storage and heat on antimicrobial proteins in human milk. Arch Dis Child. 1978;53(3):239–241. doi: 10.1136/adc.53.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.