Abstract
Aim
Organ fibrosis is a common pathological outcome of persistent tissue injury correlated with organ failure and death. Although current antifibrotic therapies have led to unprecedented successes, only a minority of patients with fibrosis benefit from these treatments. There is an urgent need to identify new targets and biomarkers that could be exploited in the diagnosis and treatment of fibrosis.
Methods
Macrophages play a dual role in the fibrogenesis across different organs either by promoting pro‐inflammatory or anti‐inflammatory responses. Noncoding RNAs (ncRNAs) have been demonstrated to play key roles in macrophage functions by manipulating macrophage polarization. Therefore, understanding the mechanism of ncRNA‐associated macrophage polarization is important to move toward therapeutic interventions.
Results
In this review, we provide an overview of recent insights into the role of ncRNAs in different fibrotic diseases by modulating macrophage phenotypic plasticity and functional heterogeneity. We also discuss the potential mechanisms of different ncRNAs integrate heterogeneous macrophages in fibrogenesis,including regulatory signatures, networks, and reciprocal interactions.
Conclusions
A broader understanding of how ncRNA‐directed macrophage phenotype transition in immunity and fibrosis might promote the development of a novel strategy for antifibrotic treatment.
Keywords: circular RNA, fibrosis, long noncoding RNA, macrophage polarization, microRNA
Macrophages play a dual role in the fibrogenesis across different organs either by promoting pro‐inflammatory or anti‐inflammatory responses. Noncoding RNAs have been demonstrated to play key roles in macrophage functions by manipulating macrophage polarization.
Abbreviations
- AKI
acute kidney injury
- ALD
alcoholic liver diseases
- ALF
acute liver failure
- ALI
acute lung injury
- AM
alveolar macrophage
- BM‐MSC
bone marrow‐derived mesenchymal stem cell
- CAD
coronary artery disease
- ceRNA
competing endogenous RNA
- CF
cardiac fibrosis
- CHB
chronic hepatitis B
- COPD
chronic obstructive pulmonary disease
- circRNA
circular RNA
- CKD
chronic kidney diseases
- CM
cardiac macrophages
- CS
cigarette smoke
- DN
diabetic nephropathy
- DNMT3b
DNA methyltransferase 3b
- DSS
dextran sulfate sodium
- ECM
extracellular matrix
- EMT
epithelial mesenchymal transition
- EV
extracellular vesicle
- HCC
hepatocellular carcinoma
- HF
hepatic fibrosis
- HSC
hepatic stellate cell
- IFN‐γ
interferon‐γ
- IL
interleukin
- IM
infiltrated macrophage
- IR
ischemia‐reperfusion
- IRAK
IL‐1 receptor‐associated kinase
- KC
Kupffer cell
- lncRNA
long noncoding RNA
- LXR
liver X receptor
- MCD
methionine‐choline–deficient
- MI
myocardial infarction
- miRNA
microRNA
- NASH
nonalcoholic steatohepatitis
- ncRNA
noncoding RNA
- NF‐κB
nuclear factor kappa B
- NLRP3
nucleotide‐binding and oligomerization domain‐like receptor 3
- NSCLC
non–small‐cell lung cancer
- OSA
obstructive sleep apnea
- PCG
protein‐coding gene
- PF
pulmonary fibrosis
- PPAR
peroxisome proliferator‐activated receptor
- RF
renal fibrosis
- RIF
renal interstitial fibrosis
- RILF
radiation‐induced lung fibrosis
- RM
renal macrophage
- RNA‐Seq
RNA sequencing
- SphK
sphingosine kinase
- STAT1
signal transducer and activator of transcription 1
- T‐UCR
transcribed ultraconserved region
- TAM
tumor‐associated macrophage
- TEC
tubular epithelial cell
- TGF
transforming growth factor
- TLR
toll‐like receptor
- TMAO
trimethylamine N‐oxide
- TNF‐α
tumor necrosis factor α
- UTR
untranslated region
1. INTRODUCTION
Organ fibrosis is characterized by excessive deposition of connective tissue components and is commonly associated with high morbidity and mortality worldwide. Activated myofibroblasts are identified as the predominant effector cells and prompt the deposition of extracellular matrix (ECM). 1 However, current myofibroblast‐centered views for antifibrotic therapy are not sufficient for the treatment of the majority of patients with fibrosis. Interestingly, macrophage heterogeneity is commonly observed in the pathogenesis of fibrotic diseases and can either attenuate or exacerbate fibrosis progression.
Monocyte/macrophage plays key roles in innate immune system and is characterized by phenotypic diversity and functional plasticity. There are two principal macrophage subsets with opposite activation states are known as classical (M1) and alternative (M2) phenotypes. 2 M1/M2 polarization represents the extremes of a continuum of functional states in response to different microenvironmental signals. M1 subset is stimulated by microbial products or pro‐inflammatory cytokines, such as interferon‐γ (IFN‐γ), tumor necrosis factor α (TNF‐α), or toll‐like receptor (TLR) ligands, thereby suggesting a role of proinflammation and resistance against intracellular parasites and tumors. 3 By contrast, macrophages exposed to interleukin (IL)‐4, IL‐10, IL‐13 or transforming growth factor (TGF)‐β differentiate toward an M2 phenotype. The outcome of an M2 polarizing event is tightly linked to anti‐inflammatory response, tumor progression, tissue repair, and remodeling. 4 M1 or M2 phenotype is not fixed and can be reversed in the context of specific stimuli. For example, gene expression analysis confirmed that macrophages could undergo M1 to M2 transition after removing the inflammatory cues in the local microenvironment. 5 Heterogeneous macrophages are commonly involved in the pathogenesis of different fibrotic diseases and can function as either promoter or suppressor of fibrosis across different organ types.
Noncoding RNAs (ncRNAs) represent an important population of the transcriptome, which are comprised of a wide range of endogenous RNA‐based molecules. Different ncRNAs are emerging as a revolution in the regulation of gene expression and are involved with M1/M2 polarization. 6 Here, we summarize the phenotype and ontogeny of different macrophage subpopulations and discuss the key roles and molecular mechanisms of ncRNAs action in M1/M2 polarization in the context of fibrotic microenvironment including liver, kidney, lung, and heart. Characterization of ncRNA‐mediated macrophage heterogeneity may contribute to developing novel opportunities for their therapeutic translation for fibrotic diseases.
2. THE CLASSIFICATION AND FUNCTION OF NCRNAS
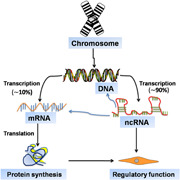
Noncoding portion of the mammalian genome, rather than its coding counterpart, is likely to explain the greater complexity of higher eukaryotes. Among all epigenetic modifications, ncRNAs are undeniably one of the best‐studied mediators of innate immune system, which are not protein‐coding genes (PCGs) and accounted for almost 90% across the human transcriptome. 7 To date, a growing number of ncRNAs is known to participate in the control of cell biology, including long noncoding RNA (lncRNA), microRNA (miRNA), and circular RNA (circRNA). Pervasive expression of different types of ncRNAs is a prominent feature of the gene regulatory networks of multicellular organisms. Given the critical role of ncRNAs in regulating gene expression, harnessing these regulatory responses promotes the dissected research field of ncRNA‐targeted therapy potency (Figure 1).
Figure 1.
Noncoding genes account for most transcription from the genome. Eukaryotic genomes are extensively transcribed, forming both messenger RNAs (mRNAs) and noncoding RNAs (ncRNAs). Of note, ncRNAs remarkably differ from their better‐known counterpart mRNAs, including transcripts numbers and functions. Although these ncRNAs that do not code for proteins, they may affect gene expression and disease progression through a variety of mechanisms
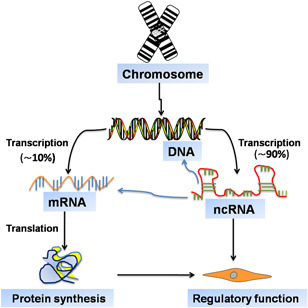
Firstly, miRNAs are small ncRNAs molecules of ∼22 nucleotides in length and are evolutionarily conserved across species. 8 They negatively regulate gene expression by sequence‐specific translation inhibition and mRNAs decay by binding 3′ untranslated regions (UTRs). In addition, lncRNAs have emerged as key components of ncRNAs and play a critical function in the gene activation and deactivation. They are generated by RNA polymerase‐mediated extragenic transcription and at least ∼200 nt in size. 9 LncRNAs could cause the cis action on the genome and chromatin, which are capable of regulating several biological phenomena, such as gene imprinting and transcriptional enhancement by acting as molecular scaffolds, architectural RNAs, or as regulatory molecules. 10 In particular, they have the ability to compete for miRNA binding by acting as a competing endogenous RNA (ceRNA) and “sponges” for miRNAs. 11 More recently, circRNAs have been attracting much interest for their potential in the maintenance of diseases and homeostasis. They are produced by circularization of specific exons of 3′ and 5′ ends covalently bonded and are highly abundant and evolutionarily conserved. However, the role of circRNAs in gene regulation is still not completely understood and some research studies imply their functions in acting as a miRNA sponge and regulating RNA‐binding proteins. 12 The coordinated activities of ncRNA‐mediated M1/M2 polarization are essential for the maintenance of tissue homeostasis and are also associated with the development of inflammatory and fibrotic disorders. 13 There is an urgent need to improve our understanding of the biological function of more potent ncRNAs. Herein, we review different ncRNAs molecules that are capable of regulating macrophage polarization in a variety of fibrotic diseases. These investigations into the mechanisms of how these ncRNAs determine specific macrophage phenotypes, hold promise for the treatment of fibrosis across different organ types (Figure 2).
Figure 2.
An expanding universe of ncRNA classification and function. Accumulating evidence has uncovered the presence and importance of ncRNAs, which includes miRNAs, lncRNAs, and circRNAs. Intensive research studies have revealed that different ncRNAs play key roles in a great variety of processes, including transcriptional regulation, chromosome replication, RNA processing and modification, mRNA stability and translation, and even protein degradation and translocation. circRNA, circular RNA; lncRNA, long noncoding RNA; mRNA, messenger RNA; miRNA, microRNA; ncRNA, noncoding RNA; RBP, RNA‐binding protein
3. HEPATIC FIBROSIS
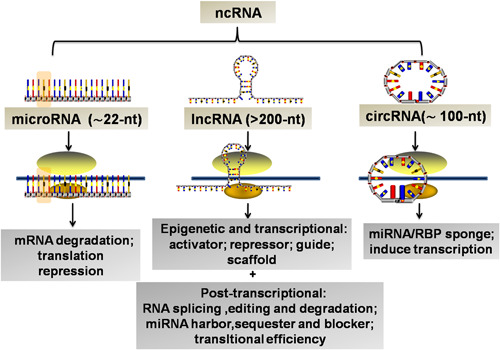
Hepatic fibrosis (HF) is the common outcome of various liver injuries, and might progress to cirrhosis and liver cancer. Liver parenchymal cells (ie, mainly hepatocytes) and nonparenchymal cells (ie, mainly hepatic stellate cells [HSCs] and various immune cells) are totally responsible for maintaining liver homeostasis and diseases. Activated HSCs have been identified as the most important promoter in the process of liver fibrogenesis by releasing abundant ECM. 14 HF is widely regarded as a reversible wound‐healing response by selectively inducing HSCs apoptosis, whereas incomplete clinical effects are obtained. Fine‐tuning of the balance between two functionally contrasted hepatic macrophage subsets is now at the heart of macrophage‐based antifibrotic therapy. Infiltrating monocyte‐derived macrophages and resident Kupffer cells (KCs) have been implicated in the pathogenesis of liver inflammation and fibrosis, either by promoting inflammatory pathways with M1 subset, or by enhancing anti‐inflammatory response with M2 subset. Furthermore, the ncRNA‐dependent M1/M2 polarization is required for causing either profibrotic or antifibrotic responses in HF microenvironment. 15
High levels of sphingosine kinase (SphK1) promotes the activation and migration of HSCs and KCs by inhibiting miR‐19b‐3p, resulting in the enhanced secretion of CCL2 and CCR2. 16 CCL2/CCR2 axis is frequently found to cause the blood monocytes' recruitment into inflamed tissues and promotes M2 polarization. Indeed, the CCR2+ macrophage pharmacologic antagonist exhibits a significant antitumor function for hepatocellular carcinoma (HCC). 17 The detection of miRNAs is a prime example of the use of macrophage activation pathways to drive the recognition of the pathophysiology of alcoholic liver diseases (ALDs). MiR‐155 is a gene inducible by many stimuli such as TLR4 and links alcohol‐induced responsiveness and inflammation of KCs. As expected, upregulation of miR‐155 in KCs of the chronic alcohol‐exposed livers contributes to the elevation of TNF‐α by nuclear factor kappa B (NF‐κB) activation and targeting C/EBPβ in ALD. 18 Conversely, miR‐155 deficiency significantly inhibits the alcohol or methionine‐choline–deficient (MCD) induced steatohepatitis and fibrosis by decreasing the number of CD163+ CD206+ infiltrating macrophages and promoting M2 KCs. 19 In addition, a predominant M2 KC profile existed in the miR‐155‐deficient mice, which could ameliorate liver ischemia‐reperfusion (IR) injury by suppressing pro‐inflammatory cytokine (TNF‐α, IL‐6, and IL‐1β) secretion and enhancing IL‐10 production. 20 These observed results seem to uncover the potential therapeutic role of miR‐155 in HF microenvironment. Another human study showed that acute alcohol binge induced the significantly increased expression of miR‐27a in monocytes, which appeared to involve attenuated M1 and enhanced M2 polarization by targeting sprouty2 and ERK pathway activation. 21 Hepatic schistosomiasis is hallmarked by the hepatic granulomas and fibrosis, which could be prevented by the elevated miR‐146a/b‐dependent M2 KC polarization through targeting signal transducer and activator of transcription 1 (STAT1). 22 The influence of the different miRNAs on the hepatic macrophage functional plasticity has been proposed as a promising landscape for disrupting liver inflammation and HF.
Consistent with the above findings, the latter cases may also demonstrate that hepatic macrophages could undergo different polarization following the changes of miRNA signaling and function in other disease conditions. Relevant to this, the miR‐15a/16−/− mice have been observed to exhibit retarded transplanted hepatic cancer (H22) cells growth and increased sensibility to dextran sulfate sodium (DSS)‐induced colitis, resulting from the M1 polarization by the coactivation of NF‐κB and STAT3. 23 NF‐κB activation has also proved effective in enhancing M1 polarization in obstructive sleep apnea (OSA), and exacerbates inflammation and fibrosis in patients with nonalcoholic steatohepatitis (NASH) by inhibiting miR‐365. 24 It is therefore that inflammatory M1 polarization might display antitumor activities. For example, a study in mice has shown that elevation of liver macrophage miR‐138 exacerbates acute liver failure (ALF) by suppressing p53 and enhancing inflammatory factors (TNF‐α, IL‐6, and IL‐1β) expression. 25 Furthermore, overexpression of MCP‐1 induced protein (MCPIP1) in KCs could alleviate the lipopolysaccharide (LPS)‐induced live injury and septic mice by negatively regulating miR‐9/SIRT1 pathway. 26 The serum and peripheral blood monocytes (PBMs) of patients with chronic hepatitis B (CHB) had decreased miR‐210 levels, which could inhibit the anti‐inflammatory macrophage activation. 27 Silencing of miR‐375 could decrease the apoptosis of KCs and the IL‐6, TNF‐α, and IL‐1β expressions by targeting astrocyte elevated gene‐1 (AEG‐1), which improves immune function in mice with ALF. 28 Adipose mesenchymal stem cell (AMSC)‐secreted exosomes (AMSC‐Exo) contained high levels of miR‐17 which could reduce ALF through suppressing nucleotide‐binding and oligomerization domain‐like receptor 3 (NLRP3) inflammasome activation in KCs by the inhibition of TXNIP. 29
Apart from miRNAs, other ncRNAs (including lncRNAs, cirRNAs) have been considered to be a putative strategy to affect the function of hepatic macrophages. Mice lacking the lncRNA AK139328 show reduced liver IR injury by the molecular events including decreased macrophage infiltration, inhibited NF‐κB activity and inflammatory cytokines expression. 30 Extracellular vesicles (EVs) can mediate the transfer of some lncRNAs, which is capable of cell‐to‐cell communication in liver disease. HCC cell‐derived exosomes contain elevated levels of lncRNA TUC339, which leads to the decreased pro‐inflammatory cytokine production and enhanced M2 polarization. 31 Microarray analysis has identified that lncRNA TUC339 promotes M2 activation caused by the decreased phagocytosis, involving TLR signaling, cytokine‐cytokine receptor interaction, chemokines and their receptor signaling pathway. 31 LncRNA COX‐2 upregulation is positively correlated with the progression of fibrotic area. LncRNA COX‐2 is known to exert profibrotic function in HF and M1 polarization mechanism might be required in this process. Indeed, Ye et al, 32 found that M1 macrophages coincubation with HCC cell could inhibit the HCC proliferation, invasion, migration, epithelial‐mesenchymal transition (EMT) dependent on the upregulated lncRNA COX‐2. Transcribed ultraconserved regions (T‐UCRs) uc.306 is a subset of lncRNAs and its deficiency is significantly associated with a shorter overall survival of HCC due to the M2 polarization. 33 These findings have prompted studies directed toward the identification of the specific patterns of lncRNA expression in mediating M1/M2 KC polarization in different liver diseases. So far, circRNAs have been suggested to represent specific modulator of macrophage inflammation in HF, such as mmu‐circ‐35216, ‐42398, ‐34116, and ‐30981. 34 In the LPS‐induced inflammatory injury model in RAW264.7 cells, one circRNA (mmu‐circ‐35216) expression is significantly increased, three circRNA (mmu‐circ‐42398, ‐34116, and ‐30981) expression is significantly decreased. These differentially expressed circRNAs are involved in the cell composition, biological processes, molecular function, and several cell signal pathways. 34
A novel ligand of CCR2, PC3‐secreted microprotein/microseminoprotein (PSMP/MSMP), which can give rise to inflammatory macrophage infiltration and pro‐inflammatory cytokines production in HF patients. 35 The antibody of PSMP is a potential therapeutic agent for the treatment of liver fibrosis. In nonalcoholic fatty liver disease patients, sialic acid‐binding immunoglobulin‐like lectin‐7 (Siglec‐7) was mainly expressed on CCR2+ macrophages in the liver and serum levels of soluble Siglec‐7 (sSiglec‐7) were increased after stimulation by pro‐inflammatory factors in macrophages, which could serve as an independent and therapeutic marker with high specificity for advanced HF in this patient population. 36 To date, most of the data are obtained from murine models, and studies employing human patients are still not enough. Further studies will be necessary to fully understand the critical roles of various ncRNA‐mediated macrophage polarization for the identification of new regulatory networks in HF and disease progression (Figure 3).
Figure 3.
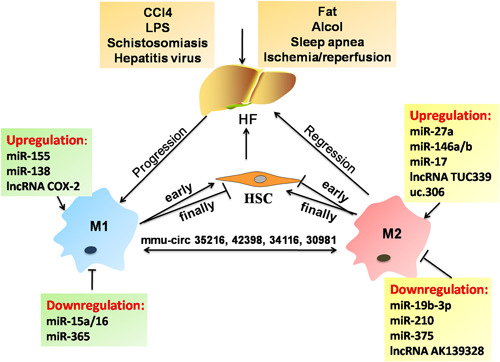
Crosstalk between heterogeneous macrophages and ncRNAs in liver fibrosis. Liver fibrosis could be induced by different etiologies (CCl4, LPS, schistosomiasis, hepatitis virus, etc). Heterogeneous macrophages (M1 and M2) contribute to the progression and regression of liver fibrosis by regulating the proliferation and activation of HSCs. HF, hepatic fibrosis; HSC, hepatic stellate cell; LPS, lipopolysaccharide; ncRNA, noncoding RNA
4. PULMONARY FIBROSIS
Pulmonary fibrosis (PF) is a chronic and highly heterogeneous respiratory disease characterized by abnormal wound‐healing condition with high mortality rates. The prevailing view has been that lung myofibroblasts are a major contributor to the aberrant deposition of ECM in PF. This long‐held view of antifibrotic therapy by directly targeting lung myofibroblasts has been challenged by evidence for a dual role of alveolar macrophages (AMs) in the pathophysiology of PF, either pro‐inflammatory or anti‐inflammatory effects. 37
Fibrotic changes in the lungs are developed from exposure to various conditions (irradiation, toxin, silica, cigarette) and are associated with M2 polarization. TGF‐β1 is extensively involved in the development of PF, stimulating ECM synthesis through a series of intracellular signaling molecules. TGF‐β1 has been implicated in mediating the differentiation and homeostasis of AMs and macrophage‐derived TGF‐β1 promotes PF. 38 Evidence suggests that bleomycin (BLM)‐induced PF in miR‐155−/− mice develop exacerbated PF, which is contributed by the liver X receptor (LXR)α deregulation and TGF‐β1 production in AMs. 39 The diverse functions of miR‐155 in different tissues fibrosis by targeting macrophage activation, remain to be explored. Next, miR‐140 has been found to be downregulated in radiation‐induced lung fibrosis (RILF), resulting in the M2 polarization and RILF progression by activating TGF‐β1/Smad3. 40 These results suggest that miRNA‐mediated TGF‐β1 activation is required for induction of AMs polarization in PF.
Recent data demonstrate that alcohol increases susceptibility to lung infection through the enhanced levels of miR‐130a/‐301a, and is capable of promoting the upregulation of TGF‐β1 by targeting peroxisome proliferator‐activated receptor (PPAR)‐γ in AMs. 41 PPARγ activation is essential for miR‐27‐3p‐mediated TLR‐2/4 signaling cascades and involves the M1‐like AMs activation and pulmonary inflammation. 42 TLR signaling molecules have been deemed as potential targets involving the fine‐tuning lung inflammatory response regulated by multiple miRNAs in AMs. Study in LPS/cigarette smoke (CS)‐treated acute lung injury (ALI) rats have shown that AMs display significant upregulation of miR‐21 and miR‐344b‐1‐3p, which could inhibit the inflammatory responses by targeting TLR‐2/4 and NF‐κB signaling pathway. 43 Microarray mRNA results indicated that AMs of smokers and chronic obstructive pulmonary disease (COPD) patients are defined by decreased M1‐ and increased M2‐regulated transcripts, along with the reduction of global miRNAs. 44 This observation agrees well with the fact that circulating miR‐320a secreted by neutrophils of smokers can modify the macrophages to an M2‐like protumorigenic phenotype through downregulation of STAT4. 45 Celecoxib ameliorated lung hyperinflammation in cystic fibrosis patients, which was caused by decreasing miR‐199a‐5p levels in the PI3K/AKT‐dependent manner in CF macrophages. 46 Furthermore, upregulated miR‐203 can alleviate lung injury in septic shock mouse models by activating AKT signaling pathway and reducing AMs levels. 47 Deletion of miR‐127 was evident in impairing M1 and enhancing M2‐biased phenotype, resulting in a decreased pulmonary inflammation and injury by the activation of JNK activity. 48 The key role of different miRNAs is well established in defining AM polarization and dissection of the molecular mechanisms may pave the way to translation. For instance, Yao et al 49 discovered the ability of exosomes to transfer overexpressing miR‐328 from M2 AMs to pulmonary interstitial fibroblasts, thereby triggering fibroblast proliferation and aggravating PF through the regulation of FAM13A. Silicosis is pathologically characterized by the diffused PF and silica‐treated macrophages induce fibroblast activation through the expression of MyD88 and Smad3 by inhibiting miR‐29b and miR‐489. 50 , 51 Distinct lncRNA signatures are associated with macrophage inflammatory response in LPS‐induced ALI, suggesting that lncRNAs might also alter the AM phenotypes. It has recently been shown that lncRNA MALAT1 could ameliorate BLM‐induced PF by suppressing M2 AMs and profibrotic genes. 52 Mechanistically, MALAT1 knockdown promotes IL‐4 induction of mitochondrial pyruvate carriers and their mediation of glucose‐derived oxidative phosphorylation (OxPhos) is critical for MALAT1‐regulated M2 polarization. 52 In consistent, MALAT1 has been known to elicit M1 activation and exacerbate the septic lung injury in mice. MALAT1 functioned as a molecular sponge for miR‐146a and activated the p38 MAPK/p65 NF‐κB signaling pathway. 53 Therefore, MALAT1‐associated M1 polarization is involved in different pulmonary pathogeneses and play opposite roles in pulmonary injury and fibrosis. There is a negative feedback loop underlying the transcript isoforms of lncRNA MEG3, transcript 4 (MEG3‐4)‐mediated inflammatory cytokines production by the sponging of miR‐138 in macrophages, which could prevent sepsis following lung infection. 54 MEG3‐4‐mediated decoy and sponging of miR‐138 in the cytoplasm increases the IL‐1β expression that subsequently induces a negative feedback mechanism mediated by NF‐κB that decreases MEG3‐4 abundance and inflammatory cytokine production. 54 M2‐derived TGF‐β1 could stimulate the upregulation of lncRNA‐ATB in lung epithelial cells and the latter exacerbated PF by promoting the EMT and targeting miR‐200c/ZEB1 axis. 55 LncRNA GNAS‐AS1 is crucial for non–small‐cell lung cancer (NSCLC) progression by directly inhibiting miR‐4319, which could target N‐terminal EF‐hand calcium‐binding protein 3 (NECAB3) to inhibit its expression and induce the tumor‐promoting M2 polarization. 56
Differentially expressed circRNAs have further amplified the unique ncRNAs functions in shaping AMs activation under physiological and pathological conditions. SiO2‐induced macrophage activation is capable of promoting fibroblast proliferation and migration via the circHECTD1/HECTD1 pathway ubiquitination 57 and circular ZC3H4 RNA/miR‐212/ZC3H4 pathway. 58 M2‐like macrophage markers (CD163 and CD204) and CD163/CD68 and CD204/CD68 cell ratios are significantly elevated in idiopathic pulmonary fibrosis (IPF) patients, associating with shorter overall survival and time‐to‐acute exacerbation in IPF patients. 59 Macrophages have a dual action in mounting a pro‐inflammatory M1‐like response to lung injury as well as in the repair of injury and profibrotic M2‐like effects in the lung. Given the crucial role of macrophage polarization in the development of PF, harnessing the ncRNA‐mediated M1/M2 responses opens up new possibilities for PF control (Figure 4).
Figure 4.
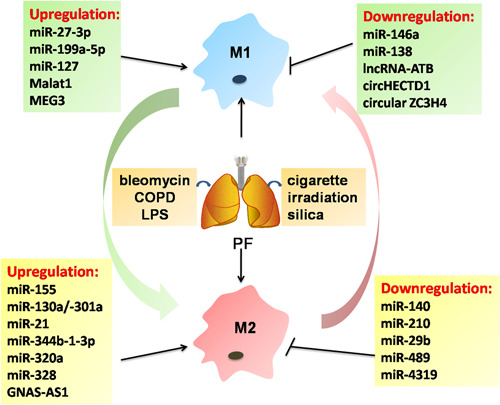
Emerging roles for ncRNAs in pulmonary fibrosis by targeting heterogeneous macrophages. Pulmonary fibrosis is now generally regarded as a consequence of multiple risk factors, such as cigarette smoke, irradiation, and silica. ncRNAs have been implicated in the pathogenesis of pulmonary fibrosis, which is associated with the function of heterogeneous macrophages. COPD, chronic obstructive pulmonary disease; lncRNA, long noncoding RNA; LPS, lipopolysaccharide; miR, microRNA; ncRNA, noncoding RNA
5. RENAL FIBROSIS
Renal fibrosis (RF) has been implicated in different chronic kidney diseases (CKDs) and is characterized by excessive ECM deposition within the glomerulus and interstitium. Activated myofibroblasts is a key driver of ECM components in RF, of which a large part is due to the complex fibroblast‐macrophage transdifferentiation and interaction. For example, TGF‐β/Smad signaling mediates the transition of bone marrow‐derived M2‐type macrophages to myofibroblasts in the renal allograft. 60 Emerging evidence suggests that M1/M2 renal macrophages (RMs) and infiltrated macrophages (IMs) are necessary in regulating kidney inflammation and fibrosis. Bone marrow‐derived mesenchymal stem cells (BM‐MSCs) could ameliorate IR injury in kidney through the induction of M2 polarization. 61 Resident adult renal/progenitor cells (ARPCs) have been recently identified as a promising population in preventing endothelial‐to‐mesenchymal transition process and promoting kidney repair in both sepsis‐ and endotoxemia‐induced acute kidney injury (AKI). 62 However, the immunomodulatory effect of ARPCs on macrophages has been still largely unknown. Interestingly, miRNAs are increasingly deemed as potential mediators in the kidney macrophage activation and function. Downregulation of miR‐376b/Atg5 suppresses renal interstitial fibrosis (RIF) by promoting RM autophagy. 63 Obesity‐induced nephropathy could be inhibited by suppressing miR‐802 or miR‐155 through NF‐κB signaling, which is associated with the reduction of IMs. 64 , 65 Silencing of miR‐21a‐5p/Notch2 receptor and overexpression of miR‐374a may be viable therapeutic options in the treatment of chronic renal allograft dysfunction and diabetic nephropathy (DN), as indicated by a reduction in IM influx. 66 Human umbilical cord‐derived MSCs attenuated RF occurring in AKI associated with reduced macrophage infiltration by downregulating miR‐29a and miR‐34a. 67 In summary, the above studies suggest that exploration of the full spectrum of miRNAs in macrophage polarization has served as a paradigm of macrophage plasticity and RF.
Under many conditions, TGF‐β1 is a critical mediator of RF and correlates with the aberrant expression of miRNAs. For example, overexpression of miR‐146a in splenic macrophage significantly inhibits the sepsis‐related renal injury. 68 Exosomes released from high glucose (HG)‐stimulated macrophages are responsible for the activation of glomerular mesangial cells and DN progression through TGF‐β1/Smad3 pathway. 69 Intriguingly, tubular epithelial cell (TEC)‐derived miRNA‐23a and miR‐19b‐3p–containing exosomes both lead to M1 macrophage activation and tubulointerstitial inflammation by targeting ubiquitin editor A20 70 and SOCS1, 71 respectively. M2‐derived legumain ameliorates the deposition of collagen and fibronectin induced by ureteral obstruction (UO) and subsequently mediates the antifibrotic effect of M2 macrophages. 72 IL‐1 receptor‐associated kinase (IRAK)‐M–deficient mice are protected from RF that is associated with decreased M2 polarization in UO. 73 MiR‐146a KO mice in a model of streptozotocin (STZ)‐induced diabetes has displayed exacerbated RF than wild‐type mice, resulting from the suppression of M1 genes (IL‐1β, IL‐18) and increased expression of M2 markers. 74 Pioglitazone could decrease renal calcium oxalate crystal formation and renal inflammation by reducing IMs and M1 RM polarization in the kidney, through a PPAR‐γ‐miR‐23‐interferon regulatory factor 1/Pknox1 axis. 75 Eventually, tumor‐associated macrophages (TAMs) plays a key role in carcinogenesis of renal cell carcinoma by inhibiting miR‐486‐5p levels in kidney cancer cells through the induction of CCL2. 76
Many lncRNAs are garnering increasing attention for their dysregulated expression in the pathogenesis of RF and disease progression, which could exert either pro‐inflammatory or profibrotic effects. Notably, the upregulation of lncRNA E330013P06 was found in monocytes from type‐2 diabetes patients and mouse macrophages treated with HG and palmitic acid. 77 It promoted a dysfunctional M2 phenotype (decreased IL‐10 levels) and enhanced M1 inflammatory response (increased IL‐6, TNF, PTGS2, and CCL2 levels) in macrophages, which could develop lncRNA‐based therapies for inflammatory diabetic complication. 77 LncRNA LRNA9884 and Erbb4‐IR are both Smad3‐dependent lncRNAs that promoted renal inflammation and fibrosis in DN by triggering MCP‐1 production and suppressing miR‐29. 78 , 79 Overexpression of lncRNA NR_038323 ameliorates the RF in STZ‐induced DN via miR‐324‐3p/DUSP1/p38MAPK and ERK1/2 pathway. 80 LncRNA Mirt2 functions as a checkpoint to prevent aberrant activation of inflammation and inhibit endotoxemia‐induced fatality and multiorgan dysfunction including kidney and liver. 15 The treatment of obstructive kidneys in mice with quercetin, decreases the levels of iNOS and IL‐12, as well as the proportion of F4/80+/CD11b+/CD86+ macrophages by downregulating NF‐κB and thereby inhibits the M1 polarization. 81 Moreover, quercetin also inhibits the polarization of F4/80+/CD11b+/CD206+ M2 macrophages by antagonizing the TGF‐β1/Smad2/3 signaling, which may have therapeutic potential for patients with kidney injury and fibrosis. 81 Thus, future clinical studies will need to address whether the above ncRNA‐based approaches to promote the M1/M2 polarization in humans may generate new therapeutic strategies for RF (Figure 5).
Figure 5.
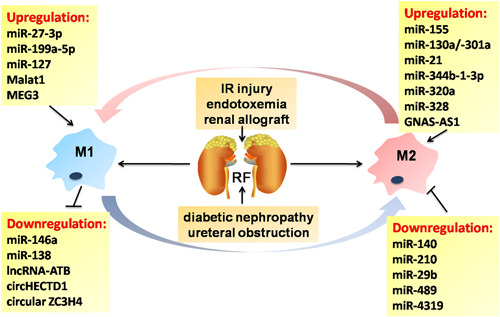
Noncoding RNA‐mediated macrophage phenotypic regulation in renal fibrosis. Renal fibrosis contributes greatly to end‐stage renal failure, characterized by the excessive ECM deposition in the interstitium of kidney. In response to the different injuries, infiltrating and resident macrophages could undergo M1 or M2 polarization, which is largely dependent on the regulation of multiple noncoding RNAs. ECM, extracellular matrix; IR, ischemia‐reperfusion; RF, renal fibrosis
6. CARDIAC FIBROSIS
Cardiac fibrosis (CF) is central to various heart diseases and is characterized by a net accumulation of ECM in the cardiac interstitium. Consistent with the role of myofibroblasts in other tissues, cardiac myofibroblasts are the predominant ECM‐producing effector cells and are responsible for the development of CF. The emerging role of infiltrating macrophages and resident cardiac macrophages (CM) in the activation of fibroblasts, suggests that distinct macrophage lineages represent promising targets for cardiac injury, recovery, and remodeling.
Trimethylamine N‐oxide (TMAO), a gut microbe‐derived metabolite, leads to the deteriorated CF through accelerating the transformation of fibroblasts into myofibroblasts and macrophage activation by targeting TGF‐β/Smad pathway. 82 TGF‐β/Smad3 activation in macrophages protects the infarcted heart from adverse remodeling by promoting an anti‐inflammatory M2 phenotype. 83 Differentiation of M1/M2 macrophages in the myocardium has been associated with the development of CF and the underlying mechanisms have also been a topic of intensive research. There is evidence that miRNAs regulate macrophages polarization and infiltration (miR‐21, 84 miR‐133a 85 ) and is involved in CF. Firstly, inhibition of miR‐155 decreases myocardial infarction (MI)‐induced sympathetic neural remodeling by repressing M1 polarization‐dependent on the SOCS1/NF‐κB pathway. 86 Hearts of microRNA‐155(−/−) mice are shown to the decreased susceptibility to viral myocarditis and improved cardiac function by modulating M2 polarization. 87 Tail vein injection of miR‐155 inhibitor, miR‐155‐AuNP, could reduce cell apoptosis, CF, and restore the cardiac function by enhancing M2 ratio in ovariectomized diabetic mice. 88 Local delivery of a miR‐21 mimic using nanoparticle or ultrasound‐targeted microbubbles into lesion sites attenuates post‐MI remodeling, heart failure, and atherosclerosis by switching macrophage phenotype from pro‐inflammatory M1 to reparative M2. 89
In addition, lncRNAs have attracted great interest as biomarkers and targets for preventing cardiac remodeling and fibrosis by modulating macrophage inflammatory functions. 90 For instance, levels of lncRNA H19 in peripheral blood mononuclear cells (PBMCs) are elevated in the coronary artery disease (CAD) patients and considered as potential biomarker for CAD diagnosis and prognosis. 91 Sallam et al 92 indicated that loss of lncRNA MeXis in mouse bone marrow cells damaged LXR‐dependent genes transcription and accelerated the development of atherosclerosis. Interestingly, lncRNA MALAT1 and NEAT1 have been found to serve as novel immunoregulators affecting monocyte‐macrophage functions and their disruption may contribute to identifying high risk in post‐MI and atherosclerosis patients. 93 , 94 In the treatment of LPS, NEAT1−/− bone marrow‐derived macrophages (BMDMs) displayed increased reactive oxygen species production and disturbed phagocytic activity following altered transcriptomes, along with aberrant chemokine/chemokine receptor expression, increased baseline phagocytosis, and attenuated proliferation. Finally, monocyte‐macrophage differentiation was deregulated in NEAT1−/− bone marrow and blood. Finally, monocyte‐macrophage differentiation was deregulated in NEAT1−/− bone marrow and blood. 94 MALAT1‐deficient ApoE−/− mice display atherosclerosis and their BMDMs responded to LPS show enhanced pro‐inflammatory cytokines expression including TNF and inducible NO synthase (NOS2). 93 It is likely that the direct interactions between MALAT1 and NEAT1 through the enzymatically MALAT1‐derived mascRNA might promote the development of atherosclerosis. Knockdown of lncRNA Mirt1 attenuates acute MI injury which could be attributed to the reduced inflammatory macrophage infiltration through inhibition of the NF‐κB pathway. 95 Recent studies have highlighted that circRNA may represent a potential new therapeutic target in cardiovascular disease 96 ; however, their function and molecular mechanism correlated with macrophages remain largely unknown and await further detailed study. Inflammation and fibrosis are the major risks for heart failure with preserved ejection fraction (HFPEF) patients, evidenced by the increased M1 and M2 numbers in HFPEF, and the HFPEF patient‐derived sera could promote healthy donor monocytes into M2 macrophage. 97 Neutrophils are another type of innate immune cell that is involved in cardiac repair after MI by polarizing macrophages toward a reparative M2 phenotype. 98 Increased fibrosis was found in neutrophil‐depleted mice subjected to MI and the phenotype of macrophage can be changed by administration of neutrophil secretome or neutrophil gelatinase‐associated lipocalin. 98 These experimental models and clinical successes have led to a macrophage‐centered view of antifibrotic approach in CF. Considering recent reports on the control of macrophage polarization by ncRNAs provided via the internal and external stimuli, various ncRNAs might be identified as candidate targets for therapeutic intervention in the CF microenvironment (Figure 6).
Figure 6.
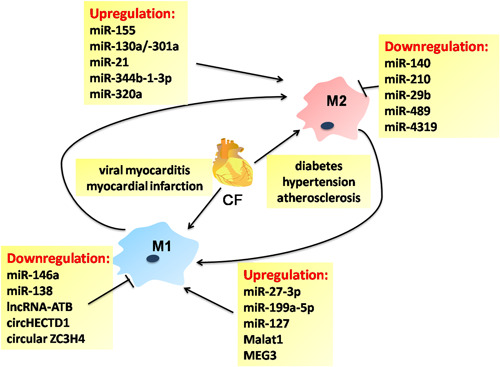
Noncoding RNA‐associated macrophage polarization regulatory networks in cardiac fibrosis. Cardiac fibrosis (CF) is considered as an important and early risk factor for cardiac development and diseases, triggered by variable etiology. Noncoding RNAs play an important role in the pathogenesis of CF by targeting M1 or M2 polarization
7. CONCLUSIONS AND PERSPECTIVE
As is known to all, the development and progression of fibrosis involve the interaction of distinct and overlapping mechanisms which orchestrate the roles and actions of multiple residents and recruited monocyte/macrophages. On the one hand, M1 macrophages initiate tissue inflammation that underlies the predominant and protective responses to tissue injury. On the other hand, prolonged inflammation promotes the maladaptive tissue remodeling and fibrosis process, which leads to chronic pathology, partially mediated by M2 macrophages. Accumulating evidence identifies multiple types of ncRNAs as key mechanistic regulators of persistent M1 and/or M2‐dependent tissue damage and fibrosis in a wide variety of organ systems. 99 Distinct ncRNAs‐regulatory modalities may be required for effective reprogramming of macrophage polarization in the specific fibrotic conditions. For this purpose, there is an urgent need to improve our understanding of the internal connections between different ncRNAs and different organs. Additionally, studies in animal models did not fully reflect the identity of humans, additional confirmatory studies would be necessary for elucidating the specific ncRNAs‐mediated M1/M2 gene expression profiles and transcriptional regulatory pathways in humans. To date, most of the new macrophage‐centered strategies have been tested in animal or early clinical trials that are not sufficient to fully reflect the clinical values in fibrotic patients therapy. Thus, further large translational studies and clinical trials, based on the interplay between ncRNAs and macrophage polarization, could be a way to identify more efficiently promising treatments for fibrotic diseases.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
DZ designed and planned the work, and revised the manuscript. YW and SW performed the literature search and interpretation, and manuscript drafting. JL and JJL revised the manuscript.
ETHICS STATEMENT
The study was approved by the Institutional Review Board of Wannan Medical College, Wuhu, China. We certify that this manuscript is original and has not been published and will not be submitted elsewhere for publication while being considered by Immunity, Inflammation and Disease. No data, text, or theories by others are presented as if they were our own. The submission has been received explicitly from all co‐authors. And authors whose names appear on the submission have contributed sufficiently to the scientific work and, therefore, share collective responsibility and accountability for the results.
ACKNOWLEDGMENTS
This study was supported by grants from the Key Laboratory of Non‐Coding RNA Transformation Research of Anhui Higher Education Institution (Wannan Medical College) (No: RNA201907), the Natural Science Foundation of Anhui province (No: 2008085QH355), and the General Program of National Natural Science Foundation of China (No: 81970534).
Zhou D, Wu Y, Wang S, Li J, Luan J. Harnessing noncoding RNA‐based macrophage polarization: Emerging therapeutic opportunities for fibrosis. Immun Inflamm Dis. 2020;8:793–806. 10.1002/iid3.341
REFERENCES
- 1. Kisseleva T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology. 2017;65:1039‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou D, Huang C, Lin Z, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192‐197. [DOI] [PubMed] [Google Scholar]
- 3. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677‐686. [DOI] [PubMed] [Google Scholar]
- 4. Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344‐346. [DOI] [PubMed] [Google Scholar]
- 5. Du RH, Sun HB, Hu ZL, Lu M, Ding JH, Hu G. Kir6.1/K‐ATP channel modulates microglia phenotypes: implication in Parkinson's disease. Cell Deat Dis. 2018;9:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun D, Yu Z, Fang X, et al. LncRNA GAS5 inhibits microglial M2 polarization and exacerbates demyelination. EMBO Rep. 2017;18:1801‐1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. St Laurent G, Wahlestedt C, Kapranov P. The landscape of long noncoding RNA classification. Trends Genet. 2015;31:239‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20:21‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arun G, Diermeier SD, Spector DL. Therapeutic targeting of long non‐coding RNAs in cancer. Trends Mol Med. 2018;24:257‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quinn JJ, Chang HY. Unique features of long non‐coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47‐62. [DOI] [PubMed] [Google Scholar]
- 11. Liang L, Xu J, Wang M, et al. LncRNA HCP5 promotes follicular thyroid carcinoma progression via miRNAs sponge. Cell Death Dis. 2018;9:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pamudurti NR, Bartok O, Jens M, et al. Translation of circRNAs. Mol Cell. 2017;66:9‐21. e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34:216‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ma L, Yang X, Wei R, et al. MicroRNA‐214 promotes hepatic stellate cell activation and liver fibrosis by suppressing Sufu expression. Cell Death Dis. 2018;9:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Du M, Yuan L, Tan X, et al. The LPS‐inducible lncRNA Mirt2 is a negative regulator of inflammation. Nat Commun. 2017;8:2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lan T, Li C, Yang G, et al. Sphingosine kinase 1 promotes liver fibrosis by preventing miR‐19b‐3p‐mediated inhibition of CCR2. Hepatology. 2018;68:1070‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bartneck M, Schrammen PL, Mockel D, et al. The CCR2(+) macrophage subset promotes pathogenic angiogenesis for tumor vascularization in fibrotic livers. Cell Mol Gastroenterol Hepatol. 2019;7:371‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bala S, Csak T, Kodys K, et al. Alcohol‐induced miR‐155 and HDAC11 inhibit negative regulators of the TLR4 pathway and lead to increased LPS responsiveness of Kupffer cells in alcoholic liver disease. J Leukoc Biol. 2017;102:487‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Csak T, Bala S, Lippai D, et al. MicroRNA‐155 deficiency attenuates liver steatosis and fibrosis without reducing inflammation in a mouse model of steatohepatitis. PLOS One. 2015;10:e0129251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Y, Ma D, Wang Z, Yang J. MicroRNA‐155 deficiency in Kupffer cells ameliorates liver ischemia‐reperfusion injury in mice. Transplantation. 2017;101:1600‐1608. [DOI] [PubMed] [Google Scholar]
- 21. Saha B, Bruneau JC, Kodys K, Szabo G. Alcohol‐induced miR‐27a regulates differentiation and M2 macrophage polarization of normal human monocytes. J Immunol. 2015;194:3079‐3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He X, Tang R, Sun Y, et al. MicroR‐146 blocks the activation of M1 macrophage by targeting signal transducer and activator of transcription 1 in hepatic schistosomiasis. EBioMedicine. 2016;13:339‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jia X, Hu X, Han S, et al. Increased M1 macrophages in young miR‐15a/16(−/−) mice with tumour grafts or dextran sulphate sodium‐induced colitis. Scand J Immunol. 2018;88:e12703. [DOI] [PubMed] [Google Scholar]
- 24. Schaefer E, Wu W, Mark C, et al. Intermittent hypoxia is a proinflammatory stimulus resulting in IL‐6 expression and M1 macrophage polarization. Hepatol Commun. 2017;1:326‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang YQ, Lan YY, Guo YC, Yuan QW, Liu P. Down‐regulation of microRNA‐138 improves immunologic function via negatively targeting p53 by regulating liver macrophage in mice with acute liver failure. Biosci Rep. 2019;39:BSR20190763. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Han S, Li Z, Ji P, et al. MCPIP1 alleviated lipopolysaccharide‐induced liver injury by regulating SIRT1 via modulation of microRNA‐9. J Cell Physiol. 2019;234:22450‐22462. [DOI] [PubMed] [Google Scholar]
- 27. Li F, Bian H, Wang W, et al. HBV infection suppresses the expression of inflammatory macrophage miR210. Mol Med Rep. 2019;19:1833‐1839. [DOI] [PubMed] [Google Scholar]
- 28. Ke QH, Chen HY, He ZL, et al. Silencing of microRNA‐375 affects immune function in mice with liver failure by upregulating astrocyte elevated gene‐1 through reducing apoptosis of Kupffer cells. J Cell Biochem. 2019;120:253‐263. [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Lou G, Li A, et al. AMSC‐derived exosomes alleviate lipopolysaccharide/d‐galactosamine‐induced acute liver failure by miR‐17‐mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine. 2018;36:140‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Z, Jia S, Li D, et al. Silencing of long noncoding RNA AK139328 attenuates ischemia/reperfusion injury in mouse livers. PLOS One. 2013;8:e80817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li X, Lei Y, Wu M, Li N. Regulation of macrophage activation and polarization by HCC‐derived exosomal lncRNA TUC339. Int J Mol Sci. 2018;19:2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ye Y, Xu Y, Lai Y, et al. Long non‐coding RNA cox‐2 prevents immune evasion and metastasis of hepatocellular carcinoma by altering M1/M2 macrophage polarization. J Cell Biochem. 2018;119:2951‐2963. [DOI] [PubMed] [Google Scholar]
- 33. Luo HL, Chen J, Luo T, et al. Downregulation of macrophage‐derived T‐UCR uc.306 associates with poor prognosis in hepatocellular carcinoma. Cell Physiol Biochem. 2017;42:1526‐1539. [DOI] [PubMed] [Google Scholar]
- 34. Zhou Y, Lv X, Qu H, et al. Differential expression of circular RNAs in hepatic tissue in a model of liver fibrosis and functional analysis of their target genes. Hepatol Res. 2019;49:324‐334. [DOI] [PubMed] [Google Scholar]
- 35. She S, Wu X, Zheng D, et al. PSMP/MSMP promotes hepatic fibrosis through CCR2 and represents a novel therapeutic target. J Hepatol. 2019;72:506‐508. [DOI] [PubMed] [Google Scholar]
- 36. Sakamoto Y, Yoshio S, Doi H, et al. Serum soluble sialic acid‐binding immunoglobulin‐like lectin‐7 concentration as an indicator of liver macrophage activation and advanced fibrosis in patients with non‐alcoholic fatty liver disease. Hepatol Res. 2019;50:466‐477. [DOI] [PubMed] [Google Scholar]
- 37. Misharin AV, Morales‐Nebreda L, Reyfman PA, et al. Monocyte‐derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214:2387‐2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu X, Buttgereit A, Lelios I, et al. The cytokine TGF‐β promotes the development and homeostasis of alveolar macrophages. Immunity. 2017;47:903‐912.e904. [DOI] [PubMed] [Google Scholar]
- 39. Kurowska‐Stolarska M, Hasoo MK, Welsh DJ, et al. The role of microRNA‐155/liver X receptor pathway in experimental and idiopathic pulmonary fibrosis. J Allergy Clin Immunol. 2017;139:1946‐1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duru N, Zhang Y, Gernapudi R, et al. Loss of miR‐140 is a key risk factor for radiation‐induced lung fibrosis through reprogramming fibroblasts and macrophages. Sci Rep. 2016;6:39572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yeligar SM, Mehta AJ, Harris FL, Brown LA, Hart CM. Peroxisome proliferator‐activated receptor γ regulates chronic alcohol‐induced alveolar macrophage dysfunction. Am J Respir Cell Mol Biol. 2016;55:35‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang D, He S, Liu B, Liu C. MiR‐27‐3p regulates TLR2/4‐dependent mouse alveolar macrophage activation by targetting PPARγ. Clin Sci. 2018;132:943‐958. [DOI] [PubMed] [Google Scholar]
- 43. Zhu WD, Xu J, Zhang M, Zhu TM, Zhang YH, Sun K. MicroRNA‐21 inhibits lipopolysaccharide‐induced acute lung injury by targeting nuclear factor‐κB. Exp Ther Med. 2018;16:4616‐4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Graff JW, Powers LS, Dickson AM, et al. Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLOS One. 2012;7:e44066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fortunato O, Borzi C, Milione M, et al. Circulating mir‐320a promotes immunosuppressive macrophages M2 phenotype associated with lung cancer risk. Int J Cancer. 2019;144:2746‐2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang PX, Cheng J, Zou S, et al. Pharmacological modulation of the AKT/microRNA‐199a‐5p/CAV1 pathway ameliorates cystic fibrosis lung hyper‐inflammation. Nat Commun. 2015;6:6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ling L, Lu HT, Wang HF, Shen MJ, Zhang HB. MicroRNA‐203 acts as a potent suppressor in septic shock by alleviating lung injury via inhibition of VNN1. Kidney Blood Pres Res. 2019;44:565‐582. [DOI] [PubMed] [Google Scholar]
- 48. Ying H, Kang Y, Zhang H, et al. MiR‐127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J Immunol. 2015;194:1239‐1251. [DOI] [PubMed] [Google Scholar]
- 49. Yao MY, Zhang WH, Ma WT, Liu QH, Xing LH, Zhao GF. microRNA‐328 in exosomes derived from M2 macrophages exerts a promotive effect on the progression of pulmonary fibrosis via FAM13A in a rat model. Exp Mol Med. 2019;51:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lian X, Chen X, Sun J, et al. MicroRNA‐29b inhibits supernatants from silica‐treated macrophages from inducing extracellular matrix synthesis in lung fibroblasts. Toxicol Res. 2017;6:878‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu Q, Han L, Yan W, et al. miR‐489 inhibits silica‐induced pulmonary fibrosis by targeting MyD88 and Smad3 and is negatively regulated by lncRNA CHRF. Sci Rep. 2016;6:30921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cui H, Banerjee S, Guo S, et al. Long noncoding RNA Malat1 regulates differential activation of macrophages and response to lung injury. JCI Insight. 2019;4:e124522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dai L, Zhang G, Cheng Z, et al. Knockdown of LncRNA MALAT1 contributes to the suppression of inflammatory responses by up‐regulating miR‐146a in LPS‐induced acute lung injury. Connect Tissue Res. 2018;59:581‐592. [DOI] [PubMed] [Google Scholar]
- 54. Li R, Fang L, Pu Q, et al. MEG3‐4 is a miRNA decoy that regulates IL‐1β abundance to initiate and then limit inflammation to prevent sepsis during lung infection. Sci Signal. 2018;11:eaao2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu Y, Li Y, Xu Q, et al. Long non‐coding RNA‐ATB promotes EMT during silica‐induced pulmonary fibrosis by competitively binding miR‐200c. Biochim Biophys Acta. 1864;2018:420‐431. [DOI] [PubMed] [Google Scholar]
- 56. Li Z, Feng C, Guo J, Hu X, Xie D. GNAS‐AS1/miR‐4319/NECAB3 axis promotes migration and invasion of non‐small cell lung cancer cells by altering macrophage polarization. Funct Integr Genomics. 2019;20:17‐28. [DOI] [PubMed] [Google Scholar]
- 57. Zhou Z, Jiang R, Yang X, et al. circRNA mediates silica‐induced macrophage activation via HECTD1/ZC3H12A‐dependent ubiquitination. Theranostics. 2018;8:575‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang X, Wang J, Zhou Z, et al. Silica‐induced initiation of circular ZC3H4 RNA/ZC3H4 pathway promotes the pulmonary macrophage activation. FASEB J. 2018;32:3264‐3277. [DOI] [PubMed] [Google Scholar]
- 59. Nouno T, Okamoto M, Ohnishi K, et al. Elevation of pulmonary CD163(+) and CD204(+) macrophages is associated with the clinical course of idiopathic pulmonary fibrosis patients. J Thorac Dis. 2019;11:4005‐4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang YY, Jiang H, Pan J, et al. Macrophage‐to‐myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J Am Soc Nephrol. 2017;28:2053‐2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wise AF, Williams TM, Kiewiet MB, et al. Human mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia‐reperfusion injury. Am J Physiol Renal Physiol. 2014;306:F1222‐F1235. [DOI] [PubMed] [Google Scholar]
- 62. Sallustio F, Stasi A, Curci C, et al. Renal progenitor cells revert LPS‐induced endothelial‐to‐mesenchymal transition by secreting CXCL6, SAA4, and BPIFA2 antiseptic peptides. FASEB J. 2019;33:10753‐10766. [DOI] [PubMed] [Google Scholar]
- 63. Yang S, Abdulla R, Lu C, Zhang L. Inhibition of microRNA‐376b protects against renal interstitial fibrosis via inducing macrophage autophagy by upregulating Atg5 in mice with chronic kidney disease. Kidney Blood Pres Res. 2018;43:1749‐1764. [DOI] [PubMed] [Google Scholar]
- 64. Sun D, Chen J, Wu W, et al. MiR‐802 causes nephropathy by suppressing NF‐κB‐repressing factor in obese mice and human. J Cell Mol Med. 2019;23:2863‐2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zheng C, Zhang J, Chen X, et al. MicroRNA‐155 mediates obesity‐induced renal inflammation and dysfunction. Inflammation. 2019;42:994‐1003. [DOI] [PubMed] [Google Scholar]
- 66. Yang Z, Guo Z, Dong J, et al. miR‐374a regulates inflammatory response in diabetic nephropathy by targeting MCP‐1 expression. Front Pharmacol. 2018;9:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rodrigues CE, Capcha JM, de Braganca AC, et al. Human umbilical cord‐derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury. Stem Cell Res Ther. 2017;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Funahashi Y, Kato N, Masuda T, et al. miR‐146a targeted to splenic macrophages prevents sepsis‐induced multiple organ injury. Lab Invest. 2019;99:1130‐1142. [DOI] [PubMed] [Google Scholar]
- 69. Zhu QJ, Zhu M, Xu XX, Meng XM, Wu YG. Exosomes from high glucose‐treated macrophages activate glomerular mesangial cells via TGF‐β1/Smad3 pathway in vivo and in vitro. FASEB J. 2019;33:9279‐9290. [DOI] [PubMed] [Google Scholar]
- 70. Li ZL, Lv LL, Tang TT, et al. HIF‐1α inducing exosomal microRNA‐23a expression mediates the cross‐talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019;95:388‐404. [DOI] [PubMed] [Google Scholar]
- 71. Lv L‐L, Feng Y, Wu M, et al. Exosomal miRNA‐19b‐3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 2019;27:210‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang D, Xiong M, Chen C, et al. Legumain, an asparaginyl endopeptidase, mediates the effect of M2 macrophages on attenuating renal interstitial fibrosis in obstructive nephropathy. Kidney Int. 2018;94:91‐101. [DOI] [PubMed] [Google Scholar]
- 73. Steiger S, Kumar SV, Honarpisheh M, et al. Immunomodulatory molecule IRAK‐M balances macrophage polarization and determines macrophage responses during renal fibrosis. J Immunol. 2017;199:1440‐1452. [DOI] [PubMed] [Google Scholar]
- 74. Bhatt K, Lanting LL, Jia Y, et al. Anti‐inflammatory role of microRNA‐146a in the pathogenesis of diabetic nephropathy. J Am Soc Nephrol. 2016;27:2277‐2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen Z, Yuan P, Sun X, et al. Pioglitazone decreased renal calcium oxalate crystal formation by suppressing M1 macrophage polarization via the PPAR‐γ‐miR‐23 axis. Am J Physiol Renal Physiol. 2019;317:F137‐F151. [DOI] [PubMed] [Google Scholar]
- 76. He Y, Liu J, Wang Y, et al. Role of miR‐486‐5p in regulating renal cell carcinoma cell proliferation and apoptosis via TGF‐β‐activated kinase 1. J Cell Biochem. 2019;120:2954‐2963. [DOI] [PubMed] [Google Scholar]
- 77. Reddy MA, Chen Z, Park JT, et al. Regulation of inflammatory phenotype in macrophages by a diabetes‐induced long noncoding RNA. Diabetes. 2014;63:4249‐4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sun SF, Tang PMK, Feng M, et al. Novel lncRNA Erbb4‐IR promotes diabetic kidney injury in db/db mice by targeting miR‐29b. Diabetes. 2018;67:731‐744. [DOI] [PubMed] [Google Scholar]
- 79. Yy Z, Pm T, Pc T, et al. LRNA9884, a novel Smad3‐dependent long noncoding RNA, promotes diabetic kidney injury in db/db mice via enhancing MCP‐1‐dependent renal inflammation. Diabetes. 2019;68:1485‐1498. [DOI] [PubMed] [Google Scholar]
- 80. Yanni G, Juan W, Dengke W, et al. lncRNA NR_038323 suppresses renal fibrosis in diabetic nephropathy by targeting the miR‐324‐3p/DUSP1 axis. Mol Ther Nucleic Acids. 2019;17:741‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lu H, Wu L, Liu L, et al. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem Pharmacol. 2018;154:203‐212. [DOI] [PubMed] [Google Scholar]
- 82. Nanto‐Hara F, Kanemitsu Y, Fukuda S. The guanylate cyclase C agonist linaclotide ameliorates the gut‐cardio‐renal axis in an adenine‐induced mouse model of chronic kidney disease. Nephrol Dialysis Transplant. 2020;35:250‐264. [DOI] [PubMed] [Google Scholar]
- 83. Chen B, Huang S, Su Y, et al. Macrophage Smad3 protects the infarcted heart, stimulating phagocytosis and regulating inflammation. Circ Res. 2019;125:55‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bejerano T, Etzion S, Elyagon S, Etzion Y, Cohen S. Nanoparticle delivery of miRNA‐21 mimic to cardiac macrophages improves myocardial remodeling after myocardial infarction. Nano Lett. 2018;18:5885‐5891. [DOI] [PubMed] [Google Scholar]
- 85. Besler C, Urban D, Watzka S, et al. Endomyocardial miR‐133a levels correlate with myocardial inflammation, improved left ventricular function, and clinical outcome in patients with inflammatory cardiomyopathy. Eur J Heart Fail. 2016;18:1442‐1451. [DOI] [PubMed] [Google Scholar]
- 86. Hu J, Huang CX, Rao PP, et al. Inhibition of microRNA‐155 attenuates sympathetic neural remodeling following myocardial infarction via reducing M1 macrophage polarization and inflammatory responses in mice. Eur J Pharmacol. 2019;851:122‐132. [DOI] [PubMed] [Google Scholar]
- 87. Zhang Y, Zhang M, Li X, et al. Silencing microRNA‐155 attenuates cardiac injury and dysfunction in viral myocarditis via promotion of M2 phenotype polarization of macrophages. Sci Rep. 2016;6:22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jia C, Chen H, Wei M, et al. Gold nanoparticle‐based miR155 antagonist macrophage delivery restores the cardiac function in ovariectomized diabetic mouse model. Int J Nanomedicine. 2017;12:4963‐4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bejerano T, Etzion S, Elyagon S, Etzion Y, Cohen S. Nanoparticle delivery of miRNA‐21 mimic to cardiac macrophages improves myocardial remodeling after myocardial infarction. Nano Lett. 2018;18:5885‐5891. [DOI] [PubMed] [Google Scholar]
- 90. Zhang H, Xue C, Wang Y, et al. Deep RNA sequencing uncovers a repertoire of human macrophage long intergenic noncoding RNAs modulated by macrophage activation and associated with cardiometabolic diseases. J Am Heart Assoc. 2017;16:e007431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bitarafan S, Yari M, Broumand MA, et al. Association of increased levels of lncRNA H19 in PBMCs with risk of coronary artery disease. Cell J. 2019;20:564‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sallam T, Jones M, Thomas BJ, et al. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nature Med. 2018;24:304‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gast M, Rauch BH, Nakagawa S, et al. Immune system‐mediated atherosclerosis caused by deficiency of long non‐coding RNA MALAT1 in ApoE−/− mice. Cardiovasc Res. 2019;115:302‐314. [DOI] [PubMed] [Google Scholar]
- 94. Gast M, Rauch BH, Haghikia A, et al. Long noncoding RNA NEAT1 modulates immune cell functions and is suppressed in early onset myocardial infarction patients. Cardiovasc Res. 2019;115:1886‐1906. [DOI] [PubMed] [Google Scholar]
- 95. Li X, Zhou J, Huang K. Inhibition of the lncRNA Mirt1 attenuates acute myocardial infarction by suppressing NF‐κB activation. Cell Physiol Biochem. 2017;42:1153‐1164. [DOI] [PubMed] [Google Scholar]
- 96. Wang L, Shen C, Wang Y, et al. Identification of circular RNA Hsa_circ_0001879 and Hsa_circ_0004104 as novel biomarkers for coronary artery disease. Atherosclerosis. 2019;286:88‐96. [DOI] [PubMed] [Google Scholar]
- 97. Glezeva N, Voon V, Watson C, et al. Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: evidence of M2 macrophage activation in disease pathogenesis. J Card Failure. 2015;21:167‐177. [DOI] [PubMed] [Google Scholar]
- 98. Horckmans M, Ring L, Duchene J, et al. Neutrophils orchestrate post‐myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38:187‐197. [DOI] [PubMed] [Google Scholar]
- 99. Zhou YX, Zhao W, Mao LW, et al. Long non‐coding RNA NIFK‐AS1 inhibits M2 polarization of macrophages in endometrial cancer through targeting miR‐146a. Int J Biochem Cell Biol. 2018;104:25‐33. [DOI] [PubMed] [Google Scholar]


