Abstract
Lecithins (E 322) were re‐evaluated in 2017 by the former EFSA Panel on Food Additives and Nutrient sources added to Food (ANS). As follow‐up to that assessment, the Panel on Food Additives and Flavourings (FAF) was requested to assess the safety of lecithins (E 322) for uses as food additive in food for infants below 16 weeks of age belonging to food categories 13.1.1 and 13.1.5.1 and as carry over in line with Annex III to Regulation (EC) No 1333/2008. In addition, the FAF Panel was requested to address the issues identified during the re‐evaluation of the food additive (E 322). The process involved the publication of a call for data to allow the interested business operators to provide the requested information to complete the risk assessment. Based on the information submitted in response to the call for data, the FAF Panel considered it feasible to amend the EU specifications, in particular for the toxic elements arsenic, lead, mercury and introduce new specifications for cadmium and microbiological criteria. The safety issue identified by the ANS Panel in 2017 concerned potential neurodevelopmental effects. For the reason that choline is a precursor of the neurotransmitter acetylcholine, the Panel considered it appropriate to address the safety of lecithins (E 322) as food additive in infant formula used in infants below the age of 16 weeks by comparing the concentration of choline in human milk with that in the formula. The Panel concluded that the intake of lecithins (E 322) as a food additive in infant formula belonging to FC 13.1.1 or in food for special medical purposes belonging to FC 13.1.5.1 does not raise safety concerns up to the maximum permitted level (MPL) of lecithins (E 322).
Keywords: Lecithins, E 322, food additive, infants
Summary
In accordance with Regulation (EU) No 257/2010, the European Food Safety Authority (EFSA) is currently re‐evaluating the safety of food additives already permitted in the Union before 20 January 2009 and issuing scientific opinions on their safety when used in food as per Annexes II and III to Regulation (EC) No 1333/2008. The risk assessment approach followed in the re‐evaluation has not covered the use of food additives in food for infants below 12 weeks of age. Additionally, while re‐evaluating the safety of food additives referred to above, EFSA identified some concerns, namely (1) data gaps that have triggered recommendations in the published scientific opinions; and/or (2) data gaps that have increased uncertainties linked to the risk assessment and/or which prevented the Panel from concluding on some aspects of it.
On 31 May 2017, EFSA published a guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age, thus enabling EFSA to assess the safety of food additive used in food for infants below this age. The age up to 16 weeks was selected in the guidance because infants are exposed to formula feeding until this age as the only source of food since complementary feeding is not supposed to be introduced before.
As follow‐up of the above, this Opinion addresses the data gaps previously identified during the re‐evaluation of lecithins (E 322) as food additive, the recommendations expressed by the ANS Panel and the safety in the special subpopulation of infants below 16 weeks of age.
The process followed involved the publication of a dedicated call for data allowing all interested business operators to provide the requested information a) for closing the data gaps and addressing the recommendations and b) for completing the assessment and to confirm that the additive is present as food additive in food for infants below 16 weeks of age belonging to food categories 13.1.1 (infant formulae as defined by Commission Delegated Regulation (EU) 2016/127) and 13.1.5.1 (dietary foods for infants for special medical purposes and special formulae for infants) and as carry over in line with Annex III to Regulation (EC) No 1333/2008.
The data submitted in response to the call for data on lecithins (E 322) included technical information, information on the levels of use and literature studies. In addition, the interested business operator provided a comparison between the levels of choline and phospholipids in the infant formulae and in the maternal milk.
Lecithins (E 322) are identified as mixtures or fractions of phosphatides obtained by physical procedures from animal or vegetable foodstuffs. Specifications for lecithins (E 322) have been defined in Commission Regulation (EU) No 231/2012.
The Panel considered it feasible to amend the EU specifications based on the information submitted in response to the call for data. This refers to lowering existing limits for the toxic elements arsenic, lead and mercury as well as introducing new specifications for cadmium and microbiological criteria.
According to Annex II of Regulation (EC) No 1333/2008, lecithins (E 322) are permitted in food categories 13.1.1 of infant formulae and 13.1.5.1 of dietary foods for infants for special medical purposes and special formulae (FSMP) for infants at a maximum level of 1,000 mg/L. Lecithins (E 322) are also authorised as food additives in all nutrients intended to be used in foods for infants and young children according to Annex III, Part 5, Section B of Regulation (EC) No 1333/2008 provided that the maximum level defined in Part E of Annex II is not exceeded.
Based on data submitted by SNE members, lecithins (E 322) are used in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions at levels of up to 1,000 mg/L, and are thus in compliance with maximum permissible levels in the EU.
For infants below 16 weeks of age consuming infant formulae (FC 13.1.1) or infant food for special medical purposes (FSMP) (FC 13.1.5.1), in the regulatory maximum level exposure assessment scenario, mean exposure to lecithins (E 322) was estimated to be 200 mg/kg body weight (bw) per day while the high level was estimated at 260 mg/kg bw per day. In the refined scenario, exposure was the same using the maximum reported levels by the interested business operators (for both FC 13.1.1 and 13.1.5.1); while using the mean reported levels by the interested business operators, refined exposure is estimated at 62 mg/kg bw per day at the mean (81 mg/kg bw per day at the p95) for FC 13.1.1 and at 180 mg/kg bw per day at the mean (234 mg/kg bw per day at the p95) for FC 13.1.5.1.
In the re‐evaluation of lecithins, the acute toxicity of lecithins (E 322) in mice, rats and rabbits was considered as low. In addition, the available subchronic and chronic toxicity studies did not report any adverse effect, even at the highest doses tested. No carcinogenic effects were reported in rats, even at the highest dose tested. The ANS Panel considered the available genotoxicity data on lecithins (E 322) to be sufficient to conclude that there is no concern with respect to genotoxicity. Adverse effects were not observed in the developmental toxicity studies performed in mice, rat and rabbits up to the highest dose tested. However, the Panel noted that reproductive toxicity studies were not available. The ANS Panel considered the available data addressing neurodevelopment inadequate and concluded that a study with lecithins (E 322) in compliance with the current OECD TG 426 would be warranted.
The main safety issue identified in the EFSA ANS Opinion concerned indications that the development of the brain was altered at concentrations in the diet of 5% soya lecithins and higher during gestation, lactation and post‐weaning period observed in toxicity studies. It has to be noted, however, that these studies had limitations which precluded firm conclusions by the ANS Panel. The studies provided during the call for data did not contribute to assessment of potential neurodevelopmental effects because this endpoint has not been addressed in the studies. Specific studies in infants were not provided and the data from the post‐marketing surveillance studies did not allow to make firm conclusions on the safety of lecithins (E 322) in infant formula. In line with the recommendations from the ANS Panel former assessment, the FAF Panel considered that the appropriate approach to be taken for the safety assessment of lecithins (E 322) as food additive in infant formula used in infants below the age of 16 weeks was to compare the concentration of choline in human milk with that in the formula.
From the information provided by the interested business operator, the mean content of total choline in formula (225 mg/L) is around 62% higher than that in human milk (138 mg/L). However, the mean content of choline released from lecithins in infant formula (12 mg/L) represents only 8.7% of the mean content of total choline in mature human milk. The same conclusion applies at the MPL of 1,000 mg/L which could contribute to 21 mg/L of choline. Most (nearly 75%) of the total choline content in infant formula is derived from the addition of choline as nutrient. The mean content of choline in the formulae is in the range of the adequate intake (AI).
Considering the data submitted by interested business operators and the considerations from the Panel, a revision of the existing EU specifications for lecithins (E 322) has been recommended (see Table 12).
Table 12.
Proposal for a revised version of the existing EU Specifications for Lecithins (E 322)
| Commission Regulation (EU) No 231/2012 | Comment/justification for revision | |
|---|---|---|
| Definition | for brevity, see Table 1 | Unchanged |
| Assay | see Table 1 | Unchanged |
| Description | see Table 1 | Unchanged |
| Identification | see Table 1 | Unchanged |
| Purity | see Table 1 | Unchanged |
| Loss on drying | see Table 1 | Unchanged |
| Toluene‐insoluble matter | see Table 1 | Unchanged |
| Acid value | see Table 1 | Unchanged |
| Peroxide value | see Table 1 | Unchanged |
| Arsenic | Not more than 3 mg/kg | Lowered on the basis of the information provided and based on the considerations of the panel |
| Lead | Not more than 2 mg/kg | Lowered on the basis of the information provided and based on the considerations of the panel |
| Mercury | Not more than 1 mg/kg | Lowered on the basis of the information provided and based on the considerations of the panel |
| Cadmium | not presently specified | To be included on the basis of the information provided and based on the considerations of the panel |
| Microbiological criteria | ||
| Cronobacter (Enterobacter) sakazakii | not presently specified | Microbiological criteria should be included on the basis of the information provided; Negative in 10 g |
The choline released from lecithins (E 322) is a minor contributor (around 8.7% as the mean) of the total content of choline of the formula and does not raise concern at the current use levels including the MPL for lecithins (E 322). Most of the choline in the formula is due to the choline added as a nutrient. In addition, it can be stated that the mean content of choline is in the range of the adequate intake level (AI). The Panel concluded that the intake of lecithins (E 322) as a food additive in infant formula belonging to FC 13.1.1 or in food for special medical purposes belonging to FC 13.1.5.1 does not raise safety concerns up to the MPL of lecithins (E 322).
1. Introduction
The present opinion deals with:
the risk assessment of lecithins (E 322) in food for infants below 16 weeks of age in the food categories 13.1.1 (Infant formulae as defined by Commission Delegated Regulation (EU) 2016/127/EC1) and 13.1.5.1 (Dietary foods for infants for special medical purposes and special formulae for infants) as defined in Annex II and III to Regulation (EC) No 1333/2008 on food additives
the follow‐up on issues that have been expressed in the conclusions and recommendations of the Scientific Opinion on the re‐evaluation of lecithins (E 322) as a food additive (EFSA ANS Panel, 2017)
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The composition of food intended for infants and young children, as defined by Regulation (EU) No 609/20132, is regulated at EU level and such rules include requirements concerning the use of substances as food additives.
The use of food additives is regulated by Regulation (EC) No 1333/2008 on food additives. Only food additives that are included in the Union list, in particular in Annex II and III to that Regulation, may be placed on the market and used in food under the conditions of use specified therein.
In accordance with Regulation (EU) No 257/20103, EFSA is currently re‐evaluating the safety of food additives already permitted in the Union before 20 January 2009 and issuing scientific opinions on their safety when used in food as per Annexes II and III to Regulation (EC) No 1333/2008. However, the risk assessment approach followed until now has not covered the use of food additives in food for infants below 12 weeks of age. Consequently, EFSA published several scientific opinions on the re‐evaluation of the safety of food additives permitted in food category 13.1 but not addressing their use in food for infants below 12 weeks of age.
In addition, in these opinions EFSA identified some concerns, namely 1) Data gaps that have triggered recommendations in the (to be) published scientific opinions, and/or; 2) Data gaps that have increased uncertainties linked to the risk assessment and/or which prevented the EFSA from concluding on some aspects of it.
On 31 May 2017, EFSA published a guidance document (EFSA Scientific Committee, 2017) on the risk assessment of substances present in food intended for infants below 16 weeks of age, thus enabling EFSA to assess the safety of food additives used in food for infants below 12 weeks of age.4 Now EFSA is expected to launch dedicated calls for data to be able to perform such risk assessments.
The EC considers it is more effective that EFSA, in the context of these dedicated calls for data, also addresses all the issues and data gaps already identified in the relevant (to be) published scientific opinions on the re‐evaluation of the safety of food additives permitted in food category 13.1.
In accordance with the current EC approach for the follow‐up of EFSA's scientific opinions on the re‐evaluation of the safety of permitted food additives for which some concerns have been identified, a specific call for data would be published by the EC on DG SANTE's website5 on food additives and additional (missing) information would then be provided by interested business operators to the EC.
However, for those scientific opinions on the re‐evaluation of the safety of permitted food additives in food category 13.1 for which the risk assessment does not address their uses in food for infants below 12 weeks of age and for which some concerns have been identified by EFSA, the EC considers that for the sake of efficiency it would be appropriate to streamline the approach as described above.
Therefore, the EC requests EFSA to address all the issues and data gaps already identified in the relevant published scientific opinions of those food additives (or groups of additives that can be addressed simultaneously) as part of the upcoming work on the safety assessment of food additives uses in food for infants below 12 weeks of age.
This follow‐up aims at completing the re‐evaluation of the food additives in question for all food categories, and includes calls for data covering the actual use and usage levels of food additives in food for both infants below 12 or 16 weeks of age as well as for older infants, young children and other groups of the population for which EFSA has already finalised its assessment.
The future evaluations of EFSA should systematically address the safety of use of food additives for all age groups, including the infants below 12 or 16 weeks of age.
1.1.2. Terms of Reference
In accordance with Article 29(1)(a) of Regulation (EC) No 178/20026, and as part of EFSA’s work in completing its risk assessments concerning the use of food additives in food for infants below 12 weeks of age,5 covered by the re‐evaluation programme and its terms of reference, the European Commission requests the European Food Safety Authority to address all the data gaps specified in the recommendations made in this scientific opinions on the re‐evaluation of the safety of food additives permitted in food category 13.1 (food for infants and young children) of Annex II to Regulation (EC) No 1333/2008.
1.1.3. Interpretation of Terms of reference
Before the publication of the EFSA Scientific Committee Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA Scientific Committee, 2017), EFSA has taken 12 weeks as a cut off age for the applicability of the safety assessment. However, according to EFSA Scientific Committee (2017), the assessment will include infants up to 16 weeks of age because they are exposed to formula feeding until this age as the only source of food since complementary feeding is not supposed to be introduced before this age (see EFSA Scientific Committee, 2017).
This risk assessment addresses the use of lecithins (E 322) in their technological function as food additive and not as a nutritional source of choline. However, it should be considered that choline is released from E 322 adding to the content of total choline in the formula.
1.2. Previous evaluations of lecithins (E 322) for use in foods for infants
Lecithins (E 322) have been evaluated by the Scientific Committee on Food (SCF) in 1981 (SCF, 1982) and 1997 (SCF, 1997). In its assessment from 1981, SCF discussed hydrolysed lecithins and their comparability to lecithins stating that ‘hydrolysed lecithin is produced in the gut as a result of normal digestion. There appears to be no specific toxicological effect in rats due to feeding of hydrolysed lecithins. This substance can therefore be regarded metabolically and toxicologically as an alternative to lecithin’. In 1997, the SCF concluded that ‘the issue of lecithins and choline in infant formulae should be considered further. However, in the context of carry‐over levels of only 0.5 mg/kg, the use of lecithins in nutrient preparations for infant formulae is acceptable and not likely to be of concern’ (SCF, 1997). The SCF additionally noted that ‘In an earlier report (SCF, 1983 ) the Committee considered lecithins as acceptable technological additives at levels up to 5 g/L. However, the Directive on Additives Other Than Colours and Sweeteners lists the maximum level as 1 g/L. This reduction in the maximum level was agreed during the negotiations on the draft Directive in response to a report (UK Ministry of Agriculture Fisheries and Food, 1992 ) which recommended that the maximum level of lecithins in infant formulae should be restricted to that of human milk (1 g/L). This recommendation was based on studies which claimed neurobehavioural effects in the offspring of rats fed high doses of lecithin. Although these studies were of poor quality, the report noted that large increases in plasma choline could affect neurotransmission in the brain and that particular caution was needed in the infant since the brain was still actively developing’.
Lecithins (E 322) were evaluated also by JECFA in 1974 (JECFA, 1974a,b); JECFA did not specify a numerical acceptable daily intake (ADI) (ADI ‘not limited’).
In 2014, the EFSA Panel on Dietetic Products, Nutrition and Allergies (EFSA NDA Panel, 2014) prepared a scientific opinion on the evaluation of allergenic foods and food ingredients for labelling purposes where the allergenicity of egg and soya lecithins were considered. The possibility of residual allergenicity in food products manufactured using egg lecithins has been reported in a double‐blind placebo‐controlled food challenge (DBPCFC). The prevalence of clinically confirmed soya allergy in unselected populations in Europe appears to be low, although few studies are available. Soybeans and eggs and products thereof (including lecithins) are listed in the Annex II of the Regulation No 1169/2011 as substances or products causing allergies or intolerances for which indication as allergens is mandatory.
1.3. Previous evaluations of choline
Dietary lecithins are known to be hydrolysed and liberate choline in humans.
In its scientific opinion on dietary reference values (DRV) for choline, the EFSA NDA Panel (2016) considered dietary choline including choline compounds (e.g. glycerophosphocholine, phosphocholine, phosphatidylcholine,7 sphingomyelin). The NDA Panel concluded that average requirements (ARs) and population reference intakes (PRIs) for choline could not be derived for adults, infants and children, and therefore defined adequate intakes (AIs).
Considering that there is no evidence for an insufficient choline intake of fully breast‐fed infants during the first 6 months of life, the amount of choline provided in human milk is considered to be adequate (EFSA NDA Panel, 2016). Considering a choline concentration of 145 mg/L (average of two studies on full‐term infants) and assuming a mean milk transfer of 0.8 L/day during the first 6 months of lactation in exclusively breastfeeding women, the estimated choline intake of fully breast‐fed infants during the first 6 months of life would be 116 mg/day, rounded up to 120 mg/day.
The SCF did not consider choline when setting tolerable upper intake levels (ULs) for vitamins and minerals (2006). The US Institute of Medicine (IOM, 1998) set a UL for choline for adults, but no UL was established for infants 0–12 months.
Choline is listed in Commission Delegated Regulation 2016/1278 on specific compositional and information requirements for infant formula and follow‐on formula and as regards requirements on information relating to infant and young child feeding. The minimal and maximal content of choline in infant formula is 20 and 50 mg/100 kcal, respectively. No minimal and maximal content of choline has been set for follow‐on formula.
1.4. Summary of the previous EFSA re‐evaluation of lecithins (E 322) for uses in food for all population groups except for infants below 12 weeks of age9
In its scientific opinion, the ANS Panel (EFSA ANS Panel, 2017) reviewed available technical, biological and toxicological data on lecithins (E 322) when used as a food additive. They concluded that there was no need for a numerical ADI for lecithins (E 322). The ANS Panel took into account uses and use levels reported by the interested business operators and derived refined exposure scenarios. They concluded that there was no safety concern for the general population (from more than 1 year of age) for lecithins (E 322) food additive itself and for exposure to the choline that derives from lecithins (E 322). Similarly, they concluded that there was no safety concern for lecithins (E 322) nor for choline derived therefrom for infants (from 12 weeks up to 11 months of age). Concerning infants above 12 weeks and young children consuming foods for special medical purposes, the ANS Panel also concluded that there was no safety concern for lecithins (E 322) or for choline derived therefrom.
The ANS Panel, however, considered that the conclusions reached on the re‐evaluation of the food additive were not applicable to the use of lecithins (E 322) in food for infants under the age of 12 weeks.10 They considered that these uses would require a specific risk assessment.
In addition, the ANS Panel drafted the following recommendations which are relevant for this assessment:
recommended that the maximum limits for the impurities of toxic elements (lead, mercury and arsenic) in the EU specification for lecithins (E 322) should be revised in order to ensure that lecithins (E 322) as a food additive will not be a significant source of exposure to those toxic elements in food. The Panel also recommended that a limit for cadmium should be included in the specifications.
recommended the content of residual proteins in lecithins (E 322) should be reduced as much as possible, since there are some case reports of hypersensitivity reactions associated with soya and egg lecithins and the Panel considered that this hypersensitivity is due to the presence of proteins.
found the data available and the relevance of existing neurodevelopmental studies to be inadequate and concluded that a study with lecithins (E 322) in compliance with the current OECD TG 426 would be warranted.
recommended that in case the food additive lecithins (E 322) is used in infant formulae and follow‐on formulae supplemented with choline or choline salts, the intake of choline from all sources including the use of the food additive lecithins (E 322) via infant formulae (category 13.1.1), follow‐on formulae (category 13.1.2) or other food should be in the order of the adequate intake values defined by the EFSA NDA Panel (2016a).
In 2020, the FAF Panel issued a scientific opinion on the safety of a proposed amendment of the specifications of the food additive lecithins (E 322) to include oat lecithin, an oat oil derived from water and ethanol extraction of oat grain seeds and composed of polar lipids (≥ 35% w/w) and non‐polar lipids (55–65% w/w). In its scientific opinion, the Panel, however, recommended that the European Commission considers including specifications for oat lecithin as a new food additive in Commission Regulation (EU) No 231/2012 rather than amending the existing specifications of lecithins (E 322) (EFSA FAF Panel, 2020).
2. Data and methodologies
2.1. Data
EFSA launched a public call for data11 to collect relevant information from the interested business operators (see also Appendix A).
The Panel based its assessment on information submitted to EFSA following the public call for data, information from previous evaluations and additional available literature up to 10 September 2020.
To verify the use of the food additive lecithins (E 322) in food products, the Mintel's GNPD was used. This database is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 3 million food and beverage products of which more than 1,100,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 24 out of its 27 Member Countries, and Norway and UK presented in the Mintel GNPD.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee and in particular the EFSA Guidance of the Scientific Committee on the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA Scientific Committee, 2017).
In order to conclude on the safety of lecithins (E 322) for all population groups and to address data gaps identified during the re‐evaluation, the FAF Panel assessed the information provided:
for the follow‐up on issues that have been raised in the conclusions and recommendations of the Scientific Opinion on the re‐evaluation of lecithins (E 322) as a food additive (EFSA ANS Panel, 2017); and
the risk assessment of lecithins (E 322) in food for infants below 16 weeks of age in the food categories 13.1.1 (infant formulae as defined by Commission Delegated Regulation (EU) 2016/127/EC) and 13.1.5.1 (dietary foods for infants for special medical purposes and special formulae for infants) as defined in Annexes II and III to Regulation (EC) No 1333/2008 on food additives
When in animal studies, the test substance was administered in the feed or in drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake is calculated by the panel using the relevant default values. In case of rodents, the values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012) are applied. In the case of other animal species, the default values by JECFA (2000) are used. In these cases, the dose was expressed as ‘equivalent to mg/kg bw per day’. If a concentration in feed or drinking water was reported and the dose in mg/kg bw per day was calculated (by the authors of the study report or the Panel) based on these reported concentrations and on reported consumption data for feed or drinking water, the dose was expressed as ‘equal to mg/kg bw per day’. When in human studies in adults (aged above 18 years), the dose of the test substance administered was reported in mg/person per day, the dose in mg/kg bw per day was calculated by the Panel using a body weight of 70 kg as default for the adult population as described in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012).
Dietary exposure to lecithins (E 322) from its use as a food additive in foods for infants below 16 weeks of age was estimated combining the mean and high level consumption values reported for the period of 14–27 days of life which corresponds to 200 and 260 mL/kg bw per day (EFSA Scientific Committee, 2017), respectively, with the maximum levels according to Annex II and Annex III, Part 5 Section B to Regulation (EC) No 1333/2008 and reported use levels submitted to EFSA following a call for data. Different scenarios were used to calculate exposure (see Section 3.3.1). Uncertainties on the exposure assessment were identified and discussed.
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
According to Commission Regulation (EU) No 231/201212, the food additive E 322 is named as lecithins and the additive is identified as mixtures or fractions of phosphatides obtained by physical procedures from animal or vegetable foodstuffs. They also include the corresponding hydrolysed products. Commission Regulation No 231/2012 includes both types of lecithins (non‐hydrolysed and hydrolysed) under the same food additive designation (E 322). JECFA differentiates between them and treats them as different food additives (INS 322i and INS 322ii) with distinct specifications (JECFA, 2007a,b).
The fatty acids in lecithins have variable carbon chain lengths; therefore, an exact molecular formula and a molecular weight can only be given for individual components (see EFSA ANS Panel, 2017 and Figure 1).
Figure 1.
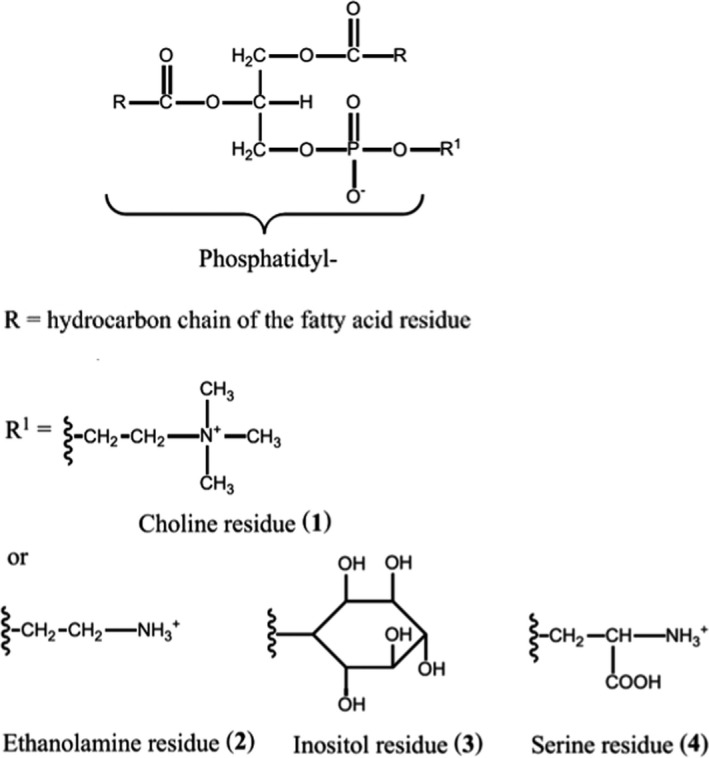
Structures of the main phospholipids in lecithins (E 322): phosphatidylcholine (1), phosphatidylethanolamine (2), phosphatidylinositol (3), phosphatidylserine (4). If R1 = H, the compound is phosphatidic acid (from EFSA ANS Panel, 2017). For more information on the physical properties and the chemical composition and structure of lecithins (E 322) from various sources such as soya bean oil, sunflower oil, rape seed oil, egg yolk, etc., the reader is referred to the ANS Panel opinion (EFSA ANS Panel, 2017)
3.1.2. Specifications
The specifications for lecithins (E 322) as defined in the Commission Regulation (EU) No 231/2012 are listed in Table 1.
Table 1.
Specifications for lecithins (E 322) according to Commission Regulation (EU) No 231/2012
| Commission Regulation (EU) No 231/2012 | |
|---|---|
| Definition | Lecithins are mixtures or fractions of phosphatides obtained by physical procedures from animal or vegetable foodstuffs; they also include hydrolysed products obtained through the use of harmless and appropriate enzymes. The final product must not show any signs of residual enzyme activity. The lecithins may be slightly bleached in aqueous medium by means of hydrogen peroxide. This oxidation must not chemically modify the lecithin phosphatides |
| Assay | Lecithins: not less than 60.0% of substances insoluble in acetone Hydrolysed lecithins: not less than 56.0% of substances insoluble in acetone |
| Description |
Lecithins: brown liquid or viscous semi‐liquid or powder Hydrolysed lecithins: light brown to brown viscous liquid or paste |
| Identification | |
| Tests for choline, for phosphorus and fatty acids | Passes test |
| Test for hydrolysed lecithin | To a 800‐mL beaker, add 500 mL of water (30–35°C). Then slowly add 50 mL of the sample with constant stirring. Hydrolysed lecithin will form a homogeneous emulsion. Non‐hydrolysed lecithin will form a distinct mass of about 50 g |
| Purity | |
| Loss on drying | Not more than 2.0% (105°C, 1 h) |
| Toluene‐insoluble matter | Not more than 0.3% |
| Acid value | Lecithins: not more than 35 mg of potassium hydroxide per gram Hydrolysed lecithins: not more than 45 mg of potassium hydroxide per gram |
| Peroxide value | Equal to or less than 10 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 1 mg/kg |
The revisions of the existing EU specifications proposed by the panel are provided under Section 3.5.
3.1.2.1. Analytical data from commercial samples of the food additive
3.1.2.1.1. Toxic elements
The call for data, requested:
analytical data on current levels of lead, mercury, cadmium and arsenic in commercial samples of the food additive,
the lowest technologically achievable level for lead, mercury, cadmium and arsenic in order to adequately define their maximum limits in the specifications.
Analytical data were provided by one interested business operator (documentation provided to EFSA n.1) for levels of lead, mercury, cadmium and arsenic in samples of lecithins (E 322). The data provided gives ranges only, said to be based on the analysis of more than 100 samples of different types of lecithins. The sample types (source of the lecithins) were not specified. A variety of analytical methods had been used and the limit of detection (LOD) and limit of quantification (LOQ) values differ even for the same element. It seems possible that different test laboratories had been used at different times, using different methods and equipment with different performance characteristics. Acid digestion followed by ICP‐MS gave the lowest LOD/LOQ values for Pb, Cd and As, but the specialised method of combustion‐amalgamation‐cold vapour‐AAS gave the lowest LOD/LOQ values for Hg.
For lead, the range of values was < 0.05–0.12 mg/kg. For mercury, the range of values was 0.0017 – < 0.02 mg/kg. For cadmium, the range of values was < 0.01–0.12 mg/kg. For arsenic, the range of values was < 0.1–0.11 mg/kg. Since only the ranges were provided, the submitted data do not give information as to the distribution of results, how many samples were non‐detects etc. Although the data reporting is limited, it was noted that the analytical methods used for toxic elements are up‐to‐date and they were presented in a summarised way (usually including a reference to the Standard or Norm used for sample preparation and for analytical determination) that is acceptable.
This interested business operator stated that ‘the lowest technically achievable level for a trace metal element depends mainly on the levels that are achievable for the raw materials used. During the manufacturing of lecithins there are no specific processes that are designed to reduce heavy metal levels. Therefore, a reasonable specification that can be met with a high level of confidence for standard food grade lecithin has to reflect what can be achieved routinely for all lecithins independent of the raw material used’. The interested business operator indicated the values in Table 2 as the lowest achievable levels of lead, mercury, cadmium and arsenic in lecithins are safe and suitable levels for lecithin used in foods for all population groups including infants.
Table 2.
Lowest achievable levels of the toxic elements Pb, Hg, Cd and As for lecithins (E 322), as proposed by one interested business operator for all population groups, including infants below 16 weeks of age, independently of the raw material used as source (documentation provided to EFSA n. 1)
| Pb | Hg | Cd | As |
|---|---|---|---|
| 0.5 mg/kg | 0.5 mg/kg | 0.25 mg/kg | 1 mg/kg |
The Panel noted that based on the results from more than 100 samples (see above), the lowest achievable levels proposed by the interested business operator in Table 2 are multiples of the highest values reported: Pb is four times (0.5 vs 0.12), Hg is 294 times (0.5 vs 0.0017), Cd is two times (0.25 vs 0.12) and As is nine times higher (1 vs 0.11). Whereas allowance for some ‘headroom’ is to be expected, the value proposed for Hg seems very much out of line with the reported results. The proposed values would only be a modest improvement on the current EU specifications which are Pb = 2, Hg = 1 and As = 3 mg/kg. Currently for Cd, there is no limit in the EU specifications.
3.1.2.1.2. Residual protein
The call for data, requested:
analytical data on current levels of residual proteins in commercial samples of the food additive,
the lowest technologically achievable level for residual proteins to reduce their content as much as possible in view of case reports on hypersensitivity reactions associated with soya and egg lecithins.
Information was received from one interested business operator (documentation provided to EFSA n. 1). They stated that the residual protein content in lecithins (E 322) is defined by the amount of toluene‐insoluble (TI) or hexane‐insoluble (HI) matter. The presence of this insoluble matter is indicative of the presence of residual impurities such as protein and carbohydrate‐containing extraction residues and mechanical contaminants. Since for E 322, the EU specifications are no more than 0.3% of toluene‐insoluble matter, then this limits the possible protein content to a maximum of 0.3%. The interested business operator further stated that according to EU legislation, protein content is analysed as total nitrogen (Kjeldahl method) and since lecithins (E 322) contain non‐protein nitrogen in its phospholipid group, this analysis determines a value which will always be higher than the actual level of protein‐bound nitrogen present in lecithins (E 322). There is no recognised quantifiable routine analytical method for analysing protein, as such, in lecithins. The interested business operator concluded that TI or HI matter is the most appropriate parameter for ensuring a low, maximum level of residual protein in lecithin and a limit of 0.3% is the level that is currently achievable.
The Panel noted that no analytical data were provided by the interested business operator on the levels of toluene‐insoluble matter, nor on the measurement of total nitrogen in commercial samples of E 322. Notwithstanding that both approaches would tend to overestimate the protein content, such information could be of use. In the absence of data, the Panel assumes that protein could be as high at 0.3% w/w in E 322. It is noted that in the EFSA re‐evaluation of lecithins (E 322) information on protein content were provided by an interested business operator (EFSA ANS Panel, 2017). The Panel noted that the source of lecithins e.g. egg, soya may contain proteins that can be responsible for food allergy (EFSA NDA Panel, 2014).
3.1.2.1.3. Choline
The call for data, requested:
information on the percentage of phosphatidylcholine content (mass %) in specific lecithins preparations used as food additive E 322 in the infant formulae for infants below 16 weeks of age (FC 13.1.1), as well as in special formulae for infants of that age under special medical conditions (FC 13.1.5.1).
One interested business operator provided following background information, regarding total choline (documentation provided to EFSA n. 2).
Total choline levels in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions, as indicated on product labels (mg/100 mL) are summarised below in Table 3. The total choline content relates to the level present in the final product (as required to Article 7 of Commission Delegated Regulation (EU) No 2016/127) and corresponds to choline from all potential sources, i.e. lecithins, choline added as a nutrient (e.g. free choline or choline salts), and choline from natural sources (e.g. raw materials). As regulatory requirements for choline are expressed on an energy basis, total choline content was also calculated on a mg/100 kcal basis based on the energy content (kcal/100 mL) of the respective infant formula. Total choline levels in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions containing lecithins ranges from 13 to 26 mg/100 mL (25–39 mg/100 kcal). These levels are in accordance with the compositional requirements for choline in infant formula of 25–50 mg/100 kcal, as per Commission Delegated Regulation (EU) 2016/127.
Table 3.
Total choline in infant formulae for infants below 16 weeks of age and in food for special formulae for infants of that age under special medical conditions as identified on the product labels (documentation provided to EFSA n. 2)
| Type of lecithins (E 322) | Total choline (mg/L), product label | Total choline (mg/100 kcal) * | |
|---|---|---|---|
| Infant formulae for infants below 16 weeks of age | Soya lecithins liquid | 130–260 | 25–39 |
| Sunflower lecithins liquid | 260 | 39 | |
| Infant formulae for infants below 16 weeks of age under special medical conditions | Soya lecithins liquid | 220 | 33 |
Calculated as: Total choline in mg/100 kcal = [(100 kcal)/ (× kcal/100 mL) × (× mg choline/100 mL)].
The choline levels provided in Table 3 correspond to the level declared on the label of the products placed on the EU market (documentation provided to EFSA n. 2,3).
On the question of the choline content in the E 322 used, one interested business operator (documentation provided to EFSA n. 2,3) provided the following commentary and data on the phospholipid composition of lecithins used in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions.
The same interested business operator stated that soya lecithins liquid used in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions contains approximately 41–51% total phospholipids, and its phospholipid composition is similar to that of commercial soya lecithins liquid for use as a food additive in all foods reported by the European Lecithin Manufacturers Association (ELMA), previously evaluated by EFSA (EFSA ANS Panel, 2017; Table 2). Specifically, the phosphatidylcholine content of soya lecithins liquid used in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions is reported to range between 11.0% and 16.0% (see Table 4), whereas phosphatidylcholine levels for this type of lecithins were previously reported to range between 12.7% and 16.7% (EFSA ANS Panel, 2017; Table 2). Assuming the content of choline that can be theoretically released from phosphatidylcholine containing two linoleate groups is 13.2% (EFSA ANS Panel, 2017), up to 2.1% choline (i.e. 13.2% of 16.0% w/w) can be theoretically released from the amount of soya lecithins liquid used in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions.
Table 4.
Phospholipids composition of lecithins (E 322) used in infant formulae for infants below 16 weeks of age and in food for special formulae for infants of that age under special medical conditions as identified on the product labels (documentation provided to EFSA n. 2)
| Type of lecithins | Total PL % | PC % | PI % | PE % | PA % | |
|---|---|---|---|---|---|---|
| Infant formulae for infants below 16 weeks of age | Soya lecithins liquid | 41.1–50.6 | 11.0–16.0 | 8.0–12.6 | 7.5–14.0 | 2.0–7.0 |
| Sunflower lecithins liquid | 39.7–47.5 | 14.8–18.2 | 12.0–15.6 | 5.4–8.1 | 1.7–4.6 | |
| Infant formulae for infants below 16 weeks of age under special medical conditions | Soya lecithins liquid | 41.1–50.6 | 11.0–16.0 | 8.0–12.6 | 7.5–14.0 | 2.0–6.0 |
PA: phosphatidic acid; PC: phosphatidylcholine; PE: phosphatidylethanolamine; PI: phosphatidylinositol; PL: phospholipids.
Further, the interested business operator stated that sunflower lecithin liquid used in infant formulae for infants below 16 weeks of age contains approximately 40–48% total phospholipids, and also has a similar phospholipid composition to commercial sunflower lecithin liquid as considered by EFSA previously. Specifically, the phosphatidylcholine content of sunflower lecithins liquid used in infant formulae for infants below 16 weeks of age is reported to range between 14.8 and 18.2% (see Table 4), whereas phosphatidylcholine levels for this type of lecithin were previously reported to range between 14.3 and 17.2% (EFSA ANS Panel, 2017; Table 2). As a result, up to 2.4% choline (i.e. 13.2% of 18.2% w/w) can be theoretically released from the amount of sunflower lecithin liquid used in infant formulae for infants below 16 weeks of age.
This information was found to be satisfactory and was considered under Section 3.6.2.5 with respect to the possible contribution to choline intake in the diet.
3.1.2.1.4. Cronobacter (Enterobacter) sakazakii in the food additive
The call for data requested:
data should be provided for foods for infants below 16 weeks of age demonstrating the absence of Cronobacter (Enterobacter) sakazakii in the food additive.
One interested business operator provided information on tests for Cronobacter (Enterobacter) sakazakii in lecithins used in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions (documentation provided to EFSA n.2). The samples were tested according to ISO 22964:2017 (ISO, 2017) using a quantitative real‐time polymerase chain reaction (qPCR) method. Cronobacter (Enterobacter) sakazakii was absent in five non‐consecutive lots of soya lecithins liquid, where the test result for each of the five samples was recorded as ‘Absent in 180 g’. Cronobacter (Enterobacter) sakazakii was also absent in five non‐consecutive lots of sunflower lecithin liquid, where the test result for each of the five samples was recorded as ‘Absent in 10 g’.
The Panel noted that in a publication on an inter‐lab trial of the ISO 22964:2017 method (De Benito et al., 2018), the sensitivity value, expressed as LOD‐50 corresponding to the level of detection for which 50% of tests give a positive result was calculated for three food items (two powdered infant formulas and one starch) with values between 0.8 and 1.1 colony‐forming unit (CFU)/sample. This sensitivity is adequate.
The Panel noted the limited number of E 322 samples reported by the interested business operator (five non‐consecutive lots).
3.1.3. Information on particular specifications in the additive for use in infant formulae
The interested business operator proposed specifications for lecithins (E 322) used in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions and compared and contrasted them with the specifications for lecithins (E 322) laid out in Commission Regulation (EU) No 231/2012 of 9 March 2012 (Table 5).
Table 5.
EU specifications and proposed revisions by the interested business operator for lecithins (E 322) used in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions (documentation provided to EFSA n. 2)
| Specification parameter | Regulation (EU) No 231/2012 | Infant formulae for infants below 16 weeks of age | Special formulae for infants of that age under special medical conditions | |
|---|---|---|---|---|
| Soya lecithins liquid | Sunflower lecithins liquid | Soya lecithins liquid | ||
| Assay | Not less than 60% of substance insoluble in acetone | Not less than 60% of substance insoluble in acetone | Not less than 60% of substance insoluble in acetone | Not less than 60% of substance insoluble in acetone |
| Description | Brown liquid or viscous semi‐liquid or powder | Brown opalescent, viscous syrup or tan to amber liquid | Yellow‐brown viscous liquid | Brown, opalescent, viscous syrup |
| Identification | ||||
| Test for phosphorous | Passes test13 | Passes test13 | Passes test13 | Passes test13 |
| Test for choline | Passes test13 | Passes test13 | Passes test13 | Passes test13 |
| Test for fatty acids | Passes test13 | Passes test13 | Passes test13 | Passes test13 |
| Purity | ||||
| Loss on drying | Not more than 2.0% (105°C, 1 h) | Not more than 0.8% | Not more than 1.5% | Not more than 0.8% |
| Toluene‐insoluble matter | Not more than 0.3% | Not more than 0.3% | Not more than 0.3% | Not more than 0.3% |
| Acid value | Not more than 35 mg KOH/g | Not more than 30 mg KOH/g | Not more than 35 mg KOH/g | Not more than 30 mg KOH/g |
| Peroxide value | Not more than 10 | Not more than 5 | Not more than 10 | Not more than 5 |
| Heavy metals | ||||
| Arsenic | Not more than 3 mg/kg | Not more than 0.2 mg/kg | Not more than 0.2 mg/kg | Not more than 0.2 mg/kg |
| Lead | Not more than 2 mg/kg | Not more than 0.25 mg/kg | Not more than 0.25 mg/kg | Not more than 0.25 mg/kg |
| Mercury | Not more than 1 mg/kg | Not more than 0.1 mg/kg | Not more than 0.1 mg/kg | Not more than 0.1 mg/kg |
KOH: potassium hydroxide.
The same interested business operator proposed lower maximum limits for As, Hg and Pb than the existing EU specifications and than those proposed in Table 2. The justification for this distinction was the differences in the sources of lecithins used; only sunflower and soya in the case of use in food for infants below 16 weeks of age (see Tables 2 and 5). In a clarification follow‐up (documentation provided to EFSA n. 4), that business operator confirmed that their members are currently using only soya and sunflower lecithins for E 322 intended for infants below 16 weeks of age but stated that they could not speak for the whole industry.
The Panel noted that different specifications are proposed for lecithins from soya and sunflower. They differ slightly on ‘loss on drying’, ‘acid value’ and ‘peroxide value’. The specifications proposed for soya lecithins used for ‘normal’ formulae and ‘formulae for special medical conditions’ are the same except a small difference in ‘description’ where ‘tan to amber liquid’ appears only once. This is of no consequence. The specifications proposed for As, Pb and Hg are 15, 8 and 10 times lower than the Regulation limit values. No specification is proposed for Cd. The specifications proposed for As, Pb and Hg are lower than the ‘Lowest achievable levels’ stated (for all E 322 uses) above (Section 3.1.2.1.1). This could be due to the fact that in Table 5, the focus is on lecithins from soya and sunflower whereas in Section 3.1.2.1.1, the values were put forward for lecithins from all sources.
Regarding other possible contaminants, the interested business operator made the observation that, in addition to specifications laid out in Commission Regulation (EU) No 231/2012 for lecithins, additional contaminants and toxic elements (e.g. mycotoxins, persistent organic pollutants, pesticides etc.) are controlled for in finished products for infants and young children by various regulations. The maximum levels established for finished products take into consideration all potential sources of these substances, including all additives and ingredients.
3.1.4. Stability of the substance and reaction and fate in food
The call for data, requested:
information on the fate and the reaction products of lecithins (E 322) in the infant formulae for infants below 16 weeks of age, as well as in special formulae for infants of that age under special medical conditions.
One interested business operator stated that: ‘Lecithins are a mixture of acetone‐insoluble phosphatides (primarily phosphatidylcholine), combined with various amounts of other substances such as triglycerides, fatty acids, and carbohydrates. These substances are considered stable under the manufacturing conditions of infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions. As a result, no significant change in composition is expected during the manufacturing process. In the finished product matrix, the lecithin ingredient itself is likely to dissociate into these individual components.’
The Panel noted that this is a viewpoint and no new information or data has been provided. There is also only limited information on stability available in the EFSA ANS Panel opinion (2017) on E 322. The Panel finds that the assumption is reasonable, that there are no concerns about stability of E 322 in infant formula.
3.2. Authorised uses and use levels
Maximum levels of lecithins (E 322) in foods for infants below 16 weeks of age are defined in Regulation (EC) No 1333/2008 on food additives, as amended. In this opinion, these levels are termed maximum permitted levels (MPLs).
According to Regulation (EC) No 1333/2008, Annex II, part E, lecithins (E 322) is authorised as a food additive in infant formulae as defined by Commission Delegated Regulation (EU) 2016/127 (FC 13.1.1) and in dietary foods for infants for special medical purposes and special formulae for infants (FC 13.1.5.1); and according to Regulation (EC) No 1333/2008, Annex III, part 5, section B, lecithins (E 322) is authorised to be added as a food additive in (preparations of) all nutrients intended to be used in foods for infants and young children, see Tables 6 and 7.
Table 6.
MPLs of lecithins (E 322) in foods for infants below 16 weeks of age according to Annex II, Part E to Regulation (EC) No 1333/2008
| Food category number | Food category name | E‐number/group | Restrictions/exception | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|---|
| 13.1.1 | Infant formulae as defined by Commission Delegated Regulation (EU) 2016/127 | E 322 | 1,000a | |
| 13.1.5.1 | Dietary foods for infants for special medical purposes and special formulae for infants | E 322 | 1,000a |
If more than one of the substances E 322, E 471, E 472c and E 473 are added to a foodstuff, the maximum level established for that foodstuff for each of those substances is lowered with that relative part as is present of the other substances together in that foodstuff.
Table 7.
MPLs of lecithins (E 322) in foods for infants below 16 weeks of age according to Annex III, Part 5, Section 5 to Regulation (EC) No 1333/2008
| E number | Name of the food additive | Maximum permitted level | Nutrient to which the food additive may be added | Food category |
|---|---|---|---|---|
| E 322 | Lecithins | For uses in nutrient preparations under the condition that the maximum level in foods mentioned in point 13.1 of Part E of Annex II is not exceeded | All nutrients | Foods for infants and young children |
3.3. Exposure data
Some food additives are authorised in the EU in infants’ formulae as defined by Commission Delegated Regulation (EU) 2016/127 (FC 13.1.1) and in dietary foods for infants for special medical purposes and special formulae for infants (FC 13.1.5.1) at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, actual use levels are required for performing a more realistic exposure assessment.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued a public call14 for technical and toxicological data on lecithins (E 322) as a food additive for uses in foods for all population groups including infants below 16 weeks of age. In response to this public call, information on the actual use levels of lecithins (E 322) in infant formulae for infants below 16 weeks of age (FC 13.1.1) and in special formulae for infants of that age under special medical conditions (FC 13.1.5.1) was made available to EFSA by the interested business operators. No analytical data on the concentration of lecithins (E 322) those foods were made available by the Member States.
3.3.1. Reported use levels in food categories 13.1.1 and 13.1.5.1 according to Annex II
The interested business operators provided EFSA with use levels (n = 20) of lecithins (E 322) used in FC 13.1.1 and FC 13.1.5.1 (documentation provided to EFSA n. 2 and 3). For infant formulae as defined by Commission Delegated Regulation (EU) 2016/127 (FC 13.1.1), typical and maximum levels (n = 16; 15 soya lecithins type and one sunflower lecithin type) ranged between 129 and 1,000 mg/L. For dietary foods for infants for special medical purposes and special formulae for infants (FC 13.1.5.1), typical and maximum levels (n = 4, all soya lecithins type) ranged between 652 and 990 mg/L.
3.3.2. Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 3 million food and beverage products of which more than 1,100,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 24 out of its 27 member countries, Norway and UK presented in the Mintel GNPD.15
For the purpose of this Scientific Opinion, Mintel's GNPD16 was used for checking the labelling of food and beverage products and food supplements for lecithins (E 322) within the EU's food market as the database contains the compulsory ingredient information on the label.
According to Mintel's GNPD, lecithins (E 322) was labelled on 229 baby food products (i.e. baby formulae for infants between 0 and 6 months) between January 2015 and June 2020. This represents 65% out of the total number of baby food products in the GNPD database during the same period of time.
3.4. Exposure estimates for infants below 16 weeks
Exposure to lecithins (E 322) from its uses as a food additive in formulae for infants below 16 weeks was estimated. This scenario is based on the recommended consumption levels from SC Guidance (EFSA Scientific Committee, 2017). This guidance ‘recommends values of 200 and 260 mL [formula]17 /kg bw per day as conservative mean and high level consumption values to be used for performing the risk assessments of substances which do not accumulate in the body present in food intended for infants below 16 weeks of age’. These recommended consumption levels correspond to 14‐ to 27‐day‐old infants consumption.
3.4.1. Dietary exposure to lecithins (E 322) from infant formulae
Table 8 summarises the estimated exposure to lecithins (E 322) from its use as a food additive both in FC 13.1.1 and FC 13.1.5.1 for infants below 16 weeks of age.
Table 8.
Dietary exposure to lecithins (E 322) in foods for infants below 16 weeks of age according to Annex II, Part E to Regulation (EC) No 1333/2008 (in mg/kg bw per day)
| Infants (< 16 weeks of age) | |
|---|---|
| Regulatory maximum level exposure assessment scenario (1,000 mg/kg) | |
|
200 |
|
260 |
| Refined estimated exposure assessment scenario | |
| Scenario using maximum use level reported by industry (1,000 mg/kg) for both FC 13.1.1 and FC 13.1.5.1 | |
|
200 |
|
260 |
| Scenario using mean use level reported by industry (310 mg/kg) for FC 13.1.1 | |
|
62 |
|
81 |
| Scenario using mean use level reported by industry (900 mg/kg) for FC 13.1.5.1 | |
|
180 |
|
234 |
The refined estimated exposure assessment scenario using the maximum use level reported by industry (the result is equal to the regulatory maximum level exposure assessment scenario) was used in the assessment, the mean occurrence scenario is reported and indicates that there are products on the market giving lower exposure levels.
3.4.2. Uncertainty analysis
In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainty have been considered and summarised in Table 9.
Table 9.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: one reference point only to estimate exposure during the period of up to 16 weeks of age | +/– |
|
Regulatory maximum level exposure assessment scenario: – exposure calculations based on the MPL according to Annex II Part E to Regulation (EC) No 1333/2008 |
+ |
|
Refined exposure assessment scenarios: – exposure calculations based on the maximum levels (reported use from industry) |
+ |
| – exposure calculations based on the mean levels (reported use from industry) | +/– |
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Lecithins (E 322) is authorised in FC 13.1.1 and FC 13.1.5.1 according to Annex II, Part E to Regulation (EC) No 1333/2008 and in foods for infants (FC 13.1) according to Annex III, Part 5, Section B of the same Regulation (EC) No 1333/2008. For the regulatory maximum level exposure assessment scenario an overestimation may result in some cases. However, based on the assumption that carers of children would be brand loyal to an infant formula (FC 13.1.1) or infant formulae for special medical purposes (FC 13.1.5.1), this exposure assessment scenario (Table 9) would in general result in a reliable estimation of exposure.
It should be noted that the use according to Annex III to Regulation No 1333/2008 was taken into account in the regulatory maximum level exposure assessment scenario. The maximum level authorised according to the Annex III is ‘For uses in nutrient preparations under the condition that the maximum level in foods mentioned in point 13.1 of Part E of Annex II is not exceeded’.
3.5. Proposed revision to existing EU Specifications for lecithins (E 322)
The Panel noted that the occurrence data submitted by the interested business operators are substantially lower than the current limits in the EU specifications. The Panel considered that the maximum limits in the EU specifications for toxic elements should be established based on actual levels measured in the food additive. If the European Commission decides to revise the current limits in the EU specifications, the estimates of toxic elements intake as described below could be considered.
One interested business operator provided lowest achievable levels for As, Cd, Hg and Pb for the additive intended for use in food for the general population (including infants below 16 weeks of age; documentation provided to EFSA n. 1), for the purpose of defining appropriate specifications. A second interested business operator proposed lower maximum limits for As, Hg and Pb for E 322 when used in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions (documentation provided to EFSA n. 2). The justification for this distinction was the differences in the sources of lecithins used; only sunflower and soya in the case of use in food for infants below 16 weeks of age (see Tables 2 and 5). In a clarification follow‐up (documentation provided to EFSA n. 4), that business operator confirmed that their members are currently using only soya and sunflower lecithins for E 322 intended for infants below 16 weeks of age but stated that they could not speak for the whole industry.
The Panel agreed to consider these proposed values as a starting point to characterise the risk of exposure to toxic elements derived from the consumption of the food additive. The potential exposure to these toxic elements can be calculated by:
assuming that the contamination of the additive may be up to the lowest achievable levels (1 (As), 0.25 (Cd), 0.5 (Pb) and 0.5 (Hg) mg/kg), as proposed by an interested business operator for all age groups (documentation provided to EFSA n. 1),
assuming that the contamination of the additive may be up to the maximum limits (0.2 (As), 0.25 (Pb) and 0.1 (Hg) mg/kg), as proposed by another interested business operator for the EU specifications for infants below 16 weeks of age (documentation provided to EFSA n. 2,4),
and then by calculation pro rata to the dietary exposure to the food additive itself for the two different groups.
With regard to the dietary exposure to the food additive for infants below 16 weeks of age, the Panel considered the refined estimated exposure assessment scenario based on maximum use levels (95th percentile), at 260 mg/kg bw per day (see Table 8). For the general population, the exposure resulting from the refined brand‐loyal scenario (95th percentile) was highest for children aged 3–9 years, at 187 mg/kg bw per day (see EFSA ANS Panel, 2017).
The maximum limits for toxic elements referred to above could result in an exposure which can be compared with the following reference points, or health‐based guidance values (HBGVs), for the four toxic elements: a BMDL01 of 0.3–8 μg/kg bw per day for arsenic (EFSA CONTAM Panel, 2009a,b), a total weekly intake (TWI) of 2.5 μg/kg bw for cadmium (EFSA CONTAM Panel, 2009a,b), a BMDL01 of 0.5 μg/kg bw per day for lead (EFSA CONTAM Panel, 2010) and a TWI of 4 μg/kg bw for mercury (EFSA CONTAM Panel, 2012).
The outcome of such an exercise (see Tables 10 and 11) illustrates the health impact that would result if the proposed maximum limits for toxic elements were to be used.
Table 10.
Risk assessment for toxic elements based on the lowest achievable levels in lecithins (E 322) for use in food for all age groups as proposed by an interested business operator (Documentation provided to EFSA n. 1)
| Exposure to the additive (mg/kg bw per day) | MOS/MOE for As | MOS/MOE for Pb | % of the TWI for Cd | % of the TWI for Hg |
|---|---|---|---|---|
| 1 mg/kg | 0.5 mg/kg | 0.25 mg/kg | 0.5 mg/kg | |
| 187* | 1.6–43 | 5.4 | 13% | 16% |
| 260 (see Table 8)** | 1.2–31 | 3.9 | 18% | 23% |
Exposure to E 322 in children aged 3–9 years (refined, brand‐loyal scenario, 95th percentile), the worst‐case exposure scenario for the population above 16 weeks of age (see EFSA ANS Panel, 2017).
Exposure to E 322 in infants below 16 weeks of age (high‐level consumption, 95th percentile, 260 mL/kg bw per day).
Table 11.
Risk assessment for toxic elements based on the maximum limits/lowest achievable levels for toxic elements in lecithins (E 322) for use in food for infants below 16 weeks of age
| Exposure to the additive (mg/kg bw/day) | MOS/MOE for As | MOS/MOE for Pb | % of the TWI for Cd | % of the TWI for Hg |
|---|---|---|---|---|
| 0.2 mg/kg# | 0.25 mg/kg# | 0.25 mg/kg** | 0.1 mg/kg# | |
| 260 (see Table 8)* | 5.8–154 | 7.7 | 18% | 5% |
High‐level consumption (95th percentile, 260 mL/kg bw per day).
According to the lowest achievable levels in lecithins (E 322) for use in food for all age groups as proposed by an interested business operator (Documentation provided to EFSA n. 1), see Table 2.
Based on the maximum limits for toxic elements in lecithins (E 322) as proposed by an interested business operator using only soya and sunflower as sources (Documentation provided to EFSA n. 2).
The resulting figures show, in both exposure scenarios described, that the exposure to toxic elements from the consumption of E 322 is substantial. The Panel noted that the MOS/MOE for arsenic and lead is very low, considering that for lead, the reference point is based on perturbation of intellectual development in children (who have the highest exposure), and for arsenic, the reference point is based on carcinogenicity. It is further noted that the exposure to toxic elements from the consumption of E 322 in infants below 16 weeks of age based on the existing EU specifications would be even higher and would result in MOS/MOE of 0.38–10 for As and 0.96 for Pb and in % of the TWI of 46% for Hg. Similarly, for the remaining populations groups, the resulting MOS/MOE would be 0.53–14 for As and 1.34 for Pb and the resulting % of the TWI would be 33% for Hg. This supports the ANS Panel recommendation to decrease the current maximum limits set for arsenic, lead and mercury, considering also other sources of exposure to these toxic elements, and to introduce a maximum limit for cadmium.
The Panel emphasises that setting the maximum limits for toxic elements in the specifications is in the remit of risk management.
With regard to the levels of residual proteins in commercial samples of E322 (expressed as percentage of toluene‐insoluble matter), and the related lowest technologically achievable levels, no analytical data were provided by the interested business operators. In the absence of data, the Panel assumes that protein could be as high at 0.3% w/w as currently laid down in EU specifications for E 322. The Panel considers that the existing EU specification on the levels of proteins should be retained, i.e. percentage of toluene‐insoluble matter. This is based on the data on post marketing surveillance provided by the interested business operator (Documentation provided to EFSA n. 2,3) showing rare cases of food allergy/intolerance‐related symptoms.
On the basis of the information provided, the Panel consider that criteria should be included for Cronobacter (Enterobacter) sakazakii.
Overall, based on the analytical data provided by the interested business operators in response to EFSA call for data (footnote) (Documentation provided to EFSA n. 1 and 2) and the relative above considerations, the Panel recommends the following revisions of the existing EU specifications for Lecithins (E 322) as listed in Table 12.
3.6. Biological and Toxicological data
3.6.1. Previous evaluation by ANS Panel (2017)
The following text (in italics) is from the opinion published in 2017 (EFSA ANS Panel, 2017). New information and assessments related to the specific age group below 16 weeks of age are added in the following paragraphs.
Absorption, distribution, metabolism and excretion
Overall, studies using radiolabelled phosphatidylcholine in animals and humans clearly indicated that, following oral administration, phosphatidylcholine is absorbed unchanged or as lysophosphatidylcholine or choline after intestinal hydrolysis. In intestinal mucosa cells, lysophosphatidylcholine would be reacylated into phosphatidylcholine or hydrolysed to glycerophosphocholine and free fatty acids. The fatty acids would be further utilised for the reassembly of triacylglycerides and phosphatidylcholine found in the chylomicrons. In humans, the absorbed phosphatidylcholine would be incorporated preferentially into the HDL fraction of plasma. Peak levels of phosphatidylcholine in blood are reached within 6 h.
Besides the intestinal wall, the major target organ for distribution and metabolism of lecithins is the liver. Only minor amounts of radioactivity were excreted via urine and faeces demonstrating that the administered lecithins would undergo metabolism as for endogenous phospholipids. From the current database, the Panel noted that only minor levels of choline labelling radioactivity were detected in the brain. In humans, dietary lecithins are known to be hydrolysed by phospholipases to liberate choline, which is rapidly absorbed by a carrier‐mediated saturable transport system and appears in plasma predominantly as free choline. Consequently, an increased plasma‐free choline concentration has been described as a consequence of increased dietary intake of lecithins. Moreover, a significant increase in breast milk concentrations of free choline was observed in lactating women receiving a phosphatidylcholine supplement in comparison with the placebo group.
Acute, subchronic, genotoxicity, chronic, developmental, reproductivity and neurotoxicity studies
The acute toxicity of lecithins (E 322) in mice, rats and rabbits is low. The Panel noted that in these studies the test substance is not always characterised.
Subchronic toxicity studies in rats and dogs did not report any adverse effect, even at the highest doses tested (3,750 mg EPL (see Section 3.1.1 )/kg bw per day, 1,000 mg soya phosphatidylinositol or EPL 18 /kg bw per day in rats and dogs, respectively, and 5,460 mg lecithins/kg bw per day in rats).
The Panel considered the available genotoxicity data on lecithins (E 322) to be sufficient to conclude that there is no concern with respect to genotoxicity.
Chronic toxicity studies in rats did not report any adverse effects, even at the highest dose tested (3,750 mg EPL/kg bw per day). No carcinogenic effects were reported in rats, even at the highest dose tested (1,470 and 2,280 mg soya lecithin/kg bw per day in males and females, respectively) for 2 years.
The Panel considered that no adverse effects were observed in the developmental toxicity studies performed in mice, rat and rabbits up to the highest dose tested. However, the Panel noted that no reproductive toxicity studies were available.
Against the background that choline availability as a precursor of acetylcholine may possibly influence neurotransmitter systems, several neurodevelopmental toxicity studies were conducted with lecithin. The Panel noted that the neurodevelopmental toxicity studies of Gozzo et al. (1982) in mice and the studies of Bell and co‐workers in rats (Bell and Lundberg, 1985; Bell and Slotkin, 1985; Bell et al., 1986) had several limitations, such as the number of pregnant animals, the number of litters, and the sex of the pups in the control and treated groups not being described in sufficient detail. In addition, the length of gestation and pup weight at birth, as well as during the tests, were not presented in all publications. Therefore, the Panel concluded that the relevance of the studies is limited but, at concentrations of 5% soya lecithin and higher in the diet during the gestation, lactation and the post‐weaning period, there were indications for alterations in the development of the brain.
3.6.2. Newly available data
3.6.2.1. Absorption, distribution, metabolism and excretion
No new data were submitted by the interested business operators or found in the provided literature search (documentation provided to EFSA n. 1 and 2).
The Panel considered that the physiological processes involved in absorption, distribution, metabolism and excretion of lecithins described in the opinion published in 2017 (EFSA ANS Panel, 2017) are relevant also for infants below 16 weeks of age.
3.6.2.2. Toxicological data
None of the studies identified in the literature by the Panel and submitted by the interested business operators aimed to investigate toxicity of lecithins as food additive (E 322) in juvenile animals. The studies did not follow any specific OECD test guideline and were not performed according to the good laboratory practice (GLP) principles.
Effects of soya lecithins during the pre‐ and post‐natal period
A study evaluating effect of diet supplementation with soya lecithins on growth and reproductive performance during summer and winter was conducted in rabbits (Attia et al., 2018). FV‐line female rabbits (6 months old, nulliparous at the start of the study; n=10/group) were kept on a pelleted diet added 0% (control), 0.5%, 1% or 1.5% soya lecithins (equivalent to 0 and approximately 150, 300 or 450 mg/kg bw per day;) starting one month before mating until day 35 of the third lactation. The does were mated three times during each season (summer or winter) with males kept on the control diet. Pregnancy was checked on day 10 after pairing. Non‐pregnant does were mated until pregnancy was achieved. Body weight of does was recorded immediately after mating and after parturition. Feed intake was measured from mating up to the end of the first week after parturition (5 weeks), in each of pregnancy periods. Milk production was measured from birth to the 35th day after parturition. Reproductive performance was evaluated by receptivity rate, conception rate and the number of matings required to induce pregnancy at the 10th day after mating. Live litter size and pup weight at birth and at 35 days of age were reported. No does died during the study. Body weight of does on diet added 1% or 1.5% soya lecithins was statistically significantly higher and daily feed intake statistically significantly lower than in the controls. In comparison to the group on the control diet, milk production was statistically significantly higher in groups receiving diets with 1% and 1.5% soya lecithins at all time points measured. Receptivity and conception rates were statistically significantly higher for does on diet added 1% or 1.5% soya lecithins as compared with controls. The number of matings was similar in all groups fed the soya lecithins added diets and was statistically significantly lower than in the controls. The litter sizes at birth and at the weaning were statistically significantly greater in all soya lecithins dietary groups than in the control group. At the same time, the litter sizes were similar for 1% or 1.5% soya lecithins dietary groups being statistically significantly greater than in 0.5% soya lecithin dietary group. Statistically significantly higher than in controls pup body weights were recorded for all soya lecithins dietary groups at birth (+8%, +15% and +15% for 0.5, 1 and 1.5% SL groups, respectively) and for 1% (+7%) and 1.5% (+8%) soya lecithins dietary groups at the end of weaning. The Panel noted that these findings have no toxicological relevance. The interaction of soya lecithins addition to the diet with a season influenced the size of the offspring at birth and at weaning for 1% and 1.5% soya lecithins in the diet. There was no effect of interaction between soya lecithins addition to the diet and the season on body weight and feed intake of does but a statistically significant effect was recorded for the size of offspring at birth and at weaning, and on composition of milk regarding the content of saturated and unsaturated fatty acids. The Panel considered that dietary doses up to 1.5% soya lecithins (equivalent to 450 mg/kg bw per day) had no adverse effect on body weight and survival of does, milk production on reproductive indices (receptivity and conception rates and number of services), litter size and pup weights.
A study evaluating effect of soya lecithins and similar products on reproductive performance was performed in pregnant sows (Cao et al., 2018). Pregnant binary hybrid sows (approximately 30 in each group, body weight not reported) were fed basal diet (control) or received 50 g/animal per day of soya lecithins or two other products in basal diet (twice a day 25 g) from day 100 of gestation until day 7 after birth. Daily feed intake of sows from soya lecithins group (3.64 kg ± 0.03) was slightly but statistically significantly increased compared to that in the controls (3.54 kg ±0.03). The following reproductive indices were statistically significantly higher in the soya lecithins group as compared to the controls: the litter size (11. 31 ± 0.23 vs 10.69 ± 0.27), life litter size (10.71 ± 0.22 vs. 10.00 ± 0.26) and qualified litter size (piglets with birth weight greater than 0.8 kg) (10.32 ± 0.22 vs. 9.56 ± 0.26). Piglet birth weight in soya lecithins (1.48 kg ± 0.02) and control (1.45 kg ± 0.02) groups was similar.
Another study in pregnant sows evaluated effects of soya lecithins (SL) on reproductive performance, colostrum and milk composition and immunoglobulin levels in milk and plasma from sows and offspring (Shi et al., 2019). Four groups of pregnant sows (crossbred Large White × Landrace, n = 12/group) were fed for 28 days i.e. from the 107th day of gestation until day 21 of lactation (weaning) either a control diet with 3% soybean oil (SO, Habin Gushi A&H, digestive energy 36.31 MJ/kg) (equivalent to 1,200 mg SO/kg bw per day) or the diets in which SO was replaced by 1%, 2% or 3% SL (Habin Gushi A&H, digestive energy 306.29 MJ/kg) (equivalent to 400, 800 or 1,200 mg SL/kg bw per day). Replacements of SO with SL had no statistically significant effects on body weight, body weight gain and mean daily feed intake of sows. Reproductive parameters like number of piglets born/alive per litter, number of piglets at weaning per litter, survival rate of piglets, piglet weight at birth were comparable between all four groups. The body weights and mean body weight gain of piglets at weaning were statistically significantly higher (+16 and +20%, respectively) in 3% SL group compared with the control group (3% SO). The composition of colostrum and milk with regard to the content of lactose, fat, protein and total solids was comparable between all groups, but statistically significant differences with the control group were recorded in immunoglobulin concentrations. In colostrum, IgA was higher in all SL groups and IgM in 2% and 3% SL groups. In milk, IgA was higher in 3% SL group. Content of phosphatidylcholine and total phospholipids was statistically significantly higher and fat globules were smaller in milk from 2% and 3% SL groups compared with the control group. The addition of SL did not produce toxicologically relevant changes in the plasma levels of immunoglobulins in sows and piglets. The Panel noted that this study provides limited evidence that lecithins as food additive have no adverse effects on the parameters measured in the piglets.
Effects of egg lecithins on immunological parameters
A study investigating effects of egg lecithins (PC, lipid‐soluble phosphatidylcholine, egg lecithins; no further data on the origin of the tested lecithins were provided) and free choline (FC, choline bitartrate) in the maternal diet on the development of the offspring's immune system was performed in rats (Lewis et al., 2016). Pregnant Sprague‐Dawley dams (n = 10) were kept on a standard diet from the 14th gestation day until 14–48 h after parturition. Thereafter, the dams were divided in two groups (five in each) and fed ad libitum either a diet containing PC or FC. Both diets provided 1 g/kg diet (0.1%; equivalent to 50 mg/kg bw per day) of total choline and had a similar fat content (wt/wt %) and fatty acid composition. The litter size was standardised at birth to 10 pups (five males and five females when possible) per dam. At weaning (day 21), two pups (sex not described) from each litter were weighed, killed (CO2 asphyxiation), liver and spleen were weighed, splenocytes harvested. Stomach content, liver, splenocytes and plasma were used to measure their choline concentration. Immune cell subsets in splenocytes were identified. Cytokine concentration was measured in plasma. Splenocytes were used for assays of ex vivo cytokine production after stimulation with concanavalin A (ConA) or lipopolysaccharide (LPS). The daily feed intake of dams on the PC or FC diets during the weaning was 40 g/animal. In pups from PC or FC dietary groups body and organ weights, intestinal length, relative number of splenocytes (number of splenocytes per gram of spleen) and total choline concentrations in the stomach or plasma were comparable. PC concentrations in plasma and spleen of pups from PC fed dams were higher than in the pups from FC fed dams. In pups from the PC fed dams as compared to pups from FC fed dams, the differences in splenocyte immune cell phenotype included a statistically significantly lower proportion of total B cells (Ig+ cells, OX12+), proportion of activated macrophages (CD68+CD11b/c+) and total cells expressing major histocompatibility complex class II (OX6+). In the ex vivo assay splenocytes from pups of PC group produced 54% more interleukin (IL)‐2, 163% more IL‐6 and 107% more IFN‐γ than splenocytes from pups of FC group after ConA stimulation and 110% more IL‐6 and 43% more TNF‐α than those from pups of FC group after stimulation with LPS. The large variations and the absence of a well‐defined control group preclude a clear conclusion on this study.
Effect of egg phospholipids and soya lecithins during post‐natal period
Effect on general health of algal/fungal triglyceride oils (TG) or egg phospholipids (PL) as sources of long‐chain polyunsaturated fatty acids was investigated in piglets when administered from postnatal day (PND) 1–16 (Mathews et al., 2002). One‐day‐old piglets (no information on strain or sex) from 13 sows were assigned to one of the following dietary treatment groups:
piglet formula without arachidonic acid (AA) or docosahexaenoic acid (DHA (control, n = 10),
piglet formula from egg phospholipids (PL, n = 10, 85 g/kg formula),
piglet formula from the fungal and algal triglyceride oils (TG, n = 10),
piglet formula from the fungal and algal triglyceride oils with additional choline (10.6 g/kg formula), cholesterol (4.5 g/kg) and soya lecithins (34 g/kg)) (TG + PL, n = 10) to match the PL formula,
piglet formula deficient in essential fatty acids (EFA) (EFAD, n = 8). An additional control consisted of 13 sow‐reared piglets from two litters (a natural fed control).
According to the authors, the control, PL, TG and TG + PL formulas contained adequate amounts of linoleic (LA) and α‐linolenic (LN) acids. PL, TG and TG + PL formulas contained AA and DHA in the same amounts.
Body weight, body weight gain, daily feed intake, feed efficiency, ALT, BUN and cholesterol plasma levels, liver weight or vacuolation of cytoplasm in hepatocytes did not differ between the control formula, PL, TG and TG + PL groups. In PL group, aspartate aminotransferase (AST) was statistically significantly higher than in TG + PL group (being comparable to that in the control formula and TG groups) and plasma glucose concentration was statistically significantly lower than in the control formula, TG, TG + PL groups. The PL group had a lower ileal apparent dry matter digestibility in comparison to the control formula or TG groups. Jejunal or ileal lactase specific activity was comparable between control formula, PL, TG, TG + PL groups. Jejunal villi height was comparable between control formula, PL, TG, TG + PL groups, but ileal villi height in PL group was lower than in the control formula group. Jejunal and ileal villi width and crypt depth were comparable between control formula PL, TG, TG + PL groups. Apparent dry matter digestibility was 10% greater in controls, TG and TG + PL groups compared with PL piglets. The Panel considered that this study did not show toxicologically relevant effects on growth, feed intake and feed efficiency, clinical chemistry, liver weight or lipid and glycogen accumulation in piglets fed with formulae containing powdered soya lecithins (no estimate for the lecithins intake in mg/kg bw per day can be made) compared to feeding a control formula.
3.6.2.3. Clinical data
No clinical studies relevant for the safety evaluation of lecithins (E 322) in infants below 16 weeks were submitted by interested business operators or found in a literature search.
3.6.2.4. Post‐marketing surveillance data
Post‐marketing data were obtained from one interested business operator in the form of a summary table of adverse events of two different companies (documentation provided to EFSA n. 2 and 3). The first company provided data collected between November 1, 2017 and October 31, 2019 on lecithin‐containing formula products available on the market in the United States, during which period several millions of individual items were sold. From 450 reported adverse events, the terms most often mentioned were gas (178 events), spitting up (119 events), fussy (106 events), crying (52 events), vomiting (41 events), constipation (39 events), refused (34 events), stomach upset (32 events), diarrhoea (30 events) and discomfort (24 events). The second company provided data collected in 2016 and 2017 on lecithin‐containing products. Among several millions of individual items sold, between the 44 adverse events reported, 39 were described as ‘unspecific nutritional disorders (e.g. diarrhoea, vomiting, obstipation)’ and 5 as ‘allergic reaction/intolerance’.
The Panel considered that the relationship between the events and the intake of lecithins (E 322) is not confirmed and that the post‐marketing surveillance data do not show specific alerts except for the very rare symptoms of ‘allergic reaction/intolerance’.
3.6.2.5. Comparative data on the content of lecithins in human milk and in infant formula
Total Phospholipids in maternal milk
The interested business operators collected several studies reporting on maternal milk levels of total phospholipids in mg/L (Hibberd et al., 1982; Bitman et al., 1986; Sala‐Vila et al., 2005; Garcia et al., 2011, 2012; Giuffrida et al., 2013; Thakkar et al., 2013; Ma et al., 2017; Cheong et al., 2018; Jiang et al., 2018; Ingvordsen Lindahl et al., 2019; McJarrow et al., 2019; Wei et al., 2019; Wu et al., 2019; documentation provided to EFSA n. 2 and 3).
The studies were performed in various countries (China, France, Germany, Ireland, Malaysia, Singapore, Spain, United Arab Emirates, the U.S. and the UK) and the participating women were not a random selection of the population of those countries. Because of various factors, the data are heterogeneous and variable (influence of diet on milk composition; Ballard and Morrow, 2013). Various analytical methods were used for separation and quantification introducing variability.
From the original study results, the interested business operators calculated 1) the average of the reported means; 2) the maximum reported mean level; 3) median levels; and 4) the maximum reported level (documentation provided to EFSA n. 3). The interested business operators presented data on colostrum, transitional milk and mature milk; however, this assessment focused on mature milk. These values are presented in Appendix B.
Phospholipids and choline levels in infant formulae
According to data provided by SNE members and confirmed by ELMA, lecithins (E 322) used in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions are composed of 39.7–50.6% total phospholipids, see Appendix B (documentation provided to EFSA n. 2 and 3).
Choline levels in maternal milk
Mean concentration and SD of choline in maternal milk were also calculated based on the data from the literature (Documentation provided to EFSA n. 3), see Appendix B.
Contents of phospholipids and choline in human milk and in formula
The content of phospholipids was calculated from the lecithins content in the formula, using the data given in Table 4 (see Table 13). The levels of phospholipids in the formula and the level of total phospholipids in mature milk are not directly comparable. The Panel focused on the comparison of the total choline.
Table 13.
Contents of phospholipids and choline in human milk and in formula
| Mature Milk | Formula using soya lecithins | Formula using sunflower lecithins | |
|---|---|---|---|
| Total phospholipids mg/L | 248 ± 91# | 239.7 ± 216.7* | 202.3 ± 182.9** |
| Total choline (mg/L) | 138.3 ± 15.9# | 225 ± 26# , *** | |
| Choline added as nutrient (mg/L) | 166 ± 37# | ||
| Choline from lecithins (mg/L) | 12 ± 8# | ||
| Choline from other ingredient sources (mg/L) | 50 ± 17# | ||
Calculated by the Panel from lecithins content in formula using a factor of 0.506 (see Table 4).
Calculated by the Panel from lecithins content in formula using a factor of 0.475 (see Table 4).
Includes choline added as nutrient, from lecithins and from other ingredients sources.
Calculated by the interested business operator (Documentation provide to EFSA n. 2, 3).
Comparison of the levels of choline in human milk and in infant formula
When comparing the total choline content of formula with that of human milk a difference is observed. Nearly 75% of the total choline in the formula is added as nutrient whereas only around 5% of total choline are present due to release from lecithins (E 322) and additional 22% from other ingredient sources (Table 13).
3.7. Discussion
The Panel considered it feasible to amend the EU specifications based on the information submitted in response to the call for data. This refers to lowering existing limits for toxic elements, as well as introducing new specifications for cadmium and microbiological criteria for the food additive (see Table 12).
No new data on absorption, distribution, metabolism and excretion of lecithins were submitted by the interested business operators or found in a literature search. The Panel considered that the physiological processes involved in absorption, distribution, metabolism and excretion of lecithins described in the opinion published in 2017 (EFSA ANS Panel, 2017) are present and taking place also in infants below 16 weeks of age.
In the re‐evaluation of lecithins (EFSA ANS Panel, 2017), the acute toxicity of lecithins (E 322) in mice, rats and rabbits was considered as low. In addition, ANS Panel concluded that subchronic toxicity studies in rats and dogs did not report any adverse effect, even at the highest doses tested (3,750 mg EPL/kg bw per day, 1,000 mg soya phosphatidylinositol or EPL/kg bw per day in rats and dogs, respectively, and 5,460 mg lecithins/kg bw per day in rats). The ANS Panel considered the available genotoxicity data on lecithins (E 322) to be sufficient to conclude that there is no concern with respect to genotoxicity. Chronic toxicity studies in rats did not report any adverse effects, even at the highest dose tested (3,750 mg EPL/kg bw per day). No carcinogenic effects were reported in rats, even at the highest dose tested (1,470 and 2,280 mg soya lecithins/kg bw per day in males and females, respectively) for 2 years. The ANS Panel considered that no adverse effects were observed in the developmental toxicity studies performed in mice, rat and rabbits up to the highest dose tested. However, the ANS Panel noted that no reproductive toxicity studies were available.
The main safety concern identified in the EFSA Opinion (EFSA ANS Panel, 2017) regarded indications for alterations in the development of the brain at concentrations of 5% soya lecithins and higher in the diet during the gestation, lactation and the post‐weaning period in the neurodevelopmental toxicity studies of Gozzo et al. (1982) in mice and in the studies of Bell and co‐workers in rats (Bell and Lundberg, 1985; Bell and Slotkin, 1985; Bell et al., 1986). It has to be noted, however, that these studies had limitations which precluded firm conclusions by the ANS Panel.
The newly provided studies (Attia et al., 2018; Cao et al., 2018; Shi et al., 2019; Lewis et al., 2016; Mathews et al., 2002) did not contribute to the assessment of potential neurodevelopmental effects because this endpoint has not been addressed in the studies. Specific studies in infants were not provided and the data from the post‐marketing surveillance studies do not allow to make firm conclusions on the safety of lecithins (E 322) in infant formula.
For the reason that choline is a precursor of the neurotransmitter acetylcholine the ANS Panel considered appropriate to address the safety of lecithins (E 322) as food additive in infant formula used in infants below the age of 16 weeks by comparing the exposure by infant formula with the adequate intake (AI) set by the EFSA NDA Panel (EFSA NDA Panel, 2016a). In following the proposed approach, the FAF Panel considered that it is appropriate to compare the concentration of choline in human milk with that in the formula, because the AI is based on the choline content of human milk.
From the information provided by the interested business operator, the mean content of total choline in formula (225 mg/L) is around 62% higher than that in human milk (138 mg/L). However, the mean content of choline released from lecithins used as a food additive (E 322) in infant formula (12 mg/L) represents only 8.7% of the mean content of total choline in mature human milk. The same conclusion applies at the MPL of 1,000 mg/L for E 322, in accordance with Annex II, Part E to Reg 1333/2008, which could contribute to 21 mg/L of choline (see Section 3.1.2.1.3). Most (nearly 75%) of the total choline content in infant formula is derived from the addition of choline as nutrient.
The minimum and maximum total content of choline in infant formula is regulated (Regulation 2016/127) at, respectively, 25 and 50 mg/100 kcal, i.e. around 175–350 mg/L. (assuming a maximum energy content for infant formula of 700 kcal/L). Therefore, the mean content of choline released from lecithins in infant formula represents only 6.9% and 3.4% (see Table 13) of the minimum and maximum content of choline regulated in infant formula. The assessment of choline added as nutrient is not in the remit of this Opinion.
According to Annex II, Part E of Regulation (EC) No 1333/20082, lecithins (E 322) are permitted in food categories 13.1.1 ‘infant formulae’ and 13.1.5.1 ‘dietary foods for infants for special medical purposes and special formulae’ (FSMP) for infants at a maximum level of 1,000 mg/L. Lecithins (E 322) are also authorised as food additive in (preparations of) all nutrients intended to be used in foods for infants and young children according to Annex III, Part 5, Section B of Regulation (EC) No 1333/2008 provided that the maximum level defined in Annex II, Part E is not exceeded.
Based on data submitted by SNE members, lecithins (E 322) are used in infant formulae for infants below 16 weeks of age and in special formulae for infants of that age under special medical conditions at levels of up to 1,000 mg/L, and are thus in compliance with maximum permitted levels in the EU.
For infants below 16 weeks of age consuming infant formulae (FC 13.1.1) or infant food for special medical purpose (FSMP) (FC 13.1.5.1), in the regulatory maximum level exposure assessment scenario, mean exposure to lecithins (E 322) was estimated to be 200 mg/kg bw per day while the high level was estimated at 260 mg/kg bw per day. In the refined scenario, exposure was the same using the maximum reported levels by the interested business operators (for both FC 13.1.1 and 13.1.5.1); while using the mean reported levels by the interested business operators, refined exposure is estimated at 62 mg/kg bw per day at the mean (81 mg/kg bw per day at the p95) for FC 13.1.1 and at 180 mg/kg bw per day at the mean (234 mg/kg bw per day at the p95) for FC 13.1.5.1.
4. Conclusions
Taking into account the data submitted by interested business operators and the considerations from the Panel, a revision of the existing EU specifications for lecithins (E 322) has been recommended (see Table 12).
The choline released from lecithins (E 322) is a minor contributor (around 8.7% as the mean) of the total content of choline of the formula and does not raise concern at the current use levels including the MPL for lecithins (E 322). Most of the choline in the formula is due to the choline added as a nutrient. In addition, it can be stated that the mean content of choline is in the range of the adequate intake level (AI). The Panel concluded that the intake of lecithins (E 322) as a food additive in infant formula belonging to FC 13.1.1 or in food for special medical purposes belonging to FC 13.1.5.1 does not raise safety concerns up to the MPL of lecithins (E 322).
5. Recommendation
The Panel recommends the European Commission to consider revising the current specifications for the food additive lecithins (E 322) on the basis of the information provided and based on the considerations of the Panel, see Table 12.
6. Documentation as provided to EFSA
European Lecithin Manufacturers Association (ELMA), 2019. Submission of data in response to the call for technical and toxicological data on lecithins (E 322) for uses as a food additive in foods for all population groups including infants below 16 weeks of age. Submitted on December 2019.
Specialised Nutrition Europe (SNE), 2019. Submission of data in response to the call for technical and toxicological data on lecithins (E 322) for uses as a food additive in foods for all population groups including infants below 16 weeks of age. Submitted on December 2019.
Specialised Nutrition Europe (SNE), 2020. Clarification on the data in response to the call for technical and toxicological data on lecithins (E 322) for uses as a food additive in foods for all population groups including infants below 16 weeks of age. Submitted on June 2020.
Specialised Nutrition Europe (SNE), 2020. Clarification on the data in response to the call for technical and toxicological data on lecithins (E 322) for uses as a food additive in foods for all population groups including infants below 16 weeks of age. Submitted on July 2020.
Abbreviations
- ADI
acceptable daily intake
- AI
adequate intake level
- ANS
Panel EFSA Panel on Food Additives and Nutrient Sources added to Food
- bw
body weight
- CAS
Chemical Abstract Service
- DBPCFC
double‐blind placebo‐controlled food challenge
- EPL
essential phospholipid
- FAF Panel
Panel on Food Additives and Flavourings
- FAO/WHO
Food and Drug Organisation/World Health Organisation
- FC
Food category
- FSMP
Food for special medical purposes
- GLP
Good Laboratory Practices
- IOM
Institute of Medicine
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- Mintel
GNPD Mintel's Global New Products Database
- MOE
margin of exposure
- MOS
margin of safety
- MPL
maximum permitted levels
- NDA
Panel EFSA Panel on Nutrition, Novel Foods and Food Allergens
- OECD
Organization for Economic Co‐operation and Development
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PI
phosphatidylinositol
- PL
phospholipids
- SCF
Scientific Committee on Food
- TWI
total weekly intake
- UL
tolerable upper intake level
Appendix A – Data requested in the call for data (call for technical and toxicological data on lecithins (E 322) for uses as a food additive in foods for all population groups including infants below 16 weeks of age19
1.
| Kind of data | Data requested in the call for data | Responses from the interested business operators | Comment |
|---|---|---|---|
| A. Information regarding the follow‐up of the conclusions and the recommendations of the EFSA ANS Panel opinion on the safety of lecithins (E 322) as food additive | |||
| 1.Technical data |
|
Received | Assessed, no further follow‐up |
| 2.Toxicological data | – | – | – |
| 3. Literature searches | Literature searches should be conducted relevant for the safety evaluation of lecithins (E 322) for all uses in foods for all population groups from 23/11/2016 up to the date of the data submission, as described in the Guidance for submission for food additive evaluations (see its Section 5.3) | Received | Assessed, no further follow‐up |
| B. Information required for the risk assessment of lecithins (E 322) as food additive for use in foods for infants below 16 weeks of age | |||
| 1. Technical data |
In addition, data should be provided demonstrating the absence of Cronobacter (Enterobacter) sakazakii in the food additive. |
Received | Assessed, no further follow‐up |
| 2. Toxicological data |
A developmental neurotoxicity study according to current OECD TG 426 is required. In the study, the concentration of metabolites of lecithins (e.g. choline) in the dams milk should be determined. Conclusions on the possible need of additional studies within the frame of the EFSA Guidance of the Scientific Committee on the risk assessment of substances present in food intended for infants below 16 weeks of age can be drawn as soon as the results of the OECD TG 426 are made available Furthermore, the following information on the toxicological properties of lecithins (E 322) and its adverse effects relevant for its use in formulae and foods for special medical purposes (FSMP) for infants below 16 weeks is required:
Additional existing studies, including confidential reports, if available:
|
Literature studies were submitted. Those studies did not contribute to assessment of potential neurodevelopmental effects because this endpoint has not been addressed in the studies Specific studies in infants were not provided and the data from the post‐marketing surveillance studies do not allow to make firm conclusions on the safety of lecithins (E 322) in infant formula Examining the available information, and in line with the recommendations from the ANS Panel former assessment, the FAF Panel considered that the appropriate approach to be taken for the safety assessment of lecithins (E 322) as food additive in infant formula used in infants below the age of 16 weeks was to compare the concentration of choline in human milk with that in the formula |
No further follow‐up |
| 3. Literature searches | Literature searches should be conducted relevant for the safety evaluation of lecithins (E 322) when used in foods for infants below 16 weeks of age up to the date of the data submission, as described in the Guidance for submission for food additive evaluations (see its Section 5.3) | Received | Assessed, no further follow‐up |
Appendix B – Phospholipids and choline levels in infant formulae and maternal milk (Documentation provided to EFSA n. 2,3)
1.
Phospholipids and choline levels in infant formulae
The mean concentration and standard deviation (SD) of phospholipids in maternal milk were calculated based on data from the literature (Hibberd et al., 1982; Bitman et al., 1986; Sala‐Vila et al., 2005; Garcia et al., 2011, 2012; Giuffrida et al., 2013; Thakkar et al., 2013; Ma et al., 2017; Cheong et al., 2018; Jiang et al., 2018; Ingvordsen Lindahl et al., 2019; McJarrow et al., 2019; Wei et al., 2019; Wu et al., 2019; Documentation provided to EFSA n. 2,3). Overall, the total phospholipid levels reported at the mean in colostrum, transitional milk and mature milk were calculated to average at 450, 382 and 248 mg/L, respectively, whereas maximum reported levels at the mean were of 1,173, 955 and 575 mg/L, respectively, see Table B.1.
Table B.1.
Total phospholipid levels in maternal milk
| Lactation stage | Total phospholipids (mg/L) | |||
|---|---|---|---|---|
| Mean (avg)a | Mean (max)b | Median | Max | |
| Colostrum | 450 | 1,173 | 330 | 1,600 |
| Transitional Milk | 382 | 955 | 285 | 900 |
| Mature Milk | 248 | 575 | 242 | 815 |
Avg: average; Max: maximum.
Average of the reported mean maternal milk levels across studies.
Maximum reported mean maternal milk level across studies.
For all three stages of lactation, the maximum mean level for total phospholipids was reported in preterm infants in the same study (Ingvordsen Lindahl et al., 2019). Median total phospholipid levels in maternal milk were reported in a single study (Giuffrida et al., 2016), at 330, 285 and 242 mg/L in colostrum, transitional and mature milk, respectively. Maximum total phospholipids levels reported to occur in colostrum, transitional and mature milk were of 1,600, 900 and 815 mg/L, respectively. In all studies that evaluated total phospholipids in maternal milk during the various stages of lactation, total phospholipids generally decreased in maternal milk as lactation progressed (Bitman et al., 1986; Sala‐Vila et al., 2005; Garcia et al., 2011; Thakkar et al., 2013; Giuffrida et al., 2016; Ma et al., 2017; Ingvordsen Lindahl et al., 2019; McJarrow et al., 2019; Wei et al., 2019). Contradictory results are reported with regard to total phospholipid levels by gestational age, whereby Ingvordsen Lindahl et al. (2019) reported significantly higher levels in the milk of mothers who delivered preterm vs term infants during all stages of lactation, whereas Wei et al. (2019) reported no significant difference.
Phospholipids and choline levels in infant formulae
The content of phospholipids and choline levels in infant formulae is reported in Table B.2, (from Documentation provided to EFSA n. 3).
Table B.2.
Mean concentration and SD of lecithins and choline in formulae (n = 20)
| Lecithins (E 322) | Choline | |||||
|---|---|---|---|---|---|---|
| Typical (mg/L) | Maximum (mg/L) | Total (mg/L) | Choline added as nutrient (mg/L) | Choline from lecithins (mg/L) | Choline from other ingredient sources (mg/L) | |
| Overall mean | 428 | 450 | 225 | 166 | 12 | 50 |
| Overall SD | 387 | 388 | 26 | 37 | 8 | 17 |
Choline levels in maternal milk
Mean concentration and SD of choline in maternal milk were also calculated by one interested business operator based on the data from the literature, see Table B.3.
Table B.3.
Mean concentration and SD of phospholipid and choline in maternal milk (documentation provided to EFSA n. 2 and 3)
| Mature milk (mg/L) | |
|---|---|
| Total phospholipids (n = 14 * ) | 248 ± 91 |
| Total choline (n = 6 ** ) | 138.3 ± 15.9 |
| Free choline (n = 10 *** ) | 16.0 ± 6.9 |
n: number of references considered.
Hibberd et al. (1982), Bitman et al. (1986), Sala‐Vila et al. (2005), Garcia et al. (2011, 2012), Giuffrida et al. (2013), Thakkar et al. (2013), Ma et al. (2017), Cheong et al. (2018), Jiang et al. (2018), Ingvordsen Lindahl et al. (2019), McJarrow et al. (2019), Wei et al. (2019), Wu et al. (2019).
Davenport et al. (2015), Holmes et al. (2000), Ilcol et al. (2005), Moukarzel et al. (2017), Moukarzel et al. (2019), Weideman et al. (2018).
Davenport et al. (2015), Fischer et al. (2010), Holmes et al. (2000), Holmes‐McNary et al. (1996), Ilcol et al. (2005), Moukarzel et al. (2017), Moukarzel et al. (2019), Weideman et al. (2018), Wu et al. (2016), Zeisel et al. (1986).
References
Bitman J, Freed LM, Neville MC, Wood DL, Hamosh P and Hamosh M, 1986. Lipid composition of prepartum human mammary secretion and postpartum milk. Journal of Pediatric Gastroenterology and Nutrition, 5, 608–615.
Cheong L‐Z, Jiang C, He X, Song S and Lai O‐M, 2018. Lipid Profiling, Particle size determination, and in vitro simulated gastrointestinal lipolysis of mature human milk and infant formula. Journal of Agricultural and Food Chemistry, 66, 12042–12050.
Davenport C, Yan J, Taesuwan S, Shields K, West AA, Jiang X, Perry CA, Malysheva OV, Stabler SP, Allen RH and Caudill MA, 2015. Choline intakes exceeding recommendations during human lactation improve breast milk choline content by increasing PEMT pathway metabolites. The Journal of Nutritional Biochemistry, 26, 903–911.
Fischer LM, da Costa KA, Galanko J, Sha W, Stephenson B, Vick J and Zeisel SH, 2010. Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. The American Journal of Clinical Nutrition, 92, 336–346.
Garcia C, Lutz NW, Confort‐Gouny S, Cozzone PJ, Armand M and Bernard M, 2012. Phospholipid fingerprints of milk from different mammalians determined by phosphorus‐31 NMR: Towards specific interest in human health. Food Chemistry, 135, 1777–1783.
Giuffrida F, Cruz‐Hernandez C, Flück B, Tavazzi I, Thakkar SK, Destaillats F and Braun M, 2013. Quantification of phospholipids classes in human milk. Lipids, 48, 1051–1058.
Garcia C, Millet V, Coste TC, Mimoun M, Ridet A, Antona C, Simeoni U and Armand M, 2011. French mothers’ milk deficient in DHA contains phospholipid species of potential interest for infant development. Journal of Pediatric Gastroenterology and Nutrition, 53, 206–212.
Hibberd CM, Brooke OG, Carter ND, Haug M and Harzer G, 1982. Variation in the composition of breast milk during the first 5 weeks of lactation: implications for the feeding of preterm infants. Archives of Disease in Childhood, 57, 658–662.
Holmes HC, Snodgrass GJ and Iles RA, 2000. Changes in the choline content of human breast milk in the first 3 weeks after birth. European Journal of Pediatrics, 159, 198–204.
Holmes‐McNary MQ, Cheng W‐L, Mar M‐H, Fussell S and Zeisel SH, 1996. Choline and choline esters in human and rat milk and in infant formulas. The American Journal of Clinical Nutrition, 64, 572–576.
Ilcol YiO, Ozbek R, Hamurtekin E and Ulus IH, 2005. Choline status in newborns, infants, children, breast‐feeding women, breast‐fed infants and human breast milk. The Journal of Nutritional Biochemistry, 16, 489–499.
Ingvordsen Lindahl IE, Artegoitia VM, Downey E, O'Mahony JA, O'Shea C‐A, Ryan CA, Kelly AL, Bertram HC and Sundekilde UK, 2019. Quantification of human milk phospholipids: the effect of gestational and lactational age on phospholipid composition. Nutrients, 11, 222 [14 pp].
Jiang C, Ma B, Song S, Lai O‐M and Cheong L‐Z, 2018. Fingerprinting of phospholipid molecular species from human milk and infant formula using HILIC‐ESI‐IT‐TOF‐MS and discriminatory analysis by principal component analysis. Journal of Agricultural and Food Chemistry, 66, 7131–7138.
Ma L, MacGibbon AKH, Mohamed HJBJ, Loy S, Rowan A, McJarrow P and Fong BY, 2017. Determination of phospholipid concentrations in breast milk and serum using a high performance liquid chromatography‐mass spectrometry‐multiple reaction monitoring method. International Dairy Journal, 71, 50–59.
McJarrow P, Radwan H, Ma L, MacGibbon AKH, Hashim M, Hasan H, Obaid RS, Naja F, Mohamed HJJ, Al Ghazal H and Fong BY, 2019. Human milk oligosaccharide, phospholipid, and ganglioside concentrations in breast milk from United Arab Emirates mothers: results from the MISC Cohort. Nutrients, 11, 2400 [14 pp].
Moukarzel S, Soberanes L, Dyer RA, Albersheim S, Elango R and Innis SM, 2017. Relationships among different water‐soluble choline compounds differ between human preterm and donor milk. Nutrients, 9, 369 [10 pp].
Moukarzel S, Wiedeman AM, Soberanes LS, Dyer RA, Innis SM and Lamers Y, 2019. Variability of Water‐Soluble Forms of Choline Concentrations in Human Milk during Storage, after Pasteurization, and among Women. Nutrients, 11, 3024 [11pp].
Sala‐Vila A, Castellote AI, Rodríguez‐Palmero M, Campoy C and López‐Sabater MC, 2005. Lipid composition in human breast milk from Granada (Spain): changes during lactation. Nutrition (Burbank, CA), 21, 467–473.
Thakkar SK, Giuffrida F, Cristina CH, De Castro CA, Mukherjee R, Tran LA, Steenhout P, Lee le Y and Destaillats F, 2013. Dynamics of Human Milk Nutrient Composition of Women from Singapore with a Special Focus on Lipids. American Journal of Human Biology, 25, 770–779.
Wei W, Yang J, Yang D, Wang X1, Yang Z, Jin Q, Wang M, Lai J and Wang X, 2019. Phospholipid Composition and Fat Globule Structure I: Comparison of Human Milk Fat from Different Gestational Ages, Lactation Stages, and Infant Formulas. Journal of Agriculture and Food Chemistry, [Epub ahead of print – December 10, 2019].
Wiedeman AM, Whitfield KC, March KM, Chen NN, Kroeun H, Sokhoing L, Sophonneary P, Dyer RA, Xu Z, Kitts DD, Green TJ, Innis SM and Barr S, 2018. Concentrations of water‐soluble forms of choline in human milk from lactating women in Canada and Cambodia. Nutrients, 10, 381 [10 pp, plus supplementary tables].
Wu J, Domellöf M, Zivkovic AM, Larsson G, Öhman A and Nording ML, 2016. NMR‐based metabolite profiling of human milk: a pilot study of methods for investigating compositional changes during lactation. Biochemical and Biophysical Research Communications, 469, 626–632.
Wu K, Gao R, Tian F, Mao Y, Wang B, Zhou L, Shen L, Guan Y and Cai M, 2019. Fatty acid positional distribution (sn‐2 fatty acids) and phospholipid composition in Chinese breast milk from colostrum to mature stage. The British Journal of Nutrition, 121, 65–73 [plus supplementary tables].
Zeisel SH, Char D and Sheard NF, 1986. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. The Journal of Nutrition, 116, 50–58.
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gürtler R, Husøy T, Manco M, Mennes W, Moldeus P, Passamonti S, Shah R, Waalkens‐Berendsen I, Wölfle D, Wright M, Dusemund B, Mortensen A, Turck D, Barmaz S, Tard A, Vianello G and Gundert‐Remy U, 2020. Scientific Opinion on the re‐evaluation of lecithins (E 322) as a food additive in foods for infants below 16 weeks of age and follow‐up of its re‐evaluation as food additive for uses in foods for all population groups. EFSA Journal 2020;18(11):6266, 37 pp. 10.2903/j.efsa.2020.6266
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00094
Panel members: EFSA Panel on Food Additives and Flavourings (FAF), Maged Younes, Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Ursula Gundert‐Remy, Rainer Gürtler, Trine Husøy, Melania Manco, Wim Mennes, Peter Moldeus, Sabina Passamonti, Romina Shah, Ine Waalkens‐Berendsen, Detlef Wölfle and Matthew Wright.
Adopted: 23 September 2020
Notes
Commission Delegated Regulation (EU) 2016/127/EC of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow‐on formula and as regards requirements on information relating to infant and young child feeding. OJ L 25, 2.2.2016, p. 1–29.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009. OJ L 181, 29.6.2013, p. 35–56.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a program for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
See Section 1.1.3.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
A major component of lecithins (E 322).
Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow‐on formula and as regards requirements on information relating to infant and young child feeding (Text with EEA relevance) OJ L 25, 2.2.2016, p. 1–29.
According to the EFSA Scientific Committee Guidance (EFSA Scientific Committee, 2017) this opinion will include infants up to 16 weeks of age.
According to the EFSA Scientific Committee Guidance (EFSA Scientific Committee, 2017), this opinion will include infants up to 16 weeks of age.
Call for technical and toxicological data on lecithins (E 322) as a food additive for uses in foods for all population groups including infants below 16 weeks of age. Published: 18 July 2018. Available online: https://www.efsa.europa.eu/en/consultations/call/call-technical-and-toxicological-data-lecithins-e-322-uses-foods
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council Text with EEA relevance. OJ L 83, 22.3.2012, p. 1–295.
Call for technical and toxicological data on lecithins (E 322) for uses as a food additive in foods for all population groups including infants below 16 weeks of age. Available online: http://www.efsa.europa.eu/sites/default/files/consultation/callsfordata/180718_lecithins_0.pdf
Missing Cyprus, Luxembourg and Malta.
http://www.gnpd.com/sinatra/home/ accessed on 16/6/2020.
The term ‘formula’ was added here.
essential phospholipid.
References
- Attia YA, Abd El‐Hamid AE, de Oliveira MC, Kamel KI, Qota EM, Al‐Harthi MA and Sadaka TA, 2018. Soya lecithin and season affect the productive performance, nutrient digestibility and blood constituents of growing rabbits.Animal Science Papers and Reports, 36, 193–203. [Google Scholar]
- Ballard O and Morrow AL, 2013. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am., 60, 49–74. 10.1016/j.pcl.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitman J, Freed LM, Neville MC, Wood DL, Hamosh P and Hamosh M, 1986. Lipid composition of prepartum human mammary secretion and postpartum milk. Journal of Pediatric Gastroenterology and Nutrition, 5, 608–615. [DOI] [PubMed] [Google Scholar]
- Cao Y, Liu K, Wei H, Mend L, Li L, Liu D and Zhang S, 2018. [Effect of soybean lecithin on the reproduction performance of sows]. Swine Production, 1, 6–8 [Chinese, English abstract].
- Cheong L‐Z, Jiang C, He X, Song S and Lai O‐M, 2018. Lipid profiling, particle size determination, and in vitro simulated gastrointestinal lipolysis of mature human milk and infant formula. Journal of Agricultural and Food Chemistry, 66, 12042–12050. [DOI] [PubMed] [Google Scholar]
- de Benito A, Gnanou Besse N, Desforges I, Gerten B, Ruiz B and Tomás D, 2018. Validation of standard method EN ISO 22964:2017 ‐ Microbiology of the food chain ‐ Horizontal method for the detection of Cronobacter spp. International Journal of Food Microbiology, 288, 47–52. 10.1016/j.ijfoodmicro.2018.03.025 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Lambré C, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Oskarsson A, Parent‐Massin D, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Brimer L, Altieri A, Christodoulidou A, Lodi F and Dusemund B, 2017. Scientific opinion on the re‐evaluation of lecithins (E 322) as a food additive. EFSA Journal 2017;15(4):4742, 74 pp. 10.2903/j.efsa.2017.4742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (Panel on Contaminants in the Food Chain), 2009a. Scientific opinion on Cadmium in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (Panel on Contaminants in the Food Chain), 2009b. Scientific opinion on Arsenic in food. EFSA Journal 2009;7(3):980, 139 pp. 10.2903/j.efsa.2009.980 [DOI] [Google Scholar]
- EFSA CONTAM Panel (Panel on Contaminants in the Food Chain), 2010. Scientific opinion on Lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (Panel on Contaminants in the Food Chain), 2012. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 15 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings), Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gürtler R, Gundert‐Remy U, Husøy T, Mennes W, Moldeus P, Oskarsson A, Shah R, Waalkens‐Berendsen I, Wölfle D, Degen G, Leblanc J‐C, Giarola A, Rincon AM, Tard A and Castle L, 2020. Scientific Opinion on the safety of use of oat lecithin as a food additive. EFSA Journal 2020;18(1):5969, 22 pp. 10.2903/j.efsa.2020.5969 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2014. Scientific Opinion on the evaluation of allergenic foods and food ingredients for labelling purposes. EFSA Journal 2014;12(11):3894, 286 pp. 10.2903/j.efsa.2014.3894 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2016. Scientific opinion on Dietary Reference Values for choline. EFSA Journal 2016;14(8):4484, 70 pp. 10.2903/j.efsa.2016.4484 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessment carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(11):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA Journal 2017;15(5):4849, 58 pp. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C, Millet V, Coste TC, Mimoun M, Ridet A, Antona C, Simeoni U and Armand M, 2011. French mothers’ milk deficient in DHA contains phospholipid species of potential interest for infant development. Journal of Pediatric Gastroenterology and Nutrition, 53, 206–212. [DOI] [PubMed] [Google Scholar]
- Garcia C, Lutz NW, Confort‐Gouny S, Cozzone PJ, Armand M and Bernard M, 2012. Phospholipid fingerprints of milk from different mammalians determined by phosphorus‐31 NMR: towards specific interest in human health. Food Chemistry, 135, 1777–1783. [DOI] [PubMed] [Google Scholar]
- Giuffrida F, Cruz‐Hernandez C, Flück B, Tavazzi I, Thakkar SK, Destaillats F and Braun M, 2013. Quantification of phospholipids classes in human milk. Lipids, 48, 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida F, Cruz‐Hernandez C, Bertschy E, Fontannaz P, Masserey Elmelegy I, Tavazzi I, Marmet C, Sanchez‐Bridge B, Thakkar SK, De Castro CA, Vynes‐Pares G, Zhang Y and Wang P, 2016. Temporal changes of human breast milk lipids of Chinese Mothers. Nutrients, 8, 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd CM, Brooke OG, Carter ND, Haug M and Harzer G, 1982. Variation in the composition of breast milk during the first 5 weeks of lactation: implications for the feeding of preterm infants. Archives of Disease in Childhood, 57, 658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvordsen Lindahl IE, Artegoitia VM, Downey E, O'Mahony JA, O'Shea C‐A, Ryan CA, Kelly AL, Bertram HC and Sundekilde UK, 2019. Quantification of human milk phospholipids: the effect of gestational and lactational age on phospholipid composition. Nutrients, 11, 222 [14 pp]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine), 1998. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Food and Nutrition Board. National Academy Press, Washington, DC. [PubMed]
- ISO (International Organization for Standardization), 2017. Microbiology of the food chain — Horizontal method for the detection of Cronobacter spp., ISO 22964:2017. [DOI] [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1974a. 299. Lecithin. WHO Food Additives Series 5.2 pp.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1974b. 297. Hydroxylated Lecithin. WHO Food Additives Series 5. 2 pp.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2007a. Lecithin. Published in FAO JECFA Monographs 4. Joint FAO/WHO Expert Committee on Food Additives. 4 pp.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2007b. Lecithin, Partially Hydrolyzed. Published in FAO JECFA Monographs 4. Joint FAO/WHO Expert Committee on Food Additives. 4 pp
- Jiang C, Ma B, Song S, Lai O‐M and Cheong L‐Z, 2018. Fingerprinting of phospholipid molecular species from human milk and infant formula using HILIC‐ESI‐IT‐TOF‐MS and discriminatory analysis by principal component analysis. Journal of Agricultural and Food Chemistry, 66, 7131–7138. [DOI] [PubMed] [Google Scholar]
- Lewis ED, Richard C, Goruk S, Dellschaft NS, Curtis JM, Jacobs RL and Field CJ, 2016. The form of choline in the maternal diet affects immune development in suckled rat offspring. The Journal of Nutrition, 146, 823–830. [DOI] [PubMed] [Google Scholar]
- Ma L, MacGibbon AKH, Mohamed HJBJ, Loy S, Rowan A, McJarrow P and Fong BY, 2017. Determination of phospholipid concentrations in breast milk and serum using a high performance liquid chromatography‐mass spectrometry‐multiple reaction monitoring method. International Dairy Journal, 71, 50–59. [Google Scholar]
- Mathews SA, Oliver WT, Phillips OT, Odle J, Diersen‐Schade DA and Harrell RJ, 2002. Comparison of triglycerides and phospholipids as supplemental sources of dietary long‐chain polyunsaturated fatty acids in piglets. The Journal of Nutrition, 132, 3081–3089. [DOI] [PubMed] [Google Scholar]
- McJarrow P, Radwan H, Ma L, MacGibbon AKH, Hashim M, Hasan H, Obaid RS, Naja F, Mohamed HJJ, Al Ghazal H and Fong BY, 2019. Human milk oligosaccharide, phospholipid, and ganglioside concentrations in breast milk from United Arab Emirates Mothers: results from the MISC Cohort. Nutrients, 11, 2400 [14 pp]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala‐Vila A, Castellote AI, Rodríguez‐Palmero M, Campoy C and López‐Sabater MC, 2005. Lipid composition in human breast milk from Granada (Spain): changes during lactation. Nutrition (Burbank, CA), 21, 467–473. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1982. Reports of the Scientific Committee for Food (13th series). Opinion expressed 1981. Food Science and Techniques.
- SCF (Scientific Committee for Food), 1983. First Report of the Scientific Committee for Food on the essential requirements of infant formulae and follow‐up milks based on cows’ milk proteins (14th series). Opinion expressed 27 April 1983.
- SCF (Scientific Committee for Food), 1997. Reports from the Scientific Committee for Food (40th series). Opinion expressed 7 June 1996. Food Science and Techniques.
- Shi B, Wang C, Teng T, Liu T, Zhang X and Shan A, 2019. Effects of dietary soybean lecithin oil on the immunoglobulin level and fat globule size of milk in lactating sows. Food and Agricultural Immunology, 30, 774–785. [Google Scholar]
- Thakkar SK, Giuffrida F, Cristina CH, De Castro CA, Mukherjee R, Tran LA, Steenhout P, Lee le Y and Destaillats F, 2013. Dynamics of Human Milk Nutrient Composition of Women from Singapore with a Special Focus on Lipids. Amercian Journal of Human Biology, 25, 770–779. [DOI] [PubMed] [Google Scholar]
- UK Ministry of Agriculture Fisheries and Food , 1992. Food Advisory Committee Report on the Review of the Use of Additives in Foods Specially Prepared for Infants and Young Children. (FdAC/REP/12). HMSO, London.
- Wei W, Yang J, Yang D, Wang X1, Yang Z, Jin Q, Wang M, Lai J and Wang X, 2019. Phospholipid composition and fat globule structure I: comparison of human milk fat from different gestational ages, lactation stages, and infant formulas. Journal of Agriculture and Food Chemistry, [Epub ahead of print – Dec. 10, 2019]. [DOI] [PubMed] [Google Scholar]
- Wu K, Gao R, Tian F, Mao Y, Wang B, Zhou L, Shen L, Guan Y and Cai M, 2019. Fatty acid positional distribution (sn‐2 fatty acids) and phospholipid composition in Chinese breast milk from colostrum to mature stage. The British Journal of Nutrition, 121, 65–73 [plus supplementary tables]. [DOI] [PubMed] [Google Scholar]


