Abstract
l-Dopa has continued to be a mainstay in the symptomatic treatment of Parkinson’s disease (PD). However, extensive peripheral metabolism, a short systemic circulation half-life and development of motor complications called dyskinesia prevents its long-term utilization as a PD therapeutic. Herein, we report a series of phosphoramidate derivatives of l-Dopa and controlled release of l-Dopa at pH 7.4 and 3. The kinetic data for the release of l-Dopa support our hypothesis that a proximal carboxylic acid can promote the pH-triggered hydrolysis of the phosphoramidate P–N bond. As expected, esterification of the proximal carboxylic acid protects the scaffold from rapid release at low pH. This latter observation is particularly noteworthy as it suggests that the phosphoramidate-based l-Dopa–conjugate scaffold can be adapted for oral administration as an ester prodrug.
Keywords: Phosphoramidate, l-Dopa, Prodrug, Parkinson’s disease
Parkinson’s disease (PD) is a progressive neurodegenerative disorder of the central nervous system (CNS) that affects the motor system.1,2 It is the 2nd-most common neurodegenerative disease, affecting about 1% of the population above the age of 65.3 Though a definite etiology has yet to be identified, the main pathological characteristics of PD are the continual loss of dopamine-secreting neurons in the substantia nigra pars compacta and the accumulation of Lewy bodies in many of the remaining neurons. The markedly low secretion of dopamine in the midbrain leads to the motor dysfunctions and non-motor related symptoms.3
Levodopa (l-Dopa) was first used to treat PD in the 1960s and remains the primary treatment option to elevate dopamine level in the CNS.4 However, l-Dopa suffers from poor pharmacokinetic properties as both the gastrointestinal absorption and brain uptake of l-Dopa at these sites compete with endogeneous amino acid substrates for transporters.5,6 Furthermore, it is extensively metabolized by peripheral Dopa decarboxylase (DCC), catechol-O-methyltransferase (COMT) and monoamine oxidase-B (MOA-B), and cleared rapidly from circulation leading to a short circulating half-life (approximately 1 h). Inhibitors of DCC (e.g. carbidopa), COMT (e.g. entacopone, tolcapone) and MOA-B (e.g. selegiline, rasagiline) are routinely co-administered with l-Dopa to help increase its circulation half-life, but it is only extended by an additional 30–90 min.7 Collectively, the erratic absorption and premature metabolism of l-Dopa result in waning and fluctuating levels in the brain, giving rise to the intermittent stimulation of dopaminergic neurons upon its conversion to dopamine. This pulsatile neuronal stimulation contributes to the development of disabling dyskinesias and motor fluctuations in patients after long-term use of l-Dopa.8,9 It is believed that approaches that maintain a steady therapeutic level of l-Dopa in systemic circulation, and hence continuous dopaminergic stimulation, could significantly reduce or delay the incidence of motor complications.9
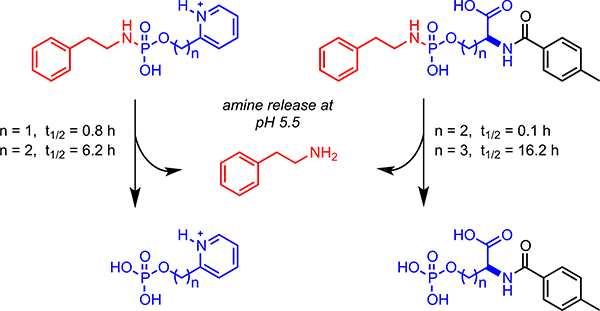
We recently reported a novel phosphoramidate scaffold that can be tuned for the controlled and pH-triggered release of amine-containing drugs.10,11 With this scaffold, control over the release of the drug was dependent upon the proximity of a neighboring acidic group (e.g. carboxylic acid or pyridinium) to the phosphorus center (Figure 1). In this present study, we hypothesized that a prodrug of l-Dopa based on a phosphoramidate scaffold could be developed for the controlled and sustained release l-Dopa at physiological conditions. Thus, the focus of this proof-of-concept study was aimed at developing a library of l-Dopa prodrugs 1, and representative ethyl ester l-Dopa double-prodrugs 2, to identify compounds with desirable properties for the controlled release of l-Dopa.
Figure 1.
Phosphoramidate-based pH-triggered cleavable scaffold.
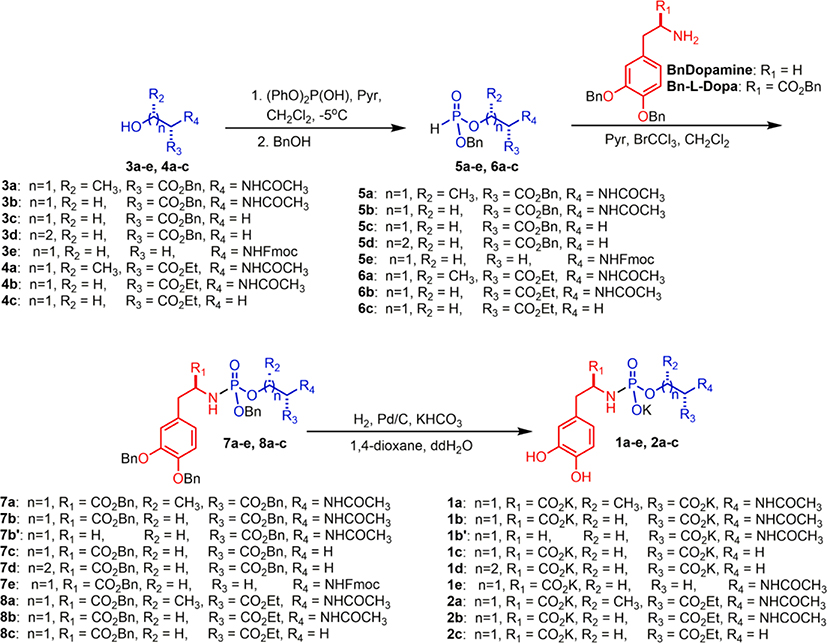
The synthesis of l-Dopa phosphoramidates was achieved via a parallel synthetic methodology, which was amenable to late stage diversification (Scheme 1). Alcohols (3 and 4) were prepared from commercially available amino acids. The protected H-phosphonates (5 and 6) and phosphoramidate intermediates (7 and 8) were synthesized as previously described11 with minor modifications (see Supplemental Information). Global deprotection via Pd/C-catalyzed hydrogenolysis yielded the final l-Dopa prodrugs 1 and 2.
Scheme 1.
Synthesis of l-Dopa Phosphoramidate-Based Prodrugs.
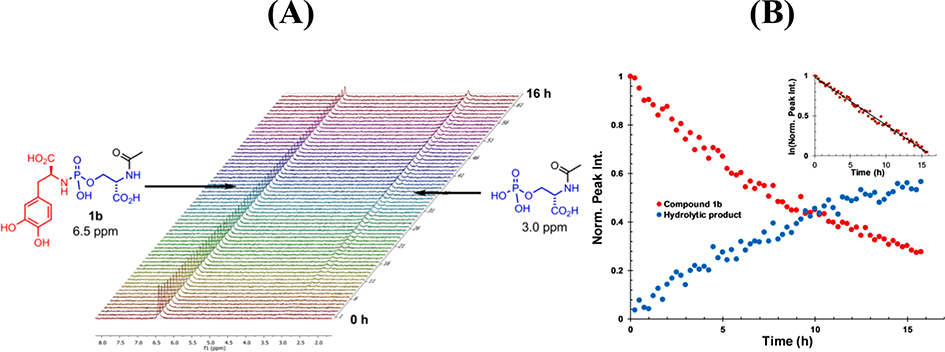
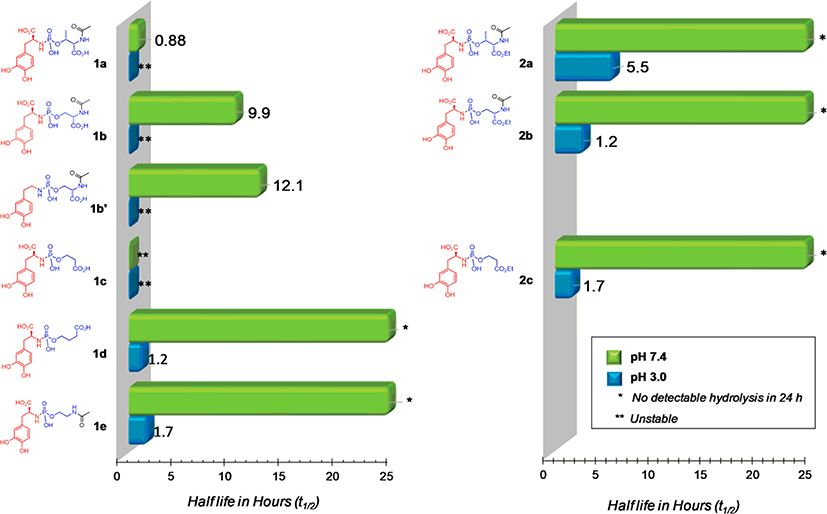
Once prepared, the stability of the l-Dopa prodrugs 1a-e and 2a-c at pH 3.0 and 7.4 were monitored by 31P NMR over 16 h (see representative example in Figure 2A) and referenced to an internal standard (triphenylphoshine oxide, TPPO). With the exception of the compounds that were stable, the rate of hydrolysis for these l-Dopa prodrugs followed first-order kinetics (see representative example in Figure 2B) as we have observed previously for simple model systems. Stability data for the complete library of compounds at pH 3.0 and 7.4 is shown in Figure 3.
Figure 2.
(A) Raw-stacked 31P NMR data for the hydrolysis of 1b (6.5 ppm) at pH 7.4 and formation of its hydrolytic product (3.0 ppm). (B) Compiled and fitted data for the integration of 1b (red) normalized to the internal standard and its hydrolytic product (blue). Inset figure: semi-log transformation for the decay of 1b illustrating first-order kinetics.
Figure 3.
Stability of l-Dopa phosphoramidates at pH 3.0 (blue) and 7.4 (green) at 37 °C.
Consistent with our previous findings,10,11 all of the prodrugs 1a – 1e were relatively unstable at pH 3 compared to pH 7.4. Also similar to our earlier observations with a model phosphoramidate linker platform, the hydrolysis rates of the l-Dopa phosphoramidates in this study were influenced by the presence of a carboxylic acid moiety and its proximity to the phosphorus center.11 For example, the half-life of prodrug 1b was observed to be approximately 10 h at pH 7.4 while the analogous compound 1e, which lacked a proximal carboxylate, was stable. Similarly, it was observed that the hydrolytic stability of compounds 1a-1c was considerably enhanced when the carboxylates were protected as the ethyl esters (2a-2c). This enhanced stability of 2a-2c at pH 3 may be sufficient for oral administration of these l-Dopa prodrugs, thus providing sustained release of l-Dopa in vivo.
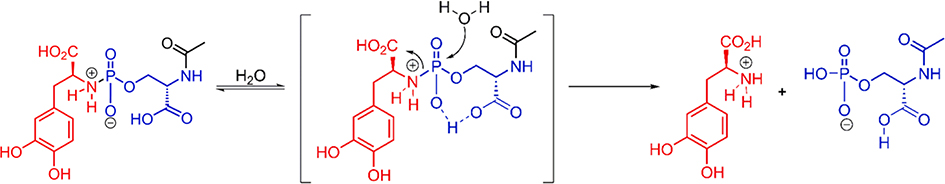
It is interesting to note that compound 1c, which lacked the amide motif, was unstable compared to 1b suggesting that the amide of the serine residue of 1b attenuated the effect of the proximal carboxylate on the phosphoramidate P–N bond. Our initial studies on this phosphoramidate platform, suggested that the hydrolysis of the P–N bond may proceed through intramolecular general-acid mechanism (Scheme 2). Based on Taft σ* constants,12 the presence of the proximal amide in 1b should decrease the pKa of the serine carboxylic acid thereby diminishing its effect on the hydrolysis of the phoshoramidate P–N bond. Therefore, it is likely that the pKa of the carboxylic acid in serine, and consequently the hydrolytic stability of the P–N bond, could be further tuned with substituted benzamides on the N-terminus of serine instead of the acetamide used in this study. In addition, it is apparent that the carboxylic acid moiety of l-Dopa itself exerts only a modest effect on the hydrolysis of the P–N bond as evidenced by comparing 1b (t1/2 = 9.9 h) to 1b’ (t1/2 = 12.1 h). Again based on Taft σ* constants,12 it is likely that the zwitterionic nitrogen (Scheme 2) has a stabilizing effect on the alpha carboxylate of l-Dopa, thereby reducing its pKa and diminishing its effect on the hydrolytic stability of the P–N bond. In addition to alterations of the pKa and proximity of the carboxylic acid to the phosphoramidate center, we also observed rate enhancement for hydrolysis of the P–N bond presumably due to the Thorpe-Ingold effect. For example, the threonine analog 1a was considerably less stable than the serine analog 1b, which is consistent with our previous observations with phosphoramidates of simple amines.11
Scheme 2.
Proposed mechanism for the hydrolysis of l-Dopa phosphoramidates.
In summary, we have demonstrated that various l-Dopa phosphoramidate-based prodrugs can be prepared to possess a wide range of stabilities for the controlled release of l-Dopa. The controlled release of l-Dopa, or other amine-containing drugs, from this phosphoramidate prodrug platform is tunable, as predicted by Taft σ* constants, and in all likelihood could be attenuated further by conveniently selecting suitably substituted benzamides of serine or threonine instead of the N-terminus acetamide used in this study. The ester-based double-prodrugs such as compounds 2a-2c demonstrate sufficient stability at pH 3 to support the feasibility of oral administration. The choice of particular esters in such compounds could provide an additional level of control based on rates of esterase-mediated hydrolysis. Such investigations are currently underway and will be reported in due course.
Supplementary Material
Acknowledgements
The authors extend their gratitude for technical assistance to G. Helms and W. Hiscox (Washington State University Center for NMR Spectroscopy) and Gerhard Munske (Washington State University Laboratory of Biotechnology and Bioanalysis).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bmcl.2019.08.005.
References
- 1.Davie CA. A review of Parkinson’s disease. Br Med Bull. 2008;86:109–127. [DOI] [PubMed] [Google Scholar]
- 2.Lang AE. The progression of Parkinson disease: a hypothesis. Neurology. 2007;68:948–952. [DOI] [PubMed] [Google Scholar]
- 3.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. [DOI] [PubMed] [Google Scholar]
- 4.Nagatsu T, Sawada M. L-Dopa therapy for Parkinson’s disease: Past, present, and future. Parkinsonism Relat. Disord 2009;15:S3–S8. [DOI] [PubMed] [Google Scholar]
- 5.Di Stefano A, Sozio P, Cerasa LS, Iannitelli A. L-Dopa prodrugs: an overview of trends for improving Parkinson’s disease treatment. Curr Pharm Des. 2011;17:3482–3493. [DOI] [PubMed] [Google Scholar]
- 6.Poewe W, Antonini A. Novel formulations and modes of delivery of levodopa. Mov Disord. 2015;30:114–120. [DOI] [PubMed] [Google Scholar]
- 7.Kianirad Y, Simuni T. Novel Approaches to Optimization of Levodopa Therapy for Parkinson’s Disease. Curr. Neurol. Neurosci. Rep 2016;16:34. [DOI] [PubMed] [Google Scholar]
- 8.Thanvi B, Lo N, Robinson T. Levodopa-induced dyskinesia in Parkinson’s disease: clinical features, pathogenesis, prevention and treatment. Postgr. Med J 2007;83:384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG. Levodopa-induced dyskinesias. Mov Disord. 2007;22:1379–1389. [DOI] [PubMed] [Google Scholar]
- 10.Choy CJ, Geruntho JJ, Davis AL, Berkman CE. Tunable pH-Sensitive Linker for Controlled Release. Bioconjug Chem. 2016;27:824–830. [DOI] [PubMed] [Google Scholar]
- 11.Choy CJ, et al. Second-Generation Tunable pH-Sensitive Phosphoramidate-Based Linkers for Controlled Release. Bioconjug Chem. 2016;27:2206–2213. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Kleinöder T, Gasteiger J. Prediction of p K a Values for Aliphatic Carboxylic Acids and Alcohols with Empirical Atomic Charge Descriptors. J Chem Inf Model. 2006;46:2256–2266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.