Abstract
Background
Antibacterial agents are an integral part of chemotherapy and play a critical role in the prophylaxis and treatment of bacterial infections. However, prescribing errors such as incomplete prescriptions that do not adhere to good prescribing practice have become a contemporary concern in hospitals in resource-limited settings. Therefore, this study aimed to assess antibacterial prescribing and its completeness among prescriptions dispensed at four governmental hospitals in Eastern Ethiopia.
Methods
A cross-sectional study was employed to assess a total of 1308 prescription encounters containing at least one antibacterial agent obtained with simple random sampling from annual antibacterial-containing prescription data of four hospitals. The data were collected retrospectively using a structured checklist.
Results
A total of 2,855 drugs were prescribed from 1308 prescribing encounters with 1496 (52.39%) being antibacterial agents. The name, age, sex, and diagnosis of the patients were written in 1158 (88.3%), 815 (62.31%), 796 (60.58%), and 183 (13.99%) prescriptions, respectively. Besides, the route of administration, strength, duration, quantity, dose, and dosage form of the drug were recorded in 2322 (81.33%), 2118 (74.19%), 1516 (53.10%), 1525 (53.42%), 746 (26.13%) and 563 (19.72%) prescriptions, respectively. Nearly 50% of the prescribing encounters were documented without a prescriber name. Dispenser name and signature were also obtained in less than 10% of the prescriptions. Combining the data of all hospitals, amoxicillin, ceftriaxone, and ciprofloxacin were identified as the top three prescribed antibacterial drugs, whereas diclofenac, paracetamol, and tramadol were the most frequently co-indicated drugs. Regarding the pharmacologic class of antibiotics, penicillins were the most commonly prescribed antibiotics (n = 596, 39.77%) followed by cephalosporins (n = 318, 21.26%) and fluoroquinolones (n=285, 19.05%).
Conclusion
Incomplete information about patient-related factors and major diagnosis, medication regimens, prescribers and dispensers was identified as a potential prescribing error and did not adhere to good prescribing practice. This can be considered as one part of the inappropriate use of antibacterial agents, a driving force for the emergence of antimicrobial resistance. This problem requires immediate and sustained action from the management of the hospitals to ensure the accountability of health professionals involved in the medication use process and to establish antimicrobial stewardship programs in such resource-limited settings.
Keywords: prescribing practice, completeness, antibacterial drugs, Eastern Ethiopia
Introduction
Antimicrobials are an essential part of modern medicine and play a major role both in the prophylaxis and treatment of infectious diseases. However, many people perish from infectious diseases that are curable but for which either we are not providing the right treatments or the available treatments are no longer effective. There is alarmingly increasing antimicrobial resistance (AMR) primarily attributable to the misuse of these agents.1–3 The importance of antibacterial agents, in particular, is notable in developing countries where bacterial infectious diseases are highly prevalent.4 In concert with a plethora of factors, inappropriate use of antibacterial agents has been a driving factor for the emergence and spread of drug resistance and we are facing a real problem and heading into a post-antibiotic era. In addition, inappropriate use of antibacterial agents is resulting in failure of treatment, escalation of health-care costs, and rising morbidity and mortality.1 The high burden of AMR in previously effective drugs leads to the use of less effective, more toxic, and more expensive second-line agents which may not be affordable by the majority of patients in developing countries.5,6
Observational studies as well as a systematic review of prescribing practices frequently revealed a diverse pattern among prescribers in the treatment of even the most common infectious diseases. Besides, the common medicine use problems observed were polypharmacy, selecting the wrong drug, prescribing the incorrect dose, prescribing medicines that cause adverse drug reactions (ADRs) or potential interactions, and using more expensive medicines in the presence of less expensive and equally or more effective alternatives.3,7,8
Incomplete and poor-quality prescriptions have become a common prescribing error making up a considerable part of medication errors. In resource-limited settings where integrated computer systems are not readily available, introducing a structured prescription form may improve the quality of handwritten prescriptions in terms of completeness as well as legibility.9 To this end, the prevalence of prescription errors at tertiary health-care facilities in the Hail region, Saudi Arabia was alarmingly high.10 Studies examining completeness and legibility of handwritten prescriptions in Sana’a, Yemen indicated that the quality and completeness of prescriptions was found to be very poor.11 Likewise, a study conducted in Asmara, Eritrea, found the overall completeness of prescriptions to be 78.63%.12 A study conducted at Tikur Anbesa Specialized Hospital, Addis Ababa, Ethiopia indicated that about 25% of prescribing encounters had patient information such as age and sex and less than 10% of the prescription contained dosage forms as a part of the medication regimen.13 In contrast, good prescribing practice manuals developed by the Food Medicine Healthcare Administration and Control Authority (FMHACA), currently renamed as Ethiopian Food and Drug Administration (EFDA), and World Health Organization (WHO)14,15 emphasized that information on patient-related factors, major diagnosis or international classification of diseases (ICD) code, medication regimen components and responsible health professionals should be duly and legibly recorded on prescription papers prepared with a preprinted format. Given the alarming rate of antibacterial resistance and high antibacterial consumption, such prescribing errors will catalyze the devastating consequences for patients and health-care systems of resource-limited settings. Hence, controlling prescribing errors is one of the important check-points.16,17 Though the inappropriate use of antibacterial agents is highly complex, the current study was aimed at assessing hospital-level antibacterial prescribing and its completeness in four governmental hospitals, Eastern Ethiopia.
Methods
Study Setting, Design and Period
The study was conducted in four government hospitals of Harar town, namely Hiwot Fana Specialized University Hospital (HFSUH), Jugal Hospital (JH), Southeast Command III Hospital (SECIIIH), and Federal Harar Police Hospital (FHPH). Harar, the capital of Harari region, is located 526 km from the capital of Ethiopia, Addis Ababa, to the east. In the region, there are four governmental hospitals, one non-governmental organization and two private hospitals, and eight health centers. HFSUH is a tertiary care teaching hospital of Haramaya University and hosts the majority of patient attendees per day from Harar town and other eastern parts of the country. In the last two decades, the hospital has been a teaching facility for health and medical sciences students of Haramaya University. Currently, the hospital has seven wards (medical, surgical, pediatric, nutrition, obstetrics, gynecology, and psychiatric). It had a total of 233 beds for admission in its various wards and is being markedly expanded to accommodate 1000 beds. JH is a regional hospital of Harari regional state. It is the oldest hospital in the town. The hospital has six wards (medical, surgical, obstetrics, gynecology, pediatric, and ophthalmology) with a total of 125 beds. FHPH and SECIIIH are special government hospitals that were established to meet the health-care demands of police and military wings and primarily serve police and military clients and their relatives. Over time, they expanded their health-care services to the general public. A hospital-based retrospective cross-sectional study was employed to assess the overall prescribing practice from October 1 to November 31, 2017.
Study Population
All outpatient prescriptions dispensed from January 1, 2016 to December 31, 2016 in each hospital and containing at least one antibacterial agent were considered as the study population. Prescriptions that were found to be illegible or were brought from outside of the selected hospitals were excluded from the study.
Sample Size Determination and Sampling Techniques
The sample size was calculated using a single population proportion formula (n= Zα2pq/d2). Where: n = sample size, Z = confidence level, p = success rate, W = maximum width tolerable error, q=1-p, failure rate. To determine the sample size, we considered the Z statistic value = 97% (2.17) with two-sided tolerable error (α) (W=3% or 0.03) to increase the representativeness of sample size and P= 50% (0.5). Since the size of the study population was found to be greater than 10,000 (N= 121,025), the adjustment formula was not applied.
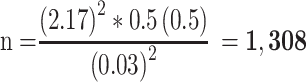 |
Hence, 1308 antibacterial-containing prescribing encounters were included in the study. Proportional allocation of sample size was carried out based on the size of the study population in each hospital (HFSUH=50,850, JH=42,747, FHPH=10,778, SECIIIH=16,650). Accordingly, the numbers of prescriptions sampled were found to be 550, 462, 180, and 116 for HFSUH, JH, SECIIIH and FHPH, respectively. A simple random sampling technique was applied to obtain study units in each hospital. In this regard, the prescriptions containing antibacterial agents were chronologically arranged and given a numerical code in each hospital. Computer-generated random numbers were used to retain the required sampling units from the sampling frame.
Data Collection Process
Data were collected retrospectively using a structured checklist adapted from the good prescribing practice manuals of FMHACA and WHO for prescriptions containing antibacterial agents at each hospital.14,15 A pretest was conducted at Haramaya Hospital to consider revision of the checklist. The checklist was designed to extract information on the three major components of the prescription (patient, medication regimen, and health professionals related information), the name, and number of antibacterial agents prescribed along with the frequency distribution of co-administered drugs. To this end, the magnitude of each drug regimen information (dose, strength, route of administration, frequency, duration and quantity of drugs) was also appropriately collected out of the total drugs prescribed.
Data Processing and Analysis
Univariate analysis was computed using Statistical Package for Social Sciences (SPSS) version 20 (IBM statistics, Armonk, NY, USA). Tabular presentation was used to summarize the findings.
Result
A total of 1308 prescription papers containing at least one antibacterial agent were analyzed from four governmental hospitals of Harar town. The total number of drugs prescribed was 2855 with the average number of drugs per encounter to be 2.18 (±0.17). The total number of antibacterial agents prescribed was 1496 (52.39% of drugs) with the average per encounter being 1.13 (±0.036). The name of the patient was written in 1158 (88.3%) prescribing encounters ranging from 76.84% at JH to 98.54% at HFSUH. Surprisingly, diagnosis or ICD code for any presumed/confirmed bacterial infection was written only in 13.99% of prescriptions with zero values recorded at SECIIIH. Except HFSUH where nearly one-third of the prescriptions contained a diagnosis, less than 5% of prescriptions were documented with a diagnosis in the rest of the hospitals. Moreover, only 60.58% of prescriptions had the age of the patient with the lowest (11.11%) and highest value (86.91%) observed in SECIIIH and HFSUH, respectively. The practice of writing a prescriber name ranged from 22.51% at JH to 59.09% at HFSUH, with the overall recording practice being less than half of all prescriptions (43.81%). Dispenser name and signature were totally neglected components of prescription in three hospitals with an overall prevalence of less than 10%. Only 26.13% of prescriptions contained doses, with the poorest recording practice observed at JH (15.79%). Moreover, the route and frequency of doses were each stated in 2322 (81.33%) drugs. Duration of therapy was recorded in 53.10% prescriptions (Table 1).
Table 1.
Overall Prescribing Pattern in Antibacterial-Containing Prescriptions Dispensed from January 2016 to December 2016 at Four Governmental Hospitals of Harar Town, Eastern Ethiopia
| Overall Prescribing Behavior | Frequency (%) | |||||
|---|---|---|---|---|---|---|
| HFSUH (n=550) | FHPH (n=116) | JH (n= 462) | SECIIIH (n=180) | Overall (n=1308) | ||
| Patient-related information | Name | 542 (98.54) | 110 (94.83) | 355 (76.84) | 151 (83.88) | 1158 (88.3) |
| Age | 478 (86.91) | 28 (24.14) | 270 (58.44) | 20 (11.11) | 796 (60.58) | |
| Sex | 485 (88.18) | 42 (36.21) | 277 (59.96) | 11 (6.11) | 815 (62.31) | |
| Weight | 20 (3.64) | 1 (0.01) | 0 | 1 (0.6) | 22 (1.68) | |
| Diagnosis | 176 (32) | 5 (4.31) | 2 (1.72) | 0 | 183 (13.99) | |
| Prescribed drugs | Total number of drugs Degree of Polypharmacy |
1078 1.96 |
323 2.78 |
1070 2.13 |
384 2.31 |
2855 2.18(±0.17) |
| Total antibacterial agents | 631 | 132 | 537 | 196 | 1496 | |
| Co-administered drugs | 447 | 191 | 533 | 188 | 1359 | |
| Average number of antibacterial agent per prescription | 1.15 (± 0.016) | 1.14 (±0.032) | 1.16 (±.019) | 1.08 (± 0.020) | 1.13(±0.036) | |
| Health professional-related information | Prescriber name | 325 (59.09) | 59 (50.86) | 104 (22.51) | 85 (47.22) | 573 (43.81) |
| Prescriber signature | 526 (95.64) | 107 (92.24) | 374 (80.95) | 179 (99.44) | 1186 (90.67) | |
| Dispenser name | 72 (13.09) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 72 (5.50) | |
| Dispenser signature | 130 (23.64) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 130 (9.93) | |
| Components of drug regimens (per total number of drugs in each hospital) |
Dose | 342 (31.72) | 101 (31.27) | 169 (15.79) | 134 (34.90) | 746 (26.13) |
| Strength | 684 (63.45) | 237 (73.37) | 868 (81.12) | 329 (85.68) | 2118 (74.19) | |
| Dosage Form | 123 (11.41) | 66 (20.43) | 65 (6.07) | 309 (80.47) | 563 (19.72) | |
| Route | 892 (82.74) | 238 (73.68) | 863 (80.65) | 329 (85.68) | 2322 (81.33) | |
| Frequency | 769 (71.33) | 301 (93.19) | 599 (55.98) | 357 (92.97) | 2322 (81.33) | |
| Duration | 499 (46.28) | 104 (32.20) | 635 (59.35) | 278 (72.40) | 1516 (53.10) | |
| Quantity | 534 (49.53) | 226 (69.97) | 393 (36.73) | 372 (96.88) | 1525 (53.42) | |
Abbreviations: HFSUH, Hiwot Fana Specialized University Hospital; FHPH, Federal Harar Police Hospital; JH, Jugal Hospital; SECIIIH, Southeast Command III Hospital.
Regarding specific antibacterial agents, amoxicillin was the antibacterial agent most commonly prescribed in FHPH, JH, and SECIIIH, while ceftriaxone was the most frequently prescribed drug in HFSUH. Ciprofloxacin was the second top prescribed drug in HFSUH (14.14%), FHPH (19.69%), and JH (16.20%) whereas it was the third agent in SECIIIH (13.78%). Overall, the top three antibacterial agents were amoxicillin, ceftriaxone, and ciprofloxacin with prevalence of 24.13%, 17.51%, and 16.00%, respectively (Table 2). Regarding the pharmacologic class of antibacterial agents, penicillins were by far the most commonly prescribed antibacterial agents (n=596, 39.77%), followed by cephalosporins (n= 318, 21.26%) and fluoroquinolones (n=285, 19.05%). Three out of five antibacterial agents prescribed were β-lactam antibiotics (61.03%) (Table 3).
Table 2.
Frequency Distribution of Antibacterial Agents in Antibacterial-Containing Prescriptions Dispensed from January 2016 to December 2016 at Four Governmental Hospitals of Harar Town, Eastern Ethiopia
| Name of Antibacterial Agents | Frequency (%) | ||||
|---|---|---|---|---|---|
| HFSUH (n=550) | FHPH (n=116) | JH (n= 462) | SECIIIH (n=180) | Overall (n= 1308) | |
| Augmentin | 21 (3.33) | 0 | 8 (1.49) | 9 (4.59) | 38 (2.54) |
| Amoxicillin | 73 (11.57) | 48 (36.36) | 148 (27.56) | 92 (46.94) | 361 (24.13) |
| Ampicillin | 44 (6.97) | 0 | 13 (2.42) | 5 (2.55) | 62 (4.14) |
| Azithromycin | 18 (2.85) | 0 | 5 (0.93) | 0 | 23 (1.54) |
| Benzathine PG | 4 | 0 | 0 | 0 | 4 (0.27) |
| Cefixime | 0 | 0 | 1 (0.19) | 0 | 1 |
| Ceftazidime | 0 | 0 | 5 (0. 93) | 0 | 5 (0.33) |
| Ceftriaxone | 182 (28.84) | 9 (6.81) | 67 (12.48) | 4 (2.04) | 262 (17.51) |
| Cephalexin | 13 (2.06) | 1 | 35 (6.52) | 1 (0.51) | 50 (3.34) |
| Chloramphenicol | 0 | 4 (3.03) | 7 (1.30) | 2 (1.02) | 13 (0.87) |
| Ciprofloxacin | 93 (14.74) | 26 (19.69) | 87 (16.20) | 27 (13.78) | 233 (16.00) |
| Clarithromycin | 2 | 1 | 4 (0.74) | 0 | 7 (0.48) |
| Cloxacillin | 43 (6.81) | 11 (8.33) | 46 (8.57) | 8 (4.08) | 108 (7.22) |
| Cotrimoxazole | 33 (5.23) | 10 (7.57) | 29 (5.40) | 29 (14.80) | 101 (6.75) |
| Crystalline Penicillin | 22 (3.49) | 0 | 0 | 0 | 22 (1.47) |
| Doxycycline | 24 (3.80) | 20 (15.15) | 43 (8.01) | 7 (3.57) | 94 (6.28) |
| Erythromycin | 4 | 0 | 3 (0.56) | 1 (0.51) | 8 (0.54) |
| Gentamicin | 29 (4.59) | 1 | 2 (0.37) | 0 | 32 (2.14) |
| Norfloxacin | 11 (1.74) | 0 | 32 (5.96) | 9 (4.59) | 52 (3.48) |
| Tetracycline | 15 (2.37) | 1 | 2 (0.37) | 2 (1.02) | 20 (1.34) |
| Total | 631 | 132 | 537 | 196 | 1496 |
Abbreviations: HFSUH, Hiwot Fana Specialized University Hospital; FHPH, Federal Harar Police Hospital; JH, Jugal Hospital; SECIIIH, Southeast Command III Hospital.
Table 3.
Frequency Distribution of Classes of Antibacterial Agents in Antibacterial-Containing Prescriptions Dispensed from January 2016 to December 2016 at Four Governmental Hospitals of Harar Town, Eastern Ethiopia
| Classes of Antibacterial Agents |
Name of Antibacterial Agents | Frequency (%) | Per group Frequency (%) |
|---|---|---|---|
| Penicillins | Amoxicillin + clavulanic acid | 38 (2.54) | 596 (39.77%) |
| Amoxicillin | 361 (24.13) | ||
| Ampicillin | 62 (4.14) | ||
| Benzathine penicillin G | 4 (0.27) | ||
| Crystalline Penicillin | 22 (1.47) | ||
| Cloxacillin | 108 (7.22) | ||
| Cephalosporins | Cefixime | 1 | 318 (21.26%) |
| Ceftazidime | 5 (0.27) | ||
| Ceftriaxone | 262 (17.51) | ||
| Cephalexin | 50 (3.34) | ||
| Macrolides | Azithromycin | 23 (1.54) | 38 (2.54%) |
| Clarithromycin | 7 (0.48) | ||
| Erythromycin | 8 (0.54) | ||
| Aminoglycosides | Gentamicin | 32 (2.14) | 32 (2.14%) |
| Tetracyclines | Doxycycline | 94 (6.28) | 114 (7.62%) |
| Tetracycline | 20 (1.34) | ||
| Fluoroquinolones Miscellaneous agents |
Ciprofloxacin | 233 (16.00) | 285 (19.05%) |
| Norfloxacin | 52 (3.48) | ||
| Chloramphenicol | 13 (0.87) | 13 (0.87%) | |
| Cotrimoxazole | 101 (6.75) | 101 (6.75%) | |
| Total | 1496 | 100 |
From all prescribing encounters, 1359 drugs were co-prescribed with antibacterial agents. Overall, analgesic and anti-inflammatory agents took the largest percentage share, totaling 43.12%. Specifically, diclofenac, paracetamol, and tramadol were the top three co-prescribed medications with rates of 18.91%, 13.24%, and 7.65%, respectively (Table 4).
Table 4.
Frequency Distribution of Co-Administered Drugs with Antibacterial Agents from January 2016 to December 2016 at Four Governmental Hospitals of Harar Town, Eastern Ethiopia
| HFSUH (n=550) | FHPH (n=116) | JH (n= 462) | SECIIIH (n=180) | Overall (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Co-Administered Drugs | No (%) | Co-Administered Drugs |
No (%) | Co-Administered Drugs |
No (%) | Co-Administered Drugs | No (%) | Drugs (Top Eight Only) |
No (%) |
| Metronidazole | 61(13.64) | Diclofenac | 35 (18.32) | Diclofenac | 132 (24.77) | Paracetamol | 63 (33.51) | Diclofenac | 257 (18.91) |
| Diclofenac | 58(12.98) | Paracetamol | 30 (15.70) | Tramadol | 60 (11.26) | Diclofenac | 32 (17.02) | Paracetamol | 180 (13.24) |
| Paracetamol | 43(9.62) | Chlorpheniramine | 21 (10.99) | Paracetamol | 44 (8.26) | Omeprazole | 12 (6.38) | Tramadol | 104 (7.65) |
| Tramadol | 40(8.95) | Cough syrup | 11 (5.76) | Omeprazole | 39 (7.32) | Ibuprofen | 9 (4.79) | Metronidazole | 100 (7.35) |
| Furosemide | 19(4.25) | Omeprazole | 10 (5.24) | Metronidazole | 33 (6.19) | Metronidazole | 9 (4.79) | Omeprazole | 78 (5.74) |
| Omeprazole | 17(3.80) | Metronidazole | 7 (3.67) | Ibuprofen | 25 (4.69) | Salbutamol | 7 (3.72) | Ibuprofen | 45 (3.31) |
| Metformin | 10(2.24) | Ketoconazole | 6 (3.14) | Cimetidine | 18 (3.38) | Multivitamin | 6 (3.19) | Furosemide | 33 (2.43) |
| Metoclopramide | 10(2.24) | Diphenhydramine | 5 (2.62) | Furosemide (Lasix) | 14 (2.63) | Mebendazole | 5 (2.66) | Cough syrup | 26 (1.91) |
| Tinidazole | 10(2.24) | Salbutamol | 4 (2.09) | Metoclopramide | 13 (2.44) | Cough syrup | 4 (2.13) | Metoclopramide | 23 (1.69) |
| Cimetidine | 8(1.79) | Tramadol | 4 (2.09) | Cough Syrup | 11 (2.06) | ORS | 4(2.13) | —- | —- |
| Ibuprofen | 8(1.79) | Ibuprofen | 3 (1.57) | Nifedipine | 9 (1.69) | TAT | 4(2.13) | —— | —- |
| Others* | 163(36.47) | Others* | 55 (28.8) | Others* | 135 (25.33) | Others* | 33 (17.55) | —- | —- |
| Total | 447 | Total | 191 | Total | 533 | Total | 188 | 1359 | |
Notes: *Variable across hospitals. Others include mebendazole, albendazole, pethidine, ranitidine, metformin, hydrocortisone, simvastatin, bisacodyl, hyoscine, glibenclamide, aspirin, fluconazole, propylthiouracil.
Abbreviations: HFSUH, Hiwot Fana Specialized University Hospital; FHPH, Federal Harar Police Hospital; JH, Jugal Hospital; SECIIIH, Southeast Command III Hospital; ORS, oral rehydration solution; TAT, tetanus antitoxin.
Discussion
The WHO recommends that information on prescriptions must be accurately and legibly written to identify patient-related characteristics including diagnosis, components of the medication(s) regimen, prescribers and dispensers.2 In this study, the analyses indicated that patient-related information such as name, age, sex, and weight of the patient were written in 88.3%, 60.58%, 62.31%, and 1.68% of prescriptions, respectively. Nearly 25% of prescriptions did not contain a patient name at JH. Better patient-related information was obtained from prescriptions dispensed at HFSUH. This might be due to the presence of a higher number of trained medical practitioners (specialists) since it is a tertiary care teaching hospital of Haramaya University. In this regard, a better finding was reported by Getachew et al. on antibiotic prescribing patterns in Northeast Ethiopia where 99.86%, 62.20%, and 88.83% of the prescriptions contained name, sex, and age of the patients, respectively.18 Our finding was also much lower than a study conducted in Asmara, Eritrea where the percentage of prescriptions with patient name, age, and sex were 99.7%, 83.4%, and 89.6%, respectively.12
Full patient-related components including age and body weight are essential for consideration of dose adjustment and recognition of possible contraindications. To this end, the study showed a profound gap in documenting the required patient information. Providing the therapeutic purpose (primary diagnosis) of the prescription can also assist patients in organizing and understanding their medications.2 Therefore, prescriptions with unknown indications vividly indicate the shallow nature of diagnosis and lack of self-confidence in putting forward the ultimate purpose of therapy they have assessed despite the fact that there is a significant shortage of facilities and diagnostic aids in such resource-limited settings.19 Besides, one antibacterial agent might have several indications making it compulsory to differentiate among the purposes of prescription to dispense the drug with the right dose, frequency, and duration of therapy. In this regard, the appearance of a diagnosis in prescription papers was scarce (13.95%) with a zero value observed at SECIIIH. Studies conducted in other areas showed much better findings about prescribing practice of antibiotics. For instance, a study conducted by Woldu et al. indicated that more than 50% of patients received antibiotics without clear indications.20 Findings from six hospitals of Lesotho indicated that no diagnosis was stated in 34.8% of encounters.21 It is further supported by a study conducted by Biswas et al. in three cities in Bangladesh, where the diagnosis was lacking and illegible in 15.1% and 18.9% of prescriptions, respectively.22 Although good diagnostic practice could not be explored in this study, proper recording of diagnosis by itself is an essential part of prescribing information. This is particularly important for the treatment of infectious diseases in which the emergence and spread of AMR might have been catalyzed due to such prescribing errors. In the majority of health-care settings, the prescribing practice primarily depends on empirical knowledge.
Regarding the drug-related information, route and frequency of medicines were the most stated information with equivalent rates, whereas dosage form was the least documented component of drug regimens. What is more, dose and duration of therapy were recorded for about 25% and 50% of the prescriptions, respectively. This finding also showed how important drug-regimen components are disregarded despite the occurrence of adverse health-related consequences including AMR. In a study conducted by Zavaleta-Bustos et al. at Primary Care University of Mexico, prescription error was observed in more than 50% of cases despite the fact that rational prescribing is the first and critical component of rational drug use.2,23 Better prescribing practice was reported from Asmara, Eritrea, where information on prescribed drugs such as dose, frequency, and duration were present in 83.7, 87.7, and 95.1%, respectively.12 Since one of the limiting factors in the effectiveness of antibacterial agents is the concentration of the drug attained in the site of action,24 which in turn depends on the dose administered, neglecting this information might lead to the failure of achieving positive treatment outcomes.
Dispensers can ascertain the legality of the prescription by checking the presence of prescriber name and signature upon receiving it from the patient.14 However, prescriber name was mentioned in less than half of the encounters. This situation has created a great gap in shouldering responsibility. It was also noticed that SECIIIH did not have standard prescription papers at all, whereas JH had a trend of prescribing drugs on pieces of paper, deviating from EFDA and WHO guidelines which recommend prescription papers should be provided in preprinted format with the necessary descriptions.14,15 Not only prescriber but also dispenser identification is also important to ensure the accountability of professionals involved in the medication use process. Surprisingly, this study revealed only a small percentage of prescriptions contained dispenser’s name and/or signature from HFSUH while the rest of the hospitals did not have such record at all. In this regard, assuring the availability of adequate and trained pharmacists and providing standard prescription papers would improve the overall prescribing quality. Pharmacy professionals must also play a pivotal role in detecting, monitoring, and intervening in prescription errors to maximize patient care.25
Among the antibacterial drugs, the overall prescription pattern indicated that amoxicillin, ceftriaxone, and ciprofloxacin were the top three antibacterial agents. Regarding the pharmacologic class of antibacterial agents, penicillins were by far the most commonly prescribed agents followed by cephalosporins and fluoroquinolones. It is now evident that frequent prescribing and misuse of these agents has become associated with increased levels of AMR.26 A similar prescription pattern was observed in Malaysia where antibacterial categories prescribed in descending order of preference were penicillins, cephalosporins, macrolides, quinolones, and tetracyclines.26 In addition, a study from Bangladesh showed that cephalosporins (31.78%), macrolides (27.33%), quinolones (16.33%), and penicillins (7.11%), were found to be the most frequently prescribed antibacterial groups.22 Another study conducted by Baktygul et al. in the Kyrgyz Republic showed that the frequently prescribed antibiotics were penicillins (36.2%), aminoglycosides (20.1%), cephalosporins (18.0%), tetracyclines (3.7), and quinolones 6 (1.4).27 The difference in the prescribing pattern of antibacterial agents might partly be attributable to sociodemographic characteristics, prevalence and nature of infectious diseases in the health-care delivery system. This finding had results similar to a study conducted in a specialized setting in Dakar, Senegal where the most prescribed antibacterial agents were β-lactams (55.43%) and the first antibacterial combination use was amoxicillin-clavulanate (37.66%). Compliance rate of antibacterial prescription to national recommendations was 74.7%.28
Moreover, analgesics and anti-inflammatory drugs including diclofenac, paracetamol, and tramadol were the most commonly co-prescribed drugs with antibacterial agents when the data of all hospitals were combined. A related finding was also reported in a pharmaco-epidemiological study of prescription patterns in southwestern Saudi Arabia.29 This might be a result of the need to alleviate pain and inflammation associated with infectious diseases for which the antibacterial drugs were prescribed. The prescriber should also consider the possibility of drug-drug interactions (e.g. diclofenac with ciprofloxacin and gentamicin) although this study could not explore this aspect.
The Ethiopian Federal Ministry of Health prepared a practical guide to establish and implement an antimicrobial stewardship program in Ethiopian hospitals. The guideline emphasizes the point-of-care interventions including clinical indication, route of administration, dose, frequency, and duration of therapy of antimicrobials, among others.30 To lower prescription writing errors, prescribers should write in compliance with the national and international prescription guidelines and the concerned bodies should strengthen the existing laws and regulations.12 Evidence showed that antibiotic prescribing is influenced by psychosocial factors including lack of accountability, clinician workload, and habit.31 In this regard, continuous professional development programs are recommended to improve the quality of prescription writing among prescribers. This also highlights the need for introducing computerized prescription order entries into general practice.11 Interventions such as effective communication among health-care providers, implementation of electronic prescription systems, physician education on prescription and pharmacists’ involvement in the prescription process should be implemented to improve medication use.10,32
Conclusion
Insufficient patient, drug, and health professional-related information was identified in the prescriptions dispensed at selected hospitals. The study indicated that the prescribing practice of these hospitals did not adhere to the good prescribing manuals of EFDA and WHO. This problem may have resulted in an inappropriate use of antibiotics. This problem requires immediate and sustained action from the management of the hospitals to ensure the accountability of all parties involved in the medication use process. The overall prescription pattern showed amoxicillin, ceftriaxone, and ciprofloxacin to be the three leading prescribed agents. Moreover, analgesics and anti-inflammatory drugs were the most commonly co-prescribed drugs. Generally, frequent and indiscriminate use of antibacterials could result in an increased level of drug resistance, which may necessitate researchers to conduct detailed drug utilization reviews and antibiogram reports in these hospital settings and the establishment of antimicrobial stewardship programs. In addition, pharmacy professionals should receive adequate training in detecting and reporting prescription errors and documenting their interventions, thereby reducing prescription errors.
Limitations of the Study
This research has tried to address the extent of prescription completeness as well as provide an overview of antibacterial utilization pattern in selected hospitals. However, the study was not without limitations. This is a descriptive cross-sectional study which could not address the underlying factors causing this problem. Issues related to good diagnostic practice, antibacterial culture, and drug sensitivity testing could not be explored in this study. This study was the benchmark for a large project that includes in-depth drug use evaluation and antibiogram study.
Acknowledgment
The authors thank data collectors and staffs of all hospitals for their substantial help to realize this research work. The authors also extend their thanks to Haramaya University for granting this research work.
Funding Statement
The authors disclosed reception of financial support from Haramaya University for conducting this research work.
Data Sharing Statement
All the data used for the study are contained within the manuscript.
Ethical Consideration
This study was conducted in accordance with the Declaration of Helsinki. Before starting data collection and preliminary study, the investigating team obtained ethical approval from the Institutional Health Research Ethical Review Committee (IHRERC) of College of Health and Medical Sciences, Haramaya University with reference number: IHRERC/078/2017. Permission letters were also received from Harari regional health office and respective hospital administrators (HFSUH, JH, FHPH and SECIIIH) to conduct this study. Voluntary, informed, written and signed consent was obtained from the administrator of each hospital. Since the study was conducted from secondary data (prescription records), informed consent was not sought from the patients or health-care professionals themselves. However, the confidentiality of patients and health professionals’ information was maintained in such a way that the data abstraction format (checklist) was kept anonymous and data obtained from the hospitals were solely used for this study.
Author Contributions
All authors contributed to conception of the original idea, study design, data analysis, drafting or critically revising the manuscript, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have declared that there is no conflict of interest.
References
- 1.Felmingham D, Cantón R, Jenkins SG. Regional trends in β-lactam, macrolide, fluoroquinolone and telithromycin resistance among Streptococcus pneumoniae isolates 2001–2004. J Infect. 2007;55:111–118. doi: 10.1016/j.jinf.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 2.WHO. Promoting Rational Use of Medicines. WHO Regional Office for South-East Asia; 2011. [Google Scholar]
- 3.WHO. World Medicine Situation Report 2011. 2016.
- 4.Denys GA, Koch KM, Dowzicky MJ. Distribution of resistant gram-positive organisms across the census regions of the United States and in vitro activity of tigecycline, a new glycylcycline antimicrobial. Am J Infect Control. 2007;35:521–526. doi: 10.1016/j.ajic.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 5.Bilal AI, Osman ED, Mulugeta A. Assessment of medicines use pattern using World Health Organization’s prescribing, patient care and health facility indicators in selected health facilities in eastern Ethiopia. BMC Health Serv Res. 2016;16:144. doi: 10.1186/s12913-016-1414-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan FA, Singh VK, Sharma S, Singh P. A prospective study on the antimicrobial usage in the medicine department of a tertiary care teaching hospital. J Clin Diagnostic Res. 2013;7:1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayinalem GA, Gelaw BK, Belay AZ, Linjesa JL Drug use evaluation of ceftriaxone in medical ward of Dessie Referral Hospital, North East Ethiopia. 2013.
- 8.Hamishehkar H, Ebrahimi D, Mahmoodpoor A, Mashayekhi S, Asgharian P, Rezaee H. Drug utilization evaluation of vancomycin in a teaching hospital in Tabriz-Iran. Pharm Sci. 2015;21:25. doi: 10.15171/PS.2015.13 [DOI] [Google Scholar]
- 9.Raza UA, Latif S, Naseer A, Saad M, Zeeshan MF, Qazi U. Introducing a structured prescription form improves the quality of handwritten prescriptions in limited resource setting of developing countries. J Eval Clin Pract. 2016;22:714–720. [DOI] [PubMed] [Google Scholar]
- 10.Altebenaui A, Alrashedi M, Alshammari T, Aljofan M. Completeness of medications prescriptions: prescription errors study in hail region (PeSHR). Pharmacoepidemiol Drug Saf. 2015;24:101. [Google Scholar]
- 11.Al-Worafi YM, Patel RP, Zaidi STR, et al. Completeness and legibility of handwritten prescriptions in Sana’a, Yemen. Med Principles Pract. 2018;27:290–292. doi: 10.1159/000487307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weldemariam DG, Amaha ND, Abdu N, Tesfamariam EH. Assessment of completeness and legibility of handwritten prescriptions in six community chain pharmacies of Asmara, Eritrea: a cross-sectional study. BMC Health Serv Res. 2020;20:1–7. doi: 10.1186/s12913-020-05418-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assefa T, Abera B, Bacha T, Beedemariam G. Prescription completeness and drug use pattern in the University Teaching Hospital, Addis Ababa, Ethiopia. J Basic Clin Pharm. 2018;9:90–95. [Google Scholar]
- 14.FMHACA. Manual for medicines good prescribing practice. Food, Medicine and Healthcare Administration and Control Authority of Ethiopia. 2012. Available from: http://apps.who.int/medicinedocs/documents/s22353en/s22353en.pdf.
- 15.WHO. Guide to Good Prescribing - A Practical Manual:World Health Organization Action Programme on Essential Drugs. WHO/DAP/94.11 Geneva: 1994. [Google Scholar]
- 16.Van der Meer J, Gyssens I. Quality of antimicrobial drug prescription in hospital. Clin Microbiol Infect. 2001;7:12–15. doi: 10.1046/j.1469-0691.2001.00079.x [DOI] [PubMed] [Google Scholar]
- 17.Umeokonkwo CD, Madubueze UC, Onah CK, et al. Point prevalence survey of antimicrobial prescription in a tertiary hospital in south East Nigeria: a call for improved antibiotic stewardship. J Global Antimicrob Resist. 2019;17:291–295. doi: 10.1016/j.jgar.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 18.Getachew E, Aragaw S, Adissie W, Agalu A. Antibiotic prescribing pattern in a referral hospital in Ethiopia. Afr J Pharm Pharmacol. 2013;7:2657–2661. doi: 10.5897/AJPP12.505 [DOI] [Google Scholar]
- 19.Asongalem EA, Monekosso GL, Mbam LA. Indications and patterns of antibiotic prescription in the Buea Regional Hospital of Cameroon. Health Sci Dis. 2015;16. [Google Scholar]
- 20.Woldu MA, Suleman S, Workneh N, Berhane H Retrospective Study of the Pattern of Antibiotic Use in Hawassa University Referral Hospital Pediatric Ward, Southern Ethiopia. 2013.
- 21.Ntšekhe M, Tjipura D Antibiotic Prescribing Patterns at Six Hospitals in Lesotho. Submitt to US Agency International Development by Strength Pharmaceuticals System Program. Arlington, VA: Management Science Health Strength; 2011. [Google Scholar]
- 22.Biswas M, Roy DN, Tajmim A, et al. Prescription antibiotics for outpatients in Bangladesh: a cross-sectional health survey conducted in three cities. Ann Clin Microbiol Antimicrob. 2014;13:15. doi: 10.1186/1476-0711-13-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zavaleta-Bustos M, Castro-Pastrana LI, Reyes-Hernández I, López-Luna MA, Bermúdez-Camps IB. Prescription errors in a primary care university unit: urgency of pharmaceutical care in Mexico. Revista Brasileira De Ciências Farmacêuticas. 2008;44:115–125. doi: 10.1590/S1516-93322008000100013 [DOI] [Google Scholar]
- 24.Li J, Xie S, Ahmed S, et al. Antimicrobial activity and resistance: influencing factors. Front Pharmacol. 2017;8:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadam A, Ganachari M, Bhise S, Gurunath S. Medication errors related to antibiotics in medical intensive care unit in a Tertiary Care Teaching Hospital in South India: a prospective study. J Pharm Res. 2009;2:1245–1248. [Google Scholar]
- 26.Ab Rahman N, Teng CL, Sivasampu S. Antibiotic prescribing in public and private practice: a cross-sectional study in primary care clinics in Malaysia. BMC Infect Dis. 2016;16:208. doi: 10.1186/s12879-016-1530-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baktygul K, Marat B, Ashirali Z, Harun-Or-Rashid M, Sakamoto J. An assessment of antibiotics prescribed at the secondary health-care level in the Kyrgyz Republic. Nagoya J Med Sci. 2011;73:157. [PMC free article] [PubMed] [Google Scholar]
- 28.Dia N, Ismail Y, Lakhe N, Diop B, Seydi M. Evaluation of the quality of prescription of antibiotics with curative intent in a specialized department in Dakar, Senegal. Int J Med Biomed Res. 2018;7:21–31. [Google Scholar]
- 29.Al-Homrany MA, Irshaid YM. Pharmacoepidemiological study of prescription pattern of analgesics, antipyretics, and nonsteroidal anti-inflammatory drugs at a tertiary health care center. Saudi Med J. 2007;28:369–374. [PubMed] [Google Scholar]
- 30.FMoH. A Practical Guide to Antimicrobial Stewardship Program in Ethiopian Hospitals. Addis Ababa: Federal Ministry of Health, Ethiopia; 2018. Available at:https://www.ghsupplychain.org/sites/default/files/2019-03/Guide%20to%20Antimicrobial%20Stewardship%20Program%20in%20Hospitals.pdf. Accessed September 30, 2020. [Google Scholar]
- 31.King LM, Fleming-Dutra KE, Hicks LA. Advances in optimizing the prescription of antibiotics in outpatient settings. BMJ. 2018;363:k3047. doi: 10.1136/bmj.k3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alwhaibi M, Balkhi B, Alshammari TM, et al. Measuring the quality and completeness of medication-related information derived from hospital electronic health records database. Saudi Pharm J. 2019;27:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]


