Abstract
Small bowel neuroendocrine tumors (SBNETs) are rare cancers originating from enterochromaffin cells of the gut. Research in this field has been limited because very few patient derived SBNET cell lines have been generated. Well-differentiated SBNET cells are slow growing and are hard to propagate. The few cell lines that have been established are not readily available, and after time in culture may not continue to express characteristics of NET cells. Generating new cell lines could take many years since SBNET cells have a long doubling time and many enrichment steps are needed in order to eliminate the rapidly dividing cancer-associated fibroblasts. To overcome these limitations, we have developed a protocol to culture SBNET cells from surgically removed tumors as spheroids in extracellular matrix (ECM). The ECM forms a 3-dimensional matrix that encapsulates SBNET cells and mimics the tumor micro-environment for allowing SBNET cells to grow. Here, we characterized the growth rate of SBNET spheroids and described methods to identify SNBET markers using immunofluorescence microscopy and immunohistochemistry to confirm that the spheroids are neuroendocrine tumor cells. In addition, we used SBNET spheroids for testing the cytotoxicity of rapamycin.
Keywords: Small bowel neuroendocrine tumors, spheroids, synaptophysin, chromogranin A, SSTR2, immunofluorescence, immunohistochemistry, extracellular matrix, rapamycin
SUMMARY:
Neuroendocrine tumors (NETs) originate from neuroendocrine cells of the neural crest. They are slow growing and challenging to culture. We present an alternative strategy to grow NETs from the small bowel by culturing them as spheroids. These spheroids have small bowel NET markers and can be used for drug testing.
INTRODUCTION:
Small bowel neuroendocrine tumors (SBNETs) originate from enterochromaffin cells of the small intestine. Although SNBETs are generally known to grow slowly, they commonly metastasize to the liver1. While the surgical removal or tumor ablation can be considered in many cases, recurrence is nearly universal, and, therefore, medical therapy plays an important role in management. Tremendous efforts have been invested to generate new SBNET cell lines for drug testing. However, there has been very little success. Only 6 SBNET cell lines (KRJ-I, CND2, GOT1, P-STS, L-STS, H-STS) have been reported2–5; and unfortunately one cell line no longer expresses NET markers6 and three other SBNET cell lines (KRJ-I, L-STS, H-STS) were determined to be derived from transformed lymphoblasts instead of NETs7. In order to accelerate the identification of drugs for targeting SBNETs, alternative methods for in vitro drug testing are needed.
Here, we take an advantage of the availability of resected SBNETs and have established a way to culture these patient derived SBNETs as spheroids growing in ECM. The overall goal of this manuscript is to describe a method to culture SBNET as a three-dimensional (3D) culture and outline procedures to characterize these spheroids for the retention of SBNET markers by immunofluorescence staining and immunohistochemistry.
In addition, we demonstrate how these SBNET spheroids can be used for testing the effect of rapamycin, an anti-cancer drug for NETs8. The rationale behind this protocol is to develop a new method to grow SBNET cells in vitro and use them for drug testing. The advantage of this technique over the traditional method of establishing a SBNET cell line is that 3D cultures of SBNETs can rapidly be obtained and drug testing can be done within 3 weeks. SBNET spheroids could potentially be used as a model for performing in vitro drug screens to identify new drugs for SBNET patients. Since SBNET cell lines are not widely available, 3D cultures of SBNET spheroids can serve as a new in vitro model for studying SBNETs and can be shared among scientists in the field.
PROTOCOL:
All experiments using human neuroendocrine tumor samples have been approved by the University of Iowa Hospital and Clinics IRB committee (Protocol number 199911057). A list of all materials and equipment is described in the Table of Materials. A list of growth media and key solutions is found in Table 1.
TABLE 1.
LIST OF MATERIAL/EQUIPMENT
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
|---|---|---|---|
| Anti-rabbit FITC | Jackson ImmunoResearch | 11-095-152 | Secondary antibody couple to a green fluorophore |
| Antigen Retrieval Solution | Agilent Dako | S2367 | Solution at pH 9 for preparing slides for IHC |
| Autostainer Link 48 | Agilent Dako | Not Available | Automated system for antibody staining |
| Cell freezing container | Thermo Scientific | 5100-0001 | Container to for freezing cells |
| CellSence | Olympus | Version 1.18 | Computer software for using fluorescent microscope |
| Chromogranin A antibody | Abcam-45179 | RB-9003-PO | Antibodies for IF |
| Chromogranin A antibody (clone LK2H10) | Thermo Scientific | MA5-13096 | Antibodies for IHC |
| Collagenase | Sigma | C0130 | Enzyme for digesting tumor tissue |
| DMEM | Gibco | 11965-092 | Medium for tissue preparation |
| DMEM/F12 | Gibco | 11320-033 | Medium for organoid cultures |
| DMSO | Sigma | D8418 | Solvent for dissolving drug |
| DNAse | Sigma | DN25 | Enzyme for digesting tumor tissue |
| Ethidium Homodimer | Chemodex | CDX-E0012-T1E | DNA and RNA binding dye |
| FBS | Gibco | 16000044 | Reagent for culture media |
| Fluorescent microscope | Olympus | CKX35 | Microscope for taking pictures of SBENT spheroids |
| Glutamine | Gibco | A2916801 | Reagent for culture media |
| ImageJ | National Institutes of Health | Version 1.51 | Computer software for image analysis |
| Insulin | Sigma | I0516 | Reagent for culture media |
| Matrigel | Corning | 356235 | Matrix to embed and anchore organoids |
| Mounting medium (VECTASHIELD) | Vector Laboratories | H-1200 | Fixative for labelled-cells with a nuclear stain |
| Nicotinamide | Sigma | 72340 | Reagent for culture media |
| Paraformaldehyde | Electron Microscopy Sciences | 15710 | Reagent to fix cells |
| PEN/STREP | Gibco | 15140-122 | Reagent for culture media |
| PT Link | Agilent Dako | Not Available | Automated system to prepare slides for IHC staining |
| Rapamycin | Alfa Aesar | J62473 | Drug that can inhibit NET growth |
| Secondary antibodies for IHC | Agilent Dako | K8000 | Secondary antibodies for IHC using Polymer-based EnVision FLEX system |
| SSTR2 antibody | GeneScritp | A01591 | Antibodies for IF |
| SSTR2 antibody (clone UMB1) | Abcam | ab134152 | Antibodies for IHC |
| Synaptophysin antibody | Abcam | 32127 | Antibodies for IF |
| Synaptophysin antibody (clone DAK-SYNAP) | Agilent Dako | M7315 | Antibodies for IHC |
| TritonX | Mallinckrodt | 3555 KBGE | Reagent to permeablize cells |
| Y-2763 ROCK inhibitor | Adipogen | AG-CR1-3564-M005 | To improve SBNET spheroid viability after freeze thaw |
1. Small bowel neuroendocrine tumor (SBNET) collection and cell dissociation
1.1. Obtain resected patient SBNET samples after tumor tissues confirmation from the Surgical Pathology Core.
1.2. Cut SBNETs into 5 mm2 cubes and store in 25 mL of DMEM/F12 medium in a conical tube for the transportation to the laboratory.
1.3. Transfer the tumors in DMEM containing 1% FBS, 1% penicillin/streptomycin (Pen/Strep), 1% glutamine (Wash Medium) and incubate in this Wash Medium for 15 min.
1.4.Transfer tumors to a new dish and mince tumors to less than 1 mm pieces using sterile curved scissors.
1.5.Transfer the minced tissues to a new 50 mL tube containing 25 mL of Wash Medium.
1.6.Centrifuge the sample at 500 x g for 15 min at 4 °C.
1.7. Discard the supernatant and resuspend the pellets in 10 mL of Wash Medium containing collagenase (100 U/mL) and DNase (0.1 mg/mL).
1.8. Allow the digestion of the minced tumors to occur in a 37 °C incubator with slow shaking (50 rpm) for 1.5 h.
2. Culture of SBNETs as tumor spheroids in ECM
2.1. After digestion is completed (step 1.8), centrifuge at 500 x g for 15 min at 4 °C, discard the supernatant and resuspend the pellet in 15 mL of Wash Medium.
2.2. Place a 70 μm cell strainer on top of a new 50 mL tube and transfer 10 mL of the Wash Medium over the cell strainer. Swirl the Wash Medium to cover the side of the plastic tube to prevent NET cells from sticking to the side of the plastic tube.
2.3. Filter the cell suspension through cell strainer.
2.4. Centrifuge at 500 x g for 15 min at 4 °C, discard the supernatant, resuspend the pellet in 200 μL of Wash Medium (total volume is ~ 250 μL) and place it on ice.
2.5. Transfer 5 μL of cells to 500 μL of Wash Medium (1/100 dilution factor). Use the diluted cells for cell counting using a hemocytometer to obtain the number of cells per milliliter. Multiply by 100 to correct for the dilution factor.
2.6. Multiply the number of cells per mL obtained in step 2.5 by the total volume of cells in suspension obtained in step 2.4 (~ 250 μL).
2.7. Centrifuge at 500 x g for 15 min at 4 °C, discard the supernatant, and resuspend the cells in liquid ECM (1 x 106 cells/mL) and keep on ice.
2.8. Transfer 5-20 μL of SBNET spheroids in ECM to a 96-well plate and allow the liquid ECM to solidify by placing the plate in a 37 °C incubator for 5 min.
2.9. Add 200 μL of SBNET culture medium (DMEM/F12 + 10% FBS + 1% PEN/STREP + 1% Glutamine + 10 mM nicotinamide + 10 μg/mL insulin) to each well of the 96-well plate containing the SBNET spheroids in ECM.
2.10. Alternatively, culture SBNET organoids in stem cell media similar to what has previously been described for growing either human liver or pancreatic organoids9 and listed in the Table of Materials.
2.11. Change media every 5-7 days.
3. Quantification of SBNET spheroid size using ImageJ
3.1. Take 5-10 pictures of SBNET spheroids at Day 1, 4, 7, 14, 23 and 97 of the culture using a 10x objective.
3.2. Open saved images in ImageJ. Go to the Analyze tab, select the Set Measurements option and place a check on the Area option.
3.3. Use the oval selection tool from the tool bar to generate an ellipsoid or circle around a well-focused spheroid.
3.4. Go to the Analyze tab and select the Measure option. Repeat steps 3.4 and 3.5 in order to get area measurement from 25-50 SBNET spheroids. The values are provided in pixel square.
3.5. Convert the pixel area to μm2 by dividing the size of each pixel with the length of each pixel to μm conversion factor of the microscopy images.
NOTE: SBNET cells grow mainly as spheroids and sometime as ellipsoids.
4. Characterization of SBNETS spheroids by immunofluorescence
4.1. Transfer the organoids grown in ECM to a 1.5 mL tube using a P1000 pipette.
4.2. Centrifuge at 1,500 x g for 1 min and remove the supernatant.
4.3. Wash the organoid culture by adding 1 mL of PBS, mix, centrifuge at 1,500 x g for 1 min and remove the supernatant.
4.4. Fix the organoids by adding 500 μL of 4% paraformaldehyde and incubate for 15 min.
4.5. Wash the culture twice with 1 mL of PBS.
4.6. Permeabilize the culture by adding 500 μL of PBS + 3% BSA + 0.1% Triton X 100 for 5 min.
4.7. Then wash for three times with 1 mL of PBS + 3% BSA.
4.8. Incubate for 1 h with primary antibodies against synaptophysin (SYP)10 at 1/600 dilution or chromogranin A (CgA)11 and somatostatin receptor 2 (SSTR2)12 at 1/400 dilution in antibody buffer (2.5% bovine serum albumin, 0.1% sodium azide, 25 mM Tris pH 7.4, 150 mM sodium chloride).
NOTE: Use 300 μL or more of antibody solution per tube.
4.9. Wash three times with 1 mL of PBS + 3% BSA.
4.10. Incubate with secondary antibodies coupled to FITC at 1/500 dilution in antibody buffer for 1 h. Use 300 μL or more of antibody solution per tube.
4.11. Wash 3 times with 1 mL of PBS + 3% BSA. Make sure to aspirate and discard all the supernatant.
4.12. Add 5 μL of mounting medium containing the nuclear stain DAPI.
4.13. Use a P20 pipette to transfer 5 μL of the SBNET spheroids from step 4.12 to a glass slide and seal with a cover slip.
4.14. Take images using a fluorescent microscope using the 10x, 20x or 40x objectives.
5. SBNET spheroids characterization by immunohistochemistry (IHC)
5.1. Transfer SBNET spheroids from the culture plate to a 1.5 mL tube by using a P1000 pipette.
5.2. Centrifuge at 1,500 x g for 1 min and remove the supernatant.
5.3. Fix SBNET spheroids by adding 500 μL of 10% formalin to the tube from step 5.2 and incubate at room temperature for 2-5 days prior to paraffin embedding and cut 4 μm thick spheroid sections.
5.4. Deparaffinize, rehydrate, and use heat-induced epitope retrieval in Antigen Retrieval Solution at pH 9 by heating to 97 °C for 20 min using the 3-in-1 automated slide processing station to prepare slides for antibody incubation.
5.5. Block slides and incubate with primary antibodies against SYP10 at a 1/100 dilution for 15 min, CgA11 at a 1/800 dilution for 15 min, and SSTR212 at a 1/5,000 dilution for 30 min.
5.6. Incubate with the secondary antibody detection system for 15 min for the anti-SYP and CgA staining and 30 min for anti-SSTR2 staining. Take pictures using a 400x objective.
6. Treatment of SBNET organoids with rapamycin
6.1. Dissolve rapamycin in DMSO to obtain a 10 mM stock solution.
6.2. Prepare SBNET culture medium with DMSO (e.g., 1 mL of SBNET culture medium + 1 μL of DMSO) and SBNET culture medium with 10 μM of rapamycin (e.g., 1 mL of SBNET culture medium + 1 μL of 10 mM rapamycin stock solution).
6.3. Transfer 200 μL media with SBNET culture medium with DMSO or 10 μM rapamycin to SBNET spheroid cultures.
6.4. Incubate SBNET spheroids for 5 days in 37 °C incubator.
6.5. Add 1 μM of ethidium homodimer and incubate for 30 min. Remove the medium containing ethidium homodimer, wash with 200 μL of PBS, and transfer 200 μL of PBS to each well.
6.6. Take microscopy images of SBNET spheroids with and without drug treatment using the red filter cube with the 10x, 20x, or 40x objectives of a fluorescent microscope.
NOTE: Dead cells stained with Ethidium homodimer appears as red using the red filter cube G of a commercially available microscope (Table of Materials). Alternatively, the signal can be captured using a spectrophotometer at 535 nm excitation and 624 nm emission.
7. Splitting SBNET spheroids
NOTE: This is done for expansion and for sharing with other researchers.
7.1. Use a P1000 pipette to mechanically break the ECM and aspirate the ECM with SBNET spheroids to a sterile 1.5 mL tube.
7.2. Centrifuge at 1,000 x g at 4 °C, remove all the supernatant and place the tube on ice.
7.3. Add 2-4x the volume of new ECM to the pellet. Mix the new ECM with the old ECM and SBNET spheroids by pipetting up and down 10x. Avoiding introducing air bubbles.
7.4. Transfer 5-20 μL of the ECM and SBNET spheroids mixture to a new plate and allow ECM to solidify.
7.5. Cover with new SBNET medium and transfer to the incubator. The recovery rate is approximately 95 to 100%.
7.6. For shipping SBNET spheroids to another lab, transfer SBNET spheroids with new ECM to a T25 flask and allow ECM to solidify.
7.7. Fill the T25 flask with SBNET spheroid medium, screw on the cap tightly, and prepare shipment package.
7.8. Upon receiving an SBNET spheroid culture, remove the culture medium and perform step 7.1 to 7.5 to put SBNET spheroids back in culture.
8. Cryostorage and recovery of SBNET spheroids
8.1. Transfer SBNET spheroids from 5-10 small wells to a 15 mL tube. Centrifuge at 500 x g for 15 min at 4 °C, remove supernatant, resuspend in freezing medium (90% FBS + 10% DMSO), store in a cell freezing container, and place this at −80 °C.
8.2. Transfer to the liquid nitrogen for longer storage.
8.3. To recover SBNET spheroids, place the frozen vial on ice and wait until the content is completely thawed. Invert the tube and re-place on ice in order to speed up the thawing process.
8.4. Once the sample is thawed, placed inside a pre-chilled centrifuge and spin at 1,000 x g for 10 min.
8.5. Remove the supernatant and resuspend the spheroids in ECM. Keep the tube on ice, transfer 20 μL to a new plate, and wait for the ECM to solidify.
8.6. Transfer 200 μL of SBNET culture medium with 10 μM of ROCK inhibitor (Y-27632)13 and transfer to the incubator.
8.7. Allow SBNET spheroids to recover and grow for 1 week. Remove the old culture medium, replenish with 200 μL of SBNET culture medium and return to the incubator.
8.8. Allow SBNET spheroids to continue to grow.
NOTE: It takes at least 1 week for rapidly growing spheroids to start to recover after cryopreservation13. SBNET spheroids take a minimum of 2-4 weeks to start growing. Many SBNET cells will die within the first 2 weeks and the survival rate is less than 10%. Since this is a very time-consuming and low yield process, it is better to share with other researchers SBNET spheroids in culture flasks (as described in step 7). Cryostorage and recovery should only be used as a backup plan in case of a bacterial contamination occurs.
REPRESENTATIVE RESULTS
There are currently only 2 SBNET cell lines established and published2–5 and they are not readily available to many researchers. Here, we propose to culture SBNET as spheroids in ECM and use this as an alternative model to study SBNET drug sensitivity. Patient-derived tumor from an SBNET that metastasized to the liver was collected, digested to release SBNET cells, and mixed with liquid ECM for establishing an SBNET spheroid culture (Figure 1A). The Ki-67 of this SBNET was 4.3%. Although SBNET spheroids have a slow growth rate, their growth can be monitored by microscopic imaging (Figure 1A). It takes approximately 14 days for SBNET spheroids to double in size when the culture media is changed once a week (Figure 1B). After 14 days in culture, SBNET spheroids do not increase in size. Instead, some SBNET cells will dissociate to a neighboring location and form new spheroids. To propagate the SBNET spheroids culture, harvest the ECM containing SBNET spheroids and reseed them in new culture plate with new culture medium (Step 7).
Figure 1: Patient-derived small bowel neuroendocrine tumors (SBNETs) grown in extracellular matrix (ECM) as spheroids.
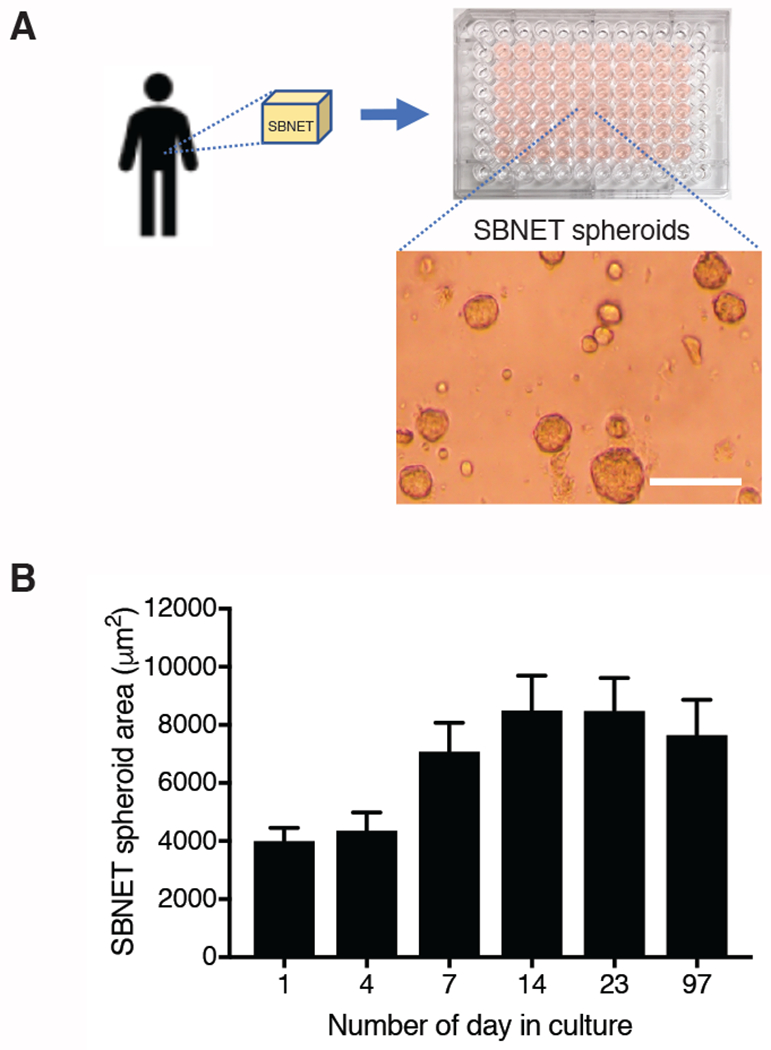
(A) Isolation of tumor cells from a resected SBNET and put in culture by mixing with ECM. Scale bar represents 100 μm. (B) Surface area of SBNET spheroids with respect to the number of days in culture quantified using ImageJ. Data were obtained from SBNET spheroids of 1 patient and are represented as the mean area ± standard error of the mean. The surface area of 30 to 60 spheroids were measured for each time point.
To confirm that the organoid cultures contain SBNET cells, we describe a simple and fast method to stain the spheroids for SBNET markers such as synaptophysin, chromogranin A, and the somatostatin receptor type 2 (SSTR2) using immunofluorescence (IF) microscopy (Step 4). Using antibodies specific against synaptophysin, chromogranin A and SSTR2, our IF data showed that these markers are localized in the cytoplasm and at the membrane of SBNET cells (Figure 2A, in green) after 1 or 9 months in culture (Figure 2B). To ensure the specificity of the SYP, CgA and SSTR2 antibodies, we performed the same staining procedures on an organoid line from pancreas tumor that does not express SYP, CgA or SSTR2 (Figure 2C) as no green signal was detected. The main advantage of this SBNET spheroid IF experiment is that it can be performed within 4 h and gives similar staining information as the immunohistochemistry (IHC; Figure 3). We provide a protocol for performing IHC of the SBNET spheroids in step 5.
Figure 2: Immunofluorescence (IF) staining of SBNET spheroids.
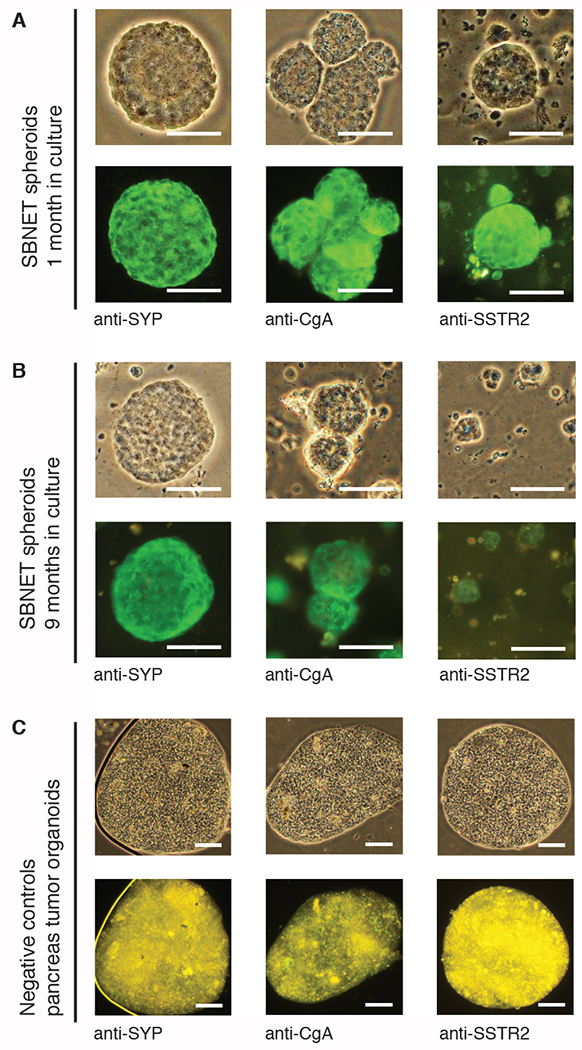
IF staining of SBNET spheroids after (A) 1 month in culture and (B) 9 months in culture. (C) IF staining of pancreas tumor organoids that do not express SBNET markers as negative controls. Tumor spheroids were fixed in 4% paraformaldehyde and stained using antibodies against synaptophysin (SYP) at 1/600 dilution, chromogranin A (CgA) at 1/400 dilution, and somatostatin receptor 2 (SSTR2) at 1/400. IF images were taken at 100 ms, 200 ms and 400 ms exposure time for SYP, CgA and SSTR2 staining, respectively using the 10x, 20x or 40x objectives. Scale bar represents 50 μm.
Figure 3: Immunohistochemistry (IHC) staining of SBNET spheroids.
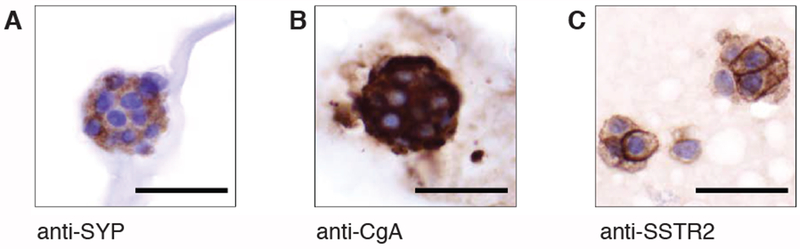
Formalin-fixed and paraffin-embedded SBNET spheroids sections were deparaffinized, rehydrated, blocked and stained with (A) SYP, (B) CgA, and (C) SSTR2 antibodies. Images were taken using the 400x objective. Scale bar represents 50 μm.
Culturing SBNET as spheroids is a valuable technique for identifying drugs that can inhibit SBNET growth. As a proof of principle, we treated SBNET spheroids with rapamycin for 5 days, an mTOR inhibitor, a class of drugs commonly used to treat NETs8. In comparison to our control SBNET spheroid, the rapamycin-treated spheroid formed a grape-like structure and became apoptotic or necrotic (Figure 4A–D). Dying cells can be detected using Ethidium homodimer to stain DNA and RNA and generate a bright signal14. This dye cannot penetrate the cell membrane of live cells.
Figure 4: Using SBNET spheroids for drug testing.
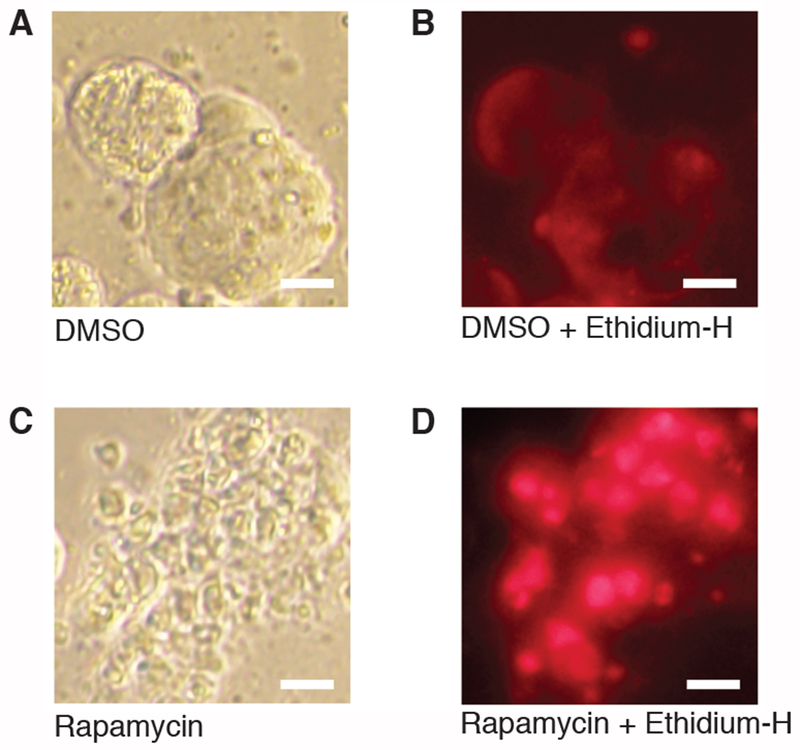
(A) Bright field image of SBNET spheroids treated with DMSO for 5 days. (B) Image of SBNET spheroids treated with DMSO and stained with Ethidium homodimer (Ethidium H). (C) Bright field image of a dead SBNET spheroid forming grape-like structure after treatment with 10 μM of rapamycin for 5 days. (D) Dead SBNET spheroid stained with Ethidium H appears as red dots. Images of Ethidium H staining were taken using the red filter cube at 100 ms exposure time. Scale bar represents 10 μm.
DISCUSSION
Tumor 3D cultures have become a valuable resource for preclinical drug testing15. Various tumor organoid biobanks have recently been established from breast cancer and prostate cancer tumors16,17. In this study, we provide a detailed protocol to culture SBNET as spheroids and a simple and fast method to validate the spheroid cultures for NET markers by immunofluorescence and test drug sensitivity. From our experience, SBNET spheroids can grow in various culture media. They grow slightly faster in stem cell media for human pancreas or liver isolation that we adapted from previously published protocols9. We chose to grow the SBNETs in DMEM/F12 medium supplemented with insulin and nicotinamide because this is less expensive than stem cell media. Increasing the percentage of fetal bovine serum is another strategy to promote the growth of SBNET organoid cultures; however, this would also increase the overall cost for culture maintenance.
Performing IF staining and imaging with a fluorescent microscopy using the 10x, 20x or 40x objectives is a quick and simple method to test for the expression SBNET markers. However, it does not give a well-defined localization in comparison to IHC (Figure 2, Figure 3). For example, the IF data showed that the membrane localization of SSTR2 is difficult to detect. We mainly detect the cytosolic SSTR2, which has previously been reported18. In order to obtain a better localization of the marker proteins, we recommend using IF and confocal microscopy. Overall, IF is a useful method to rapidly confirm SBNET markers. Our antibodies (anti-SYP, anti-CgA, anti-SSTR2) did not cross-react with the negative control organoids that do not express SYP, CgA, or SSTR2 (Figure 2C) in the IF experiment. This suggestion that the fluorescent signals that we detected are specific to SBNET spheroids.
To increase the yield of SBNET spheroids, the critical steps of this protocol are during the cell filtration step (step 2.2) and mixing the SBNET with the liquid ECM for aliquoting into tissue culture plates (step 2.8). Make sure to cover the cell strainer membrane and the collection tube with media in order to prevent the SBNET cells from sticking to the plastic. Liquid ECM will rapidly solidify if not placed on ice. Make sure to have a small ice container in the tissue culture hood in order to keep the ECM and SBNET cells cold.
The limitation of this technique is the slow growth rate of the SBNET spheroids. Experiments must be planned efficiently and avoid using an excess amount of SBNET spheroids. Another limitation is the slow recovery after freeze thaw. It takes over 1 month after thawing for the SBNET spheroids to start growing. To overcome this limitation, we suggest to continuously maintain SBNET spheroids in cultures and splitting them as needed (step 7). Even after 9 months in culture, the SBNET spheroids still maintain expression of SBNET markers (Figure 2B).
Although 3D culture of SBNET spheroids is more labor intensive than traditional 2D culture of other cancer cell lines, it is an extremely valuable model for in vitro culture of SBNET because many SBNET researchers do not have access to the existing cell lines. With this protocol, scientists and clinicians can establish SBNET spheroid cultures from resected tumors and share them with other laboratories. In addition, the SBNET spheroids could potentially be used to establish SBNET patient-derived xenograft mouse models. Overall, the techniques presented here can be adapted for culturing, characterizing and performing drug testing of other NETs such as pancreatic or lung NETs. Note that the growth rate of the organoids will vary between the different types of NETs and patient samples.
TABLE 2.
LIST OF GROWTH MEDIA AND SOLUTIONS
| Growth media or solution | Composition |
|---|---|
| Wash Medium | DMEM containing 1% FBS, 1% PEN/STREP, 1% Glutamine |
| SBNET culture medium | DMEM/F12 + 10% FBS + 1% PEN/STREP + 1% Glutamine + 10 mM nicotinamide + 10 μ/mL insulin |
| Antibody buffer | 2.5% bovine serum albumin, 0.1% sodium azide, 25 mM Tris pH 7.4, 150 mM sodium chloride |
| Freezing medium | 90% FBS + 10% DMSO |
| Human liver stem cell isolation medium | DMEM/F12 , 1% Pen/Strep, 1% GlutaMAX, 10 mM HEPES, 1/50 B27 Supplement, 1/100 N2 Supplement, 1 mM N-acetylcysteine, 200 ng/mL Rspo 1, 50ng/mL EGF, 100 ng/mL FGF10, 10 mM nicotinamide, 10 uM forskolin, 5uM A83-01 |
| Human pancreatic stem cell isolation medium | DMEM/F12 , 1% Pen/Strep, 1% GlutaMAX, 10 mM HEPES, 1/50 B27 Supplement, 1/100 N2 Supplement, 1 mM N-acetylcysteine, 200 ng/mL Rspo 1, 25 ng/mL Noggin, 50ng/mL EGF, 100 ng/mL FGF10, 10 mM nicotinamide, 10 uM forskolin, 5uM A83-01, 3 uM PGE-2 |
ACKNOWLEDGMENTS
This work was supported by NIH grants P50 CA174521 (to J.R. Howe and A.M. Bellizzi). P.H. Ear is a recipient of the P50 CA174521 Career Enhancement Program award.
Footnotes
DISCLOSURES
The authors have nothing to disclose.
REFERENCES
- 1.Maxwell JE, Sherman SK, Howe JR Translational Diagnostics and Therapeutics in Pancreatic Neuroendocrine Tumors. Clinical Cancer Research. 22, 5022–5029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfragner R et al. Establishment of a continuous cell line from a human carcinoid of the small intestine (KRJ-I). International Journal of Oncology. 8, 513–520 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Kolby L et al. A transplantable human carcinoid as model for somatostatin receptor-mediated and amine transporter-mediated radionuclide uptake. American Journal of Pathology. 158, 745–755 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Buren G et al. The development and characterization of a human midgut carcinoid cell line. Clinical Cancer Research. 13, 4704–4712 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Pfragner R et al. Establishment and characterization of three novel cell lines - P-STS, L-STS, H-STS - derived from a human metastatic midgut carcinoid. Anticancer Research. 29, 1951–1961 (2009). [PubMed] [Google Scholar]
- 6.Ellis LM, Samuel S, Sceusi E Varying opinions on the authenticity of a human midgut carcinoid cell line--letter. Clinical Cancer Research. 16, 5365–5366 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Hofving T et al. The neuroendocrine phenotype, genomic profile and therapeutic sensitivity of GEPNET cell lines. Endocrine Related Cancer. 25, 367–380 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreno A et al. Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocrine Related Cancer. 15, 257–266 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Broutier L et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nature Protocols. 11, 1724–1743 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Saito Y et al. Establishment of Patient-Derived Organoids and Drug Screening for Biliary Tract Carcinoma. Cell Reports. 27, 1265–1276 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Park SJ et al. Detection of bone marrow metastases of neuroblastoma with immunohistochemical staining of CD56, chromogranin A, and synaptophysin. Applied Immunohistochemisty and Molecular Morphology. 18, 348–352 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Clifton-Bligh RJ et al. Improving diagnosis of tumor-induced osteomalacia with Gallium-68 DOTATATE PET/CT. The Journal of Clinical Endocrinology and Metabolism. 98, 687–694 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Clinton J, McWilliams-Koeppen P Initiation, Expansion, and Cryopreservation of Human Primary Tissue-Derived Normal and Diseased Organoids in Embedded Three-Dimensional Culture. Current Protocols in Cell Biology. 82, e66 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Markovits J, Roques BP, Le Pecq JB Ethidium dimer: a new reagent for the fluorimetric determination of nucleic acids. Analytical Biochemistry. 94, 259–264 (1979). [DOI] [PubMed] [Google Scholar]
- 15.Weeber F, Ooft SN, Dijkstra KK, Voest EE Tumor Organoids as a Pre-clinical Cancer Model for Drug Discovery. Cell Chemical Biology. 24, 1092–1100 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Sachs N et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell. 172, 373–386 e310 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Puca L et al. Patient derived organoids to model rare prostate cancer phenotypes. Nature Communication. 9, 2404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh SP et al. SSTR2-based reporters for assessing gene transfer into non-small cell lung cancer: evaluation using an intrathoracic mouse model. Human Gene Therapy. 22, 55–64 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]


