Abstract
Objective
To observe whether whole-brain radiotherapy (WBRT) can bring survival benefits to patients with multiple brain metastases (BM) from non-small cell lung cancer (NSCLC) treated by epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) and determine the best time for WBRT intervention.
Methods
A retrospective analysis was performed on 148 patients diagnosed with EGFR gene-mutated NSCLC. All patients had multiple BM and received EGFR-TKI targeted therapy, which was performed to observe whether WBRT can bring survival benefits, and whether the choice of WBRT timing affects the survival of patients.
Results
Among the 148 patients with NSCLC treated with EGFR-TKI, 76 received WBRT; 72 were without WBRT. WBRT can reduce the intracranial progression rate in the patients (19.7% vs 33.3%, P=0.040), thus improving the intracranial progression-free survival (iPFS) (median iPFS: 11.9 months versus 10.2 months, P=0.039) and overall survival (OS) (median OS: 21.0 months versus 16.7 months, P=0.043). Multivariate analysis showed that WBRT (HR=0.606; 95% CI: 0.403–0.912, P=0.016) and the low Eastern Cooperative Oncology Group performance status (HR=1.884; 95% CI: 1.120–3.170, P=0.017) are independent prognostic factors in all patients. Further subgroup analysis showed that the choice of WBRT time had no effect on patient survival.
Conclusion
WBRT can improve the survival of patients with multiple BM from NSCLC receiving EGFR-TKI targeted therapy and is an independent prognostic factor. The choice of RT time has no effect on patient survival.
Keywords: epidermal growth factor receptor-tyrosine kinase inhibitors, whole-brain radiotherapy, non-small cell lung cancer, multiple brain metastases
Background
Lung cancer has the highest morbidity and mortality in China;1 brain metastases (BM) are common, especially the non-small cell lung cancer (NSCLC) type, accounting for about 20–40% and showing poor prognosis.2 Multiple BM are the common form of BM, and whole-brain radiotherapy (RT) (WBRT) is the main treatment method. However, the survival rate of patients is still unsatisfactory.3,4
Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) targeted therapy can significantly improve the survival of EGFR-mutated NSCLC patients.5,6,7 Whether EGFR-TKI alone without RT is effective in patients with BM remains controversial. Several scholars believe that although the cerebrospinal fluid permeability is low,8 and the effect of intracranial metastases control is good, brain RT causes no improvement on the survival of patients; certain patients suffer from significantly reduced quality of life due to the neurotoxicity caused by RT.3,4 Thus, clinical support exists for the use of EGFR-TKI alone in the treatment of NSCLC patients with BM.9,10,11,12 However, brain RT can improve the survival of NSCLC patients under EGFR-TKI targeted therapy.13,14,15 The correct management of EGFR mutations in NSCLC BM remains controversial, and the optimal treatment options and order have not been established.
For multiple BM with EGFR mutation, whether WBRT can bring survival benefits, related research on the best time for WBRT intervention in patients with EGFR-TKI targeted therapy is relatively limited. Therefore, we conducted a retrospective study to compare the efficacy of EGFR-TKI combined with WBRT and EGFR-TKI alone in patients with multiple NSCLC BM.
Materials and Methods
Patient Information
A total of 148 patients histologically diagnosed with NSCLC between January 2011 and June 2019 in The Second People’s Hospital of Lianyungang were considered eligible for the study. Each patient was confirmed to have multiple BM (BM ≥3) and had received EGFR-TKI targeted therapy. Clinical staging based on the 8th edition of Classification of TNM Lung Cancer, clinical information, including gender, age, smoking history, tumor type, basic disease, family history, Eastern Cooperative Oncology Group (ECOG) performance status (PS), WBRT, EGFR mutation type, extracranial metastasis, and EGFR-TKI drug treatment, were determined. The study was approved by the Medical Ethics Committee of The Second People’s Hospital of Lianyungang. All patients signed informed consent. Our study was conducted in accordance with the Declaration of Helsinki.
Therapeutic Program
All patients received EGFR-TKI targeted therapy. Gefitinib (250 mg) was administered daily, erlotinib (150 mg) was administered daily or icotinib (125 mg) was administered third daily. The total dose of WBRT was 30 Gy administered in 10 fractions, 3 Gy fractions once a day, and 5 days a week.
Efficacy and Toxicity Assessments
Tumor responses, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). All patients underwent imaging examinations after two courses of chemotherapy or every 8±1 week of EGFR-TKI treatment until disease progression. Imaging examinations included computed tomography (CT) scans of chest and abdomen, and magnetic resonance imaging (MRI) of the brain. Toxicity classification was performed using the National Cancer Institute Common Toxicity Criteria version 4.0 (CTC 4.0).
Follow-Up and Statistics
All cases were followed up to June 2020, 2 cases were lost, and the follow-up rate was 98.7%. Intracranial progression-free survival was defined as the time from using RT or EGFR-TKI until intracranial progression (iPFS). Overall survival (OS) is defined as the first day to death or the last time of treatment. The Statistical Package for the Social Sciences (SPSS), version 19.0 was used to perform the statistical analysis. The data were analyzed using chi-square test (Χ2). Survival analysis was conducted using the Kaplan–Meier method, and comparison was performed via Log rank test. Multivariate analysis was conducted using Cox proportional hazard regression model. Statistical significance was considered for a P value of less than 0.05.
Results
Patient Characteristics
A total of 148 eligible patients with multiple BM at the time of initial diagnosis of IV NSCLC were included in this study. A total of 54 patients were male, and 94 were female, with a median age of 59 years (range: 37–80 years). Exactly 135 patients had adenocarcinoma, and 13 had non-adenocarcinoma conditions. Exactly 115 patients achieved ECOG PS scores of 0–1, and 33 patients scored 2 points. In total, 41 patients reported a history of smoking, and 107 patients claimed no smoking history. Meanwhile, 39 patients had a family history of tumor, whereas 109 patients claimed no family history of tumor. A total of 74 patients were with or without the basic disease. In addition, 105 patients had extracranial metastasis, 43 had intracranial metastases. Sixty-seven had from EGFR exon 19 deletion, 72 contained EGFR exon 21 L858R mutation, and 9 cases presented other types of mutations. A total of 122 patients received EGFR-TKI as first-line treatment, the remaining 26 patients received TKI as second-line or above treatment. Patients receiving icotinib, gefitinib, and erlotinib targeted treatment totaled 116, 30, and 2 cases, respectively. The patients were divided into two groups: 76 patients received EGFR-TKI targeted therapy and WBRT (combination therapy group), and 72 patients who received EGFR-TKI targeted therapy (target drug group). The data of the two groups, including gender, age, smoking history, tumor type, basic disease, family history, ECOG performance status, EGFR mutation type, extracranial metastasis, and EGFR-TKI drug treatment were not statistically significant (all P values >0.05) and comparable. Table 1 shows the basic characteristics of patients.
Table 1.
Basic Clinical and Molecular Characteristics of Patients Characteristics
| Clinical Characteristics | All Patients (n=148) | Groups | P value* | ||
|---|---|---|---|---|---|
| Target Drug Group (n=72) | Combination Therapy Group (n=76) | ||||
| Gender | |||||
| Male | 54 (36.5) | 23 | 31 | 0.264 | |
| Female | 94 (63.5) | 49 | 45 | ||
| Age | |||||
| >60 years | 62 (41.9) | 32 | 30 | 0.540 | |
| ≤60 years | 86 (58.1) | 40 | 46 | ||
| Pathological type | |||||
| Adenocarcinoma | 135 (91.2) | 66 | 69 | 0.851 | |
| Non-adenocarcinoma | 13 (8.8) | 6 | 7 | ||
| ECOG PS | |||||
| 0–1 | 115 (77.7) | 59 | 56 | 0.228 | |
| 2 | 33 (22.3) | 13 | 20 | ||
| Smoking history | |||||
| Yes | 41 (27.7) | 19 | 22 | 0.728 | |
| No | 107 (72.3) | 53 | 54 | ||
| Family history | |||||
| Yes | 39 (26.4) | 20 | 19 | 0.701 | |
| No | 109 (73.6) | 52 | 57 | ||
| Basic disease | |||||
| Yes | 74 (50.0) | 34 | 40 | 0.511 | |
| No | 74 (50.0) | 38 | 36 | ||
| EGFR mutation: | |||||
| Exon 19 deletion | 67 (45.3) | 34 | 33 | 0.887 | |
| Exon 21L858R | 72 (48.6) | 34 | 38 | ||
| Others | 9 (6.1) | 4 | 5 | ||
| WBRT | |||||
| Yes | 76 (51.4) | - | - | - | |
| No | 72 (48.6) | - | - | - | |
| Type of EGFR-TKIs: | |||||
| Icotinib | 116 (78.4) | 53 | 63 | 0.376 | |
| Gefitinib | 30 (20.3) | 18 | 12 | ||
| Erlotinib | 2 (1.4) | 1 | 1 | ||
| Line of treatment of EGFR-TKI: | |||||
| First line | 122 (82.4) | 63 | 59 | 0.115 | |
| Second line or more | 26 (17.6) | 9 | 17 | ||
| Extracranial metastases: | |||||
| Yes | 105 (70.9) | 50 | 55 | 0.695 | |
| No | 43 (29.1) | 22 | 21 | ||
Note: *Chi-square detection values for target drug group and combination therapy group.
Efficacy and Survival Outcomes
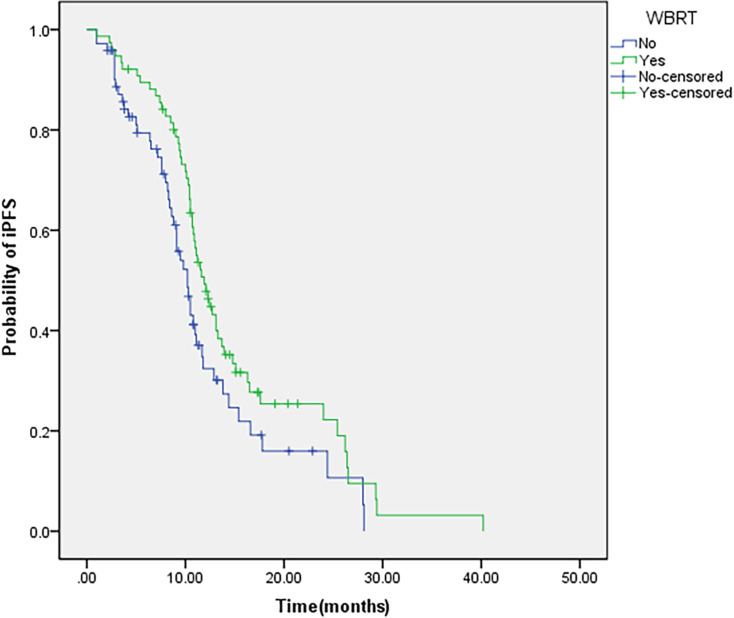
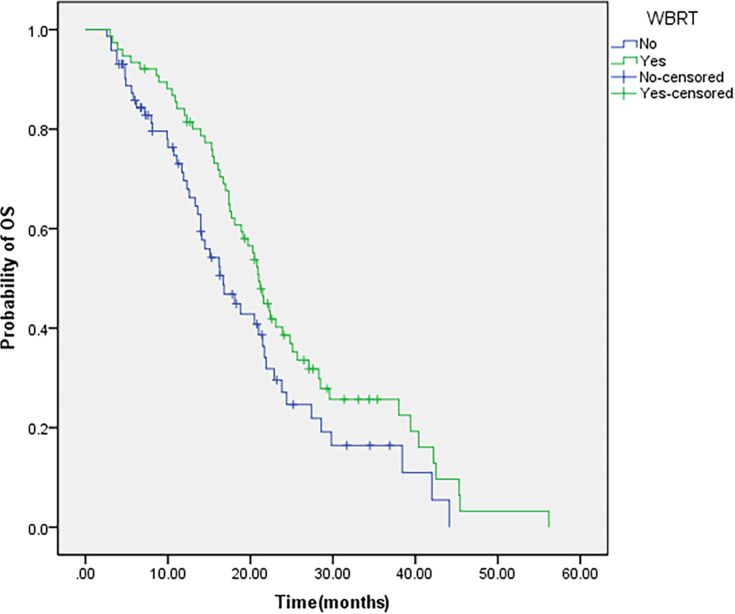
A total of 40 out of 148 patients (27.0%) developed intracranial progression, and survival analysis showed that the estimated median iPFS and median OS were 11.0 and 20.3 months, respectively. Of the 72 patients in the target drug group, 25 (33.3%) developed intracranial progression. Meanwhile, 15 (19.7%) out of 76 patients in the combined treatment group developed intracranial progression; the combined group patients had lower intracranial progression rate than target drug group patients (19.7% vs 33.3%, P=0.040). Univariate analysis showed that patients in the combined group had longer iPFS and OS than the patients in the target drug group (median iPFS: 11.9 months versus 10.2 months, P=0.039; median OS: 21.0 months versus 16.7 months, P=0.043) (Table 2; Figures 1 and 2). Multivariate analysis showed that WBRT (hazards ratio (HR)=0.606; 95% confidence interval (CI): 0.403–0.912, P=0.016) and the low Eastern Cooperative Oncology Group (ECOG) performance status (HR=1.884; 95% CI: 1.120–3.170, P=0.017) are independent prognostic factors in patients with multiple BM from NSCLC who received EGFR-TKI targeted therapy. Table 3 lists the details of the multivariate analysis.
Table 2.
Comparison of Short-Term and Long-Term Effects Between Two Groups
| Groups | No (Case) | Intracranial Progression Rate (%) | Median IPFS (Months) | Median OS (Months) |
|---|---|---|---|---|
| Combination therapy group | 76 | 19.7 | 11.9 | 21.0 |
| Target drug group | 72 | 33.3 | 10.2 | 16.7 |
| Χ2 value | – | 4.210 | 4.249 | 4.107 |
| P value | – | 0.040 | 0.039 | 0.043 |
Figure 1.
IPFS of patients with WBRT.
Figure 2.
OS of patients with WBRT.
Table 3.
Multivariate Analysis of Clinical Features and OS in Current Study
| Clinical Features | B | SE | Wald | HR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Gender | −0.663 | 0.362 | 3.355 | 0.516 | 0.254–1.047 | 0.067 |
| Age | −0.150 | 0.216 | 0.483 | 0.861 | 0.564–1.313 | 0.487 |
| Pathological type | 0.586 | 0.367 | 2.547 | 1.797 | 0.875–3.692 | 0.110 |
| Smoking history | −0.371 | 0.380 | 0.951 | 0.690 | 0.327–1.455 | 0.330 |
| Family history | −0.325 | 0.261 | 1.553 | 0.722 | 0.433–1.205 | 0.213 |
| Basic disease | 0.203 | 0.206 | 0.976 | 1.225 | 0.819–1.834 | 0.323 |
| EGFR mutation | −0.133 | 0.167 | 0.635 | 0.875 | 0.631–1.214 | 0.426 |
| Line of treatment of EGFR-TKI: | −0.231 | 0.269 | 0.733 | 0.794 | 0.468–1.346 | 0.392 |
| WBRT | −0.501 | 0.209 | 5.757 | 0.606 | 0.403–0.912 | 0.016 |
| ECOG PS | 0.633 | 0.265 | 5.696 | 1.884 | 1.120–3.170 | 0.017 |
| Extracranial metastases: | 0.099 | 0.242 | 0.166 | 1.104 | 0.687–1.774 | 0.684 |
| Type of EGFR-TKIs | 0.094 | 0.141 | 0.444 | 1.099 | 0.833–1.450 | 0.505 |
Survival of Patients with Different Treatment Modalities
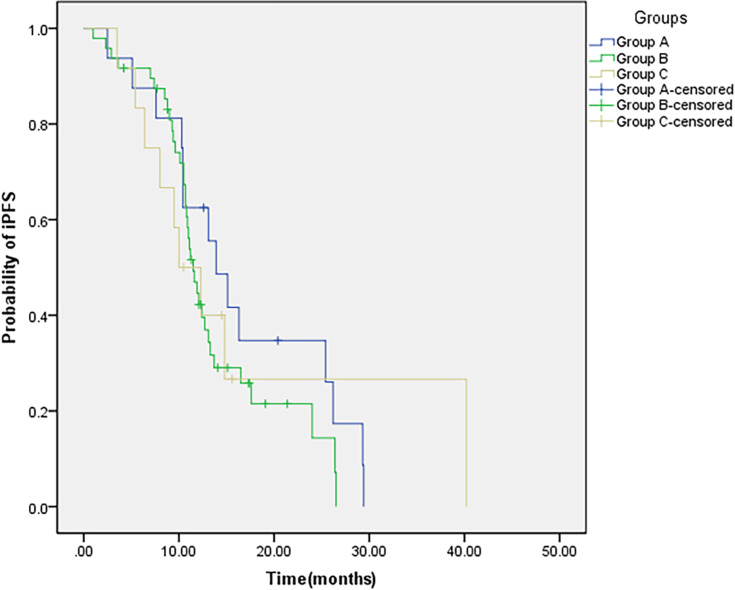
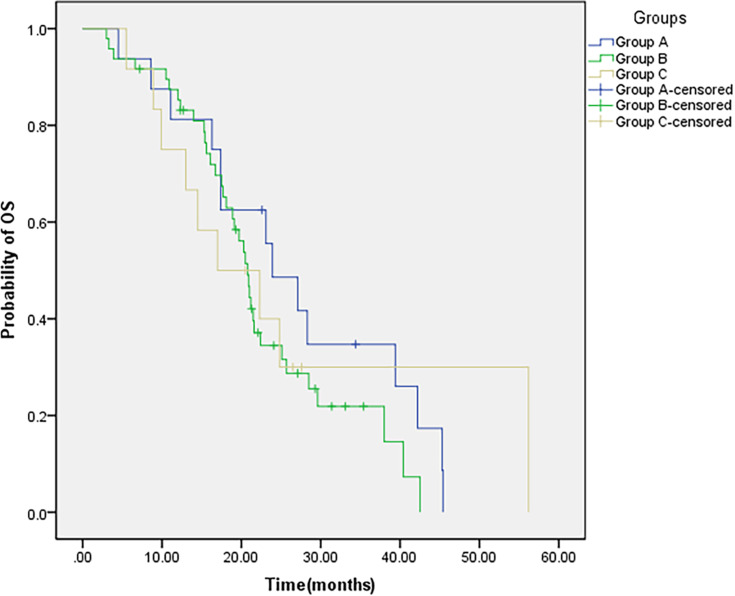
Based on the time sequence of WBRT and EGFR-TKI targeted therapy, 76 patients in the combined group were divided into three groups: group A: EGFR-TKI targeted therapy after WBRT; group B: synchronized WBRT and EGFR-TKI targeted therapy; group C: WBRT after EGFR-TKI targeted therapy. The incidence of intracranial progression was the highest in patients in group C (33.3%), followed by patients in group B (18.8%), patients in group A had the lowest intracranial progression rate (12.5%). However, no statistically significant difference was observed (P =0.376). The median iPFS of the three groups were 13.9 (group A), 11.5 (group B), and 10.0 (group C) months (P=0.604) (Figure 3). The median OS of the three groups were 23.9, 20.8, and 17.0 months (P =0.545) (Figure 4). Compared with those of the patients in group C, the iPFS and OS of the patients in groups A and B were prolonged, but the difference was not statistically significant (Table 4).
Figure 3.
IPFS of patients with different treatment modalities.
Figure 4.
OS of patients with different treatment modalities.
Table 4.
Survival of Patients with Different WBRT Times
| Groups | No (Case) | Intracranial Progression Rate (%) | Median IPFS (Months) | Median OS (Months) |
|---|---|---|---|---|
| EGFR-TKI targeted therapy after WBRT(group A) | 16 | 2 (12.5) | 13.9 | 23.9 |
| Synchronize WBRT and EGFR-TKI targeted therapy(group B) | 48 | 9 (18.8) | 11.5 | 20.8 |
| WBRT after EGFR-TKI targeted therapy(group C) | 12 | 4 (33.3) | 10.0 | 17.0 |
| Χ2 value | - | 1.959 | 1.008 | 1.214 |
| P value | - | 0.376 | 0.604 | 0.545 |
Toxicity
The most common treatment-related toxicities of the 2 groups of patients were rash, elevated transaminases, and diarrhea, which were mostly grade 1 or 2. The WBRT-related adverse events mainly included dizziness and headache. Compared with target drug group, the incidence of grade 3/4 toxicity was higher in patients in combined group (6.6% VS 4.2%), whereas no grade 4 adverse events were observed. All the patients could tolerate these toxicities after symptomatic treatment. Table 5 lists the details of treatment-related toxicities.
Table 5.
Lists the Details of Treatment-Related Toxicities
| Toxicities | Combination Therapy Group (n=76) | Target Drug Group (n=72) | ||
|---|---|---|---|---|
| Grade 1 or 2 | Grade 3 or 4 | Grade 1 or 2 | Grade 3 or 4 | |
| Rash | 25(32.9) | 3(3.9) | 24(33.3) | 2(2.8) |
| Elevated ALT/AST | 18(23.7) | 1(1.3) | 17(23.6) | 0 |
| Diarrhea | 16(21.1) | 1(1.3) | 15(20.8) | 1(1.4) |
| Dizziness | 15(19.7) | 0 | 1(1.3) | 0 |
| Fatigue | 14(18.4) | 0 | 13(18.1) | 0 |
| Headache | 14(18.4) | 0 | 1(1.3) | 0 |
| Nausea | 9(11.8) | 0 | 7(9.7) | 0 |
| Vomiting | 6(7.9) | 0 | 5(6.9) | 0 |
| Leukopenia | 5(6.6) | 0 | 4(5.5) | 0 |
| Cognitive disorder | 4(5.3) | 0 | 0 | 0 |
Discussion
Brain is a common metastatic organ in lung cancer patients, and research shows that lung cancer patients with EGFR mutations are more likely to have BM than the wild type.16,17 The prognosis of patients with BM is poor, and the median OS after BM is 3–6 months.18 The treatment and prognosis of single and multiple BM differ, and the prognosis of patients with multiple BM is poorer. EGFR-TKIs have demonstrated systemic efficacy and central nervous system activity in patients with BM from lung cancer with EGFR mutations.9,10,11,12 Although WBRT is the standard treatment option for patients with BM,19 several trials have shown that WBRT reduces intracranial recurrence but does not prolong survival and may increase the risk of impaired cognitive function.20,21 No previous study has reported whether WBRT can improve survival in patients receiving EGFR-TKI targeted therapy for multiple BM from NSCLC. We report the comparison of the efficacy of EGFR-TKI alone or combined with WBRT in patients with multiple BM from NSCLC with EGFR mutations.
Our study showed that 40 (27.0%) out of the 148 patients had intracranial progression, with median iPFS and median OS of 11.0 and 20.3 months, respectively. Our study showed that the incidence of intracranial progression was slightly higher, and survival was shorter than those in other studies;17,22–25 possibly, all the patients in the study group had multiple BM, and 105 patients (70.9%) were associated with extracranial distant metastasis. These results further confirm the poor prognosis of patients with multiple BM.
For patients with BM from lung cancer, EGFR-TKI can penetrate the blood–brain barrier (BBB).23,26 Individual EGFR-TKIs are effective in BM patients.27,28 The results of research on whether brain RT can improve the survival of NSCLC patients in EGFR-TKI targeted therapy remain controversial.9,10,13–15 Therefore, treatment strategies that use EGFR-TKI alone or combined with brain RT remain a major clinical controversy. The results of our study show that combined treatment group patients had a lower intracranial progression rate than target drug group patients (19.7% vs 33.3%, P=0.040). Univariate analysis showed that patients in the combined treatment group had longer iPFS and OS than those in the target drug group (median iPFS: 11.9 months versus 10.2 months, P=0.039; median OS: 21.0 months versus 16.7 months, P=0.043). WBRT can significantly reduce the intracranial progression rate and prolong the median iPFS and median OS; these results are consistent with those of previous studies.22,24,25,29 Multivariate analysis showed that WBRT (P=0.016) is an independent prognostic factor in patients with multiple BM from NSCLC under EGFR-TKI targeted therapy. According to our study, a comprehensive treatment model of EGFR-TKI and WBRT in lung cancer patients with multiple BM can significantly prolong the survival of patients and further consolidate the position of WBRT in BM of lung cancer. Moreover, multivariate analysis showed that ECOG PS (P=0.017) was an independent prognostic factors of OS for patients with multiple BM from NSCLC receiving EGFR-TKI targeted therapy. This finding indicates that patients with low ECOG PS scores will benefit considerably from EGFR-TKI in terms of survival. The optimal patients and treatment modalities of lung cancer multiple BM need further clinical study.
Magnuson WJ reported that in EGFR-mutant NSCLC patients with BM, the use of early EGFR-TKI and the delay of RT are related to poor OS.15 Wang W reported that delayed brain RT may lead to poor iPFS in EGFR-mutant NSCLC patients with asymptomatic BM.22 Chen H and other studies have shown that simultaneous treatment of EGFR-TKI and WBRT improves patients’ short- and long-term benefits compared with sequential or separate use.29 Liu et al showed that in EGFR-mutant NSCLC patients with BM, the timing of brain RT has no effect on OS.30 The optimal timing of brain RT for EGFR-TKI-treated lung cancer patients with BM is still inconclusive in clinical practice. Based on the time sequence of WBRT and EGFR-TKI targeted therapy, 76 patients in the combined treatment group were divided into three groups: group A: EGFR-TKI targeted therapy after WBRT; group B: synchronized WBRT and EGFR-TKI targeted therapy; group C: WBRT after EGFR-TKI targeted therapy. The incidence of intracranial progression was the highest in group C patients (33.3%), followed by patients in group B (18.8%). Patients in group A had the lowest intracranial progression rate (12.5%). However, no statistically significant difference was observed (P=0.376). The median iPFS of the three groups were 13.9 (group A), 11.5 (group B), and 10.0 (group C) months (P=0.604). The median OS of the three groups were 23.9, 20.8, and 17.0 months (P =0.545). Compared with those of the patients in group C, the iPFS and OS of the patients in groups A and B were prolonged, but the differences were not statistically significant. However, the iPFS and OS of patients who received WBRT whether upfront or concurrent EGFR-TKI targeted therapy was a prolonged trend compared with patients in the latter RT. This finding may suggest that the earlier the whole-brain radiation intervention, the more likely the patient is to survive and benefit. This condition is presumably associated with increased BBB permeability of EGFR-TKI drugs by WBRT.23
The most common treatment-related toxicities of the 2 groups of patients were rash, elevated transaminases, and diarrhea, which were mostly grade 1 or 2. Our study revealed that the majority of the patients were well tolerant to WBRT. The WBRT-related adverse events mainly included dizziness and headache. Only 4 case (5.3%) developing neurocognitive impairment, which were grade 1 or 2. The results are consistent with previous study.31
Our study encountered several limitations. The major limitation of the study is its retrospective nature. In addition, the sample size of this study was small. Although EGFR-TKI drug is limited to first generation of targeted drug, it can still provide certain clinical help for the treatment options of lung cancer patients with multiple BM receiving EGFR-TKI treatment. Thus, the results of large sample prospective studies are needed to further guide the use of the proposed treatment in clinical practice.
The results showed that WBRT can reduce the intracranial progression rate and improve the iPFS and OS of patients with multiple BM from lung cancer. The choice of WBRT time has no effect on the OS of patients.
Acknowledgments
We thank all members of The Second People’s Hospital of Lianyungang and The First Affiliated Hospital of Soochow University for their helpful advice. The authors thanks for all the patients.
Disclosure
The authors report no conflicts of interest.
References
- 1.Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30(1):1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. doi: 10.1016/S0140-6736(04)16250-8 [DOI] [PubMed] [Google Scholar]
- 4.Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a PHASE III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866–2874. doi: 10.1200/JCO.2010.33.4235 [DOI] [PubMed] [Google Scholar]
- 6.Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. doi: 10.1200/JCO.2011.36.8456 [DOI] [PubMed] [Google Scholar]
- 7.Gao G, Ren S, Li A, et al. Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: a meta-analysis from six phase III randomized controlled trials. Int J Cancer. 2012;131(5):E822–829. doi: 10.1002/ijc.27396 [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, Chen M, Zhong W, et al. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin Lung Cancer. 2013;14(2):188–193. doi: 10.1016/j.cllc.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 9.Jiang T, Su C, Li X, et al. EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol. 2016;11(10):1718–1728. [DOI] [PubMed] [Google Scholar]
- 10.Bai H, Xiong L, Han B. The effectiveness of EGFR-TKIs against brain metastases in EGFR mutation-positive non-small-cell lung cancer. Onco Targets Ther. 2017;10:2335–2340. doi: 10.2147/OTT.S129809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77(3):556–560. doi: 10.1016/j.lungcan.2012.05.092 [DOI] [PubMed] [Google Scholar]
- 12.Hyun DG, Choi CM, Lee DH, et al. Outcomes according to initial and subsequent therapies following intracranial progression in patients with EGFR-mutant lung cancer and brain metastasis. PLoS One. 2020;15(4):e0231546. doi: 10.1371/journal.pone.0231546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du XJ, Pan SM, Lai SZ, et al. Upfront cranial radiotherapy vs. EGFR tyrosine kinase inhibitors alone for the treatment of brain metastases from non-small-cell lung cancer: a meta-analysis of 1465 patients. Front Oncol. 2018;8:603. doi: 10.3389/fonc.2018.00603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, Lu X, Lyu Z, Bi N, Wang L. Comparison of up-front radiotherapy and TKI with TKI alone for NSCLC with brain metastases and EGFR mutation: a meta-analysis. Lung Cancer. 2018;122:94–99. doi: 10.1016/j.lungcan.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 15.Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35(10):1070–1077. doi: 10.1200/JCO.2016.69.7144 [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto S, Takahashi K, Iwakawa R, et al. Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer. 2006;119(6):1491–1494. doi: 10.1002/ijc.21940 [DOI] [PubMed] [Google Scholar]
- 17.Bhatt VR, D’Souza SP, Smith LM, et al. Epidermal growth factor receptor mutational status and brain metastases in non-small-cell lung cancer. J Glob Oncol. 2017;3(3):208–217. doi: 10.1200/JGO.2016.003392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a national cancer database survey. J Thorac Oncol. 2010;5(1):29–33. doi: 10.1097/JTO.0b013e3181c5920c [DOI] [PubMed] [Google Scholar]
- 19.Tsao MN, Xu W, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2018;1:CD003869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. doi: 10.1016/S1470-2045(09)70263-3 [DOI] [PubMed] [Google Scholar]
- 21.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Song Z, Zhang Y. Efficacy of brain radiotherapy plus EGFR-TKI for EGFR-mutated non-small cell lung cancer patients who develop brain metastasis. Arch Med Sci. 2018;14(6):1298–1307. doi: 10.5114/aoms.2018.78939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng YD, Liao H, Qin T, et al. Blood-brain barrier permeability of gefitinib in patients with brain metastases from non-small-cell lung cancer before and during whole brain radiation therapy. Oncotarget. 2015;6(10):8366–8376. doi: 10.18632/oncotarget.3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CH, Lee HH, Chuang HY, Hung JY, Huang MY, Chong IW. Combination of whole-brain radiotherapy with epidermal growth factor receptor tyrosine kinase inhibitors improves overall survival in EGFR-mutated non-small cell lung cancer patients with brain metastases. Cancers (Basel. 2019;11(8):1092. doi: 10.3390/cancers11081092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Yang J, Li X, et al. First-line epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor alone or with whole-brain radiotherapy for brain metastases in patients with EGFR-mutated lung adenocarcinoma. Cancer Sci. 2016;107(12):1800–1805. doi: 10.1111/cas.13079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan Y, Huang Z, Fang L, et al. A Phase II study of icotinib and whole-brain radiotherapy in Chinese patients with brain metastases from non-small cell lung cancer. Cancer Chemother Pharmacol. 2015;76(3):517–523. doi: 10.1007/s00280-015-2760-5 [DOI] [PubMed] [Google Scholar]
- 27.Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013;82(2):282–287. doi: 10.1016/j.lungcan.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 28.Wu YL, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol. 2013;24(4):993–999. doi: 10.1093/annonc/mds529 [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Wu A, Tao H, et al. Concurrent versus sequential whole brain radiotherapy and TKI in EGFR-mutated NSCLC patients with brain metastasis: a single institution retrospective analysis. Medicine (Baltimore). 2018;97(44):e13014. doi: 10.1097/MD.0000000000013014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Qiu B, Chen L, et al. Radiotherapy for asymptomatic brain metastasis in epidermal growth factor receptor mutant non-small cell lung cancer without prior tyrosine kinase inhibitors treatment: a retrospective clinical study. Radiat Oncol. 2015;10:118. doi: 10.1186/s13014-015-0421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He ZY, Li MF, Lin JH, Lin D, Lin RJ. Comparing the efficacy of concurrent EGFR-TKI and whole-brain radiotherapy vs EGFR-TKI alone as a first-line therapy for advanced EGFR-mutated non-small-cell lung cancer with brain metastases: a retrospective cohort study. Cancer Manag Res. 2019;11:2129–2138. doi: 10.2147/CMAR.S184922 [DOI] [PMC free article] [PubMed] [Google Scholar]