Abstract
Background:
Why physicians use surveillance imaging for asymptomatic cancer survivors despite recommendations against this is not known.
Methods:
Physicians surveilling head and neck cancer survivors were surveyed to determine relationships among attitudes, beliefs, guideline familiarity, and self-reported surveillance positron-emission-tomography/computed-tomography use.
Results:
Among 459 responses, 79% reported using PET/CT on some asymptomatic patients; 39% reported using PET/CT on more than half of patients. Among attitudes/beliefs, perceived value of surveillance imaging (O.R. 3.57, C.I. 2.42–5.27, P = <.0001) was the strongest predictor of high imaging, including beliefs about outcome (improved survival) and psychological benefits (reassurance, better communication). Twenty-four percent of physicians were unfamiliar with guideline recommendations against routine surveillance imaging. Among physicians with high perceived-value scores, those less familiar with guidelines imaged more (O.R. 3.55, C.I. 1.08–11.67, P = .037).
Conclusions:
Interventions to decrease routine surveillance PET/CT use for asymptomatic patients must overcome physicians’ misperceptions of its value. Education about guidelines may modify the effect of perceived value.
Keywords: head and neck cancer, PET/CT, physician decision-making, surveillance imaging, value
Précis:
Routine surveillance imaging for asymptomatic cancer survivors is not recommended in guidelines yet is commonly used. This survey found that physicians likely order these tests because of perceived value rather than reacting to external pressures or other motivations.
1 |. INTRODUCTION
Guidelines for most cancer types recommend against routine surveillance imaging,1,2 and the American Society of Clinical Oncology put surveillance imaging with positron emission tomography/ computed tomography (PET/CT) on their Choosing Wisely list of medical interventions that should be avoided for all asymptomatic cancer survivors.3 Yet these tests continue to be used,4–7 including in the setting of head and neck cancer.8 Determining why physicians use this type of imaging is important in understanding whether utilization behavior might be changed and how.
While variation in the use of surveillance imaging and testing can be partially explained by patient, geographic, and physician demographic factors,4,5,7,9,10 little is known about physicians’ decision-making in ordering these tests. Decision making is driven by the perceived benefits of a given action according to the theory of planned behavior (TPB), but behavior may also be influenced by social norms (ie, what other physicians do) and other factors that limit perceived behavioral control (ie, external pressures such as a patient’s request for imaging).11 Physician surveys have utilized the TPB to gain a conceptual understanding of the drivers of medical decision-making in other settings, showing that physician attitudes and beliefs are related to actual and reported behavior.12 Another aspect of decision making that may interact with beliefs about benefits is physicians’ knowledge, shown to be associated with the self-reported use of diagnostic tests in oncologic settings.7,13 We previously found that knowledge about guidelines was a stronger predictor of PET/CT surveillance imaging behavior than physician demographic characteristics.14
Our aim was to understand the determinants of PET/CT surveillance imaging use by physicians who treat asymptomatic survivors of head and neck squamous cell cancer. We hypothesized that physicians would report performing imaging because of beliefs about the potential benefits of routine surveillance PET/CT scans and because of external pressures. We also hypothesized that guideline familiarity would interact with imaging behavior.
2 |. METHODS
2.1 |. Study population
We surveyed members of the American Head and Neck Society (AHNS) and the American Society for Radiation Oncology (ASTRO) from July through September 2013. The former are primarily surgeons who treat head and neck cancer; all active members were invited. Radiation oncologists who are ASTRO members may treat multiple cancer types; we surveyed only those who reported an interest in head and neck cancer in their member profile.
2.2 |. Survey instrument
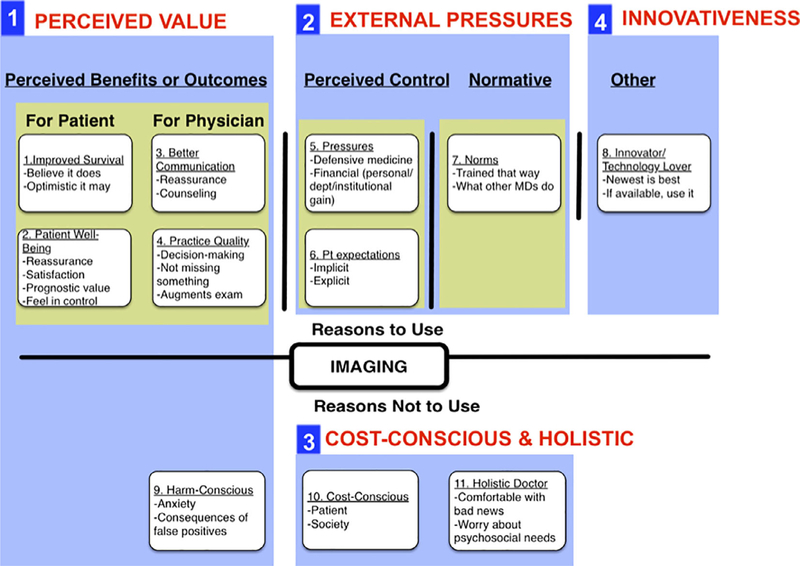
We developed a survey instrument consisting of four types of questions: (a) attitude and belief items related to the decision to use surveillance PET/CT scans, (b) knowledge of guideline recommendations related to surveillance PET/CT scan use, (c) physician self-reported use of surveillance PET/CT scans, and (d) physician demographic and practice characteristics. Categories and specific attitude and belief items were developed through semi-structured interviews with 10 surgeons and radiation-oncologists who treat head and neck cancer, as well as a review of the literature related to the TPB, decision making and overuse, and cancer surveillance practices. Many categories were aligned with TPB domains of perceived benefits or outcomes, normative beliefs, and perceived behavioral control (Figure 1). Additional categories discovered in physician interviews, including possible reasons for decreased use and innovativeness—the tendency to be interested in utilizing a technology because it is available—did not easily fit into the TPB model. The innovativeness questions were adopted from a validated 6-item scale initially developed to assess consumer domain-specific innovativeness.15 This scale has been used in healthcare to examine patient racial differences in medical innovativeness.16 The final survey comprised 40 attitude and belief items on a 5-point Likert scale from strongly disagree to strongly agree.
FIGURE 1.
Categories and domains of reasons for using surveillance PET/CT scans in asymptomatic patients. We identified 11 categories of attitudes and beliefs that were thought to influence routine surveillance PET/CT use in asymptomatic patients. Those that grouped a priori with the TPB domains of outcome expectancy, perceived control, and normative beliefs are highlighted in yellow. We identified additional categories related to innovativeness, and reasons for decreased use. The 11 categories were used to write 40 Likert attitude and belief items. Through factor analysis, four factorial domains were identified from the items (outlined and numbered), the first two of which align with TPB domains: domain 1 we call perceived value; domain 2 we call external pressures; domain 3 we call cost-consciousness and holistic; domain 4 we call innovativeness
A belief question examined physicians’ estimates of the survival benefit of routine surveillance imaging. We asked: “Routine surveillance imaging may catch recurrent cancer before symptoms develop, leading to earlier treatment of the recurrence, and possibly longer survival. In what percentage of patients who get routine surveillance imaging do you think this occurs?” Physicians could write in between 0–100%. To assess guideline knowledge, we asked after the other survey questions, “Routine surveillance imaging is recommended in the current NCCN guidelines using the following modalities [check as many as applicable]:” Answer choices were: PET/CT; MRI; CT; None of the above; Not sure. We classified physicians as incorrect about the PET/CT guidelines if they checked PET/CT scan.
Our main dependent variable was self-reported use of PET/CT scans. Physicians were asked to consider one simulated clinical scenario of a patient with advanced stage head and neck squamous cell carcinoma of any subsite, treated with any modality, who previously had a negative post-treatment PET/CT 3 months after treatment, and was presenting for routine follow-up within 2 years after treatment without any symptoms. Self-reported use was measured by their response to the item, “What percentage of your [patients with this presentation] get routine surveillance PET/CT scans?” Choices were: 0%, 1–25%, 26–50%, 51–70%, 71–90%, and 91–100%. Responders were coded as high imaging users if they reported imaging more than 50% of their patients, and low imaging users otherwise. The questionnaire did not assess reported use of MRI or CT, or beliefs about these modalities.
The questionnaire was pilot tested with the clinical experts involved in development and further refined based on their input for optimal ordering and clarity. The questionnaire took approximately 10 minutes to complete.
2.3 |. Survey administration
Two rounds of survey invitations were sent 3 weeks apart. AHNS members received email invitations to complete the survey via the REDCap electronic data capture tool.17 ASTRO members received mailed invitations because of societal rules against electronic contact, and had the option to reply via mail or REDCap. We included a lottery for an iPad Mini as incentive.
2.4 |. Analyses
We examined relationships between the level of imaging use, attitude and belief items, guideline knowledge, and physician characteristics. We used principal factor analysis with varimax rotation to reduce the 40 Likert attitude and belief items to factorial domains with eigenvalues of >1.0. We performed multivariable logistic regression to examine the association between imaging use and physicians’ factor scores relative to other physicians, while controlling for physician characteristics. To make the odds ratios of the factor scores more meaningful we scaled each factor to its SD. We excluded responders who were missing >20% of variables and those who did not respond to the outcome variable about self-reported imaging use, leaving <2% missing data in all variables. We performed two sensitivity analyses: first we performed multiple-imputation chained equations (MICE) regression18 to impute missing data, and performed factor analysis and the final logistic regression with these imputed results. Second, we performed all analyses with the dependent variable of self-reported use set as never-users vs ever-users. All statistical tests were two-sided and P-values ≤.05 were considered to be statistically significant. Analyses were conducted using Stata Statistical Software (Release 12.1; Stata Inc., College Station, TX). The University of Pennsylvania IRB approved this study.
3 |. RESULTS
3.1 |. Physician characteristics, self-reported PET/CT use, physicians’ estimates of survival benefit, and guideline familiarity
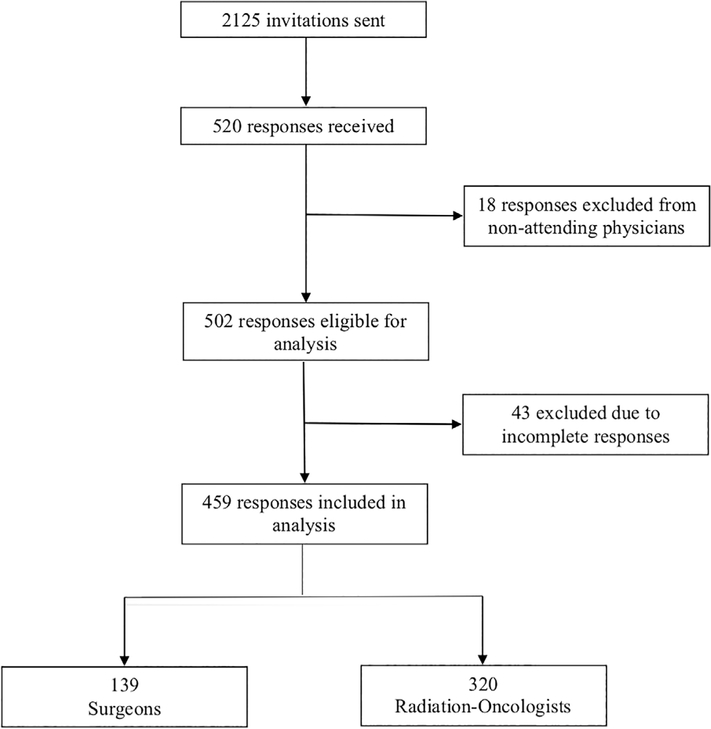
Five hundred and twenty responses were received out of 2125 invitations sent (24.5% response rate; 117 undeliverable for 25.9% cooperation rate).19 The mean age of all AHNS and ASTRO members invited was 51.7 years old, comparable to the group of responders (49.8 years). Of 520 responders, 502 were attending physicians who treat head and neck cancer and therefore eligible for analysis. Four hundred and fifty-nine physicians filled out >80% of the survey and were included in the analysis (completion rate 21.6%). Among the 43 excluded because they completed <80% of the survey, the median percentage of missing data was 85%, (IQR 85–94) and missing data was largely at the end of the survey, suggesting these responders only began the survey. The limited demographic information on these 43 physicians was similar to the information provided by those included in the analysis.
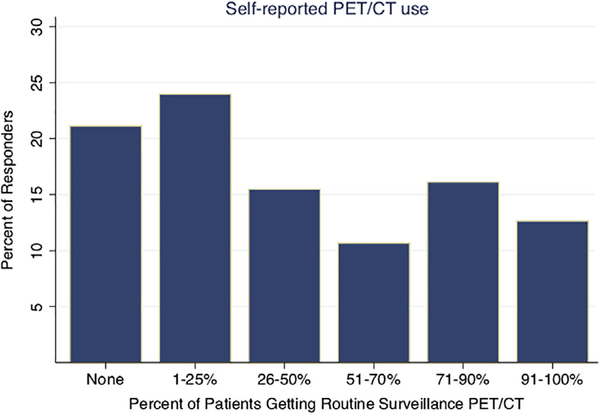
Of note, some results presented herein have been published in another manuscript focused on guideline familiarity and PET/CT use, although a slightly different analyzed cohort, and methods of analysis, were used.14 Figure 2 describes the cohort of the current manuscript in a CONSORT-type diagram. Characteristics of the analyzed sample are reported in Table 1, column 1. The majority was radiation oncologists, male, practiced in academic settings, or saw a low volume of head and neck cancer in their practice. Levels of self-reported imaging are shown in Figure 3. Seventy-nine percent of physicians reported performing routine surveillance PET/CT imaging on at least some of their asymptomatic patients; 39% reported performing imaging on more than half of their patients. Imaging use according to physician characteristics is also reported in Table 1. High imaging users were significantly more likely to be older, in private practice, or see a lower volume of head and neck cancer.
FIGURE 2.
CONSORT-type Diagram
TABLE 1.
High and low PET/CT imaging users according to physician characteristics
| Total | Low users (row %) | High users (row %) | P-valuea | |
|---|---|---|---|---|
| Responders | 459 | |||
| Physician characteristics | ||||
| Medical specialty | ||||
| Surgeon (ENT/ Gen. Surg./OMFS) | 139 | 92 (66.2%) | 47 (33.8%) | .104 |
| Radiation-oncologist | 320 | 186 (58.1%) | 134 (41.9%) | |
| Completed fellowship, surgical or radiation-oncology | ||||
| Yes | 227 | 145 (63.9%) | 82 (36.1%) | .145 |
| No | 229 | 131 (57.2%) | 98 (42.8%) | |
| Age | ||||
| Mean years (SD) | 49.8 | 48.6 (10.5%) | 51.7 (12.1%) | .004 |
| Years in practice | ||||
| Mean years (SD) | 16.8 | 15.4 (10.6%) | 18.8 (12.5%) | .002 |
| Gender | ||||
| Male | 379 | 234 (61.7%) | 145 (38.3%) | .165 |
| Female | 77 | 41 (53.2%) | 36 (46.8%) | |
| Practice setting | ||||
| Academic tertiary | 174 | 116 (66.7%) | 58 (33.3%) | .001 |
| Academic affiliate | 79 | 56 (70.9%) | 23 (29.1%) | |
| Community hospital | 102 | 57 (55.9%) | 45 (44.1%) | |
| Private practice | 99 | 45 (45.5%) | 54 (54.5%) | |
| Volume of head and neck cancer in practice | ||||
| 1–25% of practice | 240 | 131 (54.6%) | 109 (45.4%) | .010 |
| 26–50% of practice | 65 | 40 (61.5%) | 25 (38.5%) | |
| >50% of practice | 153 | 107 (69.9%) | 46 (30.1%) | |
Note: These results reflect 459 analyzed physicians, compared to our previously published manuscript reflecting 502 analyzed physicians14.
Difference between low and high imaging users calculated by chi-square, except age and years (ANOVA).
FIGURE 3.
Self-reported use of PET/CT for routine surveillance imaging in asymptomatic patients. Following a single clinical scenario, physicians were asked, “What percentage of your head and neck cancer patients get routine surveillance PET/CT scans?” n = 459
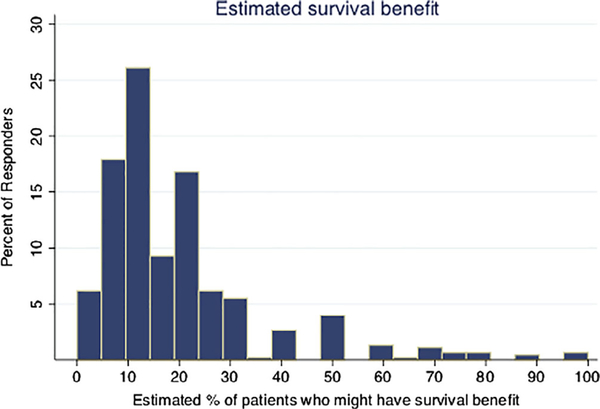
Figure 4 reports variation in physicians’ estimates of survival benefit. Physicians estimated that 19% (I.Q.R. 10–20%, SD 17%) of all asymptomatic patients receiving routine imaging might experience improved survival. Table 2 reports imaging use according to physicians’ estimates of survival benefit. Those who estimated the highest survival benefit were the most likely to be high imaging users (P < .0001). Table 2 also reports guideline familiarity and its relationship with the level of imaging use. Those who were unfamiliar with NCCN guideline recommendations against routine surveillance PET/CT scans were more likely to be high imaging users (P < .0001).
FIGURE 4.
Physicians’ estimates of survival benefit from routine surveillance PET/CT scans. Physicians were asked to estimate the percentage of asymptomatic patients getting routine surveillance imaging who might experience a survival benefit
TABLE 2.
PET/CTimaginguseaccording to guideline familiarity andphysicians’ estimates of survival benefit
| Total | Low users (row %) | High users (row %) | P-value | |
|---|---|---|---|---|
| Physicians’ estimates of survival benefit | ||||
| Responders | 452 | |||
| Estimated % patients with survival benefit | ||||
| 0–9% | 109 | 91 (83.5%) | 18 (16.5%) | <.0001 |
| 10% | 118 | 80 (67.8%) | 38 (32.2%) | |
| 11–20% | 118 | 64 (54.2%) | 54 (45.8%) | |
| > 20% | 107 | 38 (35.5%) | 69 (64.5%) | |
| Guideline Familiarty | ||||
| Responders | 450 | |||
| NCCN recommends routine surveillance imaging with PET/CT? | ||||
| No (Familiar) | 341 | 236 (69.2%) | 105 (30.8%) | <.0001 |
| Yes (Unfamiliar) | 109 | 38 (34.9%) | 71 (65.1%) |
3.2 |. Attitudes, beliefs, and factorial domains
Physician responses to individual Likert attitude and belief items and bivariate associations with imaging use are provided in Table A1. Out of 40 Likert items, 21 were significantly associated with imaging use, including 13 out of 15 from the categories related to perceived benefit. Factor analysis distinguished four domains of attitudes and beliefs among the 40 items: perceived value, external pressures, cost-consciousness and holistic, and innovativeness. The items belonging to each factorial domain and the domain’s Cronbach’s alpha (a measure of internal reliability) are provided in Table A1; the Cronbach’s alpha for the perceived value domain was 0.94 (indicating high reliability for assessing perceived value). The elements and categories of each domain are summarized visually in Figure 1.
TABLE A1.
Attitudes and beliefs
| n | High Imaging Users, n (row %) | Unadjusted OR (95% C.I.) | P-value | Category (#1–11 from Figure 1) | |
|---|---|---|---|---|---|
| Responders | 459 | 181 (39%) | |||
| Factor 1: perceived value (Cronbach’s alpha = 0.94) | |||||
| 1. Sufficient evidence exists that routine surveillance imaging improves survival. | |||||
| Agree | 77 | 59 (76.6%) | 6.99 (3.95–12.35) | <.0001 | 1 |
| Disagree or neutral | 382 | 122 (31.9%) | |||
| 2. Routine surveillance imaging may improve survival. | |||||
| Agree | 219 | 128 (58.4%) | 4.96 (3.31–7.45) | <.0001 | 1 |
| Disagree or neutral | 240 | 53 (22.1%) | |||
| 3. The prognostic information gained by routine surveillance imaging has enough value to justify its use. | |||||
| Agree | 228 | 140 (61.4%) | 7.37 (4.80–11.33) | <.0001 | 2 |
| Disagree or neutral | 231 | 41 (17.7%) | |||
| 4. Routine surveillance imaging is an important part of my patients’ satisfaction with their care. | |||||
| Agree | 287 | 159 (55.4%) | 8.36 (5.05–13.84) | <.0001 | 2 |
| Disagree or neutral | 170 | 22 (12.9%) | |||
| 5. Routine surveillance imaging helps my patients feel reassured that they remain disease-free. | |||||
| Agree | 362 | 171 (47.2%) | 7.70 (3.88–15.30) | <.0001 | 2 |
| Disagree or neutral | 96 | 10 (10.4%) | |||
| 6. Routine surveillance imaging helps my patients feel in control of their lives. | |||||
| Agree | 271 | 143 (52.8%) | 4.32 (2.81–6.64) | <.0001 | 2 |
| Disagree or neutral | 185 | 38 (20.5%) | |||
| 7. Routine surveillance imaging has value for patients who just want something done. | |||||
| Agree | 198 | 82 (41.4%) | 1.14 (0.78–1.67) | .49 | 2 |
| Disagree or neutral | 259 | 99 (38.2%) | |||
| 8. There is a higher value to reassuring cancer patients with surveillance imaging than, for instance, using imaging to reassure patients with non life-threatening conditions such as headache or low back pain. | |||||
| Agree | 220 | 111 (50.5%) | 2.47 (1.68–3.62) | <.0001 | 2 |
| Disagree or neutral | 236 | 69 (29.2%) | |||
| 9. Routine surveillance imaging helps me communicate with patients. | |||||
| Agree | 222 | 125 (56.3%) | 4.17 (2.79–6.24) | <.0001 | 3 |
| Disagree or neutral | 233 | 55 (23.6%) | |||
| 10. Routine surveillance imaging helps me reassure my patients that they remain disease-free. | |||||
| Agree | 321 | 161 (50.2%) | 5.89 (3.49–9.92) | <.0001 | 3 |
| 11. Routine surveillance imaging helps me counsel my patients if recurrence is discovered. | |||||
| Agree | 354 | 172 (48.6%) | 11.34 (5.35–24.03) | <.0001 | 3 |
| Disagree or neutral | 104 | 8 (7.7%) | |||
| 12. Routine surveillance imaging impacts decisions about care. | |||||
| Agree | 325 | 165 (50.8%) | 7.98 (4.47–14.24) | <.0001 | 4 |
| Disagree or neutral | 131 | 15 (11.5%) | |||
| 13. Routine surveillance imaging helps make sure I don’t miss something in my patients’ care. | |||||
| Agree | 270 | 144 (53.3%) | 4.83 (3.12–7.45) | <.0001 | 4 |
| Disagree or neutral | 188 | 36 (19.1%) | |||
| 14. Routine surveillance imaging augments what flexible nasopharyngoscopy provides. | |||||
| Agree | 313 | 154 (49.2%) | 4.20 (2.61–6.74) | <.0001 | 4 |
| Disagree or neutral | 144 | 27 (18.8%) | |||
| 15. I feel comfortable counseling my patients that they are disease-free without imaging. | |||||
| Agree | 281 | 78 (27.8%) | 0.28 (0.19–0.42) | <.0001 | 4 |
| Disagree or neutral | 177 | 102 (57.6%) | |||
| 16. False-positives on routine surveillance imaging, and the ensuing tests and procedures required, might outweigh its potential value. | |||||
| Agree | 236 | 56 (23.7%) | 0.24 (0.16–0.36) | <.0001 | 9 |
| Disagree or neutral | 221 | 125 (56.6%) | |||
| Factor 2: external pressures (Cronbach’s alpha = 0.73) | |||||
| 1. Some doctors order routine surveillance imaging because there is some financial benefit, either directly to themselves, or to their department or institution. | |||||
| Agree | 167 | 59 (35.3%) | 0.77 (0.52–1.14) | .192 | 5 |
| Disagree or neutral | 289 | 120 (41.5%) | |||
| 2. I have ordered routine surveillance imaging because of a direct or indirect financial benefit, either to myself, my department, or my institution. | |||||
| Agree | 4 | 1 (25%) | 0.51 (0.05–4.96) | .564 | 5 |
| Disagree or neutral | 454 | 179 (39.4%) | |||
| 3. I’ve been encouraged to do more imaging (for surveillance or otherwise) by my department or institution. | |||||
| Agree | 17 | 6 (35.3%) | 0.83 (0.30–2.28) | .717 | 5 |
| Disagree or neutral | 441 | 175 (39.7%) | |||
| 4. I have ordered routine surveillance imaging because of fear of missing something and then being sued. | |||||
| Agree | 157 | 61 (38.9%) | 0.97 (0.65–1.44) | .866 | 5 |
| Disagree or neutral | 300 | 119 (39.7%) | |||
| 5. I have ordered routine surveillance imaging because patients explicitly ask for them. | |||||
| Agree | 233 | 83 (35.6%) | 0.71 (0.49–1.03) | .0722 | 6 |
| Disagree or neutral | 221 | 97 (43.9%) | |||
| 6. I have ordered routine surveillance imaging because it sometimes seems that patients want them, even if they don’t ask. | |||||
| Agree | 81 | 34 (42%) | 1.13 (0.70–1.84) | .618 | 6 |
| Disagree or neutral | 377 | 147 (39%) | |||
| 7. I order routine surveillance imaging because that is how I was trained. | |||||
| Agree | 121 | 71 (58.7%) | 2.92 (1.90–4.48) | <.0001 | 7 |
| 8. I order routine surveillance imaging because other doctors do the same. | |||||
| Agree | 82 | 36 (43.9%) | 1.26 (0.78–2.05) | .342 | 7 |
| Disagree or neutral | 374 | 143 (38.2%) | |||
| 9. I worry that my patients will experience anxiety while waiting for the results of routine surveillance imaging. | |||||
| Agree | 198 | 75 (37.9%) | 0.88 (0.60–1.29) | .509 | 9 |
| Disagree or neutral | 259 | 106 (40.9%) | |||
| Factor 3: cost-conscious and holistic (Cronbach’s alpha = 0.60) | |||||
| 1. I am conscious of my patients’ out-of-pocket costs in my practice of medicine. | |||||
| Agree | 384 | 155 (40.4%) | 1.28 (0.76–2.14) | .356 | 10 |
| Disagree or neutral | 75 | 26 (34.7%) | |||
| 2. I am conscious of societal healthcare costs in my practice of medicine. | |||||
| Agree | 378 | 149 (39.4%) | 0.94 (0.57–1.54) | .792 | 10 |
| Disagree or neutral | 78 | 32 (41%) | |||
| 3. Physicians have a responsibility to balance the potential benefit of a medical test or treatment with its cost. | |||||
| Agree | 383 | 150 (39.2%) | 0.94 (0.57–1.54) | .791 | 10 |
| Disagree or neutral | 76 | 31 (40.8%) | |||
| 4. I feel comfortable telling my patients they do not need routine surveillance imaging even if they ask for it. | |||||
| Agree | 314 | 111 (35.4%) | 0.60 (0.40–0.90) | .0136 | 11 |
| Disagree or neutral | 143 | 68 (47.6%) | |||
| 5. I take holistic approach to my patients, making sure their psychosocial needs are met. | |||||
| Agree | 320 | 128 (40%) | 1.07 (0.71–1.61) | .749 | 11 |
| Disagree or neutral | 138 | 53 (38.4%) | |||
| 6. I feel comfortable talking to my patients about a bad prognosis. | |||||
| Agree | 422 | 168 (39.8%) | 1.17 (0.58–2.37) | .663 | 11 |
| Disagree or neutral | 36 | 13 (36.1%) | |||
| Factor 4: innovativeness (Cronbach’s alpha = 0.52) | |||||
| 1. In general, I will use a new medical test or treatment, even if I have had little experience with it. | |||||
| Agree | 66 | 28 (42.4%) | 1.18 (0.70–2.01) | .536 | 8 |
| Disagree or neutral | 388 | 149 (38.4%) | |||
| 2. Compared to my colleagues, I am rarely first to use a new medical test or treatment. | |||||
| Agree | 138 | 43 (31.2%) | 0.61 (0.40–0.93) | .0216 | 8 |
| Disagree or neutral | 319 | 136 (42.6%) | |||
| 3. In general, I am among the first of my colleagues to know about the latest medical test or treatment. | |||||
| Agree | 208 | 73 (35.1%) | 0.72 (0.50–1.06) | .0963 | 8 |
| Disagree or neutral | 248 | 106 (42.7%) | |||
| 4. I would avoid using a new medical test or treatment if I had not heard about it first from several colleagues I trust. | |||||
| Agree | 139 | 56 (40.3%) | 1.07 (0.71–1.61) | .735 | 8 |
| Disagree or neutral | 316 | 122 (38.6%) | |||
| 5. High cost medical tests and procedures should not be offered to patients when they have minimal effect on survival. | |||||
| Agree | 297 | 103 (34.7%) | 0.57 (0.39–0.84) | .00496 | 10 |
| Disagree or neutral | 162 | 78 (48.1%) | |||
| Items that did not load to specific factors (loading values ≤0.3) | |||||
| 1. Routine surveillance imaging replaces the need for flexible nasopharyngoscopy. | |||||
| Agree | 9 | 6 (66.7%) | 3.13 (0.77–12.68) | .11 | 4 |
| Disagree or neutral | 449 | 175 (39%) | |||
| 2. If I hear about a new medical test or treatment that sounds right for my patients, I am likely to use it in my practice. | |||||
| Agree | 279 | 107 (38.4%) | 0.93 (0.63–1.37) | .707 | 8 |
| Disagree or neutral | 177 | 71 (40.1%) | |||
| 3. Patients should have access to all effective treatments for their cancer regardless of the cost. | |||||
| Agree | 247 | 108 (43.7%) | 1.48 (1.01–2.16) | .0427 | 10 |
| Disagree or neutral | 212 | 73 (34.4%) | |||
| 4. I fear that in the future, insurance companies or the government will get in the way of my being able to practice medicine as I see fit. | |||||
| Agree | 367 | 159 (43.3%) | 2.36 (1.40–3.99) | .00128 | 10 |
| Disagree or neutral | 90 | 22 (24.4%) | |||
Five items had factorial complexity (loading values ≥0.3 in two domains); we used judgment to decide which domain was more conceptually compatible, giving the item to the factor with the higher loading value in 3/5 cases. Five items had negative loading values (≤ −0.3) and are conceptually reversed within the factor to which they loaded: Factor 1, items 15, 16; Factor 4 items 2, 4, 5.
3.3 |. Predictors of imaging use
On multivariable analysis controlling for physician characteristics, three of the four factors predicted the level of imaging use in expected directions (Table 3). Perceived value was the strongest predictor: for every standard-deviation increase in perceived value score, there were 3.57 increased odds of being a high imaging user compared to a low imaging user (C.I. 2.42–5.27, P < .0001). The domain holistic & cost-consciousness predicted decreased imaging use (O.R. 0.74, C.I. 0.56–0.97, P = .032). Innovativeness predicted higher imaging use (O.R. 1.48, C.I. 1.10–1.98, P = .009). The domain external pressures to perform imaging was not significant (O.R. 1.03, C.I. 0.78–1.37, P = .821). Although physicians’ estimates of survival benefit were highly correlated with imaging use on bivariate analysis and in a multivariable model with physician characteristics (data not shown), it was not significant in the presence of the perceived value factor and was therefore left out of the final model.
TABLE 3.
Multivariable regression of 4 factorial domains, guideline familiarity, and physician characteristics
| OR (95% Conf. Int.) | P-value | |
|---|---|---|
| Factorsa | ||
| 1. Perceived value | 3.57 (2.42–5.27) | <.0001 |
| 2. External pressures/ norms | 1.03 (0.78–1.37) | .821 |
| 3. Cost-conscious and holistic | 0.74 (0.56–0.97) | .032 |
| 4. Innovativeness | 1.48 (1.10–1.98) | .009 |
| Guideline familiarity | ||
| Unfamiliar with guidelines (vs familiar) | 2.78 (1.34–5.76) | .006 |
| Interaction | ||
| Guideline | 3.55 (1.08–11.67) | .037 |
| familiarity x perceived value | ||
| Physician characteristics | ||
| Surgeon (vs radiation-oncologist) | 1.23 (0.54–2.80) | .620 |
| Fellowship trained | 0.58 (0.33–1.04) | .069 |
| Female | 2.39 (1.14–5.01) | .021 |
| Every 10 additional years in practice | 1.30(1.01–1.67) | .038 |
| Practice setting | ||
| Academic tertiary | [Reference] | |
| Academic-affiliate | 0.54 (0.22–1.30) | .167 |
| Community hospital | 0.74 (0.32–1.69) | .476 |
| Private practice | 1.19 (0.52–2.72) | .683 |
| Volume of H&N cancer in practice | ||
| 1–25% of practice | [Reference] | |
| 26–50% of practice | 1.40 (0.62–3.13) | .416 |
| >50% of practice | 1.19 (0.48–2.95) | .701 |
In addition to attitudes and beliefs, being unfamiliar with guidelines was the physician characteristic most strongly predictive of high imaging use (O.R. 2.78, C.I. 1.34–5.76, P = .006). This finding is similar to our previously reported results that did not account for attitudes and beliefs.14 We tested for interactions between physician characteristics and the four factorial domains in separate models. Table 3 represents the one model with a significant interaction: Physicians who had a high perceived value score imaged less if they knew the guidelines (O.R. 3.55, C.I. 1.08–11.67, P = .037). The odds ratios and significance of other physician characteristics, factors, guideline knowledge, and estimated survival benefit were not significantly different between the model we report and the unreported models without the interaction term.
In the first sensitivity analysis, we used imputed missing data to perform factor analysis. The same four factor domains were distinguished and multivariable analyses revealed no notable differences in odds ratios or significance. In the second sensitivity analysis, the final regression model with the dependent variable was set as never-users vs ever-users. There were no notable differences in odds ratios or significance for the strong effect of guideline familiarity and perceived value, but the interaction between the two lost significance. Table A2 reports the results of four multivariable regressions that examine differences in factorial domain scores according to physician characteristics.
TABLE A2.
Multivariable regressions of physician characteristics predicting factor scores
| 1. Perceived value |
2. External pressures |
3. Cost-conscious and holistic |
4. Innovativeness |
|||||
|---|---|---|---|---|---|---|---|---|
| Factor | Coeff (95% conf. int.) | P-value | Coeff (95% conf. int.) | P-value | Coeff (95% conf. int.) | P-value | Coeff (95% conf. int.) | P-value |
| Guideline knowledge | ||||||||
| Innacurate guideline knowledge (vs accurate) | 0.27 (0.07 to 0.47) | .010 | 0.02 (−0.22 to 0.27) | .846 | −0.07 (−0.33 to 0.18) | .581 | 0.07 (−0.18 to 0.33) | .562 |
| Estimated survival benefit | ||||||||
| Estimated % patients with survival advantage | ||||||||
| <10% | (Reference group) | (Reference group) | (Reference group) | (Reference group) | ||||
| 10% | 0.61 (0.38 to 0.84) | <.0001 | 0.12 (−0.16 to 0.41) | .392 | 0.11 (−0.18 to 0.41) | .457 | −0.10 (−0.39 to 0.20) | .523 |
| 11–20% | 1.28 (1.05 to 1.51) | <.0001 | −0.04 (−0.32 to 0.24) | .767 | −0.19 (−0.48 to 0.11) | .211 | −0.05 (−0.34 to 0.24) | .739 |
| >20% | 1.40 (1.16 to 1.64) | <.0001 | 0.01 (−0.28 to 0.31) | .942 | 0.02 (−0.28 to 0.33) | .876 | 0.04 (−0.27 to 0.34) | .819 |
| Physician characteristics | ||||||||
| Surgeon (vs rad-onc) | 0.00 (−0.26 to 0.26) | .978 | −0.12 (−0.43 to 0.20) | .458 | 0.05 (−0.28 to 0.38) | .751 | −0.09 (−0.41 to 0.23) | .586 |
| Fellowship trained | 0.06 (−0.12 to 0.23) | .529 | −0.02 (−0.24 to 0.19) | .824 | −0.03 (−0.26 to 0.19) | .771 | 0.18 (−0.04 to 0.40) | .104 |
| Female | 0.13 (−0.10 to 0.35) | .273 | −0.30 (−0.58 to −0.02) | .031 | 0.23 (−0.06 to 0.52) | .116 | −0.58 (−0.86 to −0.29) | <.0001 |
| Every 10 additional years in practice | 0.06 (−0.01 to 0.14) | .095 | −0.21 (−0.29 to −0.11) | <.0001 | 0.04 (−0.06 to 0.13) | .465 | −0.01 (−0.10 to 0.09) | .902 |
| Practice setting | ||||||||
| Academic tertiary | (Reference group) | (Reference group) | (Reference group) | (Reference group) | ||||
| Academic affiliate | −0.06 (−0.32 to 0.20) | .644 | −0.04 (−0.35 to 0.28) | .815 | 0.02 (−0.31 to 0.34) | .926 | 0.04 (−0.28 to 0.36) | .820 |
| Community hospital | 0.01 (−0.25 to 0.26) | .957 | 0.24 (−0.08 to 0.55) | .137 | 0.12 (−0.20 to 0.45) | .465 | 0.22 (−0.10 to 0.54) | .179 |
| Private practice | 0.07 (−0.19 to 0.33) | .576 | 0.33 (0.02 to 0.65) | .040 | 0.16 (−0.17 to 0.48) | .354 | 0.28 (−0.05 to 0.60) | .093 |
| Volume of H&N cancer in practice | ||||||||
| 1–25% of practice (Reference group) | Reference group) | (Reference group) | (Reference group) | |||||
| 26–50% of practice | −0.08 (−0.34 to 0.17) | .524 | −0.15 (−0.46 to 0.17) | .359 | 0.11 (−0.21 to 0.44) | .495 | 0.06 (−0.25 to 0.38) | .692 |
| >50% of practice | −0.18 (−0.46 to 0.10) | .214 | 0.03 (−0.32 to 0.37) | .87 | 0.40 (0.04 to 0.76) | .029 | −0.04 (−0.40 to 0.31) | .808 |
4 |. DISCUSSION
We surveyed physicians caring for head and neck cancer survivors to determine the reasons for using surveillance PET/CT scans in asymptomatic patients. Because surveillance imaging is generally not recommended, we aimed to understand its continued use. Guideline familiarity, estimated survival benefits, and many attitudes and beliefs were related to imaging use. Among four factorial domains of attitudes and beliefs, the strongest predictor of high imaging use was perceived value.
Perceived value takes two forms. On the one hand, perceived value may be related to myriad psychological benefits of performing imaging. For example, patients have been shown to value the reassurance that comes from negative surveillance imaging.20 Literature in other fields also demonstrates the psychological value of prognostic information21 and satisfaction with care.22 For the physician, there may also be psychological value in terms of improved ability to provide reassurance or counseling to patients, or feeling reassured themselves that they are not missing something. All of these concepts were captured in our perceived value domain.
Related to the perception of value is the finding that innovativeness, or the tendency to want to be one of the first to utilize newly available technology, is associated with the level of PET/CT use. Simple availability of technology has been cited as a reason for overuse of diagnostic radiology services.23 We used a validated domain-specific innovativeness scale to examine physician behavior related to beliefs in the intrinsic value of new technology. As above, we are not able to conclude what benefits physicians believe this new technology may provide, only that a tendency to be a fast adopter or innovator is related to the reported level of use.
On the other hand, perceived value may be related to beliefs that surveillance PET/CT scans improve more tangible outcomes like survival. We captured this belief both in physicians’ estimates of survival benefit, and in some of the items making up the perceived value factorial domain. Physicians who believe in a survival advantage may be unfamiliar with current data. Two retrospective studies in head and neck cancer have not shown a survival advantage.24,25 The lack of supportive survival data is similar in many other cancer types; even possible survival advantages demonstrated in breast and colorectal cancer have recently been brought into question.26
Alternatively, physicians may extrapolate a survival advantage from other data. For instance, despite the fact that head and neck cancer recurrences amenable to surgical salvage have only a 22–39% 5-year survival (worse for nonsurgical recurrences)27 and the use of PET or PET/CT in asymptomatic head and neck cancer survivors yields a very small number of surgically salvageable cases,28–32 physicians may nonetheless misunderstand probabilities, or conclude that statistics about test accuracy and the rate of changes in management28–37 could translate into improved outcomes. This notion of extrapolation of survival benefits from other statistics relates to the debate about whether surrogate endpoints like progression-free survival are clinically meaningful.38
4.1 |. Limitations
This study has several important limitations. First, our study had a response rate of 24.5% with uneven numbers of surgeons and radiation-oncologists, and we did not include medical oncologists who may also participate in imaging decisions. For these reasons our sample may not fully represent the relevant clinician population. However, our sample represented a broad range of measured variables and our aim was not the accurate measurement of the demographics, beliefs, or behaviors of a population of physicians, but to demonstrate the possible relationships between these variables. We achieved a sample of physicians with a diverse range of beliefs, behaviors, and characteristics, and found no interactions between observable physician characteristics and our main findings.
A second limitation is that our findings rely on self-reported behavior rather than actual behavior. We have no compelling reason to believe that self -report substantially biases the associations we uncover, but our findings should be interpreted with that caution. A third limitation is that we used an unvalidated survey instrument of attitudes and beliefs. Therefore, the findings should be viewed as exploratory. Nonetheless, our aim was not to develop a psychometrically valid scale of perceived value or to validate a self-reported utilization instrument, but rather to determine if attitudes and beliefs might plausibly be related to reported behavior and, if so, which might be most strongly related. We also note the high reliability of the perceived value factor as measured by Cronbach’s alpha.
We did not evaluate for additional external influences such as ownership or partnership with imaging facilities, which may financially incentivize physicians for obtaining routine imaging for patients. Additionally, we did not evaluate the possible impact insurance companies and insurance policies may have on whether and how often physicians use surveillance imaging.
4.2 |. Implications
Despite these limitations, this is the first study to our knowledge in any cancer subsite demonstrating that physicians may be ordering surveillance PET/CT scans because of their possible psychological benefits such as reassurance, or because of perceived survival benefits, rather than because of external pressures or because of belonging to specific demographic subgroups.
Given the absence of a survival advantage from surveillance PET/CT scanning, continued use might possibly be justified by satisfactory demonstration of less tangible advantages such as reassurance, but these would have to be weighed against the known or measurable harms of routine imaging including unnecessary radiation exposure, cost, false positives, and psychological harm.
In the meantime, our findings connect the continued use of imaging with a belief in its value, and demonstrate that this association is stronger among those clinicians unfamiliar with current guidelines—suggesting benefit from broader dissemination of those guidelines. Indeed, our finding of decreased use even among those with high-perceived value, as long as they were familiar with guidelines, offers promise that such interventions might work.
5 |. CONCLUSION
Overuse of medical diagnostic tests and services is receiving increased attention as part of the societal goal of reducing health care costs and avoiding the uncompensated harm these diagnostic tests may create. Our survey suggests that overuse of routine surveillance PET/CT scans in asymptomatic cancer survivors may be driven in part by physicians’ perceptions of the tests’ psychological benefits, and misperceptions about survival benefits. To the extent we want to reduce test use, we should not only perform better outcomes research and educate physicians about the current evidence and guidelines, but also use guidelines to drive behavior change by more clearly identifying better and less costly alternatives to routine PET/CT scans. These educational efforts can be aimed at both physicians and patients.
ACKNOWLEDGMENT
This research was sponsored by the American Head and Neck Society (AHNS) Education Committee and funded in part by the University of Pennsylvania Robert Wood Johnson Foundation Clinical Scholars Program. This study was supported in part by the Cancer Center Support Grant P30 CA008748 from the National Institutes of Health/National Cancer Institute.
APPENDIX A.
Footnotes
CONFLICT OF INTEREST
The authors report no other financial disclosures or conflicts of interest.
REFERENCES
- 1.Nekhlyudov L, Lacchetti C, Davis NB, et al. Head and neck cancer survivorship care guideline: American Society of Clinical Oncology clinical practice guideline endorsement of the American Cancer Society guideline. J Clin Oncol. 2017;35(14): 1606–1621. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Guidelines for Treatment of Cancer by Site. Available from URL: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp-site.
- 3.American Society of Clinical Oncology. Choosing Wisely: 10 Things Physicians and Patients Should Question; Item #8. Available from URL: http://www.choosingwisely.org/doctorpatient-lists/american-society-of-clinical-oncology/.
- 4.Keating NL, Landrum MB, Guadagnoli E, Winer EP, Ayanian JZ. Surveillance testing among survivors of early-stage breast cancer. J Clin Oncol. 2007;25(9):1074–1081. [DOI] [PubMed] [Google Scholar]
- 5.Salloum RG, Hornbrook MC, Fishman PA, Ritzwoller DP, O’Keeffe Rossetti MC, Elston Lafata J. Adherence to surveillance care guidelines after breast and colorectal cancer treatment with curative intent. Cancer. 2012;118(22):5644–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potosky AL, Han PK, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26(12):1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han PK, Klabunde CN, Noone AM, et al. Physicians’ beliefs about breast cancer surveillance testing are consistent with test overuse. Med Care. 2013;51(4):315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myssiorek D, Roman B, Wang M, Pou A, Holsinger FC. Trends in Utilization of FDG PET Imaging in the American Head and Neck Society. AHNS Annual Meeting Oral Presentation. 2009; Phoenix, Arizona [Google Scholar]
- 9.Keating NL, Landrum MB, Lamont EB, Bozeman SR, McNeil BJ. Area-level variations in cancer care and outcomes. Med Care. 2012;50(5):366–373. [DOI] [PubMed] [Google Scholar]
- 10.Kerfoot BP, Holmberg EF, Lawler EV, Krupat E, Conlin PR. Practitioner-level determinants of inappropriate prostate-specific antigen screening. Arch Intern Med. 2007;167(13): 1367–1372. [DOI] [PubMed] [Google Scholar]
- 11.Ajzen I The theory of planned behaviour. Org Behav Human Decision Process. 1991;50:179–211. [Google Scholar]
- 12.Ramsay CR, Thomas RE, Croal BL, Grimshaw JM, Eccles MP. Using the theory of planned behaviour as a process evaluation tool in randomised trials of knowledge translation strategies: a case study from UKprimary care. Implement Sci. 2010;5:71–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray SW, Hicks-Courant K, Cronin A, Rollins BJ, Weeks JC. Physicians’ attitudes about multiplex tumor genomic testing. J Clin Oncol. 2014;32:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roman BR, Patel SG, Wang MB, et al. Guideline familiarity predicts variation in self-reported use of routine surveillance PET/CT by physicians who treat head and neck cancer. J Natl Compr Canc Netw. 2015;13:69–77. [DOI] [PubMed] [Google Scholar]
- 15.Goldsmith RE, Hofacker CF. Measuring consumer innovativeness. J Acad Mark Sci. 1991;19:209–221. [Google Scholar]
- 16.Groeneveld PW, Sonnad SS, Lee AK, Asch DA, Shea JE. Racial differences in attitudes toward innovative medical technology. J Gen Intern Med. 2006;21:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 19.American Association for Public Opinion Research. Standard Definitions: Final Disposition of Case Codes and Outcome Rates for Surveys, Revised 2011. Available at: http://aapor.org/Content/NavigationMenu/AboutAAPOR/StandardsampEthics/StandardDefinitions/StandardDefinitions2011.pdf Accessed May 22, 2014.
- 20.Papagrigoriadis S, Heyman B. Patients’ views on follow up of colorectal cancer: implications for risk communication and decision making. Postgrad Med J. 2003;79:403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asch DA, Patton JP, Hershey JC. Knowing for the sake of knowing: the value of prognostic information. Med Decis Making. 1990;10:47–57. [DOI] [PubMed] [Google Scholar]
- 22.Stearns CR, Gonzales R, Camargo CA Jr, Maselli J, Metlay JP. Antibiotic prescriptions are associated with increased patient satisfaction with emergency department visits for acute respiratory tract infections. Acad Emerg Med. 2009;16:934–941. [DOI] [PubMed] [Google Scholar]
- 23.Lysdahl KB, Hofmann BM. What causes increasing and unnecessary use of radiological investigations? A survey of radiologists’ perceptions. BMC Health Serv Res. 2009;9:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho AS, Tsao GJ, Chen FW, et al. Impact of positron emission tomography/computed tomography surveillance at 12 and 24 months for detecting head and neck cancer recurrence. Cancer. 2013;119:1349–1356. [DOI] [PubMed] [Google Scholar]
- 25.Spector ME, Chinn SB, Rosko AJ, et al. Diagnostic modalities for distant metastasis in head and neck squamous cell carcinoma: are we changing life expectancy? Laryngoscope. 2012;122:1507–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furman MJ, Lambert LA, Sullivan ME, Whalen GF. Rational follow-up after curative cancer resection. J Clin Oncol. 2013;31: 1130–1133. [DOI] [PubMed] [Google Scholar]
- 27.Zafereo ME, Hanasono MM, Rosenthal DI, et al. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009;115:5723–5733. [DOI] [PubMed] [Google Scholar]
- 28.Abgral R, Querellou S, Potard G, et al. Does 18F-FDG PET/CT improve the detection of posttreatment recurrence of head and neck squamous cell carcinoma in patients negative for disease on clinical follow-up? J Nucl Med. 2009;50:24–29. [DOI] [PubMed] [Google Scholar]
- 29.Kao J, Vu HL, Genden EM, et al. The diagnostic and prognostic utility of positron emission tomography/computed tomography-based follow-up after radiotherapy for head and neck cancer. Cancer. 2009;115:4586–4594. [DOI] [PubMed] [Google Scholar]
- 30.Krabbe CA, Pruim J, Dijkstra PU, et al. 18F-FDG PET as a routine posttreatment surveillance tool in oral and oropharyngeal squamous cell carcinoma: a prospective study. J Nucl Med. 2009;50:1940–1947. [DOI] [PubMed] [Google Scholar]
- 31.Dunsky KA, Wehrmann DJ, Osman MM, Thornberry BM, Varvares MA. PET-CT and the detection of the asymptomatic recurrence or second primary lesions in the treated head and neck cancer patient. Laryngoscope. 2013;123(9):2161–2164. [DOI] [PubMed] [Google Scholar]
- 32.Kostakoglu L, Fardanesh R, Posner M, et al. Early detection of recurrent disease by FDG-PET/CT leads to management changes in patients with squamous cell cancer of the head and neck. Oncologist. 2013;18(10):1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe VJ, Boyd JH, Dunphy FR, et al. Surveillance for recurrent head and neck cancer using positron emission tomography. J Clin Oncol. 2000;18:651–658. [DOI] [PubMed] [Google Scholar]
- 34.Lee JC, Kim JS, Lee JH, et al. F-18 FDG-PET as a routine surveillance tool for the detection of recurrent head and neck squamous cell carcinoma. Oral Oncol. 2007;43:686–692. [DOI] [PubMed] [Google Scholar]
- 35.Salaun PY, Abgral R, Querellou S, et al. Does 18fluoro-fluorodeoxyglucose positron emission tomography improve recurrence detection in patients treated for head and neck squamous cell carcinoma with negative clinical follow-up? Head Neck. 2007;29:1115–1120. [DOI] [PubMed] [Google Scholar]
- 36.Beswick DM, Gooding WE, Johnson JT, Branstetter BF. Temporal patterns of head and neck squamous cell carcinoma recurrence with positron-emission tomography/computed tomography monitoring. Laryngoscope. 2012;122:1512–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDermott M, Hughes M, Rath T, et al. Negative predictive value of surveillance PET/CT in head and neck squamous cell cancer. AJNR Am J Neuroradiol. 2013;34:1632–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Agostino RB Sr. Changing end points in breast-cancer drug approval—the Avastin story. N Engl J Med. 2011;365 (2):e2. [DOI] [PubMed] [Google Scholar]