Keywords: carcinoid, dedifferentiation, enterochromaffin, enteroendocrine, intestine, neuroendocrine tumor, reserve, stem cell
Abstract
Small intestinal neuroendocrine tumors (SI-NET) are serotonin-secreting well-differentiated neuroendocrine tumors of putative enterochromaffin (EC) cell origin. Recent studies recognize a subset of EC cells that is label-retaining at the +4 position in the crypt and functions as a reserve intestinal stem cell. Importantly, this +4 reserve EC cell subset not only contributes to regeneration of the intestinal epithelium during injury and inflammation but also to basal crypt homeostasis at a constant rate. The latter function suggests that the +4 EC cell subset serves as an active reserve stem cell via a constant rate of dedifferentiation. Characterization of early tumor formation of SI-NET, observed as crypt-based EC cell clusters in many cases of familial SI-NETs, suggests that the +4 active reserve EC cell subset is the cell of origin. This newly discovered active reserve stem cell property of EC cells can account for unique biological mechanisms and processes associated with the genesis and development of SI-NETs. The recognition of this property of the +4 active reserve EC cell subset may provide novel opportunities to explore NETs in the gastrointestinal tract and other organs.
INTRODUCTION
Small intestinal neuroendocrine tumors (SI-NET), or commonly called “ileal carcinoid or mid-gut carcinoid,” are usually well-differentiated slow-growing serotonin-producing tumors of the distal small intestine. SI-NETs account for 25% of NETs originating from the gastrointestinal tract, which comprise over 50% of total NETs. In parallel with the steady increase of NETs, the incidence of the SI-NETs has increased more than sixfold from 1973 to 2012 (12). Patients with SI-NETs are often diagnosed at an advanced stage of disease in part due to the slow-growing nature of the tumor that causes no or only mild nonspecific symptoms. Their malignant characteristics and high metastatic potential may be because of their unique biological and genetic profiles that are distinct from other endocrine tumors (11, 35). As a result, SI-NETs are the most common malignant tumors of the small intestine (35). Most cases of SI-NETs are sporadic. To date, no common genetic basis of sporadic cases has been found. CDKN1B is the most recurrently mutated gene, but accounts for only 7% of cases (16). Familial SI-NETs are rare, and similar to other hereditary gastrointestinal tumors, present with multiple synchronous primary tumors. The genetic basis of familial SI-NETs is distinct from multiple endocrine neoplasia (MEN). Although several predisposition genes such as MUTYH have been suggested by association studies (15), to date, no linkage study has detected genes linked to familial SI-NETs except a single germline mutation in inositol polyphosphate multi-kinase (IPMK) for one large family (49).
It has been widely acknowledged that SI-NETs originate from serotonin-producing enterochromaffin (EC) cells (25). However, the precise origin and development of the tumors remain uncertain. The current dominant hypothesis is that most neoplastic tumors originate from tissue-specific multipotent stem cells. Accordingly, in the intestine, accumulating evidence suggests a critical role of the intestinal stem cell (ISC) in tumorigenesis of intestinal tumors (3). In contrast, dedifferentiation theory suggests potential tumorigenicity of differentiated cells through dedifferentiation and/or reprogramming (44, 58). However, the genesis of SI-NETs does not fit well with either model but may possibly lie in between, invoking a reserve stem hypothesis (31). In this review, we aim to focus on a newly discovered subset of EC cells, it’s reserve stem cell properties and potential link with early tumor formation, observed as crypt-based EC cell clusters, to understand the genesis and development of SI-NETs.
EC CELLS CONTRIBUTE TO BASAL ISC HOMEOSTASIS AND REPAIR
The intestinal epithelium is continuously regenerated from crypt-based columnar cells (CBC) located at the crypt bottom between Paneth cells (9). CBCs specifically express Lgr5 and represent Lgr5+ actively cycling ISCs (aISCs; see Ref. 4). However, upon loss or damage of Lgr5+ aISCs during inflammation or injury, restoration of the epithelium is mediated by reserve ISCs (rISCs; see Ref. 2). The term “reserve” has been used recently because of insufficient evidence to support +4 quiescent ISC (qISC) hypothesis. The qISCs were recognized as label-retaining cells (LRCs) because of their label (i.e., [3H]thymidine, BrdU, or histone 2B)-retaining property. Because they are most frequently located at the +4 position, the qISCs are also conventionally called +4 stem cells or +4 LRCs (36). In the classical concept, the intestinal epithelium is maintained by the interplay between aISCs (or CBC) and +4 qISCs (or +4 LRC). However, following the finding that +4 LRCs belong to the secretory lineage (8), the +4 qISCs hypothesis has been gradually overtaken by +4 rISCs hypothesis.
Although the specificity of markers remains debatable (2), markers proposed to identify +4 rISC include BMI1 (42), HOPX (52), Tert (32), and Lrig1 (38). Studies using these markers and label-retaining property further confirmed that +4 LRCs belong to the secretory lineage, including secretory precursors (8, 20, 43, 57) and/or enteroendocrine cells (EECs; see Refs. 19, 23, 46, 63). In addition, recent studies found that other cell types in the intestinal epithelium, Paneth cells (41), tuft cells (61), and enterocytes (53) also possess dedifferentiation potential to restore the epithelium during severe inflammation and injury (34). Although the existence of a rare master quiescent label-retaining ISC remains unproven, these recent findings at least conceptualize the idea of reserve. However, it should be noted that only secretory precursor cells and EECs are recognized as +4 LRCs (8) and thus constitute +4 rISCs. Furthermore, secretory precursor cells and EECs are the only cell types that not only restore the epithelium during inflammation and injury but also maintain physiological ISC homeostasis (20, 43, 19, 63, 46). Therefore, the latter function provides us with a new pivotal view that basal ISC homeostasis is maintained by creating a loop of cyclical ISC dynamics using specific descendant cells that possess reserve stem cell properties (Fig. 1). Thus far, the EEC is the only cell type that has been demonstrated to dedifferentiate into ISC at a constant rate (46). In this novel view, a specific subset of +4 EECs serves as a “active reserve” cell that is ready and regularly called to action (Fig. 1).
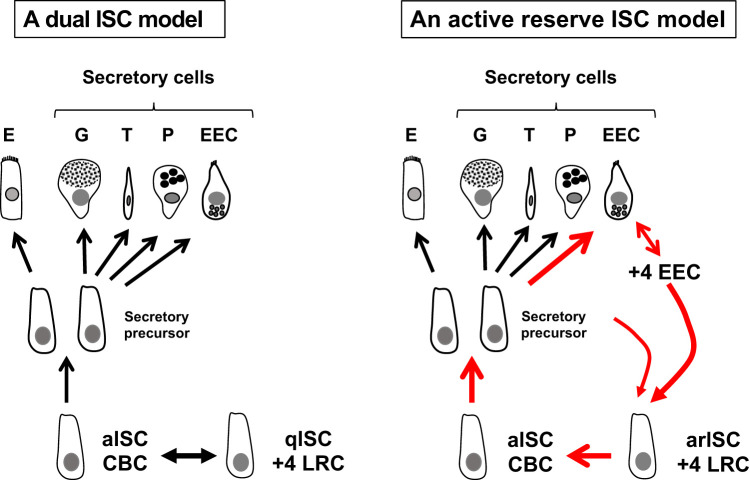
Fig. 1.
Models of small intestinal stem cell dynamics. Crypt-based columnar cells (CBCs) serve as actively cycling intestinal stem cells (aISCs) and generate all the differentiated descendants of the intestinal epithelium, including enterocytes (E), goblet cells (G), tuft cells (T), Paneth cells (P), and enteroendocrine cells (EEC). Left, a dual ISC model. ISC homeostasis is maintained by aISCs in coordination with coexisting quiescent ISCs (qISCs) identified as +4 label-retaining cells [LRCs, also recently referred to as reserve ISC (rISC)]. Right, an active reserve ISC (arISC) model. ISC homeostasis is maintained by aISCs and complemented by arISC. A subset of secretary cell precursors and +4 EECs, coincident with +4 LRCs, serve as +4 arISCs to form a dynamic loop contributing to the ISC pool.
The extent to which EECs contribute to basal ISC homeostasis, such as the rate of dedifferentiation, was estimated and characterized by lineage tracing studies using NEUROD1-CreERT transgenic mice (46). NEUROD1, a basic helix-loop-helix transcription factor that regulates post-NEUROG3 differentiation and maturation of EECs is expressed in most EECs (21). The lineage-tracing studies in NEUROD1-CreERT:Rosa26 tdTomato transgenic mice indicated that a subset of the NEUROD1-derived EECs remains at the +4 position or migrates down to the crypt bottom rather than up the villus as previously observed (6, 48). In addition, many of the +4 NEUROD1-derived EECs retained BrdU for more than 2 wk, identifying them as +4 LRCs (46), consistent with the short-term LRC reported by Li et al. (26). This subset was found in 6.7% of small intestinal crypts, similar to the 6.5% LRC frequency reported by Potten et al. (37). Dedifferentiation of EECs into fully functional ISCs was evidenced by the development of migration streams of NEUROD1-derived differentiated epithelial cells observed as ribbon-like streaks of Tomato-labeled cells starting from CBCs between Paneth cells extending continuously up the villus. Immunostaining indicated that the Tomato-labeled epithelial layers consist of mostly CD13 (aminopeptidase)-positive enterocytes and mucin 2-positive goblet cells and suggests that these non-EEC epithelial cells must have originated from NEUROD1-derived EECs. Importantly, combined lineage-tracing time-course and mathematical modeling studies indicated that NEUROD1-derived ISCs appear at a constant rate and enter in neutral drift dynamics under basal conditions such that NEUROD1-derived ISC in a crypt can give rise to clonal expansion with equal probability versus unlabeled ISCs (46).
Furthermore, the lineage tracing using tryptophan hydroxylase 1 (TPH1)-CreERT transgenic mice revealed that the EC cell was the predominant EEC type contributing to the ribbon formation in the NEUROD1-CreERT transgenic mice experiments (46). EC cells primarily express TPH1 that catalyzes the first and rate-limiting reaction of biosynthesis of serotonin (5-HT) and serves as an EC cell marker (59). The stem cell property of EC cells was first suggested by lineage-tracing studies in TPH1-CreERT:Rosa26-tdTomato transgenic mice (46). Dedifferentiation of EC cells to fully functional ISCs was evident by development of Tomato-labeled ribbon-like streaks starting from ISCs at the crypt base and continuously extending to villi. Furthermore, a comparison of lineage-tracing time-course data from TPH1-CreERT transgenic mice with the results from NEUROD1-CreERT transgenic mice revealed that, among NEUROD1-derived EECs, a subset of mostly EC cells contributed to the basal ISC homeostasis in the small intestine according to stochastic neutral drift dynamics (Fig. 2).
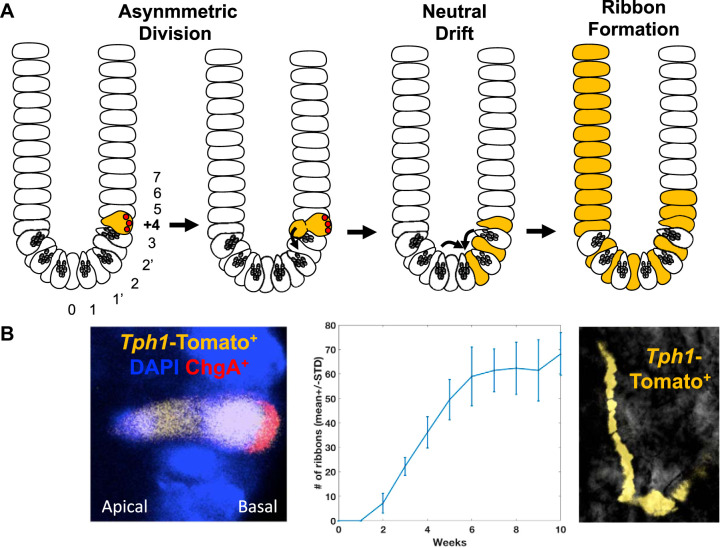
Fig. 2.
Dedifferentiation of +4 active reserve enterochromaffin cells (arECs) into actively cycling intestinal stem cells (aISCs). A: cartoon model of +4 enterochromaffin (EC)-originated generation of intestinal epithelium based on tryptophan hydroxylase 1 (TPH1) lineage-tracing studies. Following asymmetric division, a +4 EC cell dedifferentiates into an aISC and becomes subjected to neutral drift selection. Once selected, the +4 EC-originated aISC dominates the ISC population in the crypt and contributes to the generation of the epithelium. This specific subset of +4 EC cells that undergo dedifferentiation to become an aISC is termed a +4 active reserve EC cell (+4 arEC) in the text. B: Left, representative image of an asymmetric division of a TPH1-derived Tomato (orange)-labeled +4 EC cell [chromogranin (ChgA)+, basal side] initiating the subsequent formation of an aISC (ChgA−, luminal side). Middle, a computational simulation of lineage-tracing experimental data shows the time-dependent increase in Tomato-labeled ribbon formations in the small intestine following tamoxifen induction (12 mice with 600 initial +4 EC clones) that supports a neutral drift model of +4 EC cell-originated aISCs. Right, representative image of a TPH1-derived Tomato-expressing ribbon formation that originated from a Tomato-positive crypt.
The EC cells constitute the largest population of EECs and are present in all regions of the intestine (50). EC cells store and release 5-HT in response to nutrients, metabolites, and mechanical stimulation of gastrointestinal mucosa and play major roles in regulating gastrointestinal motility, secretion, nausea, visceral sensitivity, metabolism, and inflammation (5, 39, 17, 29). In addition to these functions, the above developmental data indicate that small intestinal EC cells also maintain ISC dynamics not only during injury and inflammation but also during homeostatic conditions. (Fig. 2). Importantly, this stem cell property may belong to a specific subset of EC cells that locate at the +4 position and express reserve ISC genes. The dedifferentiation of +4 TPH1-derived EC cells to ISCs was found to be mediated by asymmetric cell division (Fig. 2) as previously shown for +4 LRC (37, 40). Furthermore, in studies using a HOPX-CreERT:TPH1-CFP transgenic mouse-derived de novo organoid formation model, FACS-sorted HOPX-positive EC cells displayed significantly higher basal and reserve stem cell function compared with HOPX-negative EC cells both with and without prior irradiation. Therefore, in a mouse model, the intestinal epithelium is maintained at least in part by dedifferentiation of a subset of EC cells, likely +4 EC cells that express reserve ISC genes such as BMI1 (19, 63) and HOPX (46), to ISCs at a regular basal rate. As explained earlier (Fig. 1), this specific +4 EC cell subset commits to dedifferentiate at a constant rate and contributes to the cyclical loop of ISC dynamics. Thus, we refer to this subset as active reserve EC cells (arEC).
In normal human small intestine, we identified TPH1+ EC cells that express BMI1 and/or HOPX by RNA in situ hybridization studies using the TPH1 gene as an EC cell marker. Interestingly, the frequency of EC cells expressing BMI1 and/or HOPX genes was the highest in the TPH1+ EC cells located at the +4 position. Over 70% of the +4 EC cells expressed BMI1 and/or HOPX. Although it remains to be determined in humans, it is reasonable to speculate that BMI1- and HOPX-expressing +4 EC cells belong to a specific subset, arEC, and play a special role in ISC dynamics similar to mice.
IS THE +4 ACTIVE RESERVE EC CELL SUBSET THE CELL OF ORIGIN FOR SI-NETS?
Discovery and characterization of precursor or early lesions of neoplastic tumors are key elements in the investigation of tumorigenesis. Studies of familial SI-NETs at the National Institute of Diabetes and Digestive and Kidney Diseases (NCT00646022) have enabled the investigation of early tumor formation in tissue sections and isolated primary crypts. Early tumor formations of SI-NETs were previously recognized and described as hyperplastic nodules or aggregates of endocrine cells (28, 33). These previous studies suggested that SI-NETs originate from intraepithelial EECs and nodular hyperplasia (28, 33). However, detection of such lesions in tissue sections are rarely successful. In fact, the study by Lundqvist and Wilander (28) used 512 serial sections of a single tumor from a patient with three tumors in the ileum. To increase the chance of detecting early lesions, we studied patients with familial SI-NET suspected to have a germline origin for multiple synchronous macroscopic primary tumors and numerous microtumors. In addition, we have developed a method of crypt enrichment using free-floating isolated crypts derived from familial SI-NET patients. Abnormal crypts with micronodules of chromogranin A-positive EECs are efficiently detected by fluorescent microscopic scanning of multiple crypts. We termed such abnormal crypts “aberrant crypt containing endocrine cell clusters (ACEC).” This method is, therefore, crypt oriented and helps focus screening on early lesions of crypt-based tumor initiation. Applying this method, we investigated tissue samples from the patients with familial SI-NETs and confirmed our suspicion that ACECs were widely present in the macroscopic tumor-free mucosa throughout the resected jejuno-ileal intestine (47). In addition, mitochondrial DNA-based clonality analysis showed ACECs arose in a clonally independent manor, consistent with our suspicion for the germline origin of familial SI-NET characterized by widespread, polyclonal, and multifocal genesis of the tumors (47), similar to other tumors of germline mutations such as familial polyposis (54) or adenomatous polyposis coli-mutant mouse models (55).
Although rarely detected, ACECs offer a window into the understanding of the origin and development of tumors and their relationship with neighboring crypts. ACECs were clearly demonstrated in tumor sections as distinct early tumor formations within the epithelial layers of the crypts. For some of the ACECs, we were able to see the epithelial layer of the ACEC clearly continuous with the top epithelial layer of the villus (Fig. 3A). Villus structures were thin and deformed because of the accumulation of the tumor nests in the lamina propria and suggested impaired generation of epithelial layers from loss of ISCs. We were also able to find budding and fission of ACECs (47). These findings were consistent with earlier studies suggesting that early SI-NET formation originates from the crypt bottom, undergoes “bottom-up” morphogenesis, and grows by budding and fission (28, 33; Fig. 3B).
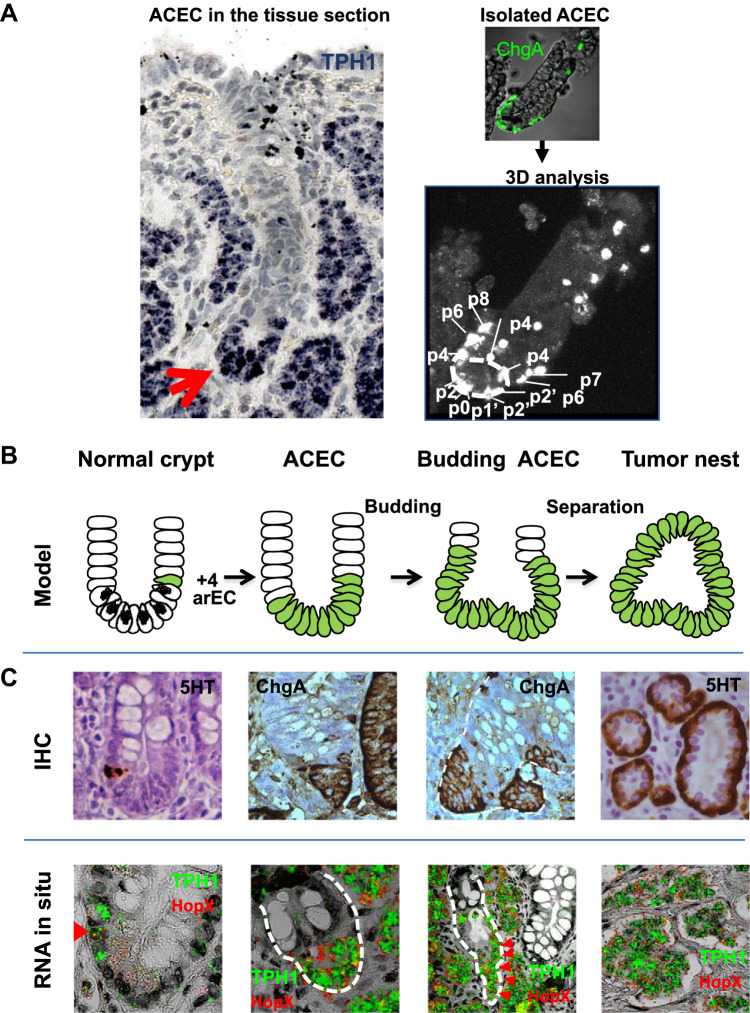
Fig. 3.
Genesis and development of small intestinal neuroendocrine tumors (SI-NETs). A: analysis of aberrant crypt containing endocrine cell clusters (ACECs). Left, representative image of an ACEC in a tryptophan hydroxylase 1 (TPH1) in situ hybridization of a tissue section from the mucosa overlying a tumor from a patient with familial SI-NET. The red arrow points to a cluster of TPH1 (blue)-expressing enterochromaffin (EC) cells at the bottom of the crypt. Top right, ACEC in a free-floating crypt isolated from the mucosa of a patient with familial SI-NET stained with chromogranin A (ChgA, green). Bottom right, confocal 3D analysis of ChgA-stained EECs in an ACEC shows the relative positions of enteroendocrine cells (EECs). B: evidence-based model for the genesis and development of SI-NETs in patients with familial SI-NET. SI-NETs are proposed to originate from +4 active reserve enterochromaffin cells (arECs) expressing reserve intestinal stem cell (ISC) genes BMI1 and HOPX (colored in green). Early tumor formation begins at the crypt bottom to form an ACEC and undergoes “bottom-up” morphogenesis. Advanced ACECs produce buds that become subepithelial tumor nests following separation from the epithelial layer. C: representative images from immunohistochemistry (IHC) staining with serotonin (5-HT) or ChgA (top) and RNA in situ hybridization with TPH1 (green) and HOPX (red) (bottom) illustrate each stage of ACEC development corresponding to the cartoon model in B. Reprinted from Ref. 47 with permission from Elsevier Inc.
Importantly, studies using serial tissue sections of ACECs along with RNA in situ hybridization provided a clue to the cell of origin and biological characteristics such as stem cell properties and markers. Earlier studies proposed that SI-NETs originate from serotonin-producing EC cells (25, 35). Indeed, clusters of EECs in ACECs were found to consist of TPH1-expressing EC cells. Further in situ hybridization studies indicated the accumulated TPH1-expressing EC cells coexpressed Tac1 but not other endocrine peptide genes such as GCG, NTS, GIP, SST, and GHRL. This suggested that clustering EC cells within ACEC belong to a subset of EC cells that coproduce 5-HT and a substance P. Finally, further characterization of gene expression indicated that the clustering EC cells in the ACECs also coexpressed reserve ISC marker genes BMI1 and HOPX. BMI1 and HOPX genes were consistently expressed in more advanced ACECs, areas of budding and submucosal tumor nests (Fig. 3C). Therefore, characterization of ACECs by RNA in situ hybridization studies suggested that the cell of origin for SI-NETs is a subset of EC cells that expresses reserve ISC genes, BMI1 and HOPX. Furthermore, the majority of these EC cells resides at the +4 position in normal ileal mucosa (47).
GENESIS AND DEVELOPMENT OF SI-NETs: INSIGHTS FROM +4 arECs BIOLOGY
In murine EC cells, +4 arECs are differentiated EECs that possess properties of +4 rISCs such as label retention (similar to +4 LRCs and qISC) and gene expression of BMI1 and HOPX. Although it remains to be shown in human tissues, it is reasonable to suggest that the +4 rISC gene-expressing subset of EC cells also functions as +4 arECs. If so, perhaps the cell of origin of SI-NETs is a +4 arEC subset of EC cells (13). This +4 arEC cell origin hypothesis would provide us new insights regarding the genesis, development, and biology of SI-NETs.
This +4 arEC origin hypothesis suggests that the SI-NETs possess properties of +4 rISCs and EECs. It accounts for the observed slow tumor growth, self-renewal potential, adaptability, and response to its environment and resistance to cell death. In fact, SI-NETs are slow-growing tumors with average Ki-67 <2.0% (10). At the same time, SI-NETs show steady growth with resistance to cell death (apoptosis index <0.01%; see Ref. 10), great adaptability to their environment (11), and capability of evolving to aggressive forms of tumors over time (10, 11).
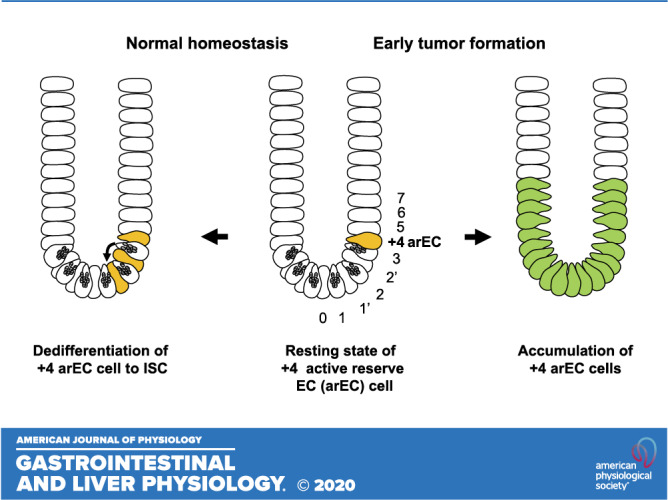
Analysis of positions and numbers of EC cells in crypts and ACECs from most patients with SI-NETs captures the progression of EC cell accumulation at the +4 and below positions in the early development of ACECs (Fig. 3). This temporal and spatial pattern of clustering fits well with +4 arISC hypothesis. As mentioned earlier, dedifferentiation of +4 arECs to ISCs was observed to occur through asymmetric cell division as previously shown for +4 LRCs (37, 40). Subsequently, the +4 arEC-derived ISCs are expected to expand by neutral drift, reside at 0, 1′, and 2′ positions between Paneth cells, and self-renew and/or differentiate by symmetric and asymmetric cell divisions in a stochastic fashion (27, 45, 51). Because the rate of dedifferentiation of +4 arECs depends on the crypt environment, it should increase upon loss of ISCs under the basal conditions of the physiological regenerative state and pathological regenerative state during injury and inflammation (46). If a high rate of aISC loss persists under some chronic conditions, the number of +4 arECs would increase to maintain homeostasis. Whether patients with SI-NETs carry any pathological conditions that cause abnormal accumulation of +4 arECs and/or whether the +4 arECs become transformed and fixed in an incompletely dedifferentiated state remain active areas of investigation. Mechanisms of interrelations between oncogenic signaling pathways and transcription factors regulating differentiation/dedifferentiation (60) are desired to be explored in future research.
Previous label-retaining studies suggested a critical role of +4 LRCs in the initiation of crypt fission in the normal regenerative state (7, 37). In support of their association with fission, EECs are frequently present at the initiation sites of crypt fission in primary crypts in both mice and humans (Y. Sei, J. Feng, X. Zhao, and S. A. Wank, unpublished observations). Notably, fission initiation sites are also hot spots for accumulation of EECs in patients with familial SI-NETs (unpublished observations). Although the fission rate in the ileal mucosa from patients has not yet been established, the observations of buddings and fissions of ACECs suggest critical roles of the fission process in the expansion of ACECs. In NEUROD1-CreERT or TPH1-CreERT mice, as evidenced in +4 LRC or BMI1+ cells (8, 56, 62), the facultative reserve function of EECs was evident in response to injury (46). It is also noteworthy that crypts form a regenerative patch originating from +4 arECs in response to injury in mice (46). Strikingly, in response to abdominal irradiation, we observed NEUROD1- or TPH1-derived epithelium that originated from patches of multiple crypts (as many as 42 crypts; see Ref. 46). The polyclonal origin of such patches was confirmed by a lineage tracing using fluorescent confetti-labeled mice (unpublished observation). Therefore, the regional patches are likely formed by rapid expansion of multiple radioresistant NEUROD1- or TPH1-derived clones (22) in multiple crypts with enhanced fission cycles (18) and with a local field effect (14), to contribute to the intestinal epithelial repair process. It seems reasonable to suggest that a patch of +4 arEC-derived ACECs could be generated similarly by cooperation of fissions and field effects via presently unknown mechanisms. As such, the generation of a patch of ACECs would accelerate the foundation for macroscopic tumor formation.
Perspectives and Significance
Recent technological advances, such as lineage tracing, organoid cultures, whole transcriptome analysis, FACS-cell sorting, and single cell analysis, have accelerated our understanding of ISCs and ISC dynamics. The findings from studies of ISCs using these techniques with new candidate marker genes shed new light on +4 LRC and provided novel insights into the “+4 reserve ISC” hypothesis (2, 31). It is now becoming clear that +4 rISCs are composed of a subset of EC cells that, despite the name reserve, actually play an active role in basal ISC dynamics and thus should more aptly be referred to as active reserve cells, arEC cells. Recent findings suggest that this subset of EC cells, +4 arECs, is the cell of origin for familial SI-NETs. Thus, SI-NETs hold properties of +4 rISCs and EEC. This understanding can account for their ability to self-renew, initiate crypt budding, and release of 5-HT. Further investigation in the role of the +4 arECs in ISC dynamics, including intestinal homeostasis, is essential to understand SI-NET tumorigenesis.
The current SI-NET +4 arEC cell origin hypothesis provides us new insights regarding the tumorigenesis of SI-NETs, and potentially other intestinal NETs. In the upper gastrointestinal tract, histamine-producing enterochromaffin-like (ECL) cell hyperplasia in stomach, gastrin-producing G cell, or somatostatin-producing D cell hyperplasia in the duodenum are recognized as putative precursor lesions of NETs and often associated with MEN1 (1, 30). The characterization of these early EEC clusters is a central approach to identify the cell of the origin and the subsequent development of these tumors (24, 30). Especially, crypt-oriented analysis of early lesions, including spatial-temporal location and gene expression of EECs, crypt budding, and fission analysis and clonality studies, will help to identify and characterize the putative cell of origin of these NETs and understand tumor initiation, growth, and development. For patients with familial NETs, examination of macroscopic-free mucosa from multiple areas has been especially informative. It would be interesting to determine whether there are subsets of active reserve ECLs, G or D cells similar to arEC cells that contribute physiologically to ISC dynamics and pathologically to the development of these NETs. What causes the constant accumulation and neoplastic transformation of these active reserve endocrine cells and how they progressively acquire malignant properties are some of the most critical questions for SI-NETS and other NETs.
GRANTS
This work was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.S., J.F., and X.Z. prepared figures; Y.S., J.F., and X.Z. drafted manuscript; Y.S. and S.A.W. edited and revised manuscript; S.A.W. approved final version of manuscript.
REFERENCES
- 1.Anlauf M, Perren A, Meyer CL, Schmid S, Saremaslani P, Kruse ML, Weihe E, Komminoth P, Heitz PU, Klöppel G. Precursor lesions in patients with multiple endocrine neoplasia type 1-associated duodenal gastrinomas. Gastroenterology 128: 1187–1198, 2005. doi: 10.1053/j.gastro.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 2.Bankaitis ED, Ha A, Kuo CJ, Magness ST. Reserve stem cells in intestinal homeostasis and injury. Gastroenterology 155: 1348–1361, 2018. doi: 10.1053/j.gastro.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457: 608–611, 2009. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 4.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 5.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O'Donnell TA, Brierley SM, Ingraham HA, Julius D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170: 185–198.e16, 2017. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. III. Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat 160: 77–91, 1981. doi: 10.1002/aja.1001600107. [DOI] [PubMed] [Google Scholar]
- 7.Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest 105: 1493–1499, 2000. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495: 65–69, 2013. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 9.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat 141: 461–479, 1974. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham JL, Grimelius L, Sundin A, Agarwal S, Janson ET. Malignant ileocaecal serotonin-producing carcinoid tumours: the presence of a solid growth pattern and/or Ki67 index above 1% identifies patients with a poorer prognosis. Acta Oncol 46: 747–756, 2007. doi: 10.1080/02841860701218659. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham JL, Janson ET. The biological hallmarks of ileal carcinoids. Eur J Clin Invest 41: 1353–1360, 2011. doi: 10.1111/j.1365-2362.2011.02537.x. [DOI] [PubMed] [Google Scholar]
- 12.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3: 1335–1342, 2017. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dempsey PJ. Are facultative reserve ISCs the cellular origin of familial small intestinal neuroendocrine tumors? Gastroenterology 151: 27–29, 2016. doi: 10.1053/j.gastro.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Dotto GP. Multifocal epithelial tumors and field cancerization: stroma as a primary determinant. J Clin Invest 124: 1446–1453, 2014. doi: 10.1172/JCI72589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumanski JP, Rasi C, Björklund P, Davies H, Ali AS, Grönberg M, Welin S, Sorbye H, Grønbæk H, Cunningham JL, Forsberg LA, Lind L, Ingelsson E, Stålberg P, Hellman P, Tiensuu Janson E. A MUTYH germline mutation is associated with small intestinal neuroendocrine tumors. Endocr Relat Cancer 24: 427–443, 2017. doi: 10.1530/ERC-17-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis JM, Kiezun A, Ramos AH, Serra S, Pedamallu CS, Qian ZR, Banck MS, Kanwar R, Kulkarni AA, Karpathakis A, Manzo V, Contractor T, Philips J, Nickerson E, Pho N, Hooshmand SM, Brais LK, Lawrence MS, Pugh T, McKenna A, Sivachenko A, Cibulskis K, Carter SL, Ojesina AI, Freeman S, Jones RT, Voet D, Saksena G, Auclair D, Onofrio R, Shefler E, Sougnez C, Grimsby J, Green L, Lennon N, Meyer T, Caplin M, Chung DC, Beutler AS, Ogino S, Thirlwell C, Shivdasani R, Asa SL, Harris CR, Getz G, Kulke M, Meyerson M. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat Genet 45: 1483–1486, 2013. doi: 10.1038/ng.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 20: 14–21, 2013. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greaves LC, Preston SL, Tadrous PJ, Taylor RW, Barron MJ, Oukrif D, Leedham SJ, Deheragoda M, Sasieni P, Novelli MR, Jankowski JA, Turnbull DM, Wright NA, McDonald SA. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc Natl Acad Sci USA 103: 714–719, 2006. doi: 10.1073/pnas.0505903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross S, Balderes D, Liu J, Asfaha S, Gu G, Wang TC, Sussel L. Nkx2.2 is expressed in a subset of enteroendocrine cells with expanded lineage potential. Am J Physiol Gastrointest Liver Physiol 309: G975–G987, 2015. doi: 10.1152/ajpgi.00244.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishibashi F, Shimizu H, Nakata T, Fujii S, Suzuki K, Kawamoto A, Anzai S, Kuno R, Nagata S, Ito G, Murano T, Mizutani T, Oshima S, Tsuchiya K, Nakamura T, Watanabe M, Okamoto R. Contribution of ATOH1+ cells to the homeostasis, repair, and tumorigenesis of the colonic epithelium. Stem Cell Reports 10: 27–42, 2018. doi: 10.1016/j.stemcr.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itkin-Ansari P, Marcora E, Geron I, Tyrberg B, Demeterco C, Hao E, Padilla C, Ratineau C, Leiter A, Lee JE, Levine F. NeuroD1 in the endocrine pancreas: localization and dual function as an activator and repressor. Dev Dyn 233: 946–953, 2005. doi: 10.1002/dvdy.20443. [DOI] [PubMed] [Google Scholar]
- 22.Itzkovitz S, Blat IC, Jacks T, Clevers H, van Oudenaarden A. Optimality in the development of intestinal crypts. Cell 148: 608–619, 2012. doi: 10.1016/j.cell.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jadhav U, Saxena M, O'Neill NK, Saadatpour A, Yuan GC, Herbert Z, Murata K, and Shivdasani RA. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell 21: 65–77.e5, 2017. doi: 10.1016/j.stem.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klöppel G, Anlauf M, Perren A. Endocrine precursor lesions of gastroenteropancreatic neuroendocrine tumors. Endocr Pathol 18: 150–155, 2007. doi: 10.1007/s12022-007-0025-5. [DOI] [PubMed] [Google Scholar]
- 25.Lembeck F. [The detection of 5-hydroxytryptamine in carcinoid metastases]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol 222: 235, 1954. doi: 10.1007/BF00249330. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Nakauka-Ddamba A, Tobias J, Jensen ST, Lengner CJ. Mouse Label-Retaining Cells Are Molecularly and Functionally Distinct From Reserve Intestinal Stem Cells. Gastroenterology 151: 298–310.e7, 2016. doi: 10.1053/j.gastro.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science 330: 822–825, 2010. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- 28.Lundqvist M, Wilander E. A study of the histopathogenesis of carcinoid tumors of the small intestine and appendix. Cancer 60: 201–206, 1987. doi:. [DOI] [PubMed] [Google Scholar]
- 29.Mawe GM, Hoffman JM. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10: 473–486, 2013. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mete O, Asa SL. Precursor lesions of endocrine system neoplasms. Pathology 45: 316–330, 2013. doi: 10.1097/PAT.0b013e32835f45c5. [DOI] [PubMed] [Google Scholar]
- 31.Mills JC, Sansom OJ. Reserve stem cells: differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal 8: re8, 2015. doi: 10.1126/scisignal.aaa7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA 108: 179–184, 2011. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moyana TN, Satkunam N. A comparative immunohistochemical study of jejunoileal and appendiceal carcinoids. Implications for histogenesis and pathogenesis. Cancer 70: 1081–1088, 1992. doi:. [DOI] [PubMed] [Google Scholar]
- 34.Murata K, Jadhav U, Madha S, van Es J, Dean J, Cavazza A, Wucherpfennig K, Michor F, Clevers H, Shivdasani RA. Ascl2-dependent cell dedifferentiation drives regeneration of ablated intestinal stem cells. Cell Stem Cell 26: 377–390.e6, 2020. doi: 10.1016/j.stem.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsson O. Profiling of ileal carcinoids. Neuroendocrinology 97: 7–18, 2013. doi: 10.1159/000343232. [DOI] [PubMed] [Google Scholar]
- 36.Potten CS, Hume WJ, Reid P, Cairns J. The segregation of DNA in epithelial stem cells. Cell 15: 899–906, 1978. doi: 10.1016/0092-8674(78)90274-X. [DOI] [PubMed] [Google Scholar]
- 37.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci 115: 2381–2388, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149: 146–158, 2012. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Psichas A, Reimann F, Gribble FM. Gut chemosensing mechanisms. J Clin Invest 125: 908–917, 2015. doi: 10.1172/JCI76309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quyn AJ, Appleton PL, Carey FA, Steele RJ, Barker N, Clevers H, Ridgway RA, Sansom OJ, Näthke IS. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell 6: 175–181, 2010. doi: 10.1016/j.stem.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Roth S, Franken P, Sacchetti A, Kremer A, Anderson K, Sansom O, Fodde R. Paneth cells in intestinal homeostasis and tissue injury. PLoS One 7: e38965, 2012. doi: 10.1371/journal.pone.0038965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40: 915–920, 2008. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol 270: 443–454, 2004. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152: 25–38, 2013. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Sei Y, Feng J, Chow CC, Wank SA. Asymmetric cell division-dominant neutral drift model for normal intestinal stem cell homeostasis. Am J Physiol Gastrointest Liver Physiol 316: G64–G74, 2019. doi: 10.1152/ajpgi.00242.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sei Y, Feng J, Samsel L, White A, Zhao X, Yun S, Citrin D, McCoy JP, Sundaresan S, Hayes MM, Merchant JL, Leiter A, Wank SA. Mature enteroendocrine cells contribute to basal and pathological stem cell dynamics in the small intestine. Am J Physiol Gastrointest Liver Physiol 315: G495–G510, 2018. doi: 10.1152/ajpgi.00036.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sei Y, Feng J, Zhao X, Forbes J, Tang D, Nagashima K, Hanson J, Quezado MM, Hughes MS, Wank SA. Polyclonal crypt genesis and development of familial small intestinal neuroendocrine tumors. Gastroenterology 151: 140–151, 2016. doi: 10.1053/j.gastro.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sei Y, Lu X, Liou A, Zhao X, Wank SA. A stem cell marker-expressing subset of enteroendocrine cells resides at the crypt base in the small intestine. Am J Physiol Gastrointest Liver Physiol 300: G345–G356, 2011. doi: 10.1152/ajpgi.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sei Y, Zhao X, Forbes J, Szymczak S, Li Q, Trivedi A, Voellinger M, Joy G, Feng J, Whatley M, Jones MS, Harper UL, Marx SJ, Venkatesan AM, Chandrasekharappa SC, Raffeld M, Quezado MM, Louie A, Chen CC, Lim RM, Agarwala R, Schäffer AA, Hughes MS, Bailey-Wilson JE, Wank SA. A hereditary form of small intestinal carcinoid associated with a germline mutation in inositol polyphosphate multikinase. Gastroenterology 149: 67–78, 2015. doi: 10.1053/j.gastro.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sjölund K, Sandén G, Håkanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology 85: 1120–1130, 1983. doi: 10.1016/S0016-5085(83)80080-8. [DOI] [PubMed] [Google Scholar]
- 51.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144, 2010. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 52.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science 334: 1420–1424, 2011. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, van Oudenaarden A, Clevers H. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 18: 203–213, 2016. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Thirlwell C, Will OC, Domingo E, Graham TA, McDonald SA, Oukrif D, Jeffrey R, Gorman M, Rodriguez-Justo M, Chin-Aleong J, Clark SK, Novelli MR, Jankowski JA, Wright NA, Tomlinson IP, Leedham SJ. Clonality assessment and clonal ordering of individual neoplastic crypts shows polyclonality of colorectal adenomas. Gastroenterology 138: 1441–1454, 2010. doi: 10.1053/j.gastro.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 55.Thliveris AT, Schwefel B, Clipson L, Plesh L, Zahm CD, Leystra AA, Washington MK, Sullivan R, Deming DA, Newton MA, Halberg RB. Transformation of epithelial cells through recruitment leads to polyclonal intestinal tumors. Proc Natl Acad Sci USA 110: 11523–11528, 2013. doi: 10.1073/pnas.1303064110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259, 2011. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens ACM, Barker N, van Oudenaarden A, Clevers H. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 14: 1099–1104, 2012. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waldum HL, Öberg K, Sørdal OF, Sandvik AK, Gustafsson BI, Mjønes P, Fossmark R. Not only stem cells, but also mature cells, particularly neuroendocrine cells, may develop into tumours: time for a paradigm shift. Therap Adv Gastroenterol 11: 1756284818775054, 2018. doi: 10.1177/1756284818775054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299: 76, 2003. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Giel-Moloney M, Rindi G, Leiter AB. Enteroendocrine precursors differentiate independently of Wnt and form serotonin expressing adenomas in response to active beta-catenin. Proc Natl Acad Sci USA 104: 11328–11333, 2007. doi: 10.1073/pnas.0702665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, Chen X, May R, Houchen CW, Fox JG, Gershon MD, Quante M, Wang TC. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest 124: 1283–1295, 2014. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA 109: 466–471, 2012. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan KS, Gevaert O, Zheng GXY, Anchang B, Probert CS, Larkin KA, Davies PS, Cheng ZF, Kaddis JS, Han A, Roelf K, Calderon RI, Cynn E, Hu X, Mandleywala K, Wilhelmy J, Grimes SM, Corney DC, Boutet SC, Terry JM, Belgrader P, Ziraldo SB, Mikkelsen TS, Wang F, von Furstenberg RJ, Smith NR, Chandrakesan P, May R, Chrissy MAS, Jain R, Cartwright CA, Niland JC, Hong YK, Carrington J, Breault DT, Epstein J, Houchen CW, Lynch JP, Martin MG, Plevritis SK, Curtis C, Ji HP, Li L, Henning SJ, Wong MH, and Kuo CJ. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell 21: 78–90.e6, 2017. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]