Keywords: dysbiosis, goblet, NHE8
Abstract
The loss of the intestinal Na+/H+ exchanger isoform 8 (NHE8) results in an ulcerative colitis-like condition with reduction of mucin production and dysbiosis, indicating that NHE8 plays an important role in intestinal mucosal protection. The aim of this study was to investigate the potential rebalance of the altered microbiota community of NHE8-deficient mice via fecal microbiota transplantation (FMT) and feeding probiotic VSL#3. We also aimed to stimulate mucin production by sodium butyrate administration via enema. Data from 16S rRNA sequencing showed that loss of NHE8 contributes to colonic microbial dysbiosis with reduction of butyrate-producing bacteria. FMT increased bacterial adhesion in the colon in NHE8 knockout (NHE8KO) mice. Periodic-acid Schiff reagent (PAS) stain and quantitative PCR showed no changes in mucin production during FMT. In mice treated with the probiotic VSL#3, a reduction of Lactobacillus and segmented filamentous bacteria (SFB) in NHE8KO mouse colon was detected and an increase in goblet cell theca was observed. In NHE8KO mice receiving sodium butyrate (NaB), 1 mM NaB stimulated Muc2 expression without changing goblet cell theca, but 10 mM NaB induced a significant reduction of goblet cell theca without altering Muc2 expression. Furthermore, 5 mM and 10 mM NaB-treated HT29-MTX cells displayed increased apoptosis, while 0.5 mM NaB stimulated Muc2 gene expression. These data showed that loss of NHE8 leads to dysbiosis with reduction of butyrate-producing bacteria and FMT and VSL#3 failed to rebalance the microbiota in NHE8KO mice. Therefore, FMT, VSL#3, and NaB are not able to restore mucin production in the absence of NHE8 in the intestine.
NEW & NOTEWORTHY Loss of Na+/H+ exchanger isoform 8 (NHE8), a Slc9 family of exchanger that contributes to sodium uptake, cell volume regulation, and intracellular pH homeostasis, resulted in dysbiosis with reduction of butyrate-producing bacteria and decrease of Muc2 production in the intestine in mice. Introducing fecal microbiota transplantation (FMT) and VSL#3 in NHE8 knockout (NHE8KO) mice failed to rebalance the microbiota in these mice. Furthermore, administration of FMT, VSL#3, and sodium butyrate was unable to restore mucin production in the absence of NHE8 in the intestine.
INTRODUCTION
NHE8 is a multifunctional Na+/H+ exchange transporter which is involved in sodium uptake, cell proliferation, and protein traffic. This protein is located at the apical membrane in the gastrointestinal tract and other tissues (42). Similar to NHE8 expression reduction in several colitis animal models [trinitrobenzesulfonic acid (TNBS) colitis, dextran sulfate sodium (DSS) colitis, and Citrobacter rodentium-infected mice] (1, 34, 36), NHE8 expression is also reduced in human ulcerative colitis patients (35). Furthermore, loss of NHE8 function leads to the decrease of Muc2 gene expression in the gastrointestinal tract, the increase of susceptibility to the development of gastric ulcer, DSS-induced colitis, and bacterial adherence in the colon of mice (36, 56, 58).
Inflammatory bowel diseases (IBD), which consist of two major forms, Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic relapsing condition that leads to significant morbidity and mortality in affected individuals. The pathology of the disease is related to the interaction involving genetic predisposition and intestinal microbial imbalance or dysbiosis (2, 16). Current treatment modalities have been used to modulate the immune system and rebalance the microbiota and promotion of intestinal homeostasis. However, a lack of efficacy has been shown.
Fecal microbiota transplantation (FMT) was used in many animal disease models and clinical trials and has gained attention in recent years (12, 15). In an experimental animal colitis model, FMT has been shown to increase mucosal healing, remodel intestinal flora, and stimulate intestinal homeostasis (25, 59). Even though evidence showed that FMT in IBD patients has been used over the last two decades, the most effective results of this intervention were observed in patients with recurrent Clostridium difficile infections (45). The mechanism of FMT may involve interaction between donor and host microbiota with subsequent mediation of gut mucosal barrier, stimulation of short-chain fatty acid production (such as butyrate) and immune system improvement (31), suggesting that FMT might be able to correct dysbiosis.
The probiotic VSL#3 also has potential in treating pouchitis in clinic. VSL#3 treatment has been associated with its anti-inflammatory properties (13). VSL#3 has also been shown to induce mucin expression in vivo and in vitro (8).
The primary defense of the intestinal mucosal layer against the microbes and pathogen present in the lumen is the mucus layer, which plays an important role in preventing the adhesion of bacterial population to the epithelial surface (29). Mucus is produced by goblet cells, and the most secreted mucin in the colon is Muc2. Muc2 knockout mice develop inflammation in the intestine from epithelial-bacterial interaction (49). Since NHE8 function is important for mucosal protection, evidenced by an impaired mucin layer integrity and UC-like inflammation in NHE8-deficient mice, we further investigated whether dysbiosis occurs in NHE8-deficient mice and the potential rebalance of the altered microbiota community and restoration of mucin production by introducing good bacteria through fecal transplantation, probiotic VSL#3 supplementation, or sodium butyrate administration.
MATERIALS AND METHODS
Animals.
NHE8 knockout (NHE8KO) mice were generated using NHE8+/− breeding pairs in a Swiss Webster background as previously described (56). Wild-type (NHE8WT) and NHE8 knockout (NHE8KO) mice were housed in separate groups in polypropylene cages with unlimited access to standard rodent food and water. Only male mice are used in our studies as male NHE8KO mice show symptoms while female NHE8KO mice are normal due to compensation from other NHE isoforms (57). All animal works in this study were approved by the University of Arizona Institutional Animal Care and Use Committee.
Cell culture.
Human intestinal goblet cells (HT29-MTX) were cultured in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) containing 4.5 g/L glucose, l-glutamine, and sodium pyruvate, 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C in a 5%CO2 atmosphere. Cells were cultured for 14 days before they were treated with various concentrations of sodium butyrate (NaB) for 24 h.
Fecal microbiota transplantation study.
Fresh stools were collected from 7-wk-old NHE8WT and NHE8KO mice (4 mice for each genotype). Stools were then resuspended in autoclaved water and passed through a 40-µm pore-size nylon filter. The filtrates were mixed with sterile glycerol at a ratio of 9:1 (vol/vol) and stored at −80°C (18). Recipient mice (8 male NHE8WT mice and 8 male NHE8KO mice, 6 wk old) were treated with a cocktail of antibiotics in drinking water for 7 days in the following concentrations: 1 g/L ampicillin, 1 g/L neomycin, 0.5 g/L vancomycin, and 1 g/L metronidazole (18). After 2 days without antibiotics, mice were transplanted with 200 µl fecal material intragastrically (gavage) once a day for 2 days. Four weeks later mice were euthanized, and colons were collected for DNA/RNA extraction and histology analysis.
VSL#3 supplement study.
NHE8WT mice and NHE8KO mice at 7 wk old (5 males for each genotype) were fed intragastrically five times per week for 3 wk with 3 × 108 colony-forming units (CFU) suspended in PBS (38). The control group received PBS only. At the end of the experiment, mice were euthanized and colons were collected for DNA/RNA extraction and histology analysis.
Butyrate supplementation study.
NHE8WT mice and NHE8KO mice at 7 wk old (6 males for each genotype) were supplemented with sodium butyrate (NaB; Sigma) via enema twice a day for 5 days with two different concentrations: 1 mM and 10 mM NaB. Control group received saline enema. After the treatment, mice were euthanized, and colons were collected for DNA/RNA extraction and histology analysis.
Intestinal microbial composition analysis.
For 16S rRNA sequencing, DNA from distal colon tissues was isolated using All/Prep DNA/RNA Mini Kit (Qiagen, Germany). The hypervariable V4 region of the 16S rRNA gene was amplified from each sample using reverse unique barcoded primers consisting of Golay barcodes, sequencing primer pads, linkers, Illumina adapters, and common forward primer (10), using MyFi (Bioline) polymerase Master Mix. Amplicons were quantified using Quant-It PicoGreen dsDNA Assay kit (Invitrogen) according to the manufacturer’s protocol. Equal amounts of 240 ng of amplified DNA from each sample were pooled and cleaned using UltraClean PCR Clean-Up Kit (MoBio). Amplicons pooled into one sequencing library were diluted and denatured, and 7 nM of the pooled library were sequenced using custom primers (10). Due to the limited sequence diversity among 16S rRNA amplicons, 5% of the PhiX control library (Illumina) made from phiX174 was added to the run. The library was subjected to the paired-end sequencing using the 2 × 150 bp MiSeq Reagent Kit V2 (Illumina). Sequencing of all samples was performed at our laboratory on Illumina MiSeq, and fastq files were generated. Sequences were demultiplexed using idemp script (https://github.com/yhwu/idemp). Filtering, dereplication, sample inference, chimera identification, merging of paired-end reads, and taxonomy assignment were done with a reference-free Divisive Amplicon Denoising Algorithm 2 (Dada2) R package (9). The taxonomy was assigned against SILVA database release 132 (https://www.arb-silva.de/documentation/release-132/). The depth of analysis was set at 22,904 reads/sample. The R vegan package (41a) was used as a tool for diversity analysis, ordination methods, analysis of dissimilarities, and statistical analysis. The obtained results were visualized with a ggplot2 package (52). For differential abundance analysis, including statistical analysis and data transformation, the DESeq2 package was used (37). Following the previously published method for the targeted bacterial group assay, qPCR was performed using primers for common intestinal bacterial groups (4, 22, 36, 39, 40, 43, 47). Total bacteria were detected with Eubac primers (EubacF: TCCTACGGGAGGCAGCAGT; EubacR: GGACTACCAGGGTATCTAATCCTGTT). Bacteria in the Bacteroidetes group were detected with Bac primers (Bac934F: GGARCATGTGGTTTAATTCGATGAT; Bac1060R: AGCTGACGACAACCATGCAG). Bacteria in the Firmicutes group were detected with Firm primers (Firm934F: GGAGTATGTGGTTTAATTCGAAGCA; Firm1060R: AGCTGACGACAACCATGCAC). Bacteria in the Lactobacillus group were detected with Lacto primers (LactoF: GAGGCAGCAGTAGGGAATCTTC; LactoR: GGCCAGTTACTACCTCTATCCTTCTTC). Bacteria in the segmented filamentous bacteria group were detected with segmented filamentous bacteria (SFB) primers (SFB736F: GACGCTGAGGCATGAGAGCAT; SFB844R: GACGGCACGGATTGTTATTCA). Fold changes were used to compare the relative abundance of the targeted bacterial groups between control mice and treated mice.
RNA purification and gene expression assay.
Total RNA was isolated from tissue and cells using Directzol kit (Genesee Scientific; El Cajon, CA). The gene expression was measured using TaqMan probes (Applied Biosystems, Foster City, CA) on LightCycler 96 (Roche). TATA-binding protein (TBP) was used as a housekeeping gene to normalize gene expression levels.
Protein extraction and dot blot.
Total protein was prepared from cells in RIPA buffer containing Halt protease and phosphatase inhibitor cocktail (Thermo Scientific). Protein concentration was measured using BCA protein assay (Thermo Scientific). Protein samples (5 µg) were dotted on a nitrocellulose membrane. The membrane was blocked using 5% nonfat milk diluted in TBS-T for 1 h and then incubated with primary antibodies for 30 min, followed by secondary antibody for 30 min. The primary antibodies are Muc2 (R-12, goat polyclonal, Santa Cruz; 1:6,000; react with human/rat/mouse mucin 2 protein) and β-actin (Mouse monoclonal, Sigma; 1:5,000; react with human/rat/mouse β-actin protein). The second antibodies are donkey anti-goat horseradish peroxidase (HRP; Santa Cruz; 1:5,000) and anti-mouse HRP (Sigma; 1:40,000). The detection was performed using EcL Chemiluminescence Western Blotting Substrate (Thermo Scientific) and Syngene G-BOX Imaging System. ImageJ software was used to perform the densitometric analysis.
Goblet cell counting and goblet cell theca measurement.
Goblet cells in the tissue were analyzed in a 5-μm-thick section stained with periodic-acid Schiff reagent (PAS). Tissue sectioning and PAS staining were done by the University of Arizona Pathology laboratory (Tucson, AZ). Images were observed under EVOS FL microscope (Life Technologies). Goblet cells and goblet cell theca were measured using an ImageJ program. At least 10 crypts from each section were used for measurements.
Cell death analysis.
HT29-MTX cells were harvested, washed in cold PBS, and centrifuged. Cells were suspended in 1× annexin-binding buffer (Invitrogen) and incubated with annexin-V (BD Pharmigene) and 7-aminoactinomycin D (7-AAD) (BD Pharmigene) following the manufacturer’s instruction. Data were collected using LSRII Fortessa (BD Biosciences) with FlowJo Software (Tree Star).
Statistical analysis.
Kruskal-Wallis H test was used to analyze 16S rRNA sequencing data. Student’s t test was used to compare data throughout the study. Data are present in means ± SE. Differences were considered statistically significant when P < 0.05.
RESULTS
Effect of NHE8 deficiency on colonic microbiome.
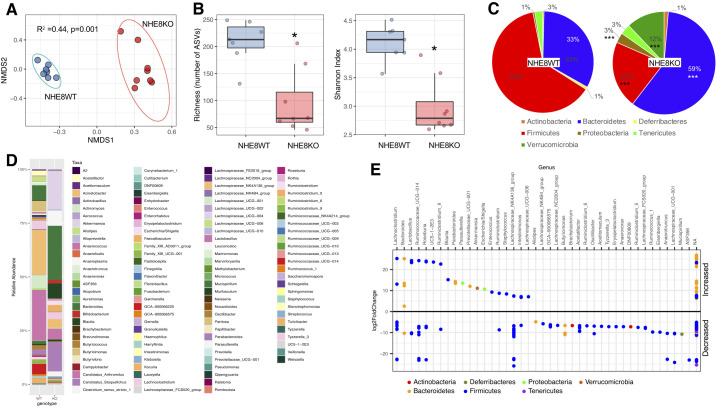
We have demonstrated that normal NHE8 function is important for maintaining mucosal homeostasis and that loss of NHE8 in mice led to increased bacterial adhesion and susceptibility to DSS and aaaazoxymethane (AOM)-induced tumor formation (36, 50, 58). To further understand whether NHE8 deficiency alters intestinal microbial composition, we isolated DNA from distal colonic mucosa and analyzed bacterial composition by 16S rRNA amplicon sequencing. Beta diversity as nonmetric multidimensional scaling (NMDS) based on Bray-Curtis distances showed a significant (Adonis, P = 0.001) difference between NHE8WT and NHE8KO mucosal microbial communities (Fig. 1A). When the community richness was calculated, the NHE8WT mice showed a significantly higher number of amplicon sequence variants (ASVs) compare with NHE8KO mice (208 vs. 95, respectively, P = 0.002). This decrease in richness is accompanied by a decreased Shannon Index (4.11 vs. 2.99, P = 0.001), which shows that evenness of the community is lower (Fig. 1B). Taxonomic analysis showed significant changes at the phylum and genus levels (Fig. 1, C and E). At phyla level, Firmicutes were decreased from 63% in NHE8WT to 21% in NHE8KO mice (P = 0.00062). Bacteroidetes were increased from 33% in NHE8WT to 59% in NHE8KO mice (P = 0.004), similarly to Proteobacteria phylum, which was increased from 1% to 3%, respectively (P = 0.009) and to Verrucomicrobia phylum, which was increased from 0.01% to 12% in NHE8KO mice (P = 0.0014). Although an increase was detected in Actinobacteria and Tenericutes phylum, the increase did not reach statistical significance. A significant difference at Genera level was seen in NHE8KO mice compared with NHE8WT, which was clearly reflected at the Phylum level (Fig. 1E) (adjusted P < 0.05). These observations suggest that loss of NHE8 leads to colonic mucosal microbial dysbiosis.
Fig. 1.
Distal colonic mucosa associated microbiome in Na+/H+ exchanger isoform 8 knockout (NHE8KO) mice. A: nonmetric multidimensional scaling (NMDS) on Bray-Curtis distances in NHE8 wild-type (NHE8WT) (blue) mice and in NHE8KO mice (red). The data ellipses were calculated with 95% confidence level. Permutational multivariate analysis of variance using the distance matrixes Adonis test was used to compare 2 genotypes. B: richness, a measure of the number of amplicon sequence variants (ASVs), and Shannon Index, a measure of evenness of the microbial populations in NHE8WT and NHE8KO mice. *P < 0.05, Mann-Whitney U test. C: distribution of major phyla in NHE8WT and NHE8KO mice. ***P < 0.001, phyla significantly different between genotypes by Mann-Whitney U test. D: microbial composition in NHE8WT (WT) and NHE8KO mice (KO) at the genus level. For visualization, all taxa with a mean value of the relative abundance <0.01% were filtered out. E: significantly different genera in NHE8KO mice (KO) in comparison with NHE8WT mice (WT) (bold line). Each dot represents a genus significantly different (DESeq2 adjusted P < 0.05) between 2 genotypes. The magnitude of difference is presented as a log2 scale of fold change comparing to WT mice.
Effect of fecal material transplantation on NKE8KO mice.
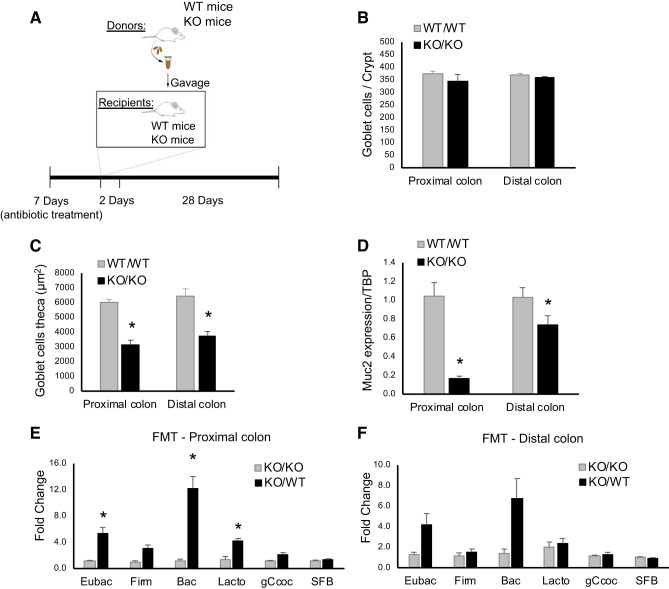
Since NHE8KO mice have an altered microbiome, we wanted to test whether the microbiome from NHE8WT mice could restore the bacterial composition in NHE8KO mice. To perform this study, donor fecal materials from NHE8WT and NHE8KO mice were collected and transplanted intragastrically (gavage) into NHE8WT- or NHE8KO-recipient mice (Fig. 2A). During the period of the FMT experiment, no changes were observed in the body weight and the consistency of the stools in these FMT mice (data not shown). Although the number of goblet cells in the colon was unchanged between NHE8WT mice receiving NHE8WT fecal material (WT/WT) and NHE8KO mice receiving NHE8KO fecal material (KO/KO) (Fig. 2B), the goblet cell theca were significantly lower in KO/KO mice than that of WT/WT mice (3,168 ± 286 in KO/KO vs. 6,019 ± 211 in WT/WT, n = 8 mice, P = 0.008 in proximal colon; 3,768 ± 284 in KO/KO vs. 6,418 ± 525 in WT/WT, n = 8 mice, P = 0.045 in distal colon) (Fig. 2C). Colonic Muc2 expression was also significantly lower in KO/KO than that of WT/WT mice (0.16 ± 0.03 in KO/KO vs. 1.05 ± 0.14 in WT/WT, n = 8 mice, P < 0.001 in proximal colon; 0.74 ± 0.10 in KO/KO vs. 1.03 ± 0.11 in WT/WT, n = 8 mice, P = 0.043 in distal colon) (Fig. 2D).
Fig. 2.
Fecal microbiota transplantation (FMT) in Na+/H+ exchanger isoform 8 knockout (NHE8KO) mice. A: schematic representation of the FMT treatment. B: number of goblet cells per crypt. C: goblet cell theca (µm2). D: Muc2 mRNA expression. E: quantitative analysis of bacterial groups in the proximal colon comparing NHE8KO mice receiving NHE8KO fecal material (KO/KO) with NHE8KO mice receiving NHE8 wild-type (NHE8WT) fecal material (KO/WT). F: quantitative analysis of bacterial groups in the distal colon comparing KO/KO with KO/WT. The fold changes are comparing relative abundance of the targeted bacterial groups between control mice and treated mice. Eubac, Eubacteria; Firm, Firmicutes; Bac, Bacteroidetes; Lacto, Lactobacillus; gCcoc, Clostridium coccoides; SFB, segmented filament bacteria. PAS-positive cells were quantified using ImageJ program. Data are represented as means ± SE from 8 mice. *P ≤ 0.05.
To understand whether FMT changes the adhered bacteria population in the colon in NHE8KO mice, quantitative PCR was used to compare the major bacterial groups between NHE8KO mice receiving NHE8KO fecal material (KO/KO) and NHE8KO mice receiving NHE8WT fecal material (KO/WT). As shown in Fig. 2E, total bacterial burden was increased by ~5 fold on the colonic mucosal surface in the proximal colon of KO/WT mice (5.40 ± 0.88 in KO/WT vs. 1.10 ± 0.16 in KO/KO, n = 8 mice, P = 0.038). The increased bacteria were mainly associated with Bacteroidetes (12.22 ± 1.798 in KO/WT vs. 1.19 ± 0.25 in KO/KO, n = 8 mice, P = 0.019) and Lactobacillus (4.13 ± 0.45 in KO/WT vs. 1.43 ± 0.39 in KO/KO, n = 8 mice, P = 0.042). In the distal colon, an increased tendency was observed in the total bacterial burden in KO/WT mice (4.14 ± 1.13 in KO/WT vs. 1.27 ± 0.24 in KO/KO), with possible increases seen in the Bacteroidetes group (6.74 ± 1.95 in KO/WT vs. 1.44 ± 0.38 in KO/KO) (Fig. 2F).
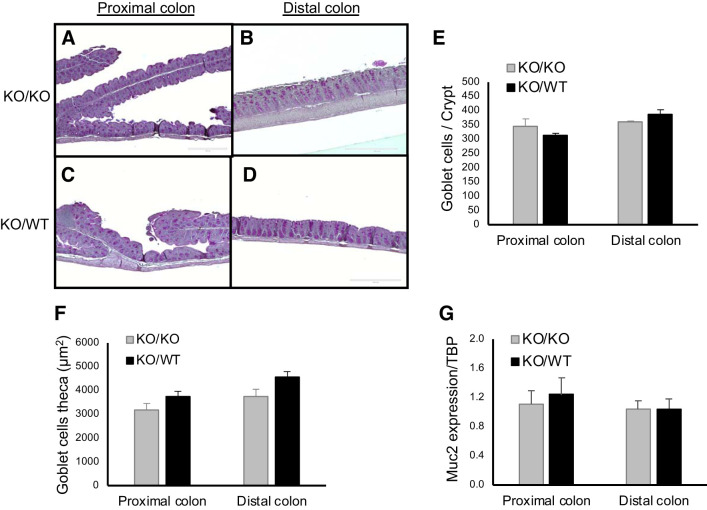
To analyze whether FMT modulates mucin production in NHE8KO mice, PAS staining was performed on tissue sections and PAS-positive cells were counted. As shown in Fig. 3, A–D, the area and the intensity of PAS staining was similar between KO/KO mice and KO/WT mice. Further quantification of goblet cell numbers per crypt confirmed that FMT did not change the number of goblet cells in NHE8KO colon (Fig. 3E). The same observation was also seen in goblet cell theca (Fig. 3F). Furthermore, the gene expression of Muc2 were similar in both proximal and distal colon in KO/WT mice compared with KO/KO mice (Fig. 3G).
Fig. 3.
Modulation of mucin production by fecal microbiota transplantation (FMT) in the proximal colon and distal colon of Na+/H+ exchanger isoform 8 knockout (NHE8KO) mice. A– D: periodic-acid Schiff (PAS) staining from the proximal colon and distal colon of NHE8KO mice receiving NHE8KO fecal material (KO/KO) comparing with NHE8KO mice receiving NHE8 wild-type (NHE8WT) fecal material (KO/WT). At least 10 crypts from each section were used for measurements, and the representative pictures were selected. E: number of goblet cells per crypt. F: goblet cell theca (µm2). G: Muc2 mRNA expression. PAS staining (×10) magnification. Periodic-acid Schiff (PAS)-positive cells were quantified using ImageJ program. Data are represented as means ± SE from 8 mice.
Effect of VSL#3 supplementation on NHE8KO mice.
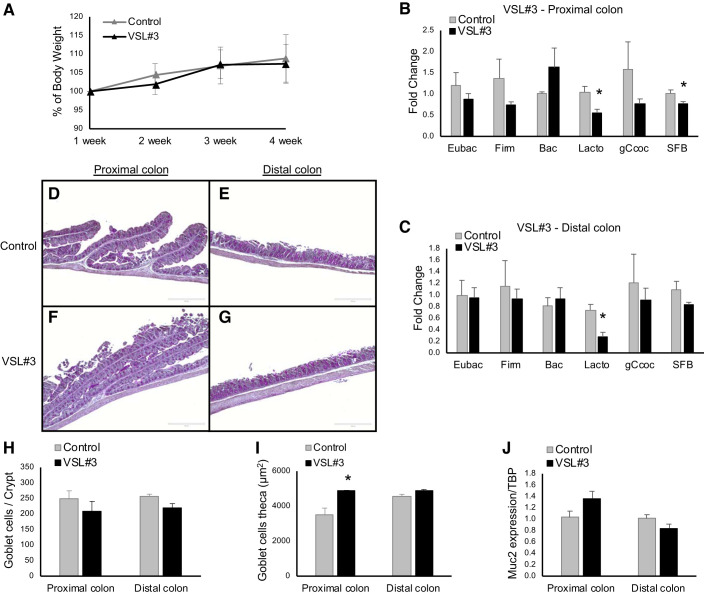
Since FMT did not correct microbiota or improve Muc2 expression in NHE8KO mice, we thought to evaluate the effect of the probiotic VSL#3. Apparently, NHE8KO mice treated with VSL#3 did not alter their body weight (Fig. 4A) or the consistency of the stools (data not shown). By analyzing the population of adherent bacteria, we found that VSL#3 treatment significantly decreased the number of Lactobacillus in the proximal colon (0.55 ± 0.09 in VSL#3 vs. 1.04 ± 0.15 in Control, n = 5 mice, P = 0.027) (Fig. 4B) and in the distal colon (0.27 ± 0.08 in VSL#3 vs. 0.74 ± 0.10 in Control, n = 5 mice, P = 0.014) (Fig. 4C). The SFB population was also decreased but only showed statistical significance in the proximal colon (0.77 ± 0.05 in VSL#3 vs. 1.02 ± 0.08 in Control, n = 5 mice, P = 0.045) (Fig. 4B).
Fig. 4.
The probiotic VSL#3 supplementation on Na+/H+ exchanger isoform 8 knockout (NHE8KO) mice. A: the body weight differences in percentage comparing control group with VSL#3 treatment. B: quantitative analysis of bacterial groups in the proximal colon comparing NHE8KO mice with VSL#3-treated NHE8KO mice. C: quantitative analysis of bacterial groups in the distal colon comparing NHE8KO mice with VSL#3-treated NHE8KO mice. The fold changes are comparing relative abundance of the targeted bacterial groups between control mice and treated mice. D–G: periodic-acid Schiff (PAS) staining from the proximal colon and distal colon of NHE8KO mice and VSL#3-treated NHE8KO mice. H: number of goblet cells per crypt. I: goblet cell theca (µm2). J: Muc2 mRNA expression. PAS staining (×10) magnification. PAS-positive cells were quantified using ImageJ program. TBP, TATA-binding protein; Eubac, Eubacteria; Firm, Firmicutes; Bac, Bacteroidetes; Lacto, Lactobacillus; gCcoc, Clostridium coccoides; SFB, segmented filament bacteria. Data are represented as means ± SE from 5 mice. *P ≤ 0.05.
To study the effect of VSL#3 on mucin production, we performed PAS staining on tissue sections and analyzed PAS-positive cells. As shown in Fig. 4, D–G, VSL#3-treated NHE8KO mice showed increased PAS staining in the proximal colon. While the number of goblet cells per crypt showed no change between VSL#3 treatment and nontreatment group (Fig. 4H), the goblet cell theca was significantly increased in the proximal colon of VSL#3-treated NHE8KO mice (4879 ± 26 in VSL#3 vs. 3508 ± 384 in Control, n = 5 mice, P = 0.044) (Fig. 4I). Furthermore, VSL#3 treatment marginally increased the expression of Muc2 gene in the proximal colon of NHE8KO mice (1.37 ± 0.12 in VSL#3 vs. 1.03 ± 0.11 in Control, n = 5 mice, P = 0.062) but had no effect on the expression of Muc2 gene in the distal colon of NHE8KO mice (Fig. 4J).
Effect of butyrate supplementation in NHE8KO mice.
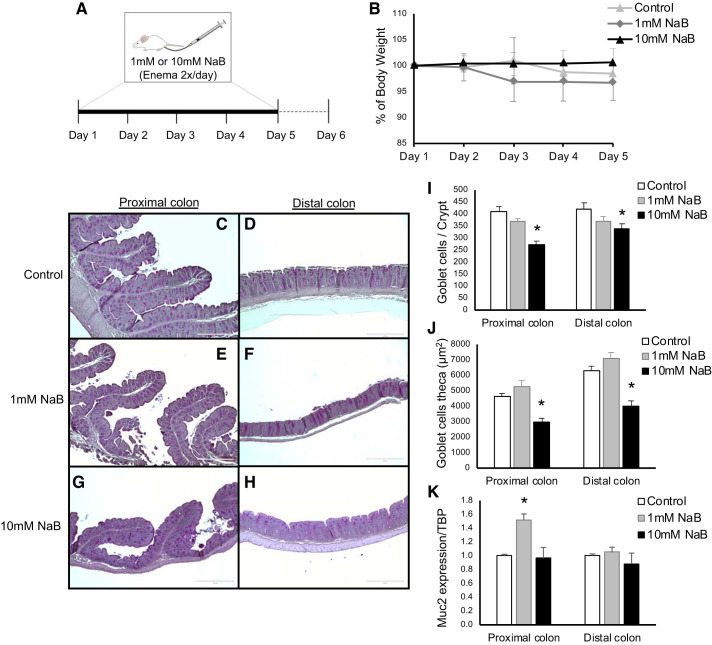
To address the effect of butyrate in the absence of NHE8 expression, NHE8KO mice were treated with various concentrations of NaB via enema twice a day for 5 days (Fig. 5A). Administration of both concentrations (1 mM and 10 mM) of NaB had no effect on body weight in NHE8KO mice (Fig. 5B). PAS stain revealed a NaB concentration-dependent mucin production alteration. While 1 mM NaB supplementation showed similar PAS-positive cells compared with control group, 10 mM NaB showed reduced PAS-positive cells both in the proximal and distal colon (Fig. 5, C–H). Quantification of goblet cells confirmed that 1 mM NaB treatment did not affect the number of goblet cells per crypt in NHE8KO mice, but 10 mM NaB treatment significantly reduced the number of goblet cells per crypt both in the proximal colon (270 ± 17 in 10 mM NaB vs. 409 ± 22 in Control, n = 6 mice, P = 0.002) and in the distal colon (337 ± 22 in 10 mM NaB vs. 422 ± 25 in Control, n = 6 mice, P = 0.036) (Fig. 5I). When comparing goblet cell theca, 1 mM NaB treatment did not change goblet cell theca, but 10 mM NaB treatment induced a significant reduction of goblet cell theca in the proximal colon (3,008 ± 212 in 10 mM NaB vs. 4,650 ± 175 in Control, n = 6 mice, P < 0.001) and in the distal colon (3,970 ± 375 in 10 mM NaB vs. 6,286 ± 303 in Control, n = 6 mice, P < 0.005) in NHE8KO mice (Fig. 5J). Although Muc2 gene expression was not altered by NaB treatment in most of the conditions, the only exception was that 1 mM NaB supplementation increased Muc2 mRNA abundancy in the proximal colon of NHE8KO mice (1.52 ± 0.09 in 1 mM NaB vs. 1.00 ± 0.02 in Control, n = 6 mice, P = 0.014) (Fig. 5K).
Fig. 5.
Sodium butyrate (NaB) supplementation in Na+/H+ exchanger isoform 8 knockout (NHE8KO) mice. A: schematic representation of the NaB treatment. B: the body weight differences in percentage comparing control group with 1 mM and 10 mM NaB-treated NHE8KO mice. C–H: periodic-acid Schiff (PAS) staining from the proximal colon and distal colon of control group and 1 mM and 10 mM NaB-treated NHE8KO mice. I: number of goblet cells per crypt. J: goblet cell theca (µm2). K: Muc2 mRNA expression. PAS staining (×10) magnification. PAS-positive cells were quantified using ImageJ program. Data are represented as means ± SE from 6 mice. *P ≤ 0.05.
Effect of sodium butyrate on Muc2 expression in cultured goblet cells.
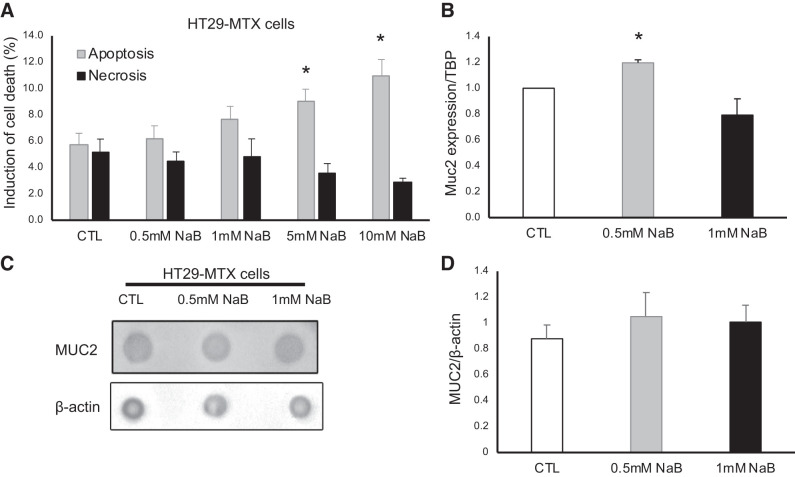
To gain more insight into butyrate effect on goblet cell function, we treated the human goblet cells HT29-MTX cells with NaB. Because butyrate is detrimental to cancer cells, we first analyzed how different concentrations of NaB affect goblet cell death. As shown in Fig. 6A, 0.5 mM and 1 mM NaB treatment did not significantly introduce cell death, but 5 mM and 10 mM NaB induced cell death from 5.7 ± 0.87% in control cells to 9.0 ± 0.91% in 5 mM NaB-treated cells (n = 3 experiments, P = 0.049) and 11.0 ± 1.23% in 10 mM NaB-treated cells (n = 3 experiments, P = 0.023). Based on the results, we chose 0.5 mM and 1 mM NaB to investigate whether butyrate stimulate MUC2 expression in goblet cells. As shown in Fig. 6B, MUC2 mRNA expression was increased in 0.5 mM NaB-treated cells (1.20 ± 0.02 in 0.5 mM NaB vs. 1.00 ± 0.00 in Control, n = 3 experiments, P = 0.001) but not in 1 mM NaB-treated cells. Dot blot did not detect the changes in MUC2 protein abundance in these cells (1.05 ± 0.18 in 0.5 mM NB and 1.01 ± 0.13 in 1 mM NB vs. 0.88 ± 0.11 in Control, n = 3 experiments, Fig. 6, C and D).
Fig. 6.
Effect of sodium butyrate (NaB) in goblet cells. A: induction of apoptosis and necrosis by different concentrations of NaB in HT29-MTX cells. B: Muc2 mRNA expression. TBP, TATA-binding protein. C: dot blot of MUC2 protein analysis. D: quantification of the relative abundance of MUC2 protein. Data are represented as means ± SE from 3 experiments. *P ≤ 0.05.
DISCUSSION
The bacteria and the intestinal tissue are separated by the mucus layer. Mucus is produced by goblet cells, and it protects the intestinal epithelial cells from the external agents, including food particles and pathogens. The most colonic secretory mucin is the heavily glycosylated gel-forming mucin Muc2. In the colon, Muc2 is responsible for forming the inner and outer mucin layers. The inner layer is well organized, firmly attached to the epithelial cells, and impenetrable to bacteria under normal physiological conditions. On the other hand, the outer layer is disorganized and is a habitat for commensal bacteria (3, 7). Loss of these mucin layers is associated with increased bacteria-epithelia interaction and therefore colitis induction (5). We have previously reported that NHE8 is located at the apical membrane in the intestinal tract and that loss of NHE8 function leads to decreased Muc2 expression, high susceptibility to development of gastric ulcers, and a significant increase in adherent bacteria in the colon (36, 56, 58). Here, we showed that loss of NHE8 function resulted in microbiota dysbiosis as Bacteroidetes were increased and Firmicutes were decreased in NHE8KO mice. Specifically, the genus Butyricimonas and genus Ruminiclostridium_5 and _6 were dramatically reduced in NHE8KO mice. The remarkable reduction in those taxonomic genera suggested a potential butyrate production defect in NHE8KO mice, since these groups of bacteria are involved in the production of butyrate (14, 41, 46). Our data also suggest a possible role of NHE8 in maintaining intestinal bacterial homeostasis. Interestingly, the similar microbiome alteration was also reported in NHE3KO mice with a reduction of butyrate-producing bacteria and an expansion of Bacteroidaceae (23, 32). Although NHE8 and NHE3 are expressed in the intestine, their physiological roles are different. NHE8 contributes to intestinal sodium absorption at the early development stage when NHE3 expression is low (54, 55). At adult age, NHE3 is mainly involved in sodium absorption regulation while NHE8 involves Muc2 expression regulation (33, 44, 58). The observations of intestinal microbiome dysbiosis in the absence of either NHE3 or NHE8 protein suggest the important roles of these intestinal sodium-hydrogen exchangers in modulating bacterial diversity.
Since NHE8KO mice display microbiota dysbiosis, we performed a fecal microbiota transplantation (FMT) study to address whether this procedure could improve the attached microbiota in NHE8KO mice by analyzing the most common intestinal bacterial groups (4, 22, 36, 39, 40, 43, 47). FMT has been used in clinical trials to treat several diseases in recent years, such as UC and refractory Clostridium difficile infection (12, 15). The cure rate of this approach varies depending on the type of disease, and it is considered as the last option for treatment. FMT intervention has achieved good results in patients with recurrent Clostridium difficile infections (45). The molecular and cellular mechanism for FMT remains unclear, but it may involve interaction between donor and host microbiota and subsequent mediation of gut mucosal barrier and the immune system (31). Even with promising results, FMT therapy still needs to be carefully monitored as a possible therapeutic strategy due to the emerging adverse events (51). According to our results, FMT from the NHE8WT donor changed the composition of the gut microbiota in NHE8KO-recipient mice, as evidenced by the significant increase in the abundance of Bacteroidetes, Lactobacillus, and segmented filamentous bacteria (SFB) in the proximal colon of NHE8KO-recipient mice. Only SFB was significantly increased in the distal colon of NHE8KO-recipient mice compared with NHE8WT mice. This indicates that the mucosal surface of NHE8KO mice is more susceptible to bacteria-epithelia interaction due to the defective mucin production. The increased bacteria adhesion might also be due to the intrinsic characteristics of the gut of NHE8KO mice. The microbial growth, metabolism, and metabolic products are fermented mainly in the cecum in mice and throughout the colon in humans, and it is associated with pH levels (26). Ilhan and coworkers, using weighted UniFrac analysis, showed that mixed community structures are selectively pressured by the gut pH (26). The fact that the mucosal surface pH of NHE8KO mice is lower than that of NHE8WT mice (~6.9 in NHE8KO mice vs. ~7.4 in NHE8WT mice) (58) might explain the failure of FMT in NHE8KO mice. Although we observed bacterial population alteration in FMT NHE8KO mice, we did not observe changes in the number of goblet cells per crypt and Muc2 expression. These results suggest that FMT cannot improve bacterial population and mucosal protection in NHE8KO mice.
Since FMT failed to improve bacterial population and Muc2 expression in NHE8KO mice, we thought to test whether VSL#3 could restore microbiome in these mice. VSL#3 is a probiotic composed of eight live freeze-dried bacterial species, including four strains of lactobacilli (Lactobacillus casei, L. plantarum, L. acidophilus, and L. delbrueckii subsp. Bulgaricus), three strains of bifidobacteria (Bifidobacterium longum, B. breve, and B. infantis) and Streptococcus salivarius subsp. thermophilus. VSL#3 has been shown to have anti-inflammatory properties and has been used in clinic to treat pouchitis (13). Studies also showed the potential effect of probiotics on epithelial barrier function and mucin production from exopolysaccharides (EPS) produced by lactobacilli and bifidobacteria (11, 60, 61). In the current study, we noticed a significant reduction of Lactobacillus and SFB in VSL#3-treated NHE8KO mice, which was different from other studies. In studies using VSL#3 in human subjects and an animal model, no bacteria species changes were detected in the mucus-associated microbiota (6, 48). The discrepancy between our study and others might be due to the altered mucosal layer in NHE8KO mice. We also observed an increase in goblet cell theca in the proximal colon in VSL#3-treated NHE8KO mice, although Muc2 expression showed a tendency to increase in the proximal colon. These data indicated that VSL#3 may have more effect on goblet cell function in the proximal colon than in the distal colon in NHE8KO mice.
With both FMT and VSL#3 supplement failing to restore microbiota and improve goblet cell function in NHE8KO mice, we examined whether sodium butyrate could improve mucin expression in NHE8KO mice. Butyrate is a short-chain fatty acid by-product of the microbial fermentation in the large intestine. Butyrate not only acts as an energy source for enterocytes but also contributes to intestinal homeostasis by stimulating Muc2 expression to promote mucus production (20, 21, 24, 28). In mice, short-chain fatty acid concentrations decrease from the proximal colon (140 mM) to the distal colon (70 mM) and the main components are acetate, propionate, and butyrate, following the ratio of 2:1:1 (17). In the current study, we demonstrated that a low concentration (1 mM) of sodium butyrate significantly increased Muc2 expression in the proximal colon of NHE8KO mice without changing the number of goblet cells and the goblet cell theca. On the other hand, administering sodium butyrate in physiologic concentration (10 mM) significantly decreased the number of goblet cells and goblet cell theca, both in the proximal and distal colon of NHE8KO mice, without changing Muc2 expression. This led us to postulate two possibilities: first, a high concentration of sodium butyrate administered via enema could be inducing cell death in NHE8KO mice and consequently reducing the goblet cells number and goblet cell theca observed in this study. In fact, high concentrations of sodium butyrate have been shown to induce apoptosis in human colonic cells (19, 53); therefore, second, the reduction of butyrate-producers observed in NHE8KO mice may function as a protective mechanism for colonic crypts, because exposure of progenitor cells to butyrate let to delay of wound repair and induced cell death (30). As NHE8KO mice have reduced Muc2 expression and a disorganized mucus layer, high doses of butyrate might be detrimental to them. Our in vitro study in goblet cells confirmed our in vivo observation that low concentrations of sodium butyrate could increase Muc2 expression while high concentrations of sodium butyrate induces cell death.
Conclusions.
In the absence of NHE8, mice displayed microbiota dysbiosis with reduction of butyrate-producing bacteria and reduced mucin production. FMT and VSL#3 failed to restore the bacterial population in NHE8KO mice. FMT, VSL#3, and NaB were not able to restore mucin production in NHE8KO mice. Altogether, our data reinforced the importance of NHE8 function in goblet cells and mucosal protection.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-3023890.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.B., H.X., and F.K.G. conceived and designed research; C.B., H.X., H.T., D.L., V.F.d.P., and L.C. performed experiments; C.B., H.X., H.T., D.L., V.F.d.P., and L.C. analyzed data; C.B., H.X., D.L., and V.F.d.P. interpreted results of experiments; C.B. and H.X. prepared figures; C.B., H.T., D.L., and V.F.d.P. drafted manuscript; H.X. and F.K.G. edited and revised manuscript; H.X. and F.K.G. approved final version of manuscript.
REFERENCES
- 1.Al-Shamali A, Khan I. Expression of Na-H exchanger-8 isoform is suppressed in experimental colitis in adult rat: lack of reversibility by dexamethasone. Scand J Gastroenterol 46: 20–29, 2011. doi: 10.3109/00365521.2010.521890. [DOI] [PubMed] [Google Scholar]
- 2.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 12: 205–217, 2015. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 3.Arike L, Holmén-Larsson J, Hansson GC. Intestinal Muc2 mucin O-glycosylation is affected by microbiota and regulated by differential expression of glycosyltranferases. Glycobiology 27: 318–328, 2017. doi: 10.1093/glycob/cww134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun 76: 907–915, 2008. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergstrom K, Liu X, Zhao Y, Gao N, Wu Q, Song K, Cui Y, Li Y, McDaniel JM, McGee S, Chen W, Huycke MM, Houchen CW, Zenewicz LA, West CM, Chen H, Braun J, Fu J, Xia L. Defective intestinal mucin-type o-glycosylation causes spontaneous colitis-associated cancer in mice. Gastroenterology 151: 152–164.e11, 2016. doi: 10.1053/j.gastro.2016.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol 100: 1539–1546, 2005. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 7.Birchenough GM, Johansson ME, Gustafsson JK, Bergström JH, Hansson GC. New developments in goblet cell mucus secretion and function. Mucosal Immunol 8: 712–719, 2015. doi: 10.1038/mi.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caballero-Franco C, Keller K, De Simone C, Chadee K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 292: G315–G322, 2007. doi: 10.1152/ajpgi.00265.2006. [DOI] [PubMed] [Google Scholar]
- 9.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13: 581–583, 2016. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6: 1621–1624, 2012. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro-Bravo N, Wells JM, Margolles A, Ruas-Madiedo P. Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Front Microbiol 9: 2426, 2018. doi: 10.3389/fmicb.2018.02426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CW, Lee HC, Li LH, Chiang Chiau JS, Wang TE, Chuang WH, Chen MJ, Wang HY, Shih SC, Liu CY, Tsai TH, Chen YJ. Fecal microbiota transplantation prevents intestinal injury, upregulation of toll-like receptors, and 5-fluorouracil/oxaliplatin-induced toxicity in colorectal cancer. Int J Mol Sci 21: 386, 2020. doi: 10.3390/ijms21020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman TM, Plosker GL, Figgitt DP. VSL#3 probiotic mixture: a review of its use in chronic inflammatory bowel diseases. Drugs 66: 1371–1387, 2006. doi: 10.2165/00003495-200666100-00006. [DOI] [PubMed] [Google Scholar]
- 14.Cheng C, Li W, Lin M, Yang ST. Metabolic engineering of Clostridium carboxidivorans for enhanced ethanol and butanol production from syngas and glucose. Bioresour Technol 284: 415–423, 2019. doi: 10.1016/j.biortech.2019.03.145. [DOI] [PubMed] [Google Scholar]
- 15.Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol 107: 1452–1459, 2012. doi: 10.1038/ajg.2012.93. [DOI] [PubMed] [Google Scholar]
- 16.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 13: 13–27, 2016. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 17.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325–2340, 2013. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ericsson AC, Personett AR, Turner G, Dorfmeyer RA, Franklin CL. Variable colonization after reciprocal fecal microbiota transfer between mice with low and high richness microbiota. Front Microbiol 8: 196, 2017. doi: 10.3389/fmicb.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung KY, Brierley GV, Henderson S, Hoffmann P, McColl SR, Lockett T, Head R, Cosgrove L. Butyrate-induced apoptosis in HCT116 colorectal cancer cells includes induction of a cell stress response. J Proteome Res 10: 1860–1869, 2011. doi: 10.1021/pr1011125. [DOI] [PubMed] [Google Scholar]
- 20.Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol 287: G1168–G1174, 2004. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- 21.Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe G, De Vos M, Boon N, Van de Wiele T. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep 7: 11450, 2017. doi: 10.1038/s41598-017-11734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol 47: 367–373, 2008. doi: 10.1111/j.1472-765X.2008.02408.x. [DOI] [PubMed] [Google Scholar]
- 23.Harrison CA, Laubitz D, Ohland CL, Midura-Kiela MT, Patil K, Besselsen DG, Jamwal DR, Jobin C, Ghishan FK, Kiela PR. Microbial dysbiosis associated with impaired intestinal Na+/H+ exchange accelerates and exacerbates colitis in ex-germ free mice. Mucosal Immunol 11: 1329–1341, 2018. doi: 10.1038/s41385-018-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatayama H, Iwashita J, Kuwajima A, Abe T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem Biophys Res Commun 356: 599–603, 2007. doi: 10.1016/j.bbrc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 25.He Y, Li X, Yu H, Ge Y, Liu Y, Qin X, Jiang M, Wang X. The functional role of fecal microbiota transplantation on dextran sulfate sodium-induced colitis in mice. Front Cell Infect Microbiol 9: 393, 2019. doi: 10.3389/fcimb.2019.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilhan ZE, Marcus AK, Kang DW, Rittmann BE, Krajmalnik-Brown R. pH-mediated microbial and metabolic interactions in fecal enrichment cultures. MSphere 2: e00047-17, 2017. doi: 10.1128/mSphere.00047-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiminez JA, Uwiera TC, Abbott DW, Uwiera RRE, Inglis GD. Butyrate supplementation at high concentrations alters enteric bacterial communities and reduces intestinal inflammation in mice infected with Citrobacter rodentium. MSphere 2: e00243-17, 2017. doi: 10.1128/mSphere.00243-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 105: 15064–15069, 2008. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165: 1708–1720, 2016. doi: 10.1016/j.cell.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol 13: 508–516, 2016. doi: 10.1038/nrgastro.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larmonier CB, Laubitz D, Hill FM, Shehab KW, Lipinski L, Midura-Kiela MT, McFadden RM, Ramalingam R, Hassan KA, Golebiewski M, Besselsen DG, Ghishan FK, Kiela PR. Reduced colonic microbial diversity is associated with colitis in NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol 305: G667–G677, 2013. doi: 10.1152/ajpgi.00189.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laubitz D, Larmonier CB, Bai A, Midura-Kiela MT, Lipko MA, Thurston RD, Kiela PR, Ghishan FK. Colonic gene expression profile in NHE3-deficient mice: evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol 295: G63–G77, 2008. doi: 10.1152/ajpgi.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei X, Cai L, Li X, Xu H, Geng C, Wang C. Up-regulation of NHE8 by somatostatin ameliorates the diarrhea symptom in infectious colitis mice model. Korean J Physiol Pharmacol 22: 269–275, 2018. doi: 10.4196/kjpp.2018.22.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Cai L, Xu H, Geng C, Lu J, Tao L, Sun D, Ghishan FK, Wang C. Somatostatin regulates NHE8 protein expression via the ERK1/2 MAPK pathway in DSS-induced colitis mice. Am J Physiol Gastrointest Liver Physiol 311: G954–G963, 2016. doi: 10.1152/ajpgi.00239.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C, Xu H, Zhang B, Johansson ME, Li J, Hansson GC, Ghishan FK. NHE8 plays an important role in mucosal protection via its effect on bacterial adhesion. Am J Physiol Cell Physiol 305: C121–C128, 2013. doi: 10.1152/ajpcell.00101.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mariman R, Tielen F, Koning F, Nagelkerken L. The probiotic mixture VSL#3 has differential effects on intestinal immune parameters in healthy female BALB/c and C57BL/6 mice. J Nutr 145: 1354–1361, 2015. doi: 10.3945/jn.114.199729. [DOI] [PubMed] [Google Scholar]
- 39.Matsuki T, Watanabe K, Fujimoto J, Miyamoto Y, Takada T, Matsumoto K, Oyaizu H, Tanaka R. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl Environ Microbiol 68: 5445–5451, 2002. doi: 10.1128/AEM.68.11.5445-5451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148: 257–266, 2002. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen TTT, Oshima K, Toh H, Khasnobish A, Fujii Y, Arakawa K, Morita H. Draft genome sequence of Butyricimonas faecihominis 30A1, isolated from feces of a japanese alzheimer’s disease patient. Microbiol Resour Announc 8: e00462, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41a.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MH, Szoecs E, Wagner H. Vegan: Community Ecology Package. 2019.
- 42.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch 447: 549–565, 2004. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- 43.Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol 97: 1166–1177, 2004. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- 44.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 45.Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology 145: 946–953, 2013. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 46.Song Y, Malmuthuge N, Steele MA, Guan LL. Shift of hindgut microbiota and microbial short chain fatty acids profiles in dairy calves from birth to pre-weaning. FEMS Microbiol Ecol 94: fix179, 2018. doi: 10.1093/femsec/fix179. [DOI] [PubMed] [Google Scholar]
- 47.Trkov M, Avgustin G. An improved 16S rRNA based PCR method for the specific detection of Salmonella enterica. Int J Food Microbiol 80: 67–75, 2003. doi: 10.1016/S0168-1605(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 48.Uronis JM, Arthur JC, Keku T, Fodor A, Carroll IM, Cruz ML, Appleyard CB, Jobin C. Gut microbial diversity is reduced by the probiotic VSL#3 and correlates with decreased TNBS-induced colitis. Inflamm Bowel Dis 17: 289–297, 2011. doi: 10.1002/ibd.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131: 117–129, 2006. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 50.Wang A, Li J, Zhao Y, Johansson ME, Xu H, Ghishan FK. Loss of NHE8 expression impairs intestinal mucosal integrity. Am J Physiol Gastrointest Liver Physiol 309: G855–G864, 2015. doi: 10.1152/ajpgi.00278.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S, Xu M, Wang W, Cao X, Piao M, Khan S, Yan F, Cao H, Wang B. Systematic review: adverse events of fecal microbiota transplantation. PLoS One 11: e0161174, 2016. doi: 10.1371/journal.pone.0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wickham H. ggplot2: Elegant Graphics For Data Analysis. New York: Springer-Verlag, 2009. doi: 10.1007/978-0-387-98141-3. [DOI] [Google Scholar]
- 53.Xiao M, Liu YG, Zou MC, Zou F. Sodium butyrate induces apoptosis of human colon cancer cells by modulating ERK and sphingosine kinase 2. Biomed Environ Sci 27: 197–203, 2014. [DOI] [PubMed] [Google Scholar]
- 54.Xu H, Chen H, Dong J, Lynch R, Ghishan FK. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8). Cell Physiol Biochem 21: 109–116, 2008. doi: 10.1159/000113752. [DOI] [PubMed] [Google Scholar]
- 55.Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36–G41, 2005. doi: 10.1152/ajpgi.00552.2004. [DOI] [PubMed] [Google Scholar]
- 56.Xu H, Li J, Chen H, Wang C, Ghishan FK. NHE8 plays important roles in gastric mucosal protection. Am J Physiol Gastrointest Liver Physiol 304: G257–G261, 2013. doi: 10.1152/ajpgi.00433.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu H, Li J, Chen R, Zhang B, Wang C, King N, Chen H, Ghishan FK. NHE2X3 DKO mice exhibit gender-specific NHE8 compensation. Am J Physiol Gastrointest Liver Physiol 300: G647–G653, 2011. doi: 10.1152/ajpgi.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu H, Zhang B, Li J, Wang C, Chen H, Ghishan FK. Impaired mucin synthesis and bicarbonate secretion in the colon of NHE8 knockout mice. Am J Physiol Gastrointest Liver Physiol 303: G335–G343, 2012. doi: 10.1152/ajpgi.00146.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou J, Zhou Z, Ji P, Ma M, Guo J, Jiang S. Effect of fecal microbiota transplantation on experimental colitis in mice. Exp Ther Med 17: 2581–2586, 2019. doi: 10.3892/etm.2019.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou X, Qi W, Hong T, Xiong T, Gong D, Xie M, Nie S. Exopolysaccharides from Lactobacillus plantarum NCU116 regulate intestinal barrier function via STAT3 signaling pathway. J Agric Food Chem 66: 9719–9727, 2018. doi: 10.1021/acs.jafc.8b03340. [DOI] [PubMed] [Google Scholar]
- 61.Zhou X, Zhang K, Qi W, Zhou Y, Hong T, Xiong T, Xie M, Nie S. Exopolysaccharides from Lactobacillus plantarum NCU116 enhances colonic mucosal homeostasis by controlling epithelial cell differentiation and c-Jun/Muc2 signaling. J Agric Food Chem 67: 9831–9839, 2019. doi: 10.1021/acs.jafc.9b03939. [DOI] [PubMed] [Google Scholar]