Abstract
The ability to replace defective cells in an airway with cells that can engraft, integrate, and restore a functional epithelium could potentially cure a number of lung diseases. Progress toward the development of strategies to regenerate the adult lung by either in vivo or ex vivo targeting of endogenous stem cells or pluripotent stem cell derivatives is limited by our fundamental lack of understanding of the mechanisms controlling human lung development, the precise identity and function of human lung stem and progenitor cell types, and the genetic and epigenetic control of human lung fate. In this review, we intend to discuss the known stem/progenitor cell populations, their relative differences between rodents and humans, their roles in chronic lung disease, and their therapeutic prospects. Additionally, we highlight the recent breakthroughs that have increased our understanding of these cell types. These advancements include novel lineage-traced animal models and single-cell RNA sequencing of human airway cells, which have provided critical information on the stem cell subtypes, transition states, identifying cell markers, and intricate pathways that commit a stem cell to differentiate or to maintain plasticity. As our capacity to model the human lung evolves, so will our understanding of lung regeneration and our ability to target endogenous stem cells as a therapeutic approach for lung disease.
Keywords: airway epithelium, basal cells, cell therapy, differentiation, pulmonary neuroendocrine cells
INTRODUCTION
The human conducting airway epithelium is composed of several functional cell types organized in a pseudostratified fashion atop a submucosal layer that transitions to a monolayer distally. The most abundantly encountered cell types include secretory cells (SCs), multiciliated cells (MCCs), and basal cells (BCs) (as shown in Fig. 1), followed by less frequent types such as neuroendocrine cells, goblet cells (GCs), brush cells, and ionocytes. The specific proximodistal distribution of cell types plays a variety of physiological roles, including providing host defense, repair, and regeneration of the airway epithelial cells. Of particular interest is this latter function provided by airway stem/progenitor cells. These cells are capable of self-renewal and generation of one or more mature cell types. Contrary to other epithelial systems, the airway epithelium is maintained by infrequent progenitor cell division as evidenced by a ciliated cell half-life of 17 months in a murine bronchus (157). A series of lineage-tracing strategies and airway injury experiments instigating a repair process has introduced a capacity to identify a whole host of surface epithelial, and some submucosal gland, cells to be responsible for epithelial or alveolar homeostasis (112, 160, 197). Newer studies involving single-cell RNA sequencing of human airway cells have provided more critical information on the stem cell subtypes, the transition states, and the intricate pathways that commit a stem cell to differentiate or to maintain plasticity (133, 149, 166).
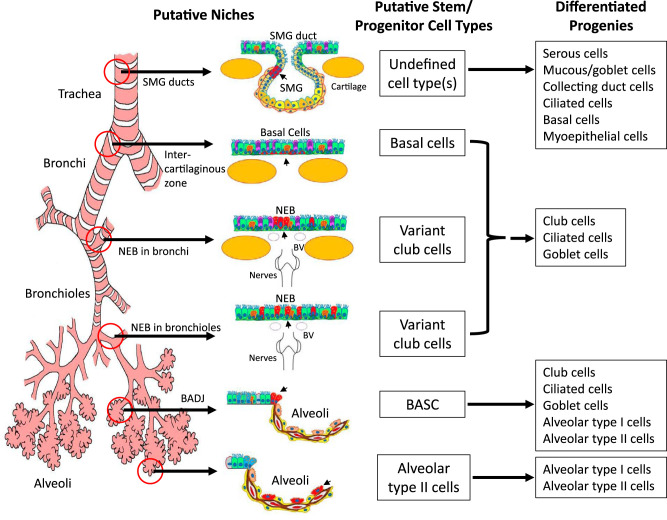
Fig. 1.
Illustration of putative stem/progenitor cells and their niches in the adult murine lung. The lung can be divided into three major levels of conducting airways (the trachea, bronchi, and bronchioles) plus the gas-exchanging alveoli. Distinct region-specific stem/progenitor cell niches are thought to exist along the proximal-distal axis of the airway. These include the submucosal gland (SMG) ducts in the proximal trachea, basal cells within intercartilaginous zones of the trachea and primary bronchi, neuroepithelial bodies (NEBs) in the intralobar bronchi and bronchioles, and the bronchioalveolar duct junction (BADJ) and alveolar spaces within the alveoli. Progenitor/stem cells (marked in red and listed) reside in their respective local niches, and these environments enable them to maintain their stem/progenitor properties and control their ability to differentiate into various progeny cell types. BV, blood vessel; BASC, bronchioalveolar stem cell. [From Lynch et al. (117), reprinted by permission from Springer Nature.]
Another facet of recent interest is the dysregulation of stem cell maintenance, which has been implicated in the pathogenesis of chronic lung diseases. The absence of KRT5+/TP63+ (cytokeratin 5 and tumor protein 63) BCs and submucosal glands (SMGs), for example, has been associated with the development of bronchiolitis obliterans (189). Similarly, the depletion of club cells in a transplanted lung also triggers the same pathology by priming the recipient immune system (105). Chronic cigarette smoke (CS) exposure creates a proinflammatory microenvironment that distorts the BC transcriptome, yielding pathologic epithelial phenotypes (167), and eventual progenitor exhaustion is responsible for chronic obstructive pulmonary disease (COPD) pathogenesis (53). RNA analysis of lung adenocarcinomas has also demonstrated a “BC signature” indicating that stem/progenitor cells can undergo malignant transformation (49, 66). We currently acknowledge that BCs, SMG progenitor cells, secretory cells, pulmonary neuroendocrine cells (PNECs), and alveolar progenitor cells can contribute to both homeostatic and pathogenic repair based on the environmental niche and local signaling. Appreciating these nuances is critical for understanding the pathophysiology of chronic lung diseases and harnessing the potential of stem/progenitor cell therapy.
ENDOGENOUS AIRWAY STEM AND PROGENITOR CELLS
The airway epithelium is a continuous sheet of cells that extends from the nasal passage through the trachea and to the alveolar sacs. The morphology and cellular composition notably change along the proximodistal axis of the airway. The proximal airway is columnar, pseudostratified, and predominantly composed of mucus-secreting goblet cells and multiciliated cells that make up the mucociliary elevator for host defense. The distal airways transition to resemble a single layer composed of secretory cells and fewer ciliated cells. In their relative positions, all cells contribute to conductance of the air to alveolar regions and to maintaining a barrier against inhaled agents. Regardless of the location in the airway, the entire epithelium is constantly exposed to the environment and subject to potential injury. Repair of epithelial injury, as well as homeostatic cellular turnover, is dependent on the resident stem/progenitor cells. Within the last decade it has been established that different populations of stem/progenitors reside in various niches of the airway. These include basal cells, submucosal gland progenitors, secretory cells, pulmonary neuroendocrine cells, and alveolar progenitor cells; a summary of these cell types and their defining markers is included in Table 1. In this review we will discuss each of these stem cell populations focusing on their species-specific identity and stem cell functions.
Table 1.
Identification of pulmonary epithelial stem and progenitor cells
| Stem/Progenitor Cell Type | Location | Defining Marker | Notes | Ref. No. |
|---|---|---|---|---|
| Basal cells (BCs) | Epithelium in trachea through bronchioles | TP63, KRT5, NGFR, ITGA6, PDPN, KRT8 (luminal), | Subpopulations of basal cells are being identified in the airways, including multipotent, secretory, and ciliated primed cells | 54, 90, 114, 127, 133, 163, 149, 166 |
| Club cells (CCs) | Epithelium in trachea through bronchioles | SCGB1A1, SCGB3A2, KRT17, KRT19 | After severe injury, CCs can acquire BC markers and contribute to regeneration of AT1/2 cells and bronchioles | 9, 63, 166, 198, 219, 220 |
| SMG myoepithelial cells (MECs) | Subepithelial surface in cartilaginous airways | SMMHC, α-SMA or ACTA2, LEF-1 | Slow-cycling progenitors capable of repopulating all cell types of the airway lumen and submucosal gland following injury | 34, 35, 114, 115, 197, 212, 212 |
| Neuroendocrine cells (PNECs) | Prominent at branching points in the bronchi and bronchioles | GRP, ASCL1, CXCR4, NCAD, ROBO | Notch signaling causes the proliferation and transdifferentiation of PNECs to SCs and MCCs | 11, 85, 104, 132, 140, 188, 215 |
| Alveolar type II cells (AT2) | Distal airway alveolar sacs | SFTPC, LAMP3, ABCA3, TM4SF1 (subpopulation) | AT2 cells can self-renew and differentiate into AT1 cells to facilitate gas exchange | 31, 82, 88, 97, 103, 116, 177, 202, 217 |
| Distal airway stem cells (DASCs) | Distal airways (bronchioles and alveolar regions) | TP63, KRT5 | Self-renewal and differentiation to alveolar and bronchial epithelial cells. Observed as KRT5 “pods” | 96, 118, 224 |
α-SMA/ACTA2, smooth muscle α-actin; ABCA3, ATP-binding cassette subfamily A member 3; ASCL1, achaete-scute homolog 1; AT1/2, alveolar type I and II cells; CXCR4, chemokine receptor; DASCs, distal airway stem cells; GRP, bombesin or gene-related peptide; ITGA6, integrin-α6; KRT5/8/17/19, cytokeratin 5/8/17/9; LAMP3, lysosomal-associated membrane protein 3; LEF-1, lymphoid enhancer factor; MCCs, multiciliated cells; NCAD, N-cadherin; NGFR, nerve factor growth receptor; PDPN, podoplanin; ROBO, roundabout receptors; SCs, secretory cells; SCGB1A1/3A2, secretoglobin family 1A member 1/3A member 2; SFTPC, surfactant protein C; SMG, submucosal gland; SMMHC, smooth muscle myosin heavy chain; TM4SF1, transmembrane 4L six family member 1; TP63, tumor protein 63.
Basal Stem Cells
BCs are multipotent stem cells residing in close proximity to the basal lamina in the pseudostratified epithelium of the tracheobronchial tree. In this position BCs anchor the overlying cells to the underlying matrix and play a dedicated role in epithelial homeostasis due to their ability to self-renew and regenerate all luminal cell types (9, 146, 161). The versatility of BCs has been demonstrated by regeneration of the airway epithelium following injury or on a decellularized matrix (55, 59, 137, 160). An absence of BCs has also been implicated in disease pathogenesis where the regenerative capacity of BCs is exhausted (56, 143, 189). As such, it is imperative that the mechanisms regulating their maintenance and lineage commitment are fully understood.
BCs are ubiquitous in the human conducting airway occupying ~31% and ~6% of the epithelium in the largest (≥4 mm) and smallest (<0.5 mm) conducting airways, respectively (9). Lineage tracing studies in mice have shown these cells are embryologically derived from bud tip progenitor cells via transient SMAD [signal transducers for receptors of the transforming growth factor-β (TGF-β)] activation (131); insulin growth factor-I (IGF-I) repression by enhancer of zeste homolog 2 (EZH2), on the other hand, prevents BC lineage specification during endoderm development (51, 156, 178, 214). In the human, rat, and mouse airways, BCs form a mostly continuous monolayer in the larger airways that transition into clusters or individual cells in the distal bronchioles (127, 138, 161). They are anchored to the subjacent basement membrane via α6β4-integrins, and luminal cell types are connected through a repertoire of cytokeratins (KRTs) to establish a structural network (90, 127, 163). For example, KRT14 interacts with KRT5 to establish a network of intermediate filaments for proliferation (163). In addition to providing structural integrity, KRT markers distinguish the functional properties and identities of BCs, defining BC subtypes and differentiated luminal cells (54, 149, 166). At steady state, airway BCs are commonly identified by KRT5 and TP63 expression. Through lineage tracing of KRT5 cells in transgenic mice, cell surface markers nerve growth factor receptor (NGFR) and integrin-α6 (ITGA6) were identified (160). An important smaller subset of unipotent and self-renewing KRT14+ basal cells, which accounts for <20% of the BC population, has also been identified to adopt multipotency in the setting of acute injury (54, 114). BCs are heterogeneous and are distributed into functional subpopulations consisting of stem cells and long-lived progenitor cells that are fated to luminal cells. The former group maintains epithelial homeostasis by self-renewal and producing progenitors. The latter group persists in the basal layer for approximately 2 weeks and upregulates KRT8 prior to differentiating into a luminal cell.
An important aspect of BC application in stem cell therapy is understanding its lineage commitments into the luminal cell types including MCCs, SCs, GCs, PNECs, brush cells, and ionocytes. Figure 2 summarizes BCs, their regulation and differentiation potential. Thanks to recent publications using single-cell RNA sequencing, basal cell intermediates and signaling pathways have been identified to further understand this process (133, 149, 166). A plethora of studies have demonstrated that Notch signaling is critical in the commitment of BCs. From lineage tracing experiments in mice we know that intracellular Notch2 (N2ICD) activation directly generates SCs following sulfur dioxide (SO2) inhalation injury. In the same murine models, c-myb activation yields MCCs. In humans, Notch1 and 3 are known to skew BCs toward a SC fate, with regulation of Notch2 and -4 having little effect on BC differentiation (58). Similarly, direct repression of the δ/Notch pathway via miR-34/449 families of microRNAs permits differentiation of ciliated cell progenitors (119). A comparable differentiation pathway is activated in inflammatory states which trigger interleukin-6 (IL-6) production and activate downstream Janus kinase (JAK)/STAT3 signaling to promote ciliogenesis via a direct inhibition of Notch1 gene expression and upregulation of forkhead box protein J1 (FOXJ1) and miR-449 (191).
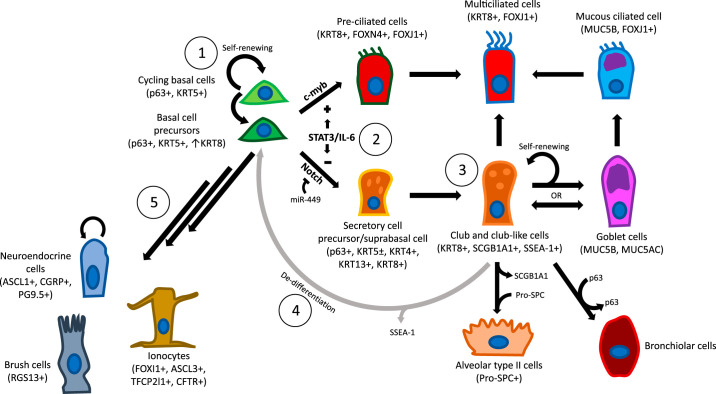
Fig. 2.
Illustration of basal stem cells and their putative differentiated progeny in the human lung. 1) Basal cells (BC) self-renew and are capable of giving rise to all cell types present on the surface airway epithelium through an intermediate BC precursor. 2) A subset of these BCs that display intracellular Notch commit to a secretory cell lineage while low-level c-myb expression in the same population will differentiate to preciliated and multiciliated cells (MCCs) subsequently. Other identified pathways that induce this lineage relationship include signal transducer and activator of transcription 3 (STAT3)/IL-6 or inhibitory miR-449. 3) club-like cells (CCs) share a common pathway with BCs when differentiating into MCCs but can also renew the MCCs through a goblet cell (GC) intermediate. Alveolar type II and bronchiolar cells are additional contributions from CCs. 4) If BCs are ablated, club-like cells (CCs) have been demonstrated to dedifferentiate to renew BCs. 5) BCs can also give rise to self-renewing neuroendocrine cells as well as brush cells and ionocytes. KRT, cytokeratin; ASCL1, achaete-scute homolog 1; CGRP, calcitonin gene-related peptide; FOX, forkhead box protein; CFTR, cystic fibrosis transmembrane conductance regulator; PGP9.5, protein gene product 9.5; RGS13, regulator of G protein signalling 13; SCGB1A1, secretoglobin family 1A member 1/club cell secretory protein/club cell 10 kDa protein secretory club cells; SP-C, surfactant protein C; MUC, mucin; TFCP2, transcription factor CP2; SSEA-1, stage specific embryonic antigen 1.
BCs, at baseline, express ligands δ-like ligand 1 (DLL1), jagged 1 and 2 proteins (JAG1 and JAG2, respectively), and notch 1 intracellular domain (N1ICD), but have no detectable targeted gene expression, which indicates an inactive Notch pathway. Upon activation, Notch signaling suppresses TP63 and geminin coiled-coil domain containing (GMNC), which is a master regulator of cell fate, and Notch activates Sam-pointed domain Ets-like factor (SPDEF). SPDEF then induces GC differentiation and production of mucins (18, 19, 132, 150, 165). Nuclear factor 1 A-type (NFIA), a regulator of Notch, was also recently identified as the first transcription factor enriched in club cells (CCs) (132). Conversely in pre-MCCs and MCC cells, target genes Notch2 and Notch3 are downregulated, while there is an increased presence of dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A), an inhibitor of Notch intracellular domain (NICD), which confirms a major inhibitory signature of Notch signaling in MCCs. In the absence of Notch, BCs differentiate into MCCs under the control of GMNC. Downstream differentiation is induced by a signaling cascade that includes activation of a transcriptional complex comprising of multiciliate differentiation and DNA synthesis associated cell cycle protein (MCIDAS), E2F transcription factor (E2F4/5), and deuterostome assembly protein 1 (DEUP1), leading to centriole amplification and microtubule organization. Activation of FOXJ1 completes transcriptional activation and assembly of ciliary proteins (5, 17, 181, 214). FOXN4 has recently been identified in a preciliated cell population that is enriched for FOXJ1, but low for markers of maturity, such as β-tubulin (TUBB4) (147).
Wnt, bone morphogenic protein (BMP), and TGF-β signaling are other important pathways that regulate BC fate decisions. Wnt target genes snail family transcriptional repressor 2 (SNAI2) and transcription factor 4 (TCF4) are indicators of active Wnt signaling, and the ligand, WNT10A, is enriched in BCs. In fact, WNT10A is an autocrine regulator that may direct BC self-renewal. However, in differentiated progeny such as MCCs, SNAI2 expression is not detected, and two ATP-dependent DNA helicases, RuvB-like AAA ATPase 1/2 (RUVBL1 and RUVBL2), that function as Wnt signaling repressors are expressed (159). This supports that MCC differentiation is contingent upon suppression of the Wnt pathway. BMP ligands are heterogeneously distributed in the airway. For example, BMP2 and BMP7 are enriched in BCs, whereas BMP3 and BMP4 are enriched in the SC/GC populations. Of note is the expression of BMP inhibitors, such as inhibitory ligand follistatin (FST) and intracellular inhibitor peptidyl-prolyl cis-trans isomerase (FKBP1A) in BCs. These findings suggest that the BMP pathway hinders BC proliferation, which is consistent with a prior study demonstrating preserved maintenance of proliferative BC potential following dual SMAD inhibition (134, 165). In regards to the TGF-β pathway, MCC populations do not express TGF-β ligands, but there is specific expression of serpin family E member 1 (SERPINE1), CCN2 or connective tissue growth factor (CTGF), cAMP-dependent transcription factor (ATF3), transforming growth factor-β receptor III (TGFBR3), and interferon regulatory factor 7 (IRF7), and prior studies suggest that regulation of motile cilia length depends on a close relationship of TGF-β and these factors (197).
Submucosal Gland Progenitor Cells
SMGs are secretory tubuloacinar structures found in the submucosa at all levels of the human cartilaginous airway. SMGs participate in a host of functions related to innate immunity and mucociliary clearance. They predominantly secrete mucin 5B (MUC5B) and mucin 5AC (MUC5AC) to a lesser extent, as well as antimicrobial peptides such as lysozyme, lactoferrin, β-defensins, and surfactant proteins SP-D and SP-A (10). Recent studies have demonstrated that SMGs also serve as a stem cell niche and contribute to airway repair. The SMG is split into four domains with varying cellular makeup: serous acini, mucous tubules, collecting ducts, and ciliated ducts listed in a distal-to-proximal fashion. The ciliated ducts are considered to be an extension of the surface airway containing similar cell types, including BCs, MCCs, and SCs. The distal components are composed of the mucous tubules and serous acini (112). Well-innervated contractile myoepithelial cells (MECs) line the mesenchymal surfaces of glands excluding the ciliated ducts. In this section, we will discuss ductal cells and MECs as SMG progenitors. Figure 3 provides a schematic identifying the putative stem and progenitor cells that have been shown to reside within the SMG ducts. Hegab et al. (70, 71) conclude that three distinctive populations of SMG duct cells exist: BCs of the duct (ITGA6+/NGFR+/tubulin−), ciliated cells of the duct (ITGA6−/NGFR−/tubulin+), and nonciliated non-BCs of the duct (ITGA6−/NGFR−/tubulin−); potentially one of these is a multipotent duct progenitor. In their murine model, isolated tumor-associated calcium signal transducer 2 (TROP-2+) SMG duct cells were capable of surviving a severe hypoxic-ischemic injury, of self-renewal, and of differentiating to express KRT5 and KRT14. Additional markers expressed to a limited extent included KRT15, KRT8, TP63, polymeric immunoglobulin receptor (PLGR, a serous cell marker), and mucus confirmed by periodic acid-Schiff (PAS) staining. RNA sequencing and quantitative PCR data from this study comparing the transcriptomes between BCs and SMG duct cells showed a substantial increase in the enrichment of genes responsible for stratified squamous epithelial development in duct cells, suggesting that these ductal cells are distinct from the surface BCs that contribute to postinjury repair.
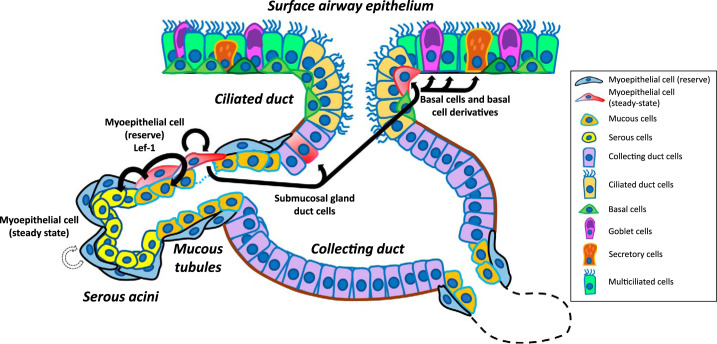
Fig. 3.
Schematic of the submucosal gland as a stem cell niche. The submucosal gland is a convoluted tubuloacinar structure beneath the surface airway epithelium beginning with an invagination that then undergoes a series of branching (dotted lines represent additional branches). Specific cells reside in the four identified domains, which are the ciliated ducts, collecting ducts, mucous tubules, and serous acini. Myoepithelial cells (MECs) are a population of self-renewing stem cells that produce glandular serous and mucous cells at steady state. Following severe airway surface injury, MECs express lymphoid enhancer factor (Lef-1) that promotes proliferation into ductal cells and migration to the surface to adopt a basal cell morphology. These cells have a lineage bias toward multiciliated cells and secretory goblet cells and are less likely to renew into secretoglobin family 1A member 1/club cell secretory protein/club cell 10 kDa protein (SCGB1A1+) secretory club cells.
MECs are another population of stem cells within the SMG niche as evidenced by slow-cycling progenitors that retain nucleotide labels following repeated injury, and they have recently been attributed to repopulating all cell types of the airway lumen and submucosal gland following injury (114, 115, 197, 212). As previously mentioned, MECs line the extraluminal surface of SMGs and express both α-smooth muscle actin (α-SMA or ACTA2) and smooth muscle myosin heavy chain 11 (SMMHC). Lineage-traced mice experiments, using these markers, have demonstrated that MECs first emerge after placode formation during the elongation phase when SMG tubules invaginate in the embryonic airway. This morphogenesis is mediated by SRY (sex determining region Y)-box 2 (SOX2) repression and Wnt/β-catenin-dependent induction of lymphoid enhancer factor (LEF-1) gene expression (34, 35, 212). A self-renewing unipotent MEC progenitor emerges by ~3–4 days following birth and contributes to the majority of MECs in the 21-day gland. Between 5 and 21 days of life, lineage-positive α-SMA-negative cells constitute 50% of the SMG tubular epithelia, suggesting that a subset of the initial MECs lose their α-SMA signature and become multipotent (112, 197).
The following recent studies strongly indicate that these MECs act as reserve stem cells following airway injury. Lineage-traced mouse models with naphthalene- and SO2-induced airway injury have exhibited emergence of α-SMA+ epithelial cells on the surface airway epithelium, indicating that these cells are derived from glandular MECs. Traced cells on the surface airway epithelium (SAE) adopt a BC phenotype with the ability to differentiate into KRT8+ luminal columnar cells. There is also gradual loss of α-SMA expression during this differentiation process. Additionally, Lef-1 was shown to be critical to the multipotent nature of the MECs. Overexpression of Lef-1 in MECs in the postinjury state promoted regeneration and differentiation into SAE BCs (KRT5+), CC [secretoglobin family 1A member 1/club cell secretory protein/club cell 10 kDa protein (SCGB1A1+)], and MCC (α-tubulin+) as well as SMG glandular duct (Trop-2+), ciliated duct (α-tubulin+), and serous [Ulex europaeus agglutinin I (UEA-1+)] cells. Interestingly, SMG-derived MECs expanded in vitro and seeded in denuded tracheal xenografts also demonstrating organization and reconstitution of SMGs and SAE (112, 197). Tata and colleagues (197) also showed that the transcription factor, Sox9, serves as a unique SMG marker that is necessary for MEC plasticity in airway regeneration. This study used a mouse model that deleted Sox9, which showed significantly less proliferation and migration of MECs to the SAE postinjury (197). Indirect evidence of the regenerative capacity of these cells is further demonstrated in diseased states where the glandular structures may be destroyed. Depletion of the SMG stem cell niche in chronic lung allograft dysfunction (CLAD), documented in both ferret and human lung transplant recipients, suggests that loss of multipotent SMG stem cells may lead to decline of surface airway basal cells and thereby lead to aberrant airway repair and fibrosis that results in obliterative bronchiolitis (OB) (189).
Secretory Cells
Airway SCs have historically been grouped by morphological and ultrastructural differences and location within the tracheobronchial tree: club cells (CCs), goblet cells (GCs), and serous cells (83, 84). However, considering advances in single-cell RNA sequencing, identification of SC subtypes and differentiation potentials have challenged this straightforward nomenclature. Serous cells are predominantly present in fetal airways and sequestered in mature adult SMGs, and serous-like cells have been identified on the SAE of human bronchioles (162). Their role as airway progenitors has not yet been elucidated, so in this section we will primarily discuss CCs and GCs. CCs, commonly identified by expression of SCGB1A1 (also known as club cell secretory protein or CCSP), are dome-shaped columnar PAS-negative cells that are largely confined to the terminal and respiratory bronchioles of the human. In addition to SCGB1A1, SCGB3A1 and SCGB3A2 are expressed in heterogeneous populations of CCs, and SCGB3A2 has been reported to be the earliest marker of commitment to this cell type. Recently, KRT17 and KRT19 have been found to be strongly enriched in CCs (9, 63, 166). Unlike CCs, GCs are PAS-reactive flask-shaped cells that are abundant in the proximal airways and largely absent from the terminal and respiratory bronchioles. In this section, we intend to highlight the versatile role of CCs in maintaining the airway and alveoli.
As described above in the section on Basal Stem Cells, BC-to-SC differentiation is regulated and maintained by the Notch pathway and NFIA; and CCs serve as a primary progenitor for both MCCs and GCs. This notion is supported by RNA sequencing of human bronchial biopsies that identified several “intermediate” cell populations expressing SCGB1A1/dynein intermediate chain 1 (DNAI1, an MCC marker) and SCGB1A1/MUC5AC (207, 225). In the absence of Notch, CCs and BCs appear to share a common differentiation pathway into MCCs; however, this has yet to be functionally validated. MCC differentiation is regulated by the chromatin-binding protein GMNC, which controls the transcriptional activity and assembly of ciliary proteins (6, 16, 183, 222). Conversely, when Notch is present, the transcription factor SPDEF is activated, which directs mucin production. The presence of FoxQ1 serves as an early GC-specific marker (18, 19, 133, 153). GC hyperplasia and metaplasia, induced by interleukin 13 (IL-13), signal transducer and activator of transcription 6 (STAT6), and forkhead box protein A3 (FOXA3) activation can be triggered by environmental factors (e.g., toxins, irritants, microorganisms, particles) and chronic lung diseases [e.g., COPD, cystic fibrosis (CF), asthma], resulting in accompanying mucus hyperproduction and recruitment of immune and stromal cells to the airway in the distal airway (18, 32, 133, 216). There appears to be minimal involvement of the BMP/TGF-β/SMAD signaling pathway as evidenced by a weak presence of phosphorylated (p)- SMAD1/5/8 and p-SMAD2/3 in MUC5AC+ luminal GCs. This is in contrast to the activated pathway during differentiation of BCs to MCCs and SCs (40).
An additional important finding is the CC’s ability to dedifferentiate in vivo into BCs postinjury. Following an inhalational doxycycline insult, mice were shown to have increased proliferation in SCGB1A1+ SCs relative to the remaining BCs, suggesting the SCs are the predominant replicating force after stem cell ablation. Lineage-labeled SCs were found to acquire BC markers (KRT5, NGFR, TP63, PDPN) and lose SC surface markers after adopting BC morphology (198). Separate studies by Zheng et al. (219, 220) provide evidence that SCGB1A1+ CCs also contribute to regeneration of alveolar type I and II (AT1 and AT2, respectively) cells and bronchioles. In a SCGB1A1-CreER lineage-traced model for CCs, murine airways were injured with influenza virus infection or bleomycin after which patches of new SCGB1A1+, TP63+ cells were observed to organize into multilayered lumen-like structures composed of columnar/cuboidal cells resembling bronchioles. These cells expressed cytochrome P-450 2F2 (CYP2f2), a marker of CC immaturity. After repair, the TP63 marker disappeared and the bronchioles transitioned to a monolayer, suggesting that SCGB1A1-derived TP63+ cells are a transient intermediate cell during bronchiolar regeneration (219). In the same lineage-traced injury model, a small fraction of CCs known to express both SCGB1A1 and pro-SPC, which is an AT2 marker, were observed to lose SCGB1A1 expression during differentiation into AT2 cells (220). This finding appears to be supported by recent single-cell RNA sequencing (scRNAseq) experiments that used induced pluripotent stem cells (iPSCs) from murine and human airways. Within a subset of human iPSC-derived SCs (SCGB1A1+ and SCGB3A2+), there was enrichment of AT2 markers. This same group also observed that blocking Wnt signaling committed iPSC-derived SCs to an AT2 fate (122). A rare slow-cycling uroplakin3a (UPK3a)-expressing club cell (U-CC) has also been identified in the murine airway. This subpopulation of CCs is intrapulmonary and enriched around neuroepithelial bodies (NEBs). Notably, they are capable of surviving naphthalene injury through the absence of CYP2f2 (62). Following bleomycin injury, U-CCs regenerate AT1 and AT2 cells, and they contribute to maintenance of uninjured airways by generating CCs and MCCs (62). Last, GCs have also demonstrated some plasticity, since RNA sequencing data have identified a hybrid cell expressing MUC5AC and FoxJ1 in human bronchial and newborn pig tracheas, suggesting a transitory state between GCs and MCCs (166). However, the cellular mechanisms that drive lineage commitment have not be identified for many of the SCs.
Pulmonary Neuroendocrine Cells
PNECs are endogenous multifunctional cells with known roles in oxygen sensing, regulation of blood flow, modulation of immune responses, and stimulation of intraepithelial vagosensory nerve fibers (104, 187). PNECs are also postulated to provide a stem cell niche during injury, and there is evidence that they have a capacity to act as a progenitor cell, giving rise to club and ciliated cells (179). PNECs are rare, detected in the airways comprising of <1% cells in the SAE (92). As such, there is limited single-cell sequencing available that defines and validates a cellular signature of primary PNECs (20, 75, 149). They are derived from endodermal progenitor cells and, in mice, identified by calcitonin gene-related peptide (CGRP) and additional neurotransmitter proteins (92). CGRP expression and distribution in PNEC, however, appears to vary among species. In humans, CGRP expression correlates to cancer phenotype (38) and has been observed to be present in only ~20% of primary lung PNEC, suggesting that it may mark a specific subpopulation of PNEC (210). In human lungs, bombesin/gene-related peptide (GRP) is more robust and widely accepted as a marker of fetal-stage PNEC (104, 132, 188). Inflated PNEC numbers have also been associated with lungs diseases such as COPD, sudden infant death syndrome (SIDS), and small-cell lung cancer (SCLC) (92, 140).
PNECs exist as solitary cells or in small innervated clusters, NEBs, in the airways (100, 140, 205). Single PNECs migrate to form nodal NEBs at the bifurcation points of branching airways or to internodal NEBs at interbifurcation points (140). Nodal NEBs have been shown to typically develop closest to a single intersection point or at the centroid of the triangle that links three intersection points (140). Single PNEC cells that are not able to reach a bifurcation point cluster into internodal NEBs that are on average smaller than their nodal counterparts (140). C-X-C motif chemokine receptor 4 (CXCR4), a chemokine receptor regulating migration, and N-cadherin, a transmembrane protein controlling cell-cell adhesion, are expressed in PNEC cells and are key components in their ability to form NEBs (140). Dense core vesicles containing bioactive neuropeptides, including GRP, and amines, like serotonin, are released by solitary and clustered PNECs in response to respiratory conditions such as oxygen level (11). Interestingly, mature NEBs are the only innervated airway epithelial cells observed in mammals: they are innervated on the basal side by vagal nerve afferents for which neurotrophin 4 (NT4) has been shown to be important for purinergic innervation (11, 145). This innervation may have significant impact on the allergic responses and inflammation in the airways; postnatal mice exposed to allergens exhibit increased NT4 leading to hyperinnervation and hyperactive sensory neurons, which lead to mucus overproduction (145). Tissue-wide immune responses can be initiated by PNECs in response to airborne stimuli, both directly and through the recruitment of type 2 innate lymphoid cells (ILC2) (92). During development, roundabout receptor (ROBO) genes are expressed in PNECs and impact their ability to cluster into NEBs (11). In addition, they have important functions in regulating immune responses as determined in studies using knockout models for both ROBO 1 and 2. These models resulted in unclustered PNEC and a notable rounding of the typical wedge-shaped PNEC. PNEC number, however, remained unchanged (3). The resulting unclustered cells lost their typical wedge shape and appeared to be more rounded; however, the total number of PNEC cells was unaffected (11). These ROBO mutant airways had increased expression of many immune response genes and elevated immune cell populations (11), suggesting important roles for ROBO gene expression in the immune response of PNEC in the airways.
Notch signaling has been identified as a crucial factor for both the development and differentiation of PNECs. Notch-hairy and enhancer of split-1 (HES1) signaling is thought to be responsible for the differentiation of single PNECs, controlling their numbers, but not location or NEB formation (140). The Notch-HES1/HEY1 (hairy/enhancer-of-split related with YRPW motif protein 1) pathway regulates the fate of lung endoderm. HES1, the target gene for Notch, is expressed in non-neuroendocrine cells but absent in PNEC, suggesting that Notch-HES1 signaling provides an inhibitory signal to maintain a fixed population of PNEC in the adult airways (92). In fact, the total number of PNECs is thought to be established during embryogenesis and maintained throughout life, since the total number of PNEC cells has been observed to remain constant after birth in both rats and humans (140). Transcription factor achaete-scute homolog 1 (ASCL1), expressed by both differentiated and precursors of PNEC, is critical for PNEC development; if ASCL1 is mutated in mice, PNEC are absent in the airways (85). Insulinoma-associated 1 (INSM1), encoding a zinc finger protein, is also important in PNEC differentiation, being expressed in both developing and mature PNEC and associated with single PNEC and NEB. IMSM1 is thought to repress HES1 by binding directly to regulatory sequences in the HES1 gene after its initial stimulation by ASCL1 (85). Mutations in INSM1 block the terminal differentiation of murine PNECs, suggesting that ASCL1 drives PNEC specification and INSM1 enables complete differentiation (85). Notch signaling is again activated in proliferating PNECs in response to epithelial lung injury (215). The elevated level of Notch signaling causes the proliferation and transdifferentiation of PNECs to CCs, MCCs, and GCs while inactivation of the Notch pathway leads to an inability to transdifferentiate after injury (215). The definitive role of Notch signaling in PNEC development and maintenance is still under investigation, however, and has only been extensively studied in mice and not widely compared across multiple species.
Alveolar Progenitor Cells
In the distal lung functional alveolar units responsible for gas exchange comprise of two cell types: squamous gas-exchanging AT1 cells and cuboidal surfactant-secreting AT2 cells (31, 107). Of these, the AT2 cells are considered as bifunctional alveolar progenitor cells that fulfill the stem cell niche while also maintaining their vital role in secreting phospholipid surfactants, such as surfactant protein C (SFTPC), to prevent the collapse of alveoli during respiration (31, 82, 88, 97, 103, 177). In addition to self-renewal, AT2 cells can differentiate into AT1 cells, which facilitate gas exchange through provision of a large surface area for gas diffusion (17, 82, 120, 150). Recent studies have indicated that these AT2 functions are regulated by BMP signaling, in which BMP4 prevents AT2 proliferation and encourages differentiation, whereas antagonists, such as Noggin, encourage proliferation (24). Following distal lung injury, other factors and signaling pathways are implicated in the self-renewal of AT2s, including yes-associated protein (YAP) activation, stromal cell-derived factor 1 (SDF1) signaling that leads to the production of growth factors, such as epithelial growth factor (EGF), and paracrine signals released by macrophages (24, 221). Although these processes are very effective in the restoration of the injured lung, AT2 cells have a limited ability to proliferate following an injury, and their expansion occurs over months (116). Alveolar epithelial cells (AECs) are identified through specific markers: for AT2 cells these include SFTPC, lysosomal-associated membrane protein 3 (LAMP3), and ATP-binding cassette subfamily A member 3 (ABCA3); and for AT1 cells podoplanin (PDPN), homeodomain-only protein (HOPX), advanced glycosylation end product-specific receptor (AGER), and aquaporin 5 (AQP5) (116, 202). Further subclassification of AT2 cells can be achieved based on divergent cellular behaviors, and, more recently, intermediary alveolar cell types have been identified by single cell transcriptomic data (46, 110, 142, 201, 202, 213). For example, in response to acute lung injury, a subpopulation of AT2 cells, denoted airway epithelial progenitors (AEPs), express transmembrane 4L six family member 1 (TM4SF1). These cells are capable of rapid proliferation and have a distinct response to Wnt and fibroblast growth factor (FGF) signaling, leading to both differentiation and proliferation (217). AEPs also differ in their chromatin structure, with distinct open chromatin located near lung development genes as opposed to housekeeping genes (217). A longitudinal analysis of murine lung regeneration after injury discovered a novel Krt8+ progenitor cell state derived from activated AT2 cells underwent a sequence of transcriptional states to its terminal AT1 cell state (182). Although the majority of single cell analysis of the distal lung has been completed in mice, a recent study has produced a molecular atlas of the human lung and, at the transcriptomic level, two clusters of AT2 cells were identified, one expressing canonical AT2 markers, such as SFTPC and ETV5 (ETS variant 5), and a second population, referred to here as signaling AT2 cells, has a number of signaling pathways activated, including wnt signaling (201). In situ analysis suggested that the two subpopulations were intermingled throughout the alveolar epithelium. It will be interesting to see whether the activated human AT2 cells reflect the KRT8 transitioning cells that are activated in injury to the murine lung.
Recently, a population of TP63+ and KRT5+ cells, denoted distal airway stem cells (DASCs), capable of self-renewal and differentiation into alveolar and bronchial epithelial cells was identified in mouse and human distal airways (118, 224). Remarkably, from studies in mice it was suggested that DASCs proliferate, migrate along the distal airways into the alveoli, and then form discrete regenerating “pods” in the damaged areas (96). Expression profiles of these KRT5 pods is thought to reflect intermediate cell type in lung regeneration (96). Although more research is needed to understand these processes in humans, using DASCs to regenerate distal airways and alveoli in damaged lungs represents a potential therapeutic strategy for treating COPD (208).
In mice, two other cell types, the airway CCs and bronchioalveolar stem cells (BASCs), are involved in airway and alveolar regeneration. Murine CCs are capable of self-renewal and generation of MCCs and AECs (57, 180). Together with AT2 cells, CCs are thought to maintain the bronchioalveolar epithelium in the mouse under steady-state conditions. Severe damage to lung epithelium on the other hand triggers expansion and differentiation of bipotent BASCs for regeneration of both AT2 and CCs (169). It remains unknown, however, whether this also applies in the human lung, where CCs are most prevalent in the small airways and respiratory bronchioles and can be generated from BCs (225). Future cell-based therapy for alveolar repair will rely heavily on understanding the process of lineage commitment for the variety of putative distal airway progenitor cells, alongside a complete dissection of the progenitor cell hierarchy, which is dependent on the level of injury to the lung. A summary of what is currently known is provided in Fig. 4. Further discovery of definitive markers for each subpopulation of alveolar progenitor cells will aid in their classification and lineage tracing and facilitate the identification of their specific functional roles within alveolar epithelial maintenance and repair.
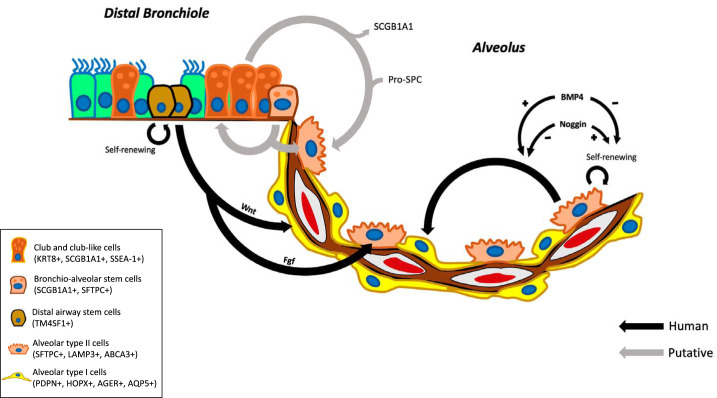
Fig. 4.
Schematic of putative progenitors of the alveolar epithelium. Alveolar type II cells (AT2) cells secrete surfactants essential for alveolar function, such as surfactant protein C (pro-SP-C). During lung homeostasis, AT2 cells also function as self-renewing alveolar progenitor cells, in addition to differentiating into AT1 cells, which facilitate gas exchange. Renewal vs. differentiation is regulated by the bone morphogenetic protein (BMP) signaling pathway. In response to acute lung injury, airway epithelial progenitors [club-like cells, bronchioalveolar stem cells (BASCs), and distal airway stem cells (DASCs)] are capable of rapid proliferation and, in response to Wnt and FGF signaling, they can differentiate into AT1 and AT2 cells, respectively (10). Studies in mice suggest that airway club cells self-renew and generate ciliated cells and alveolar cells during homeostasis, whereas severe damage to lung epithelium triggers the expansion and differentiation of BASCs, which regenerates the pool of AT2 and club cells (17) SCGB1A1, secretoglobin family 1A member 1/club cell secretory protein/club cell 10 kDa protein secretory club cells; SSEA-1, stage specific embryonic antigen 1; Fgf, fibroblast growth factor; KRT, cytokeratin; SFTPC, surfactant protein C; TM4SF1, transmembrane 4L six family member 1; LAMP3, lysosomal-associated membrane protein 3; ABCA3, ATP-binding cassette subfamily A member 3; PDPN, podoplanin; HOPX, homeodomain-only protein; AGER, advanced glycosylation end product-specific receptor; AQP5, aquaporin 5.
MAINTAINING HOMEOSTASIS AFTER LUNG INJURY
The capacity for any adult tissue to repair falls along an injury-repair/regeneration spectrum. Some tissues, such as the intestine and hematopoietic system, have a high cellular turnover and well-established stem cell hierarchy leading to rapid regeneration and restoration of homeostasis (8, 13, 36). Other organs, such as the heart (195, 204, 218), have few/no identified stem cell populations and little capacity for regeneration; repair mechanisms in these tissues often lead to scarring and some loss of function. The lung resides between these extremes with a relatively slow level of cellular turnover under tissue homeostasis but with a remarkable capacity to regenerate in response to injury. This regeneration can restore a fully functional airway or can repair with scarring leading to lung diseases. Understanding the mechanisms driving the differences between regeneration and repair differentiating from restoration of homeostasis and initiation of disease is at the forefront of regenerative medicine approaches.
Stem Cells in Chronic Obstructive Pulmonary Disease
COPD is characterized by chronic, progressive, and irreversible airflow limitations that result from a mixture of airways disease (e.g., bronchitis) and lung parenchymal destruction (emphysema) (152). COPD affected almost 400 million people in 2010, and, with effective treatment options lacking, causes approximately three million deaths annually (3, 134). The best-studied disease factor is chronic exposure to cigarette smoke (CS). CS contains more than 7,000 components, including many harmful substances, such as carbon monoxide, nicotine, oxidants, fine particulate matter, and aldehydes. Until recently, the predominant model was that recurrent epithelial injury inflicted by these toxic agents, such as loss of cell-cell junctions, DNA senescence, cell death, and DNA damage, together with chronic inflammation and immune cell infiltration, are the drivers of pathological remodeling in COPD (5, 72, 95, 141). This remodeling is characterized by the narrowing of the airways, mucociliary dysfunction, loss of small airways, and destruction of the lung parenchyma. New evidence is emerging, however, on how intrinsic lung stem cells are permanently altered by the CS/COPD microenvironment, leading to stem cell exhaustion, defective differentiation, and reduced capacity to generate normal lung epithelia even when CS exposure ends (28). Lung stem cell pathobiology therefore plays a crucial role in understanding COPD disease onset and progression. Furthermore, although clinical trials using exogenous mesenchymal stem cells to treat COPD have remained unsuccessful (186), learning how to protect, activate, and replenish the intrinsic lung stem cells might provide a new treatment option.
The resident stem cells of the central and peripheral airways, BCs, are affected by the CS/COPD microenvironment in two ways: first, in vitro data indicated that exposure to CS, or to factors released by epithelial cells in response to CS such as EGF and amphiregulin (AREG), acutely stimulate BCs to generate tissues with histological hallmarks of COPD. These hallmarks include BC hyperplasia, squamous metaplasia, GC hyperplasia, reduced ciliary length, loss of MCCS, and reduced barrier function (171, 175). Second, cigarette smoking is associated with an increased frequency of somatic mutations and genome-wide epigenetic, transcriptional, and metabolite changes in BCs (14, 28, 30), many of which have been associated with an increased risk of developing COPD in response to cigarette smoking (168, 174). Moreover, DNA hypermethylation in BCs isolated from smokers is associated with a reduced capacity of generating healthy in vitro epithelia, even in absence of CS (181). Indeed, although an increased fraction of KRT14-expressing BCs, which are indicative of proliferation and injury response, has been observed in COPD patients (161), COPD airways contain fewer numbers of KRT5/TP63-positive BCs that are able to form clones, self-renew, and differentiate properly, which is a sign of stem cell exhaustion (56). Summarizing the evidence, the COPD/CS microenvironment appears to permanently imprint airway basal cells to generate COPD-typical airway epithelia with defective barrier function, altered cell-type proportions, reduced regenerative capacity, and mucociliary dysfunction.
In contrast to the emergent role of stem cells in shaping the COPD airways, very little is known about the contributions of AT2 cells, the resident alveolar stem cells (7, 47), to the development of emphysema in human patients. Recent studies suggest that chronic CS may cause AT2 cells, which can self-renew and generate the gas-exchanging AT1 cells, to become senescent, leading to diminished repair of CS-mediated tissue damage and increased induction of harmful proteolytic enzymes and inflammatory cytokines (26). Indeed, accelerated aging, including in particular cellular senescence, is an emergent model for describing alveolar emphysema in COPD (12). Interestingly, SCGB1A1, the major secreted product of airway CCs, is protective against CS-induced lung remodeling (98), and its deficiency causes COPD-like lung pathology in mice (99). Given that SCGB1A1 expression is reduced in smokers (98) and by in vitro cigarette smoke exposure (223) and that COPD is thought to initiate in the small airways (124), further research into human club cell self-renewal and differentiation in response to chronic CS exposure might reveal key mechanisms of onset and progression of COPD.
Stem Cells in Cystic Fibrosis
CF is a genetic disease caused by mutations in the gene encoding the CF transmembrane conductance regulator (CFTR) (reviewed in Ref. 155). CFTR protein is expressed in the epithelia of many organs and primarily functions as an ion channel that controls the flow of ions and, indirectly, water across cellular membranes. In CF, the absence of functional CFTR is associated with decreased chloride and bicarbonate secretion through CFTR and increased sodium absorption by the epithelial sodium channel (ENaC). The ionic imbalance leads to increased mucus acidity and viscosity (196), mucus tethering (74), and mucus accumulation in the lung, pancreas, and other mucin-producing organs (39). Depending on the combination of the specific CFTR mutation (more than 2000 have been identified according to the CF mutation database maintained by the CF center at the Hospital for Sick Children in Toronto, http://www.genet.sickkids.on.ca/), non-CFTR gene modifiers, and environmental factors, patients exhibit highly variable disease phenotypes. Lung disease, however, remains the main causes of morbidity and mortality associated with CF (170). According to the central hypothesis, CF-associated dehydration and tethering of the mucus impairs mucociliary clearance of pathogens and noxious compounds, leading to early and recurrent respiratory infections, chronic excessive neutrophilic inflammation, structural airway changes (i.e., bronchiectasis), and progressive pulmonary obstruction that ultimately leads to respiratory failure (23, 45). Similar to COPD, CF seems to start in the small airways (200), can lead to emphysema (128), and is characterized by extensive structural remodeling of the airway and alveolar walls (199, 203). As in COPD, CF airway remodeling is characterized by BC hyperplasia and squamous cell metaplasia (148, 206), suggesting that altered proliferation and differentiation of airway stem cells play a major role in CF pathogenesis. Indeed, in vitro cultures of the CF human airway epithelial cells (HAEC) showed a delay in differentiation, a higher rate of proliferation during regeneration, and a pronounced thickness increase and basal cell hyperplasia in the differentiated CF HAEC compared with the non-CF HAEC (67). Another in vitro study found that CF HAEC exhibited BC hyperplasia and an increase in goblet cell numbers, which was associated with an hyperinflammatory phenotype (2). Interestingly, the hyperinflammation and the abnormal remodeling both vanished with time in culture, indicating a resolution of the phenotype that is not typically observed in vivo and highlighting the limitations of in vitro modeling of complex phenotypes involving multiple cell types. Furthermore, exposing non-CF HAEC to a similar mix of inflammatory stimuli recreated a similar remodeling phenotype. Recently, it was postulated that this inflammatory remodeling process may be independent of infection (159), possibly because the abnormalities of CF-mucus can be proinflammatory (106). It has also been shown that endoplasmic reticulum-associated protein degradation, induced by misprocessed F508del CFTR, might also stimulate a heightened inflammatory response (79). Taken together, these studies indicate that inflammatory states in the CF epithelium, whether due to CFTR dysfunction, infection, or a combination of both, can alter BC proliferation and differentiation in a way that favors airway remodeling and mucus accumulation. In addition, changes to BC differentiation might reduce the number of pulmonary ionocytes, a recently discovered rare cell type in the airway epithelium, that, in mice, derives from BCs and may be particularly important for CFTR-mediated functions. Ionocytes express higher levels of CFTR than any other large airway cell type in humans, and, in vitro, the relative abundance of human ionocytes indeed correlated with tissue-level CFTR function (133, 149). Future research will reveal whether human pulmonary ionocytes are derived from airway BCs and whether this differentiation pathway is impaired in the CF airways.
Stem Cells in Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive disease with unclear etiology that leads to scarring of the lung. It is progressively fatal with no curative medical therapy. Because of their pertinent role in epithelial homeostasis and repair, lung progenitor cells are of particular interest in the progression of IPF. Smirnova (176) and colleagues performed a quantitative analysis of BCs in normal human lungs and lungs from patients with IPF and noted a significant change in the distribution patterns of the various BC phenotypes in the IPF lungs. Specifically, they found a significant increase in the number of KRT5+KRT14+TP63+ BCs in the distal airways of IPF compared with the nondiseased human lungs. They also noted that KRT14+ failed to colocalize with staining for differentiation markers of bronchial/alveolar epithelial cells, including pro-SP-C, AQP5, SCGB1A1, and MUC5B, in IPF lungs (176). Jonsdottir and colleagues (86) described a mesenchymal differentiation phenotype of BCs in the fibroblastic foci of human IPF lungs, demonstrated through loss of E-cadherin, acquisition of N-cadherin, and increased vimentin expression. Using in vitro airway differentiation at the air-liquid interface (ALI, using serum substitute, Ultroser G-based media), they also demonstrated that TP63+ BCs can undergo epithelial-mesenchymal transition, suggesting a contribution of BCs to fibrosis in IPF lungs. More recently, Kobayashi and colleagues (91) have implicated a transitional state between AT2 and AT1 cells as a pre-AT1 transitional state (PATS), a distinct phenotype en route to differentiation (91). Furthermore, they noted an enrichment of the PATS-like state in human IPF lungs, suggesting that persistence of this state may be the underlying cause of fibrosis.
Because of the lack of an animal model that captures all the aspects of human IPF, the murine bleomycin injury model is most commonly used to study therapeutic strategies for IPF in the preclinical phase. Although the majority of studies have been conducted using mesenchymal stem cells and iPSC-derived AT2 cells, endogenous AT2 cells have been evaluated in preclinical trials in a rat bleomycin injury model and shown to reduce fibrosis (173) and restore surfactant levels (64). In clinical trials, AT2 cell therapy has been shown to be safe and well tolerated with a stabilization of lung function, improvement in 6-min walk distance, and decrease in dyspnea and cough in a small cohort of patients (172). Whereas the safe delivery of AT2 cells suggests feasibility of cellular therapy, it must be remembered that, in these trials, the status of engraftment and functionality of these cells remains unknown. In the preclinical trials in rats, delivery of male donor AT2 cells to female recipients was confirmed by detection of the Y chromosome 15 days after intratracheal delivery. However, even in the rat models, the long-term engraftment of the AT2 cells was not rigorously studied (173). A subsequent and similar study of AT2 cell delivery to the rat lung validated a recovery from acute lung injury and suggested that an AT2 paracrine effect on alveolar macrophages, producing an anti-inflammatory response, may have augmented the resolution of the injury (65). These studies provide proof for feasibility of the safe delivery of AT2 cells to the lung, but whether any functional engraftment is occurring remains to be verified.
Stem Cells and Lung Transplantation
Lung transplantation is an effective therapeutic option for end-stage lung diseases. However, the fate of the stem cells in the allograft was, until recently, largely unknown due to a lack of a suitable animal model that recapitulates human allograft pathology. Using a ferret model of orthotopic lung transplantation that captures the entire spectrum human allograft pathology (184), it has been shown that airway basal stem cells and submucosal glands are depleted as the allografts develop OB (189), a progressively fatal condition that develops in most recipients following lung transplantation. This finding changes the old paradigm in which OB was a disease limited to the small airways of the allografts. The stem cell depletion seen in OB is global in large cartilaginous bronchi and distal small bronchioles. Whether airway epithelial stem cell therapy can be a potential therapeutic tool to prevent or delay progression of OB remains an exciting area of further investigation. The transplanted lung provides a unique opportunity to test the potential of airway stem cell therapy due to the fact that endogenous stem cell in the allograft is depleted as part of the natural course, thereby creating room for therapeutic cells to engraft in the biological niche (111).
PROSPECTS FOR STEM CELL THERAPY FOR THE LUNG
The concept of using exogenous stem/progenitor cells to regenerate or enhance repair of the human lung has generated considerable excitement within the field. However, there are substantial limitations remaining before this can become a reality. First and foremost, access to enough autologous cells for gene editing and transplant is limited by our capacity to locate, isolate, and expand specific lung progenitor cells. Lung basal cells are routinely isolated and expanded from explanted lungs; however, serial passaging alters the cellular phenotype and results in decreased cellular function, including decreased CFTR expression and poor differentiation capacity (147). Despite the extensive discussion on lung stem cells in this review, there is still a paucity of information on the comprehensive identification and characterization that defines subpopulations of stem cells in both the small and large airways. Successfully engrafted stem/progenitor cells must be robust and long lived, and delivery of the cells into a lung stem cell niche that would support a long-term functional engraftment remains a sizable challenge. Developing new and validated in vitro and in vivo model systems will be essential to better predict longevity and differentiation potential of stem cells in the lung. Current studies of cellular engraftment indicate a relatively poor efficiency (<2%) and a lack of long-term persistence. It is important to recognize that most of these studies use rodent models for evaluation of human cell engraftment (33, 53, 118, 130, 164). Extant models provide important information but are limited in their capacity to accurately mimic human lung disease pathogenesis. Rodents have large disparities in the size, physiology, structure, and cellular composition of their airways relative to humans. Such differences limit the conversion of findings in preclinical rodent models into therapeutics in the clinic. Because the capacity to fully evaluate the conditions essential to success of cellular therapy is not possible in humans, progression toward a cellular therapy for lung disease will require a preclinical animal model system that closely resembles the physiology and function of the human lung and one that also recapitulates the pathogenesis of the human lung disease in question. Taking cystic fibrosis as an example, rodents models fail to develop significant lung disease, whereas ferrets and pigs share similar lung physiology, for example, the presence of submucosal glands throughout the cartilaginous airways, and emulate many clinical features associated with human lung diseases (4, 44, 136, 184, 185).
Given the significant safety challenges faced by stem cell-based therapies, rigorous preclinical models for evaluation must be developed and protocols ironed out to enable safe and effective cellular transplantation in the lung. A wide spectrum of stem cell-based products with potential for regenerative medicine exist, including embryonic stem cells, endogenous stem and progenitor cells, and differentiated cellular progeny. Furthermore, the introduction of induced pluripotency (iPSCs) provides increased hope for therapeutic innovation. Although the efficiency and efficacy of differentiation of iPSC toward lung lineages has improved over the past eight years, the field is limited by our relatively poor understanding of the identity and regulation of lung stem/progenitor cells. Both issues are highly active areas of research in which one should expect considerable advances to be made soon.
Pluripotent Stem Cell-Derived Lung Cells
Although the true value of pluripotent stem cells (PSCs) and their derivatives has yet to near its full potential for clinical application, these cells have become a valuable technology for understanding human lung development and disease, complimentary to more conventional approaches using animal models or immortalized cells. A new era of genetic and cell biology research was initiated by Takahashi and Yamanaka back in 2007 when they discovered that induction of pluripotency (induced PSC or iPSC) in fully differentiated/mature somatic cells could be achieved using a minimal cocktail of four transcription factors [octamer binding transcription factor 4 (OCT4), SOX2, Kruppel-like factor-4 (KLF4), and c-Myc] in combination with defined culture conditions (192). iPSC retain unique properties of continued clonal expansion and multilineage differentiation capacity; these properties have been extensively reviewed (41, 43, 50, 73, 76, 93, 125, 126, 135, 194, 209). Differentiation of iPSC toward the respiratory epithelium has proven challenging; however, recent efforts have seen the evolution of protocols to differentiate iPSC to cells of the respiratory epithelium (21, 22, 37, 42, 48, 52, 60, 68, 69, 77, 78, 80, 81, 87, 94, 108, 121–123, 129, 144, 211). These were recently reviewed (15). Although all of these methods strive to mimic the pathways of lung development, there remain limitations in recapitulation of signaling gradients of chemokines, cytokines, and growth factors along the anterior/posterior, dorso/ventral, and proximo/distal axis. In lieu of sophisticated 3D models, protocols have been able to induce the primitive streak and continue specification through mesendoderm to definitive endoderm with high efficiency (109). The efficiency of subsequent cellular fate decisions drops as cells progress through anteriorization of the ventral foregut endoderm (AFE), primordial homeobox protein Nkx-2.1 (NKX2.1)-expressing lung endodermal progenitor cells to lung BCs, and mature cells of the airways, including club, goblet, multiciliated, alveolar, and neuroendocrine cells. Specific formation of the AFE, characterized by expression of transcription factors SOX2 and FOXA2, can be assisted by inhibition of TGF-β (61) in addition to inhibition of Wnt- and BMP-signaling. As cells transition toward the primordial lung progenitor, defined by induction of transcription factor NKX2.1 (also known as TTF1) (101, 108), the precise spatial and temporal expression of FGFs, retinoic acid (RA), BMP, and sonic hedgehog (Shh) signaling defines the sequential cellular transitions toward lung fate (89, 154). iPSC-derived NKX2.1-expressing cells can be purified via flow-activated cell sorting (FACS) collecting CD47hi, CD26lo (69), or carboxypeptidase-Mhi (CPM)-expressing cells (60, 94). It should be noted that, while NKX2.1 defines the lung progenitor cell (1, 102, 165), it can also be expressed in endodermal-derived thyroid tissues [coexpressing paired box protein 8 (PAX8) (165)] and the forebrain [coexpressing paired box protein 6 (PAX6) (25, 193)].
3D cultures allow for growth and expansion in all dimensions, permitting budding and elongation of airways more akin to the in vivo conditions for lung development. Progenitor cells at the leading tip of the lung buds, forming from the lateral foregut endoderm, are capable of differentiation to both the proximal and distal lung (29, 130, 139). These progenitor cells express NKX2.1, SOX2, and SOX9 in humans, defining a cellular population that differs from its counterpart in mice, which express NKX2.1 and either SOX2 or SOX9 (130). Fate decisions of such stem cells can be studied in detail in mice using complex lineage traces in transgenic mice (7, 158, 160); however, equivalent studies are not possible in humans. Through the development of in vitro models, it may be possible to compare human and mouse lung development more closely. Specification of the distal airways and the AT2 progenitor cells can be controlled via regulation of Wnt signaling. High Wnt activation supports a distal fate decision while suppression of Wnt signaling favors a proximal fate (123). iPSC-derived AT2 cells express NKX2.1, can be continually expanded in 3D organoid culture, and have a transcriptomic profile akin to that of fetal human lung AT2 cells. Interestingly, their differentiation to AT1 cells in culture is currently challenging unlike their primary counterparts that spontaneously generate AT1 cells in 2D culture systems (81, 120).
iPSC also provide a suitable model system for genetic manipulation by current state-of-the art gene-editing technologies in both model and gene-correct genetic lung disorders (recently reviewed for CF in Ref. 151). Acquisition of sufficient primary cellular material for extensive in vitro studies on rare genetic lung disease, diseases with multiple subtypes, compound heterozygosity, or epigenetic factors impacting efficacy of current therapeutics is incredibly difficult. Self-renewing iPSC can be applied for a personalized precision medicine approach to studying human lung disease and therapeutic screening. In the respiratory field, proof-of-principle studies have demonstrated the correction of CFTR in CF patient-derived iPSC, which were subsequently differentiated into functional epithelial cells (27, 43). iPSCs, therefore, provide a valuable model system for studying human lung development, generating patient- and mutation-specific models of lung disease and providing plentiful cells for therapeutic screening and cellular therapy. As for any model, their value should be considered alongside other established systems to create a complete and accurate picture of the human lung. It will be critical for the field to now validate their iPSC-derived cells through robust functional comparisons to their primary counterparts.
CONCLUSIONS
The ability to replace defective cells in an airway with a cell that has the capacity to engraft and electromechanically integrate in an injured airway to restore a functional epithelium could potentially cure a number of diseases. While recent advances provide great promise, significant translational challenges remain. Major roadblocks moving toward cell therapy include the poor efficiency of ex vivo cell expansion and defining the conditions essential for successful in vivo cell engraftment. Progress toward the development of strategies to regenerate the adult lung, either in vivo or ex vivo targeting of endogenous stem cells or iPSC derivatives, is limited by our fundamental lack of understanding of the precise identity and function of human lung cell types and the genetic and epigenetic control of human lung fate. In this review we have summarized the current understanding of known stem/progenitor cell populations and their relative differences between rodents and humans, their roles in chronic lung disease, and their therapeutic prospects. Recent advances in lineage-traced animal models and in single-cell RNA sequencing of human airway cells, have provided new information on stem cell subtypes, transition states, identifying cell markers, and intricate pathways that commit a stem cell to differentiate or to maintain plasticity. It is not critical that the numerous cellular subtypes identified through these techniques are functionally characterized to further understand their potential in lung regeneration or their pathogenic roles in chronic lung disease.
GRANTS
This work was supported by the Hastings Foundation (A.L.R.); National Heart, Lung, and Blood Institute/NIH (NHLBI) Grant 5R01-HL139828-03 and Cystic Fibrosis Foundation Therapeutics, FIRTH17XX0 (A.L.R.) and FIRTH15XX0 (A.L.R./K.R.P.), NHLBI/NIH Grant R01-HL136370-01 (K.R.P.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.R.P., J.N., A.P., S.M.B., C.N.S., and A.L.R. prepared figures; K.R.P., J.N., A.P., S.M.B., C.N.S., and A.L.R. drafted manuscript; K.R.P., J.N., A.P., S.M.B., C.N.S., and A.L.R. edited and revised manuscript; K.R.P., A.P., S.M.B., C.N.S., and A.L.R. approved final version of manuscript.
REFERENCES
- 1.Acebrón A, Aza-Blanc P, Rossi DL, Lamas L, Santisteban P. Congenital human thyroglobulin defect due to low expression of the thyroid-specific transcription factor TTF-1. J Clin Invest 96: 781–785, 1995. doi: 10.1172/JCI118123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam D, Roux-Delrieu J, Luczka E, Bonnomet A, Lesage J, Mérol JC, Polette M, Abély M, Coraux C. Cystic fibrosis airway epithelium remodelling: involvement of inflammation. J Pathol 235: 408–419, 2015. doi: 10.1002/path.4471. [DOI] [PubMed] [Google Scholar]
- 3.Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, Nair H, Gasevic D, Sridhar D, Campbell H, Chan KY, Sheikh A, Rudan I, Global Health Epidemiology Reference G; Global Health Epidemiology Reference Group (GHERG) . Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health 5: 020415, 2015. doi: 10.7189/jogh.05.020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aizawa K, Liu C, Veeramachaneni S, Hu KQ, Smith DE, Wang XD. Development of ferret as a human lung cancer model by injecting 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Lung Cancer 82: 390–396, 2013. doi: 10.1016/j.lungcan.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoshiba K, Tsuji T, Yamaguchi K, Itoh M, Nakamura H. The danger signal plus DNA damage two-hit hypothesis for chronic inflammation in COPD. Eur Respir J 42: 1689–1695, 2013. doi: 10.1183/09031936.00102912. [DOI] [PubMed] [Google Scholar]
- 6.Arbi M, Pefani DE, Kyrousi C, Lalioti ME, Kalogeropoulou A, Papanastasiou AD, Taraviras S, Lygerou Z. GemC1 controls multiciliogenesis in the airway epithelium. EMBO Rep 17: 400–413, 2016. doi: 10.15252/embr.201540882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123: 3025–3036, 2013. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bello SA, Torres-Gutiérrez V, Rodríguez-Flores EJ, Toledo-Román EJ, Rodríguez N, Díaz-Díaz LM, Vázquez-Figueroa LD, Cuesta JM, Grillo-Alvarado V, Amador A, Reyes-Rivera J, García-Arrarás JE. Insights into intestinal regeneration signaling mechanisms. Dev Biol 458: 12–31, 2020. doi: 10.1016/j.ydbio.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med 157: 2000–2006, 1998. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- 10.Bonser LR, Erle DJ. Airway Mucus and Asthma: The Role of MUC5AC and MUC5B. J Clin Med 6: 112, 2017. doi: 10.3390/jcm6120112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branchfield K, Nantie L, Verheyden JM, Sui P, Wienhold MD, Sun X. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science 351: 707–710, 2016. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandsma CA, de Vries M, Costa R, Woldhuis RR, Königshoff M, Timens W. Lung ageing and COPD: is there a role for ageing in abnormal tissue repair? Eur Respir Rev 26: 170073, 2017. doi: 10.1183/16000617.0073-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol 169: 338–346, 2006. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buro-Auriemma LJ, Salit J, Hackett NR, Walters MS, Strulovici-Barel Y, Staudt MR, Fuller J, Mahmoud M, Stevenson CS, Hilton H, Ho MW, Crystal RG. Cigarette smoking induces small airway epithelial epigenetic changes with corresponding modulation of gene expression. Hum Mol Genet 22: 4726–4738, 2013. doi: 10.1093/hmg/ddt326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvert BA, Ryan (Firth) AL. Application of iPSC to modelling of respiratory diseases. Adv Exp Med Biol 7: 1–16, 2019. doi: 10.1007/5584_2019_430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell EP, Quigley IK, Kintner C. Foxn4 promotes gene expression required for the formation of multiple motile cilia. Development 143: 4654–4664, 2016. doi: 10.1242/dev.143859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castaldi A, Horie M, Rieger ME, Dubourd M, Sunohara M, Pandit K, Zhou B, Offringa IA, Marconett CN, Borok Z. Genome-wide integration of microRNA and transcriptomic profiles of differentiating human alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 319: L173–L184, 2020. doi: 10.1152/ajplung.00519.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, Kitzmiller J, Maeda Y, Haitchi HM, Sridharan A, Senft AP, Whitsett JA. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med 189: 301–313, 2014. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 119: 2914–2924, 2009. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen HJ, Poran A, Unni AM, Huang SX, Elemento O, Snoeck HW, Varmus H. Generation of pulmonary neuroendocrine cells and SCLC-like tumors from human embryonic stem cells. J Exp Med 216: 674–687, 2019. doi: 10.1084/jem.20181155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YW, Huang SX, de Carvalho ALRT, Ho SH, Islam MN, Volpi S, Notarangelo LD, Ciancanelli M, Casanova JL, Bhattacharya J, Liang AF, Palermo LM, Porotto M, Moscona A, Snoeck HW. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19: 542–549, 2017. doi: 10.1038/ncb3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng X, Ying L, Lu L, Galvão AM, Mills JA, Lin HC, Kotton DN, Shen SS, Nostro MC, Choi JK, Weiss MJ, French DL, Gadue P. Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell 10: 371–384, 2012. doi: 10.1016/j.stem.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chmiel JF, Davis PB. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can’t they clear the infection? Respir Res 4: 8, 2003. doi: 10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung MI, Bujnis M, Barkauskas CE, Kobayashi Y, Hogan BLM. Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development 145: dev163014, 2018. doi: 10.1242/dev.163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corbin JG, Rutlin M, Gaiano N, Fishell G. Combinatorial function of the homeodomain proteins Nkx2.1 and Gsh2 in ventral telencephalic patterning. Development 130: 4895–4906, 2003. doi: 10.1242/dev.00717. [DOI] [PubMed] [Google Scholar]
- 26.Cottage CT, Peterson N, Kearley J, Berlin A, Xiong X, Huntley A, Zhao W, Brown C, Migneault A, Zerrouki K, Criner G, Kolbeck R, Connor J, Lemaire R. Targeting p16-induced senescence prevents cigarette smoke-induced emphysema by promoting IGF1/Akt1 signaling in mice. Commun Biol 2: 307, 2019. doi: 10.1038/s42003-019-0532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crane AM, Kramer P, Bui JH, Chung WJ, Li XS, Gonzalez-Garay ML, Hawkins F, Liao W, Mora D, Choi S, Wang J, Sun HC, Paschon DE, Guschin DY, Gregory PD, Kotton DN, Holmes MC, Sorscher EJ, Davis BR. Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Reports 4: 569–577, 2015. doi: 10.1016/j.stemcr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crystal RG. Airway basal cells. The “smoking gun” of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 190: 1355–1362, 2014. doi: 10.1164/rccm.201408-1492PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danopoulos S, Alonso I, Thornton ME, Grubbs BH, Bellusci S, Warburton D, Al Alam D. Human lung branching morphogenesis is orchestrated by the spatiotemporal distribution of ACTA2, SOX2, and SOX9. Am J Physiol Lung Cell Mol Physiol 314: L144–L149, 2018. doi: 10.1152/ajplung.00379.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deeb RS, Walters MS, Strulovici-Barel Y, Chen Q, Gross SS, Crystal RG. Smoking-associated disordering of the airway basal stem/progenitor cell metabotype. Am J Respir Cell Mol Biol 54: 231–240, 2016. doi: 10.1165/rcmb.2015-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507: 190–194, 2014. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol 19: 676–680, 2007. doi: 10.1016/j.coi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Doi R, Tsuchiya T, Mitsutake N, Nishimura S, Matsuu-Matsuyama M, Nakazawa Y, Ogi T, Akita S, Yukawa H, Baba Y, Yamasaki N, Matsumoto K, Miyazaki T, Kamohara R, Hatachi G, Sengyoku H, Watanabe H, Obata T, Niklason LE, Nagayasu T. Transplantation of bioengineered rat lungs recellularized with endothelial and adipose-derived stromal cells. Sci Rep 7: 8447, 2017. doi: 10.1038/s41598-017-09115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Driskell RR, Goodheart M, Neff T, Liu X, Luo M, Moothart C, Sigmund CD, Hosokawa R, Chai Y, Engelhardt JF. Wnt3a regulates Lef-1 expression during airway submucosal gland morphogenesis. Dev Biol 305: 90–102, 2007. doi: 10.1016/j.ydbio.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan D, Yue Y, Zhou W, Labed B, Ritchie TC, Grosschedl R, Engelhardt JF. Submucosal gland development in the airway is controlled by lymphoid enhancer binding factor 1 (LEF1). Development 126: 4441–4453, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Dubrovsky G, Dunn JCY. Mechanisms for intestinal regeneration. Curr Opin Pediatr 30: 424–429, 2018. doi: 10.1097/MOP.0000000000000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD, White ES, Deutsch GH, Spence JR. In vitro generation of human pluripotent stem cell derived lung organoids (Abstract). eLife 4: e05098, 2015. doi: 10.7554/eLife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edbrooke MR, Parker D, McVey JH, Riley JH, Sorenson GD, Pettengill OS, Craig RK. Expression of the human calcitonin/CGRP gene in lung and thyroid carcinoma. EMBO J 4: 715–724, 1985. doi: 10.1002/j.1460-2075.1985.tb03688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehre C, Ridley C, Thornton DJ. Cystic fibrosis: an inherited disease affecting mucin-producing organs. Int J Biochem Cell Biol 52: 136–145, 2014. doi: 10.1016/j.biocel.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman MB, Wood M, Lapey A, Mou H. SMAD signaling restricts mucous cell differentiation in human airway epithelium. Am J Respir Cell Mol Biol 61: 322–331, 2019. doi: 10.1165/rcmb.2018-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiorotto R, Amenduni M, Mariotti V, Fabris L, Spirli C, Strazzabosco M. Liver diseases in the dish: iPSC and organoids as a new approach to modeling liver diseases. Biochim Biophys Acta Mol Basis Dis 1865: 920–928, 2019. doi: 10.1016/j.bbadis.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Firth AL, Dargitz CT, Qualls SJ, Menon T, Wright R, Singer O, Gage FH, Khanna A, Verma IM. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc Natl Acad Sci USA 111: E1723–E1730, 2014. doi: 10.1073/pnas.1403470111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Firth AL, Menon T, Parker GS, Qualls SJ, Lewis BM, Ke E, Dargitz CT, Wright R, Khanna A, Gage FH, Verma IM. Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep 12: 1385–1390, 2015. doi: 10.1016/j.celrep.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher JT, Zhang Y, Engelhardt JF. Comparative biology of cystic fibrosis animal models. Methods Mol Biol 742: 311–334, 2011. doi: 10.1007/978-1-61779-120-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flume PA. Pulmonary complications of cystic fibrosis. Respir Care 54: 618–627, 2009. doi: 10.4187/aarc0443. [DOI] [PubMed] [Google Scholar]
- 46.Frank DB, Penkala IJ, Zepp JA, Sivakumar A, Linares-Saldana R, Zacharias WJ, Stolz KG, Pankin J, Lu M, Wang Q, Babu A, Li L, Zhou S, Morley MP, Jain R, Morrisey EE. Early lineage specification defines alveolar epithelial ontogeny in the murine lung. Proc Natl Acad Sci USA 116: 4362–4371, 2019. doi: 10.1073/pnas.1813952116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujino N, Kubo H, Suzuki T, Ota C, Hegab AE, He M, Suzuki S, Suzuki T, Yamada M, Kondo T, Kato H, Yamaya M. Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab Invest 91: 363–378, 2011. doi: 10.1038/labinvest.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujita A, Uchida N, Haro-Mora JJ, Winkler T, Tisdale J. β-Globin-expressing definitive erythroid progenitor cells generated from embryonic and induced pluripotent stem cell-derived sacs. Stem Cells 34: 1541–1552, 2016. doi: 10.1002/stem.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukui T, Shaykhiev R, Agosto-Perez F, Mezey JG, Downey RJ, Travis WD, Crystal RG. Lung adenocarcinoma subtypes based on expression of human airway basal cell genes. Eur Respir J 42: 1332–1344, 2013. doi: 10.1183/09031936.00144012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fyfe I. Mutation-specific amyloid-β processing in iPSC-derived neurons. Nat Rev Neurol 15: 310, 2019. doi: 10.1038/s41582-019-0195-z. [DOI] [PubMed] [Google Scholar]
- 51.Galvis LA, Holik AZ, Short KM, Pasquet J, Lun AT, Blewitt ME, Smyth IM, Ritchie ME, Asselin-Labat ML. Repression of Igf1 expression by Ezh2 prevents basal cell differentiation in the developing lung. Development 142: 1458–1469, 2015. doi: 10.1242/dev.122077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghaedi M, Mendez JJ, Bove PF, Sivarapatna A, Raredon MS, Niklason LE. Alveolar epithelial differentiation of human induced pluripotent stem cells in a rotating bioreactor. Biomaterials 35: 699–710, 2014. doi: 10.1016/j.biomaterials.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh M, Ahmad S, White CW, Reynolds SD. Transplantation of airway epithelial stem/progenitor cells: a future for cell-based therapy. Am J Respir Cell Mol Biol 56: 1–10, 2017. doi: 10.1165/rcmb.2016-0181MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghosh M, Brechbuhl HM, Smith RW, Li B, Hicks DA, Titchner T, Runkle CM, Reynolds SD. Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells. Am J Respir Cell Mol Biol 45: 403–410, 2011. doi: 10.1165/rcmb.2010-0283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh M, Helm KM, Smith RW, Giordanengo MS, Li B, Shen H, Reynolds SD. A single cell functions as a tissue-specific stem cell and the in vitro niche-forming cell. Am J Respir Cell Mol Biol 45: 459–469, 2011. doi: 10.1165/rcmb.2010-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghosh M, Miller YE, Nakachi I, Kwon JB, Barón AE, Brantley AE, Merrick DT, Franklin WA, Keith RL, Vandivier RW. Exhaustion of airway basal progenitor cells in early and established chronic obstructive pulmonary disease. Am J Respir Crit Care Med 197: 885–896, 2018. doi: 10.1164/rccm.201704-0667OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 161: 173–182, 2002. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomi K, Arbelaez V, Crystal RG, Walters MS. Activation of NOTCH1 or NOTCH3 signaling skews human airway basal cell differentiation toward a secretory pathway. PLoS One 10: e0116507, 2015. doi: 10.1371/journal.pone.0116507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goto Y, Noguchi Y, Nomura A, Sakamoto T, Ishii Y, Bitoh S, Picton C, Fujita Y, Watanabe T, Hasegawa S, Uchida Y. In vitro reconstitution of the tracheal epithelium. Am J Respir Cell Mol Biol 20: 312–318, 1999. doi: 10.1165/ajrcmb.20.2.3062. [DOI] [PubMed] [Google Scholar]
- 60.Gotoh S, Ito I, Nagasaki T, Yamamoto Y, Konishi S, Korogi Y, Matsumoto H, Muro S, Hirai T, Funato M, Mae S, Toyoda T, Sato-Otsubo A, Ogawa S, Osafune K, Mishima M. Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Reports 3: 394–403, 2014. doi: 10.1016/j.stemcr.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Green MD, Chen A, Nostro MC, d’Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck HW. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol 29: 267–272, 2011. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guha A, Deshpande A, Jain A, Sebastiani P, Cardoso WV. Uroplakin 3a+ cells are a distinctive population of epithelial progenitors that contribute to airway maintenance and post-injury repair. Cell Rep 19: 246–254, 2017. doi: 10.1016/j.celrep.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 63.Guha A, Vasconcelos M, Cai Y, Yoneda M, Hinds A, Qian J, Li G, Dickel L, Johnson JE, Kimura S, Guo J, McMahon J, McMahon AP, Cardoso WV. Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc Natl Acad Sci USA 109: 12592–12597, 2012. doi: 10.1073/pnas.1204710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guillamat-Prats R, Gay-Jordi G, Xaubet A, Peinado VI, Serrano-Mollar A. Alveolar type II cell transplantation restores pulmonary surfactant protein levels in lung fibrosis. J Heart Lung Transplant 33: 758–765, 2014. doi: 10.1016/j.healun.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Guillamat-Prats R, Puig F, Camprubí-Rimblas M, Herrero R, Serrano-Mollar A, Gómez MN, Tijero J, Matthay MA, Blanch L, Artigas A. Intratracheal instillation of alveolar type II cells enhances recovery from acute lung injury in rats. J Heart Lung Transplant 37: 782–791, 2018. doi: 10.1016/j.healun.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 66.Hackett NR, Shaykhiev R, Walters MS, Wang R, Zwick RK, Ferris B, Witover B, Salit J, Crystal RG. The human airway epithelial basal cell transcriptome. PLoS One 6: e18378, 2011. doi: 10.1371/journal.pone.0018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hajj R, Lesimple P, Nawrocki-Raby B, Birembaut P, Puchelle E, Coraux C. Human airway surface epithelial regeneration is delayed and abnormal in cystic fibrosis. J Pathol 211: 340–350, 2007. doi: 10.1002/path.2118. [DOI] [PubMed] [Google Scholar]
- 68.Hawkins F, Kotton DN. Embryonic and induced pluripotent stem cells for lung regeneration. Ann Am Thorac Soc 12, Suppl 1: S50–S53, 2015. doi: 10.1513/AnnalsATS.201410-457MG. [DOI] [PubMed] [Google Scholar]
- 69.Hawkins F, Kramer P, Jacob A, Driver I, Thomas DC, McCauley KB, Skvir N, Crane AM, Kurmann AA, Hollenberg AN, Nguyen S, Wong BG, Khalil AS, Huang SX, Guttentag S, Rock JR, Shannon JM, Davis BR, Kotton DN. Prospective isolation of NKX2-1-expressing human lung progenitors derived from pluripotent stem cells. J Clin Invest 127: 2277–2294, 2017. doi: 10.1172/JCI89950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hegab AE, Ha VL, Darmawan DO, Gilbert JL, Ooi AT, Attiga YS, Bisht B, Nickerson DW, Gomperts BN. Isolation and in vitro characterization of basal and submucosal gland duct stem/progenitor cells from human proximal airways. Stem Cells Transl Med 1: 719–724, 2012. doi: 10.5966/sctm.2012-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hegab AE, Ha VL, Gilbert JL, Zhang KX, Malkoski SP, Chon AT, Darmawan DO, Bisht B, Ooi AT, Pellegrini M, Nickerson DW, Gomperts BN. Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells 29: 1283–1293, 2011. doi: 10.1002/stem.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heijink IH, Brandenburg SM, Postma DS, van Oosterhout AJ. Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery. Eur Respir J 39: 419–428, 2012. doi: 10.1183/09031936.00193810. [DOI] [PubMed] [Google Scholar]
- 73.Hnatiuk A, Mercola M. Stars in the night sky: iPSC-cardiomyocytes return the patient context to drug screening. Cell Stem Cell 24: 506–507, 2019. doi: 10.1016/j.stem.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 74.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, Moninger TO, Michalski AS, Hoffman EA, Zabner J, Stoltz DA, Welsh MJ. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 345: 818–822, 2014. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hor P, Punj V, Calvert BA, Castaldi A, Miller AJ, Carraro G, Stripp BR, Brody SL, Spence JR, Ichida JK, Ryan Firth AL, Borok Z. Efficient generation and transcriptomic profiling of human iPSC-derived pulmonary neuroendocrine cells. iScience 23: 101083, 2020. doi: 10.1016/j.isci.2020.101083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoshina A, Kawamoto T, Sueta SI, Mae SI, Araoka T, Tanaka H, Sato Y, Yamagishi Y, Osafune K. Development of new method to enrich human iPSC-derived renal progenitors using cell surface markers. Sci Rep 8: 6375, 2018. doi: 10.1038/s41598-018-24714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang SX, Green MD, de Carvalho AT, Mumau M, Chen YW, D’Souza SL, Snoeck HW. The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat Protoc 10: 413–425, 2015. doi: 10.1038/nprot.2015.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 32: 84–91, 2014. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hybiske K, Fu Z, Schwarzer C, Tseng J, Do J, Huang N, Machen TE. Effects of cystic fibrosis transmembrane conductance regulator and DeltaF508CFTR on inflammatory response, ER stress, and Ca2+ of airway epithelia. Am J Physiol Lung Cell Mol Physiol 293: L1250–L1260, 2007. doi: 10.1152/ajplung.00231.2007. [DOI] [PubMed] [Google Scholar]
- 80.Ikonomou L, Kotton DN. Derivation of endodermal progenitors from pluripotent stem cells. J Cell Physiol 230: 246–258, 2015. doi: 10.1002/jcp.24771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacob A, Morley M, Hawkins F, McCauley KB, Jean JC, Heins H, Na CL, Weaver TE, Vedaie M, Hurley K, Hinds A, Russo SJ, Kook S, Zacharias W, Ochs M, Traber K, Quinton LJ, Crane A, Davis BR, White FV, Wambach J, Whitsett JA, Cole FS, Morrisey EE, Guttentag SH, Beers MF, Kotton DN. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell 21: 472–488e10, 2017. doi: 10.1016/j.stem.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jain R, Barkauskas CE, Takeda N, Bowie EJ, Aghajanian H, Wang Q, Padmanabhan A, Manderfield LJ, Gupta M, Li D, Li L, Trivedi CM, Hogan BLM, Epstein JA. Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nat Commun 6: 6727, 2015. doi: 10.1038/ncomms7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeffery PK, Gaillard D, Moret S. Human airway secretory cells during development and in mature airway epithelium. Eur Respir J 5: 93–104, 1992. [PubMed] [Google Scholar]
- 84.Jeffery PK, Li D. Airway mucosa: secretory cells, mucus and mucin genes. Eur Respir J 10: 1655–1662, 1997. doi: 10.1183/09031936.97.10071655. [DOI] [PubMed] [Google Scholar]
- 85.Jia S, Wildner H, Birchmeier C. Insm1 controls the differentiation of pulmonary neuroendocrine cells by repressing Hes1. Dev Biol 408: 90–98, 2015. doi: 10.1016/j.ydbio.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 86.Jonsdottir HR, Arason AJ, Palsson R, Franzdottir SR, Gudbjartsson T, Isaksson HJ, Gudmundsson G, Gudjonsson T, Magnusson MK. Basal cells of the human airways acquire mesenchymal traits in idiopathic pulmonary fibrosis and in culture. Lab Invest 95: 1418–1428, 2015. doi: 10.1038/labinvest.2015.114. [DOI] [PubMed] [Google Scholar]
- 87.Kadzik RS, Morrisey EE. Directing lung endoderm differentiation in pluripotent stem cells. Cell Stem Cell 10: 355–361, 2012. doi: 10.1016/j.stem.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katsura H, Kobayashi Y, Tata PR, Hogan BLM. IL-1 and TNFα contribute to the inflammatory niche to enhance alveolar regeneration. Stem Cell Reports 12: 657–666, 2019. doi: 10.1016/j.stemcr.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kimura J, Deutsch GH. Key mechanisms of early lung development. Pediatr Dev Pathol 10: 335–347, 2007. doi: 10.2350/07-06-0290.1. [DOI] [PubMed] [Google Scholar]
- 90.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology 8: 432–446, 2003. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 91.Kobayashi Y, Tata A, Konkimalla A, Katsura H, Lee RF, Ou J, Banovich NE, Kropski JA, Tata PR. Persistence of a novel regeneration-associated transitional cell state in pulmonary fibrosis. Nat Cell Biol 22: 934–946, 2019. doi: 10.1038/s41556-020-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kobayashi Y, Tata PR. Pulmonary neuroendocrine cells: sensors and sentinels of the lung. Dev Cell 45: 425–426, 2018. doi: 10.1016/j.devcel.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 93.Kogut I, Roop DR, Bilousova G. Differentiation of human induced pluripotent stem cells into a keratinocyte lineage. Methods Mol Biol 1195: 1–12, 2014. doi: 10.1007/7651_2014_79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Konishi S, Gotoh S, Tateishi K, Yamamoto Y, Korogi Y, Nagasaki T, Matsumoto H, Muro S, Hirai T, Ito I, Tsukita S, Mishima M. Directed induction of functional multi-ciliated cells in proximal airway epithelial spheroids from human pluripotent stem cells. Stem Cell Reports 6: 18–25, 2016. doi: 10.1016/j.stemcr.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumar M, Seeger W, Voswinckel R. Senescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 51: 323–333, 2014. doi: 10.1165/rcmb.2013-0382PS. [DOI] [PubMed] [Google Scholar]
- 96.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang Y, Lim B, Chow VT, Crum CP, Xian W, McKeon F. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147: 525–538, 2011. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.LaCanna R, Liccardo D, Zhang P, Tragesser L, Wang Y, Cao T, Chapman HA, Morrisey EE, Shen H, Koch WJ, Kosmider B, Wolfson MR, Tian Y. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J Clin Invest 129: 2107–2122, 2019. doi: 10.1172/JCI125014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laucho-Contreras ME, Polverino F, Gupta K, Taylor KL, Kelly E, Pinto-Plata V, Divo M, Ashfaq N, Petersen H, Stripp B, Pilon AL, Tesfaigzi Y, Celli BR, Owen CA. Protective role for club cell secretory protein-16 (CC16) in the development of COPD. Eur Respir J 45: 1544–1556, 2015. doi: 10.1183/09031936.00134214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Laucho-Contreras ME, Polverino F, Rojas-Quintero J, Wang X, Owen CA. Club cell protein 16 (Cc16) deficiency increases inflamm-aging in the lungs of mice. Physiol Rep 6: e13797, 2018. doi: 10.14814/phy2.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lauweryns JM, Van Ranst L. Protein gene product 9.5 expression in the lungs of humans and other mammals. Immunocytochemical detection in neuroepithelial bodies, neuroendocrine cells and nerves. Neurosci Lett 85: 311–316, 1988. doi: 10.1016/0304-3940(88)90584-8. [DOI] [PubMed] [Google Scholar]
- 101.Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 113: 1093–1104, 1991. [DOI] [PubMed] [Google Scholar]
- 102.Lee BJ, Cho GJ, Norgren RB Jr, Junier MP, Hill DF, Tapia V, Costa ME, Ojeda SR. TTF-1, a homeodomain gene required for diencephalic morphogenesis, is postnatally expressed in the neuroendocrine brain in a developmentally regulated and cell-specific fashion. Mol Cell Neurosci 17: 107–126, 2001. doi: 10.1006/mcne.2000.0933. [DOI] [PubMed] [Google Scholar]
- 103.Lee JH, Kim J, Gludish D, Roach RR, Saunders AH, Barrios J, Woo AJ, Chen H, Conner DA, Fujiwara Y, Stripp BR, Kim CF. Surfactant protein-C chromatin-bound green fluorescence protein reporter mice reveal heterogeneity of surfactant protein C-expressing lung cells. Am J Respir Cell Mol Biol 48: 288–298, 2013. doi: 10.1165/rcmb.2011-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Linnoila RI. Functional facets of the pulmonary neuroendocrine system. Lab Invest 86: 425–444, 2006. doi: 10.1038/labinvest.3700412. [DOI] [PubMed] [Google Scholar]
- 105.Liu Z, Liao F, Scozzi D, Furuya Y, Pugh KN, Hachem R, Chen DL, Cano M, Green JM, Krupnick AS, Kreisel D, Perl AKT, Huang HJ, Brody SL, Gelman AE. An obligatory role for club cells in preventing obliterative bronchiolitis in lung transplants. JCI Insight 4: e124732, 2019. doi: 10.1172/jci.insight.124732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, Randell SH, O’Neal WK. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol 5: 397–408, 2012. doi: 10.1038/mi.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Logan CY, Desai TJ. Keeping it together: pulmonary alveoli are maintained by a hierarchy of cellular programs. BioEssays 37: 1028–1037, 2015. doi: 10.1002/bies.201500031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS, Dowton AA, Serra M, Weiss DJ, Green MD, Snoeck HW, Ramirez MI, Kotton DN. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10: 398–411, 2012. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Longmire TA, Ikonomou L, Kotton DN. Mouse ESC differentiation to Nkx2.1+ lung and thyroid progenitors. Bio Protoc 2: e295, 2012. doi: 10.21769/BioProtoc.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lv T, Jiang K, Wang J, Tang N, Dai H, Wang C. Single-cell RNA sequencing profiling of the effects of aging on alveolar stem cells. Sci China Life Sci 62: 1028–1037, 2019. doi: 10.1007/s11427-019-9583-9. [DOI] [PubMed] [Google Scholar]
- 111.Lynch TJ, Ahlers BA, Parekh KR. Lung transplantation: chronic rejection and stem cell depletion. J Thorac Dis 10: E666–E668, 2018. doi: 10.21037/jtd.2018.07.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lynch TJ, Anderson PJ, Rotti PG, Tyler SR, Crooke AK, Choi SH, Montoro DT, Silverman CL, Shahin W, Zhao R, Jensen-Cody CW, Adamcakova-Dodd A, Evans TIA, Xie W, Zhang Y, Mou H, Herring BP, Thorne PS, Rajagopal J, Yeaman C, Parekh KR, Engelhardt JF. Submucosal gland myoepithelial cells are reserve stem cells that can regenerate mouse tracheal epithelium. Cell Stem Cell 22: 779, 2018. doi: 10.1016/j.stem.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lynch TJ, Anderson PJ, Xie W, Crooke AK, Liu X, Tyler SR, Luo M, Kusner DM, Zhang Y, Neff T, Burnette DC, Walters KS, Goodheart MJ, Parekh KR, Engelhardt JF. wnt signaling regulates airway epithelial stem cells in adult murine submucosal glands. Stem Cells 34: 2758–2771, 2016. doi: 10.1002/stem.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lynch TJ, Engelhardt JF. Progenitor cells in proximal airway epithelial development and regeneration. J Cell Biochem 115: 1637–1645, 2014. doi: 10.1002/jcb.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lynch TJ, Ievlev V, Parekh KR. Heterogeneity of pulmonary stem cells. Adv Exp Med Biol 1169: 95–117, 2019. doi: 10.1007/978-3-030-24108-7_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lynch TJ, Liu XM, Wei J, Engelhardt JF. Stem cell niches in the lung. In: Stem Cell Biology and Regenerative Medicine. New York: Springer, 2015, p. 35–58. [Google Scholar]
- 118.Ma Q, Ma Y, Dai X, Ren T, Fu Y, Liu W, Han Y, Wu Y, Cheng Y, Zhang T, Zuo W. Regeneration of functional alveoli by adult human SOX9+ airway basal cell transplantation. Protein Cell 9: 267–282, 2018. doi: 10.1007/s13238-018-0506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marcet B, Chevalier B, Luxardi G, Coraux C, Zaragosi LE, Cibois M, Robbe-Sermesant K, Jolly T, Cardinaud B, Moreilhon C, Giovannini-Chami L, Nawrocki-Raby B, Birembaut P, Waldmann R, Kodjabachian L, Barbry P. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat Cell Biol 13: 693–699, 2011. doi: 10.1038/ncb2241. [DOI] [PubMed] [Google Scholar]
- 120.Marconett CN, Zhou B, Siegmund KD, Borok Z, Laird-Offringa IA. Transcriptomic profiling of primary alveolar epithelial cell differentiation in human and rat. Genom Data 2: 105–109, 2014. doi: 10.1016/j.gdata.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McCauley KB, Alysandratos KD, Jacob A, Hawkins F, Caballero IS, Vedaie M, Yang W, Slovik KJ, Morley M, Carraro G, Kook S, Guttentag SH, Stripp BR, Morrisey EE, Kotton DN. Single-cell transcriptomic profiling of pluripotent stem cell-derived SCGB3A2+ airway epithelium. Stem Cell Reports 10: 1579–1595, 2018. doi: 10.1016/j.stemcr.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCauley KB, Hawkins F, Kotton DN. Derivation of epithelial-only airway organoids from human pluripotent stem cells. Curr Protoc Stem Cell Biol 45: e51, 2018. doi: 10.1002/cpsc.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN. efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell 20: 844–857.e6, 2017. doi: 10.1016/j.stem.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, Paré PD, Sin DD, Pierce RA, Woods JC, McWilliams AM, Mayo JR, Lam SC, Cooper JD, Hogg JC. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 365: 1567–1575, 2011. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meijer M, Rehbach K, Brunner JW, Classen JA, Lammertse HCA, van Linge LA, Schut D, Krutenko T, Hebisch M, Cornelisse LN, Sullivan PF, Peitz M, Toonen RF, Brustle O, Verhage M. A single-cell model for synaptic transmission and plasticity in human iPSC-derived neurons. Cell Rep 27: 2199–2211.e6, 2019. doi: 10.1016/j.celrep.2019.04.058. [DOI] [PubMed] [Google Scholar]
- 126.Menon T, Firth AL, Scripture-Adams DD, Galic Z, Qualls SJ, Gilmore WB, Ke E, Singer O, Anderson LS, Bornzin AR, Alexander IE, Zack JA, Verma IM. Lymphoid regeneration from gene-corrected SCID-X1 subject-derived iPSCs. Cell Stem Cell 16: 367–372, 2015. doi: 10.1016/j.stem.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mercer RR, Russell ML, Roggli VL, Crapo JD. Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol 10: 613–624, 1994. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- 128.Mets OM, Roothaan SM, Bronsveld I, Luijk B, van de Graaf EA, Vink A, de Jong PA. Emphysema is common in lungs of cystic fibrosis lung transplantation patients: a histopathological and computed tomography study. PLoS One 10: e0128062, 2015. doi: 10.1371/journal.pone.0128062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miller AJ, Dye BR, Ferrer-Torres D, Hill DR, Overeem AW, Shea LD, Spence JR. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc 14: 518–540, 2019. doi: 10.1038/s41596-018-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miller AJ, Hill DR, Nagy MS, Aoki Y, Dye BR, Chin AM, Huang S, Zhu F, White ES, Lama V, Spence JR. In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Reports 10: 101–119, 2018. doi: 10.1016/j.stemcr.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miller AJ, Yu Q, Czerwinski M, Tsai YH, Conway RF, Wu A, Holloway EM, Walker T, Glass IA, Treutlein B, Camp JG, Spence JR. In vitro and in vivo development of the human airway at single-cell resolution. Dev Cell 53: 117–128.e6, 2020. doi: 10.1016/j.devcel.2020.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(-/-) mouse embryos. Dev Biol 209: 60–71, 1999. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- 133.Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, Yuan F, Chen S, Leung HM, Villoria J, Rogel N, Burgin G, Tsankov AM, Waghray A, Slyper M, Waldman J, Nguyen L, Dionne D, Rozenblatt-Rosen O, Tata PR, Mou H, Shivaraju M, Bihler H, Mense M, Tearney GJ, Rowe SM, Engelhardt JF, Regev A, Rajagopal J. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560: 319–324, 2018. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mortality GBD. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385: 117–171, 2015. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mucci A, Lopez-Rodriguez E, Hetzel M, Liu S, Suzuki T, Happle C, Ackermann M, Kempf H, Hillje R, Kunkiel J, Janosz E, Brennig S, Glage S, Bankstahl JP, Dettmer S, Rodt T, Gohring G, Trapnell B, Hansen G, Trapnell C, Knudsen L, Lachmann N, Moritz T. iPSC-derived macrophages effectively treat pulmonary alveolar proteinosis in Csf2rb-deficient mice. Stem Cell Reports 11: 696–710, 2018. doi: 10.1016/j.stemcr.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Munder A, Tümmler B. Origins of cystic fibrosis lung disease. N Engl J Med 372: 1574–1575, 2015. doi: 10.1056/NEJMc1502191. [DOI] [PubMed] [Google Scholar]
- 137.Musah S, Chen J, Hoyle GW. Repair of tracheal epithelium by basal cells after chlorine-induced injury. Respir Res 13: 107, 2012. doi: 10.1186/1465-9921-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nakajima M, Kawanami O, Jin E, Ghazizadeh M, Honda M, Asano G, Horiba K, Ferrans VJ. Immunohistochemical and ultrastructural studies of basal cells, Clara cells and bronchiolar cuboidal cells in normal human airways. Pathol Int 48: 944–953, 1998. doi: 10.1111/j.1440-1827.1998.tb03865.x. [DOI] [PubMed] [Google Scholar]
- 139.Nikolić MZ, Caritg O, Jeng Q, Johnson JA, Sun D, Howell KJ, Brady JL, Laresgoiti U, Allen G, Butler R, Zilbauer M, Giangreco A, Rawlins EL. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. eLife 6: e26575, 2017. doi: 10.7554/eLife.26575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Noguchi M, Sumiyama K, Morimoto M. Directed migration of pulmonary neuroendocrine cells toward airway branches organizes the stereotypic location of neuroepithelial bodies. Cell Rep 13: 2679–2686, 2015. doi: 10.1016/j.celrep.2015.11.058. [DOI] [PubMed] [Google Scholar]
- 141.Nyunoya T, Mebratu Y, Contreras A, Delgado M, Chand HS, Tesfaigzi Y. Molecular processes that drive cigarette smoke-induced epithelial cell fate of the lung. Am J Respir Cell Mol Biol 50: 471–482, 2014. doi: 10.1165/rcmb.2013-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Okawa S, Angarica VE, Lemischka I, Moore K, Del Sol A. A differential network analysis approach for lineage specifier prediction in stem cell subpopulations. NPJ Syst Biol Appl 1: 15012, 2015. doi: 10.1038/npjsba.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.O’Koren EG, Hogan BL, Gunn MD. Loss of basal cells precedes bronchiolitis obliterans-like pathological changes in a murine model of chlorine gas inhalation. Am J Respir Cell Mol Biol 49: 788–797, 2013. doi: 10.1165/rcmb.2012-0369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Olmer R, Engels L, Usman A, Menke S, Malik MNH, Pessler F, Göhring G, Bornhorst D, Bolten S, Abdelilah-Seyfried S, Scheper T, Kempf H, Zweigerdt R, Martin U. Differentiation of human pluripotent stem cells into functional endothelial cells in scalable suspension culture. Stem Cell Reports 10: 1657–1672, 2018. doi: 10.1016/j.stemcr.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oztay F, Brouns I, Pintelon I, Raab M, Neuhuber W, Timmermans JP, Adriaensen D. Neurotrophin-4 dependency of intraepithelial vagal sensory nerve terminals that selectively contact pulmonary NEBs in mice. Histol Histopathol 25: 975–984, 2010. doi: 10.14670/HH-25.975. [DOI] [PubMed] [Google Scholar]
- 146.Pardo-Saganta A, Law BM, Tata PR, Villoria J, Saez B, Mou H, Zhao R, Rajagopal J. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 16: 184–197, 2015. doi: 10.1016/j.stem.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peters-Hall JR, Coquelin ML, Torres MJ, LaRanger R, Alabi BR, Sho S, Calva-Moreno JF, Thomas PJ, Shay JW. Long-term culture and cloning of primary human bronchial basal cells that maintain multipotent differentiation capacity and CFTR channel function. Am J Physiol Lung Cell Mol Physiol 315: L313–L327, 2018. doi: 10.1152/ajplung.00355.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Piorunek T, Marszalek A, Biczysko W, Gozdzik J, Cofta S, Seget M. Correlation between the stage of cystic fibrosis and the level of morphological changes in adult patients. J Physiol Pharmacol 59, Suppl 6: 565–572, 2008. [PubMed] [Google Scholar]
- 149.Plasschaert LW, Žilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, Klein AM, Jaffe AB. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560: 377–381, 2018. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qiao R, Yan W, Clavijo C, Mehrian-Shai R, Zhong Q, Kim KJ, Ann D, Crandall ED, Borok Z. Effects of KGF on alveolar epithelial cell transdifferentiation are mediated by JNK signaling. Am J Respir Cell Mol Biol 38: 239–246, 2008. doi: 10.1165/rcmb.2007-0172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Quiroz EJ, Ryan Firth AL. CRISPR/Cas9 editing in induced pluripotent stem cells: a way forward for treating cystic fibrosis? In: Stem Cell-Based Therapy for Lung Disease, edited by Burgess J. New York: Springer, 2019, p. 153–178. [Google Scholar]
- 152.Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet 389: 1931–1940, 2017. doi: 10.1016/S0140-6736(17)31222-9. [DOI] [PubMed] [Google Scholar]
- 153.Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest 125: 2021–2031, 2015. doi: 10.1172/JCI79422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rankin SA, McCracken KW, Luedeke DM, Han L, Wells JM, Shannon JM, Zorn AM. Timing is everything: Reiterative Wnt, BMP and RA signaling regulate developmental competence during endoderm organogenesis. Dev Biol 434: 121–132, 2018. doi: 10.1016/j.ydbio.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primers 1: 15010, 2015. doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 136: 3741–3745, 2009. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol 295: L231–L234, 2008. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4: 525–534, 2009. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Regamey N, Jeffery PK, Alton EW, Bush A, Davies JC. Airway remodelling and its relationship to inflammation in cystic fibrosis. Thorax 66: 624–629, 2011. doi: 10.1136/thx.2009.134106. [DOI] [PubMed] [Google Scholar]
- 160.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 106: 12771–12775, 2009. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech 3: 545–556, 2010. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rogers AV, Dewar A, Corrin B, Jeffery PK. Identification of serous-like cells in the surface epithelium of human bronchioles. Eur Respir J 6: 498–504, 1993. [PubMed] [Google Scholar]
- 163.Romano RA, Ortt K, Birkaya B, Smalley K, Sinha S. An active role of the DeltaN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate. PLoS One 4: e5623, 2009. doi: 10.1371/journal.pone.0005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rosen C, Shezen E, Aronovich A, Klionsky YZ, Yaakov Y, Assayag M, Biton IE, Tal O, Shakhar G, Ben-Hur H, Shneider D, Vaknin Z, Sadan O, Evron S, Freud E, Shoseyov D, Wilschanski M, Berkman N, Fibbe WE, Hagin D, Hillel-Karniel C, Krentsis IM, Bachar-Lustig E, Reisner Y. Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat Med 21: 869–879, 2015. doi: 10.1038/nm.3889. [DOI] [PubMed] [Google Scholar]
- 165.Rossi DL, Acebrón A, Santisteban P. Function of the homeo and paired domain proteins TTF-1 and Pax-8 in thyroid cell proliferation. J Biol Chem 270: 23139–23142, 1995. doi: 10.1074/jbc.270.39.23139. [DOI] [PubMed] [Google Scholar]
- 166.Ruiz García S, Deprez M, Lebrigand K, Cavard A, Paquet A, Arguel MJ, Magnone V, Truchi M, Caballero I, Leroy S, Marquette CH, Marcet B, Barbry P, Zaragosi LE. Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development 146: dev.177428, 2019. doi: 10.1242/dev.177428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ryan BM, Wang Y, Jen J, Yi ES, Olivo-Marston S, Yang P, Harris CC. Evidence that the lung Adenocarcinoma EML4-ALK fusion gene is not caused by exposure to secondhand tobacco smoke during childhood. Cancer Epidemiol Biomarkers Prev 23: 1432–1434, 2014. doi: 10.1158/1055-9965.EPI-14-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ryan DM, Vincent TL, Salit J, Walters MS, Agosto-Perez F, Shaykhiev R, Strulovici-Barel Y, Downey RJ, Buro-Auriemma LJ, Staudt MR, Hackett NR, Mezey JG, Crystal RG. Smoking dysregulates the human airway basal cell transcriptome at COPD risk locus 19q13.2. PLoS One 9: e88051, 2014. doi: 10.1371/journal.pone.0088051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Salwig I, Spitznagel B, Vazquez-Armendariz AI, Khalooghi K, Guenther S, Herold S, Szibor M, Braun T. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J 38: 38, 2019. doi: 10.15252/embj.2019102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sanders DB, Fink AK. Background and epidemiology. Pediatr Clin North Am 63: 567–584, 2016. doi: 10.1016/j.pcl.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schamberger AC, Staab-Weijnitz CA, Mise-Racek N, Eickelberg O. Cigarette smoke alters primary human bronchial epithelial cell differentiation at the air-liquid interface. Sci Rep 5: 8163, 2015. doi: 10.1038/srep08163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Serrano-Mollar A, Gay-Jordi G, Guillamat-Prats R, Closa D, Hernandez-Gonzalez F, Marin P, Burgos F, Martorell J, Sánchez M, Arguis P, Soy D, Bayas JM, Ramirez J, Tetley TD, Molins L, de la Bellacasa JP, Rodríguez-Villar C, Rovira I, Fiblà JJ, Xaubet A; Pneumocyte Study Group . Safety and tolerability of alveolar type II cell transplantation in idiopathic pulmonary fibrosis. Chest 150: 533–543, 2016. doi: 10.1016/j.chest.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 173.Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A, Bulbena O. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 176: 1261–1268, 2007. doi: 10.1164/rccm.200610-1491OC. [DOI] [PubMed] [Google Scholar]
- 174.Shaykhiev R, Crystal RG. Early events in the pathogenesis of chronic obstructive pulmonary disease. Smoking-induced reprogramming of airway epithelial basal progenitor cells. Ann Am Thorac Soc 11, Suppl 5: S252–S258, 2014. doi: 10.1513/AnnalsATS.201402-049AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shaykhiev R, Zuo WL, Chao I, Fukui T, Witover B, Brekman A, Crystal RG. EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype. Proc Natl Acad Sci USA 110: 12102–12107, 2013. doi: 10.1073/pnas.1303058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Smirnova NF, Schamberger AC, Nayakanti S, Hatz R, Behr J, Eickelberg O. Detection and quantification of epithelial progenitor cell populations in human healthy and IPF lungs. Respir Res 17: 83, 2016. doi: 10.1186/s12931-016-0404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Smith LJ, Kaplan NB, Brody J. Response of normal and beige mouse alveolar type 2 cells to lung injury. Am Rev Respir Dis 122: 947–957, 1980. [DOI] [PubMed] [Google Scholar]
- 178.Snitow ME, Li S, Morley MP, Rathi K, Lu MM, Kadzik RS, Stewart KM, Morrisey EE. Ezh2 represses the basal cell lineage during lung endoderm development. Development 142: 108–117, 2015. doi: 10.1242/dev.116947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Song H, Yao E, Lin C, Gacayan R, Chen MH, Chuang PT. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci USA 109: 17531–17536, 2012. doi: 10.1073/pnas.1207238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Spella M, Lilis I, Pepe MA, Chen Y, Armaka M, Lamort AS, Zazara DE, Roumelioti F, Vreka M, Kanellakis NI, Wagner DE, Giannou AD, Armenis V, Arendt KA, Klotz LV, Toumpanakis D, Karavana V, Zakynthinos SG, Giopanou I, Marazioti A, Aidinis V, Sotillo R, Stathopoulos GT. Club cells form lung adenocarcinomas and maintain the alveoli of adult mice. eLife 8: e45571, 2019. doi: 10.7554/eLife.45571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Staudt MR, Buro-Auriemma LJ, Walters MS, Salit J, Vincent T, Shaykhiev R, Mezey JG, Tilley AE, Kaner RJ, Ho MW, Crystal RG. Airway basal stem/progenitor cells have diminished capacity to regenerate airway epithelium in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 190: 955–958, 2014. doi: 10.1164/rccm.201406-1167LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Strunz M, Simon LM, Ansari M, Mattner LF, Angelidis I, Mayr CH, Kathiriya J, Yee M, Ogar P, Sengupta A, Kukhtevich I, Schneider R, Zhao Z, Neumann JHL, Behr J, Voss C, Stöger T, Lehmann M, Königshoff M, Burgstaller G, O’Reilly M, Chapman HA, Theis FJ, Schiller HB. Longitudinal single cell transcriptomics reveals Krt8+ alveolar epithelial progenitors in lung regeneration (Preprint). bioRxiv 2019. doi: 10.1101/705244. [DOI]
- 183.Stubbs JL, Vladar EK, Axelrod JD, Kintner C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat Cell Biol 14: 140–147, 2012. doi: 10.1038/ncb2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sui H, Olivier AK, Klesney-Tait JA, Brooks L, Tyler SR, Sun X, Skopec A, Kline J, Sanchez PG, Meyerholz DK, Zavazava N, Iannettoni M, Engelhardt JF, Parekh KR. Ferret lung transplant: an orthotopic model of obliterative bronchiolitis. Am J Transplant 13: 467–473, 2013. doi: 10.1111/j.1600-6143.2012.04337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, Kinyon JM, Lei-Butters DC, Griffin MA, Naumann P, Luo M, Ascher J, Wang K, Frana T, Wine JJ, Meyerholz DK, Engelhardt JF. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest 120: 3149–3160, 2010. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sun Z, Li F, Zhou X, Chung KF, Wang W, Wang J. Stem cell therapies for chronic obstructive pulmonary disease: current status of pre-clinical studies and clinical trials. J Thorac Dis 10: 1084–1098, 2018. doi: 10.21037/jtd.2018.01.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sunday ME. Pulmonary neuroendocrine cells and lung development. Endocr Pathol 7: 173–201, 1996. doi: 10.1007/BF02739921. [DOI] [PubMed] [Google Scholar]
- 188.Sunday ME, Hua J, Torday J. Bombesin may play a role in fetal lung growth and maturation in utero and in lung organ culture. Chest Suppl 99: 21S, 1991. doi: 10.1378/chest.99.3_Supplement.21S. [DOI] [PubMed] [Google Scholar]
- 189.Swatek AM, Lynch TJ, Crooke AK, Anderson PJ, Tyler SR, Brooks L, Ivanovic M, Klesney-Tait JA, Eberlein M, Pena T, Meyerholz DK, Engelhardt JF, Parekh KR. Depletion of airway submucosal glands and TP63+KRT5+ basal cells in obliterative bronchiolitis. Am J Respir Crit Care Med 197: 1045–1057, 2018. doi: 10.1164/rccm.201707-1368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tadokoro T, Wang Y, Barak LS, Bai Y, Randell SH, Hogan BL. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc Natl Acad Sci USA 111: E3641–E3649, 2014. doi: 10.1073/pnas.1409781111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 193.Takahashi M, Osumi N. Pax6 regulates specification of ventral neurone subtypes in the hindbrain by establishing progenitor domains. Development 129: 1327–1338, 2002. [DOI] [PubMed] [Google Scholar]
- 194.Tan YT, Ye L, Xie F, Beyer AI, Muench MO, Wang J, Chen Z, Liu H, Chen SJ, Kan YW. Respecifying human iPSC-derived blood cells into highly engraftable hematopoietic stem and progenitor cells with a single factor. Proc Natl Acad Sci USA 115: 2180–2185, 2018. doi: 10.1073/pnas.1718446115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tang J, Li Y, Huang X, He L, Zhang L, Wang H, Yu W, Pu W, Tian X, Nie Y, Hu S, Wang QD, Lui KO, Zhou B. Fate mapping of Sca1+ cardiac progenitor cells in the adult mouse heart. Circulation 138: 2967–2969, 2018. doi: 10.1161/CIRCULATIONAHA.118.036210. [DOI] [PubMed] [Google Scholar]
- 196.Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, Choudhury B, Varki A, Stoltz DA, Welsh MJ. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest 126: 879–891, 2016. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tata A, Kobayashi Y, Chow RD, Tran J, Desai A, Massri AJ, McCord TJ, Gunn MD, Tata PR. Myoepithelial cells of submucosal glands can function as reserve stem cells to regenerate airways after injury. Cell Stem Cell 22: 668–683.e6, 2018. doi: 10.1016/j.stem.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, Medoff BD, Rajagopal J. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503: 218–223, 2013. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tiddens H, Silverman M, Bush A. The role of inflammation in airway disease: remodeling. Am J Respir Crit Care Med 162, Suppl 1: S7–S10, 2000. doi: 10.1164/ajrccm.162.supplement_1.maic-2. [DOI] [PubMed] [Google Scholar]
- 200.Tiddens HA, Donaldson SH, Rosenfeld M, Paré PD. Cystic fibrosis lung disease starts in the small airways: can we treat it more effectively? Pediatr Pulmonol 45: 107–117, 2010. doi: 10.1002/ppul.21154. [DOI] [PubMed] [Google Scholar]
- 201.Travaglini KJ, Nabhan AN, Penland L, Sinha R, Gillich A, Sit RV, Chang S, Conley SD, Mori Y, Seita J, Berry GJ, Shrager JB, Metzger RJ, Kuo CS, Neff N, Weissman IL, Quake SR, Krasnow MA. A molecular cell atlas of the human lung from single cell RNA sequencing (Preprint). bioRxiv 2020. doi: 10.1101/742320. [DOI] [PMC free article] [PubMed]
- 202.Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, Desai TJ, Krasnow MA, Quake SR. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 509: 371–375, 2014. doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ulrich M, Worlitzsch D, Viglio S, Siegmann N, Iadarola P, Shute JK, Geiser M, Pier GB, Friedel G, Barr ML, Schuster A, Meyer KC, Ratjen F, Bjarnsholt T, Gulbins E, Döring G. Alveolar inflammation in cystic fibrosis. J Cyst Fibros 9: 217–227, 2010. doi: 10.1016/j.jcf.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vagnozzi RJ, Sargent MA, Lin SJ, Palpant NJ, Murry CE, Molkentin JD. Genetic lineage tracing of Sca-1+ cells reveals endothelial but not myogenic contribution to the murine heart. Circulation 138: 2931–2939, 2018. doi: 10.1161/CIRCULATIONAHA.118.035210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.van Lommel AT, Lauweryns JM. Ultrastructure and innervation of neuroepithelial bodies in the lungs of newborn cats. Anat Rec 236: 181–190, 1993. doi: 10.1002/ar.1092360122. [DOI] [PubMed] [Google Scholar]
- 206.Voynow JA, Fischer BM, Roberts BC, Proia AD. Basal-like cells constitute the proliferating cell population in cystic fibrosis airways. Am J Respir Crit Care Med 172: 1013–1018, 2005. doi: 10.1164/rccm.200410-1398OC. [DOI] [PubMed] [Google Scholar]
- 207.Wang G, Lou HH, Salit J, Leopold PL, Driscoll S, Schymeinsky J, Quast K, Visvanathan S, Fine JS, Thomas MJ, Crystal RG. Characterization of an immortalized human small airway basal stem/progenitor cell line with airway region-specific differentiation capacity. Respir Res 20: 196, 2019. doi: 10.1186/s12931-019-1140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang Y, Lu Y, Wu Y, Sun Y, Zhou Y, Ma Q, Zheng Y, Yu Q, Cao Y, Chen G, Zhang T, Dai X, Ren T, Ma Y, Zuo W. Alveolar differentiation potency of human distal airway stem cells is associated with pulmonary pathological conditions. Stem Cells Int 2019: 7123078, 2019. doi: 10.1155/2019/7123078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ward MC, Gilad Y. A generally conserved response to hypoxia in iPSC-derived cardiomyocytes from humans and chimpanzees. eLife 8: e42374, 2019. doi: 10.7554/eLife.42374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Weichselbaum M, Sparrow MP, Hamilton EJ, Thompson PJ, Knight DA. A confocal microscopic study of solitary pulmonary neuroendocrine cells in human airway epithelium. Respir Res 6: 115, 2005. doi: 10.1186/1465-9921-6-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wong AP, Rossant J. Generation of lung epithelium from pluripotent stem cells. Curr Pathobiol Rep 1: 137–145, 2013. doi: 10.1007/s40139-013-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xie W, Lynch TJ, Liu X, Tyler SR, Yu S, Zhou X, Luo M, Kusner DM, Sun X, Yi Y, Zhang Y, Goodheart MJ, Parekh KR, Wells JM, Xue HH, Pevny LH, Engelhardt JF. Sox2 modulates Lef-1 expression during airway submucosal gland development. Am J Physiol Lung Cell Mol Physiol 306: L645–L660, 2014. doi: 10.1152/ajplung.00157.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, Wikenheiser-Brokamp KA, Perl AT, Funari VA, Gokey JJ, Stripp BR, Whitsett JA. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1: e90558, 2016. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yang Y, Riccio P, Schotsaert M, Mori M, Lu J, Lee DK, Garcia-Sastre A, Xu J, Cardoso WV. Spatial-temporal lineage restrictions of embryonic p63(+) progenitors establish distinct stem cell pools in adult airways. Dev Cell 44: 752–761.e4, 2018. doi: 10.1016/j.devcel.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yao E, Lin C, Wu Q, Zhang K, Song H, Chuang PT. Notch signaling controls transdifferentiation of pulmonary neuroendocrine cells in response to lung injury. Stem Cells 36: 377–391, 2018. doi: 10.1002/stem.2744. [DOI] [PubMed] [Google Scholar]
- 216.Yu H, Li Q, Kolosov VP, Perelman JM, Zhou X. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun Adhes 17: 83–92, 2010. doi: 10.3109/15419061.2010.551682. [DOI] [PubMed] [Google Scholar]
- 217.Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, Zhou S, Cantu E, Morrisey EE. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555: 251–255, 2018. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang L, Sultana N, Yan J, Yang F, Chen F, Chepurko E, Yang FC, Du Q, Zangi L, Xu M, Bu L, Cai CL. Cardiac Sca-1+ cells are not intrinsic stem cells for myocardial development, renewal, and repair. Circulation 138: 2919–2930, 2018. doi: 10.1161/CIRCULATIONAHA.118.035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zheng BN, Liu HT, Lin QH, Guo JY, Zheng CL. [To discuss the problems existing in the labor ability appraisal of an occupational chronic benzene poisoning incidence]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 37: 379–381, 2019. doi: 10.3760/cma.j.issn.1001-9391.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 220.Zheng D, Limmon GV, Yin L, Leung NH, Yu H, Chow VT, Chen J. Regeneration of alveolar type I and II cells from Scgb1a1-expressing cells following severe pulmonary damage induced by bleomycin and influenza. PLoS One 7: e48451, 2012. doi: 10.1371/journal.pone.0048451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhou B, Flodby P, Luo J, Castillo DR, Liu Y, Yu FX, McConnell A, Varghese B, Li G, Chimge NO, Sunohara M, Koss MN, Elatre W, Conti P, Liebler JM, Yang C, Marconett CN, Laird-Offringa IA, Minoo P, Guan K, Stripp BR, Crandall ED, Borok Z. Claudin-18-mediated YAP activity regulates lung stem and progenitor cell homeostasis and tumorigenesis. J Clin Invest 128: 970–984, 2018. doi: 10.1172/JCI90429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhou F, Narasimhan V, Shboul M, Chong YL, Reversade B, Roy S. Gmnc is a master regulator of the multiciliated cell differentiation program. Curr Biol 25: 3267–3273, 2015. doi: 10.1016/j.cub.2015.10.062. [DOI] [PubMed] [Google Scholar]
- 223.Zhu L, Di PY, Wu R, Pinkerton KE, Chen Y. Repression of CC16 by cigarette smoke (CS) exposure. PLoS One 10: e0116159, 2015. doi: 10.1371/journal.pone.0116159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, Crum CP, Xian W, McKeon F. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature 517: 616–620, 2015. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zuo WL, Shenoy SA, Li S, O’Beirne SL, Strulovici-Barel Y, Leopold PL, Wang G, Staudt MR, Walters MS, Mason C, Kaner RJ, Mezey JG, Crystal RG. Ontogeny and biology of human small airway epithelial club cells. Am J Respir Crit Care Med 198: 1375–1388, 2018. doi: 10.1164/rccm.201710-2107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]