Abstract
Anion channels in the retinal pigment epithelium (RPE) play an essential role in the transport of Cl− between the outer retina and the choroidal blood to regulate the ionic composition and volume of the subretinal fluid that surrounds the photoreceptor outer segments. Recently, we reported that the anion conductance of the mouse RPE basolateral membrane is highly selective for the biologically active anion thiocyanate (SCN−), a property that does not correspond with any of the Cl− channels that have been found to be expressed in the RPE to date. The purpose of this study was to determine the extent to which SLC26A7, a SCN− permeable-anion exchanger/channel that was reported to be expressed in human RPE, contributes to the RPE basolateral anion conductance. We show by quantitative RT-PCR that Slc26a7 is highly expressed in mouse RPE compared with other members of the Slc26 gene family and Cl− channel genes known to be expressed in the RPE. By applying immunofluorescence microscopy to mouse retinal sections and isolated cells, we localized SLC26A7 to the RPE basolateral membrane. Finally, we performed whole cell and excised patch recordings from RPE cells acutely isolated from Slc26a7 knockout mice to show that the SCN− conductance and permeability of its basolateral membrane are dramatically smaller relative to wild-type mouse RPE cells. These findings establish SLC26A7 as the SCN−-selective conductance of the RPE basolateral membrane and provide new insight into the physiology of an anion channel that may participate in anion transport and pH regulation by the RPE.
Keywords: anion permeability, RPE, SLC26A7
INTRODUCTION
Along with a broad spectrum of other functions of the retinal pigment epithelium (RPE), anion transport has long been recognized as being essential to the health and physiology of the adjacent rod and cone photoreceptors. Cl− transport across the RPE provides a major driving force for fluid reabsorption, and its disruption can lead to the accumulation of fluid in the subretinal space and, secondarily, to retinal degeneration, as occurs in patients with inherited mutations in the Cl− channel gene BEST1 (25). Transepithelial absorption of Cl− by the RPE is achieved by its uptake at the apical membrane via a Na-K-2Cl cotransporter and its exit from the cell via the relatively large Cl− conductance of the basolateral membrane. Although studies on native and cultured mammalian RPE have provided evidence for the expression of a number of Cl− channels including ANO1 (11), ANO2 (18), BEST1 (25), CFTR (5), and ClC-2 (7), our understanding of the relative contribution of these Cl− channels to the Cl− conductance of the RPE basolateral membrane is incomplete.
In our previous whole cell patch-clamp studies on acutely dissociated mouse RPE cells, we demonstrated that the RPE plasma membrane contains a conductance that is highly selective for the pseudohalide thiocyanate (SCN−), with a relative conductance for SCN− [thiocyanate-to-chloride conductance ratio (GSCN/GCl)] of ~40 (10) and relative permeability for SCN− [thiocyanate-to-chloride permeability ratio (PSCN/PCl)] of ~2,000 (9). We further showed by recording macroscopic currents from basolateral and apical membrane patches excised from mouse RPE cells that the SCN−-selective conductance resides primarily in the basolateral membrane (10). As the anion selectivity and other properties of this conductance do not match the biophysical profile of any of the aforementioned Cl− channels, our findings suggested that the RPE contains an anion channel and/or electrogenic transporter that has not been identified to date.
Transcriptome analysis identified SLC26A7, a gene with a restricted tissue expression pattern (24, 37), as being highly expressed in human RPE relative to photoreceptors (6). Originally reported to function as a Cl−/ exchanger (24), SLC26A7 was later shown to operate as a bona fide Cl− channel (20). Because SLC26A7 heterologously expressed in mammalian cells displays a very high conductance for SCN− (33), we considered the possibility that this anion transport protein underlies the SCN−-selective anion conductance of the RPE basolateral membrane.
In this work, we applied an array of techniques including quantitative RT-PCR (qPCR), indirect immunofluorescence microscopy, and patch-clamp electrophysiology on the RPE from wild-type (WT) and Slc26a7 knockout (KO) mice to demonstrate that SLC26A7 forms the SCN−-selective conductance of the RPE basolateral membrane.
MATERIALS AND METHODS
Mice
All procedures involving mice received Institutional Animal Care and Use Committee (IACUC) approval and were conducted in compliance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Slc26a7+/− mice on a C57BL/6J background, which have been described previously (43), were obtained from M. Soleimani. Slc26a7−/− (KO) or Slc26a7+/+ (WT) mice were generated by breeding male and female Slc26a7+/− mice or by breeding pairs of Slc26a7−/− or Slc26a7+/+ mice. Tail genotyping was performed using Mouse Direct PCR Kit (Bimake) and the following primer sets: KO allele, 5′-actctgattagtgcattcctc-3′ (forward) and 5′-tgcgaggccagaggccacttgtgtagc-3′ (reverse) or 5′-cacccttagcttgtgcactc-3′ (forward) and 5′-ctccagactgccttgggaaa-3′ (reverse); WT allele, 5′-cagcatagaaggagccgtctg-3′ (forward) and 5′-gtccttcgctatgctttcatc-3′ or 5′-ccaccggaaaccagctaatcc-3′ (forward) and 5′-ttctcctcgttgtacacgctc-3′ (reverse).
Tissue Isolation and Total RNA Isolation
WT mice of both sexes and ages 4–16 wk were euthanized by CO2 inhalation before enucleation or dissection of tissues. Enucleated eyes were hemisected posteriorly to the ora serrata, and the anterior segment and lens were removed. After incubating the eyecup in Hank’s balanced salt solution (HBSS) without Ca2+ or Mg2+ for 10 min, the neural retina was gently peeled away and cut at the optic nerve, and then both the eyecup and retina were rinsed three times in HBSS. Total RNA from retina was extracted using TRIzol followed by Direct-zol RNA MicroPrep (Zymo Research). Following removal of the neural retina, total RNA from the RPE was isolated using the simultaneous RPE isolation and RNA stabilization method essentially as described (42). Briefly, eyecups were rinsed in HBSS without Ca2+ or Mg2+ and then incubated for 20 min in RNAprotect (Qiagen) with intermittent agitation to dislodge RPE cells, followed by DNA digestion with DNase I (DNA-free Kit; Ambion) and total RNA extraction using Direct-zol RNA MicroPrep. RNA from the eyes of multiple mice of the same sex and similar ages was pooled and stored at −20°C until further analysis.
Heart, lung, and kidney samples (<30 mg) were dissected from freshly euthanized adult WT mice, weighed, gently ground by mortar and pestle under liquid nitrogen, dried on dry ice, and then processed sequentially with QIAshredder and RNeasy kits (Qiagen) to extract total RNA.
The quality and concentration of RNA were determined by NanoDrop One (Thermo Scientific) before being reverse transcribed into cDNA using a RETROscript kit (Ambion) according to the manufacturer’s instructions.
PCR Primers
PCR primer pairs (Table 1) were designed using Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) and synthesized by Integrated DNA Technologies. The specificity of primer pairs was tested using pooled kidney, retina, lung, and heart cDNA as template and conventional PCR [settings: cycle 1, 1 × 94°C for 3 min; cycle 2, 35 × (94°C for 30 s, 57.5°C for 30 s, and 72°C for 1 min); and cycle 3, 1 × 72°C for 10 min] followed by separation of products by 1.5% agarose gel electrophoresis to confirm the presence of a single, appropriate-sized band.
Table 1.
Target gene primer sequences and expected sizes of RT-PCR products
| Target Gene | Primer Sequence | Expected Size, bp | GenBank Accession No. |
|---|---|---|---|
| Ano1 | Forward 5′-tcgatcgtgggtgtcattgt-3′ Reverse 5′-acggtggcagggttatcaaa-3′ |
197 | NM_178642 |
| Ano2 | Forward 5′-gtcccctgtgtgacaagtcc-3′ Reverse 5′-ttcgatcccagtcaggtccc-3′ |
183 | NM_153589.2 |
| Best1 | Forward 5′-ggtgtggtttgccaacttgt-3′ Reverse 5′-cagaaactgcctcccgatca-3′ |
220 | NM_011913 |
| Best2 | Forward 5′- gcacagggctacaaagacca-3′ Reverse 5′- gctagcatggacacctgg aa-3′ |
182 | NM_001130194 |
| Cftr | Forward 5′-gcatattgttgggaatcagc-3′ Reverse 5′-acgattccgttgatgactgt-3′ |
176 | NM_021050 |
| Clcn2 | Forward 5′-taccacaggcaacatggagtc-3′ Reverse 5′-tggctcatctagctgctgttc-3′ |
217 | NM_009900 |
| Slc26a1 | Forward 5′-ccaaccagctctctgtctgt-3′ Reverse 5′-gtcggaccagtaccagtgtc-3′ |
189 | NM_174870 |
| Slc26a2 | Forward 5′-ctacataaagcctgccctga-3′ Reverse 5′-tgaaagaagcccattgctac-3′ |
182 | NM_007885 |
| Slc26a3 | Forward 5′-gtcctcaagctccacagaaa-3′ Reverse 5′-ggcagctgtagcatgaattt-3′ |
216 | NM_021353 |
| Slc26a4 | Forward 5′-aggagtgaggtcattgcgaa-3′ Reverse 5′-ctttacagtcccgaatgcga-3′ |
248 | NM_011867 |
| Slc26a5 | Forward 5′-gaaaggcccatcttcagtcatc-3′ Reverse 5′-gccacttagtgataggcaggaac-3′ |
157 | NM_030727 |
| Slc26a6 | Forward 5′-ttgctggagctgtatcttcc-3′ Reverse 5′-tgtttgccttccaaagagag-3′ |
162 | NM_134420 |
| Slc26a7 | Forward 5′-gcatgatgaaacctcgcaaca-3′ Reverse 5′-ttcattgcagttgccgttgg-3′ |
197 | NM_145947 |
| Slc26a8 | Forward 5′-ccaccggaaaccagctaatcc-3′ Reverse 5′-ttctcctcgttgtacacgctc-3′ |
123 | NM_001290320 |
| Slc26a9 | Forward 5′-atcgcctcgctcatctttgc-3′ Reverse 5′-gggaaacccttgtcggatct-3′ |
204 | NM_177243 |
| Slc26a10 | Forward 5′-ctgtggctggagtaaccgtg-3′ Reverse 5′-agaaagacgtgtagagtccga-3′ |
102 | NM_177615 |
| Slc26a11 | Forward 5′-agtgtatgcgggaacacatgc-3′ Reverse 5′-cgaaggcgtaagcaatcagag-3′ |
141 | NM_178743 |
| Actb | Forward 5′-cactgtcgagtggcgtcca-3′ Reverse 5′-tgacccattcccaccatcac-3′ |
227 | NM_007393.5 |
| B2m | Forward 5′-taagcatgccagtatggccg-3′ Reverse 5′-ttgctatttctttctgcgtgc-3′ |
155 | NM_009735 |
| Kcnj13 | Forward 5′-aatgctcctaggcctcatgc-3′ Reverse 5′-agacacgaacgttggtcaga-3′ |
191 | NM_001110227 |
Real-Time PCR Analysis
The expression of genes of interest in WT mouse RPE and retina was quantified using the iQ SYBR Green Supermix assay (Bio-Rad) on a thermocycler (iCycler iQ; Bio-Rad). The following PCR cycle parameters were used: an initial denaturation step (95°C for 3 min) followed by 40 cycles of denaturation (95°C for 10 s) and annealing/extension (61.5°C for 45 s). Fluorescence data were acquired during the annealing/extension step. To confirm amplification specificity, the PCR products from each primer pair were subjected to melting curve analysis. Melting curve analysis was performed using the following PCR cycle parameters: 95°C for 1 min and 55°C for 1 min, followed by an increase in temperature to 95°C in 0.5°C steps. Fluorescence was measured continually during this melting curve cycle. The efficiencies of the target and endogenous control amplifications were determined for all primer sets except for Slc26a5, a gene with expression restricted to the inner ear, by the standard curve method using RNA pooled from various mouse tissues including heart, kidney, lung, and retina. The amplification efficiencies for all primers ranged from 90.8 to 107.7. The data were analyzed with iCycler iQ optical system software (version 3.0a; Bio-Rad Laboratories, Hercules, CA) to determine the fractional cycle number at which threshold fluorescence was obtained [threshold cycle (CT)]. The ΔCT values for all genes in a sample were calculated by subtracting the mean of CT values for two housekeeping genes (Actb and B2m) from the CT value for each target gene. The relative quantity of mRNA for each gene in WT mouse RPE was determined by calculating the 2−ΔCt value. The relative gene expression between WT mouse RPE and WT mouse neural retina was determined by the 2−ΔΔCt method (23). All reactions were conducted in triplicate.
Antibodies
A monoclonal anti-SLC26A7 antibody raised against the hydrophobic COOH terminus of mouse SLC26A7, described and validated by Dudas et al. (12), was purchased from Santa Cruz Biotechnology (cat. no. sc-53960). Affinity-purified rabbit polyclonal antibodies raised against a synthetic peptide in the intracellular COOH-terminal region of human Kir7.1 were generated in our laboratory, and their specificity was confirmed by Western blot and immunohistochemical experiments in the absence or presence of antigenic peptide (45). Polyclonal antibodies against mouse MCT3, a gift from Nancy Philp (Jefferson University), were validated previously (30). Secondary antibodies used in this study were Alexa Fluor 488 goat anti-mouse IgG (H+L; cat. no. A-11001) and Alexa Fluor 555 goat anti-rabbit IgG (H+L; cat. no. A-21248) from Invitrogen.
Eyecup and Isolated RPE Cell Fixation
Eyes enucleated from euthanized WT or Slc26a7 KO adult mice of both sexes were pierced through the cornea with a 22-gauge hypodermic needle and then immersed for 1–2 h in 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS) at 4°C. After rinsing in chilled PBS (3 × 10 min), the cornea was cut using a razor blade, and the lens and vitreous were removed. To cryoprotect before freezing, eyecups were incubated in successive 1-h incubations in 5% and 10% sucrose solutions in PBS and then in 20% sucrose in PBS overnight at 4°C. Eyecups were embedded in optimal cutting temperature embedding medium (Tissue-Tek; Sakura Finetek, Inc., Torrance, CA) and frozen in liquid nitrogen. Cryosections (6 or 10 µm) were cut, collected on glass slides, dried at room temperature, and stored at −80°C until use. Single, enzymatically dispersed mouse RPE cells (see below) were suspended in L-15 medium, transferred to the surface of a glass coverslip coated with 0.01% poly-l-lysine hydrobromide (Sigma-Aldrich), and allowed to attach for 15 min. Cells were then fixed for 10 min with 4% PFA, washed in PBS (3 × 5 min), and stored in a humidified box at 4°C until use.
Immunofluorescence Microscopy
For immunofluorescence labeling, fixed retinal cryosections or isolated RPE cells were blocked with PBS containing 10% normal goat serum for 60 min, washed (3 × 10 min), 1% BSA, and 0.3% Triton X-100 and then incubated overnight at 4°C in PBS containing primary antibodies (anti-SLC26A7, 1:200 or 1:250 dilution; anti-Kir7.1, 1:200 dilution; and anti-MCT3, 1:10,000 dilution) plus 2% normal goat serum and 0.3% Triton X-100. Sections or cells were washed (3 × 10 min) and incubated at room temperature in the dark for 1 h with two mixed secondary antibodies (Alexa Fluor 488 goat anti-mouse and Alexa Fluor 555 goat anti-rabbit) diluted in 0.2% Triton X-100 and 2% normal goat serum in PBS to a final dilution of 1:500. After washing three times in the dark, sections (or isolated cells) were covered in mounting medium (Gel Mount; BioMeda, Toronto, ON, Canada) and secured with a coverslip (or glass slide).
Specimens were analyzed on a scanning laser confocal microscope (Leica SP5; Mannheim, Germany) using a ×40 oil immersion objective lens. Digital confocal images were collected at 16-bit resolution in 0.29-µm z-sections and analyzed by Leica LAS AF image-analysis software. The confocal immunofluorescence images of retinal sections in Fig. 2 are three-dimensional (3-D) projections generated from 4–8 consecutive z-sections, whereas the 3-D projections of the isolated RPE cells in Fig. 3, A and B, were generated from 32 or 43 consecutive z-sections following 3-D deconvolution. Files were exported for additional processing by Photoshop CC (Adobe, San Jose, CA). In experiments comparing SLC26A7 immunostaining in retinal sections from WT and Slc26a7 KO mice, laser settings, photomultiplier tube (PMT) gain, exposure time, and brightness and contrast settings were identical for both images.
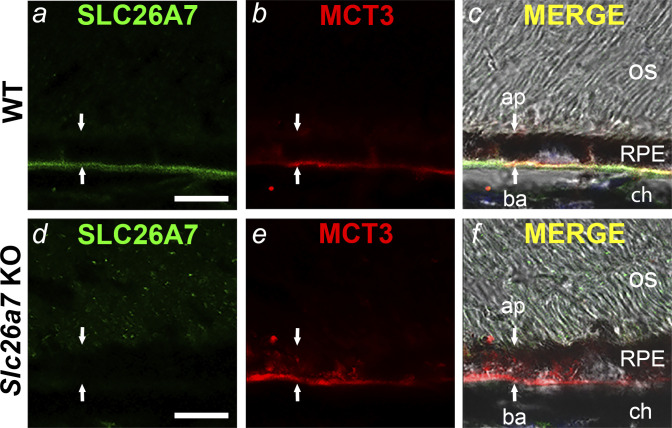
Fig. 2.
Immunolocalization of SLC26A7 in the mouse retinal pigment epithelium (RPE) basolateral membrane. Confocal and DIC images of retinal cryosections from wild-type (WT) mice (a–c) and Slc26a7 knockout (KO) mice (d–f). SLC26A7 immunostaining colocalized with the RPE basolateral membrane marker MCT3 in WT mouse RPE (c) but was absent in Slc26a7 KO mouse RPE (d). Scale bars in a and d, 10 µm; ap, apical membrane; ba, basolateral membrane; ch, choroid; os, photoreceptor outer segments. Downward and upward arrows point to the apical and basal aspects of the RPE, respectively. Retinal cryosections were immunostained with a mouse monoclonal anti-SLC26A7 antibody (17) and rabbit polyclonal anti‐MCT3 antibodies (30) followed by Alexa Fluor 448- and 555‐tagged secondary antibodies. Laser power and photomultiplier tube gain for the 488-nm (green) channel were identical in a and d. The immunolocalization of SLC26A7 to the basolateral membrane shown in a is representative of results from 5 independent experiments on retinal sections from three 3–6-wk-old WT mice of both sexes.
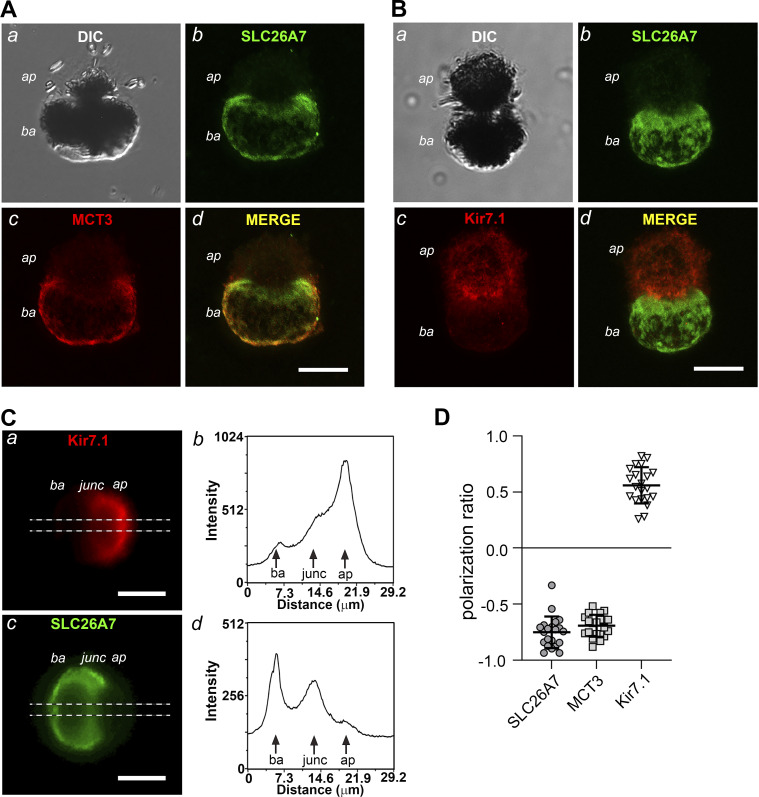
Fig. 3.
Isolated mouse retinal pigment epithelial (RPE) cells retain the polarized expression of SLC26A7 in the basolateral membrane. A and B: confocal and DIC images of fixed, isolated wild-type (WT) mouse RPE cells double labeled with anti-SLC26A7 and anti-MCT3 (A) or anti-Kir7.1 (B) antibodies. Scale bars, 10 µm; ap, apical pole; ba, basolateral pole. The results for SLC26A7, MCT3, and Kir7.1 immunolabeling are representative of 40 cells from 4 adult WT mice, 42 cells from 2 adult WT mice, and 33 cells from 2 adult WT mice, respectively. C: wide-field epifluorescence images of a fixed WT mouse RPE cell double labeled with anti-Kir7.1 (a) and anti-SLC26A7 (c) antibodies. Note that the orientation of the cell differs from that in B. The dashed horizontal lines mark the region subjected to line scan analysis, the results of which are shown in b (Kir7.1) and d (SLC26A7). Scale bars, 10 µm; junc, junction. D: quantification of the membrane polarity of SLC26A7, MCT3, and Kir7.1 expression in isolated mouse RPE cells from line scan analysis of fluorescence along the major axis as in C, b and d, using Eq. 1. Positive values indicate predominantly apical membrane expression, whereas negative values indicate predominantly basolateral membrane expression. Symbols represent measurements in individual cells (n = 21 cells for each group), and horizontal lines and error bars represent means ± SD. The cells used in this analysis were isolated from two adult WT mice.
The membrane polarity of SLC26A7, MCT3, and Kir7.1 expression was determined by analysis of images obtained from fixed and immunolabeled isolated WT RPE cells with a wide-field epifluorescence microscope (Nikon E600-FN; Nikon) and a ×40 oil immersion lens in conjunction with a cooled charge-coupled device (CCD) camera (Coolsnap HQ; Roper Scientific) using the line scan feature of MetaMorph 7.8 image analysis software (Molecular Devices). The polarization ratio was calculated from the background-corrected peak fluorescence intensities at the apical (Fap) and basolateral (Fba) poles of the cell by the equation
| (1) |
Electrophysiology
RPE cells were isolated enzymatically from the eyes of 4–40-wk-old WT or Slc26a7 KO mice of both sexes using papain as described previously (7), stored in L-15 medium containing 0.5 mM taurine and 1 mM reduced glutathione at 4°C, and used within 8 h. All solutions used for the isolation and storage of RPE cells were bubbled with 90% N2-10% O2. This measure improved cell quality significantly.
The pipette solution was an N-methyl-d-glucamine (NMDG)-Cl-based solution containing (in mM) 135 NMDG-Cl, 10 NMDG-HEPES, 5.5 EGTA-NMDG, 0.5 CaCl2, and 2 MgCl2 (pH 7.2). The standard bath solution contained (in mM) 140 NaCl, 10 Na-HEPES, 10 glucose, 1.8 CaCl2, and 1 MgCl2 (pH 7.4). In some experiments, 10 or 140 mM NaCl was replaced by an equimolar amount of sodium thiocyanate (NaSCN) or the Na+ salts of other anions. When the effects of 0.5 mM SCN− were tested, SCN− was added to 140 mM NaCl bath solution as a Na+ salt. In most experiments, nonselective cation channels were blocked by the addition of 100 µM GdCl3 to the bathing solution. All chemicals were obtained from Sigma-Aldrich except for papain, which was obtained from Worthington Biochemical Corporation.
Enzymatically dissociated mouse RPE cells were allowed to attach to the glass coverslip bottom of a recording chamber and were constantly superfused with bath solutions delivered by gravity feed. Macroscopic membrane currents were recorded using the whole cell or excised outside-out patch configuration of the patch-clamp technique. Patch pipettes were pulled from 1.65-mm-outer diameter (O.D.) glass capillary tubing (Warner Instruments) using a multistage programmable microelectrode puller (model P-97; Sutter Instruments) and had an impedance of 2–5 MΩ after fire polishing. Series resistance (Rs) was normally <10 MΩ and was compensated by 50–80%. Signals were amplified with an Axopatch 200 or Multiclamp 700B amplifier (Molecular Devices). The amplifier’s circuit ground was connected to a Ag/AgCl electrode separated from the bath by a 3 M KCl-agar bridge. All experiments were performed at room temperature (22–25°C).
Voltage-clamp commands were generated by pCLAMP 9 (Molecular Devices). In most experiments, currents were evoked from a holding potential (HP) of –60 mV by a series of 1-s voltage steps in the range of −120 to +60 mV, in 10-mV increments, with an intersweep interval of 3 s. Reversal potential (Erev) and outward slope conductance of whole cells (G) or excised membrane patches (g) were determined by linear regression analysis of current-voltage (I-V) plots. G and g were measured as the slope of the I-V curve between 0 and +50 mV. Junction potentials arising at the pipette tip and the 3 M KCl-agar bridge of the reference electrode were calculated using the Junction Potential Calculator function of pCLAMP 9. Errors in membrane potential (Vm) arising from junction potentials and the current flow across Rs were corrected by the formula
| (2) |
where Vm,uncorr is the apparent Vm, Rs,correct is the series resistance compensation setting of the voltage-clamp amplifier expressed as a decimal, and jp is the correction for junction potentials. For simplicity, command potentials are given for voltage-clamp protocols, except where noted.
The relative permeability ratio for various anions (PA/PCl) was calculated using the Goldman–Hodgkin–Katz equation
| (3) |
where ΔErev is the change in Erev produced by replacing 10 or 140 mM Cl− with a substitute anion (A), is the initial external Cl− concentration, and are the extracellular concentrations of A− and Cl−, respectively, after the substitution of A− for Cl−, z = −1, and F, R, and T have their normal thermodynamic meanings (F is Faraday’s constant, R is the ideal gas constant, and T is temperature).
In the analysis of some electrophysiological results from Slc26a7 KO mouse RPE cells (Figs. 5–7, 9, and 10), we reused data from two of our previous studies on WT mouse RPE cells (9, 10) for controls. Reuse of these data sets for this purpose minimized the number of animals used in accordance with NIH “Research Using Vertebrate Animals” and IACUC guidelines and is appropriate because the data were obtained from Slc26a7+/+ mice (littermates of the Slc26a7−/− mice) using identical conditions and methods during an overlapping time frame.
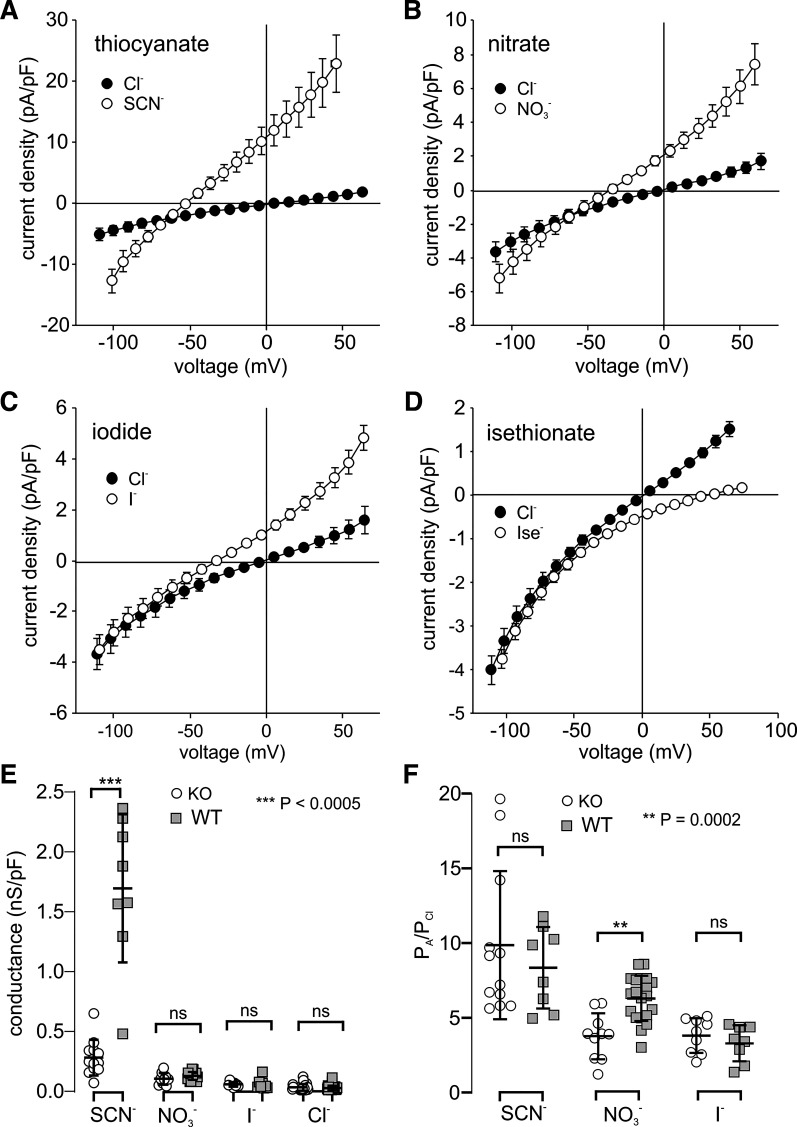
Fig. 5.
Effect of anion substitution on the whole cell current-voltage (I-V) relationship of Slc26a7 knockout (KO) mouse retinal pigment epithelium (RPE) cells. Cells were dialyzed with N-methyl-d-glucamine (NMDG)-Cl-based internal solution. A–D: currents were recorded before and after replacing 140 mM external NaCl with 140 mM sodium thiocyanate (NaSCN; A, n = 12 cells from 8 mice of both sexes, 4.6–19 wk old), NaNO3 (B, n = 10 cells from 6 mice of both sexes, 4.6–19 wk old), NaI (C, n = 9 cells from 6 mice of both sexes, 4.6–19 wk old), or Na-isethionate (D, n = 8 cells from 2 mice of both sexes, 8.6–9.2 wk old). Currents were generated by 1-s voltage ramps from −120 mV to +60 mV from a holding potential of −60 mV. Symbols represent means, and bidirectional error bars represent SE. Where not visible, error bars are smaller than the symbol. Ise−, isethionate. E: comparison of normalized outward conductance of isolated Slc26a7 KO (open circles) and wild-type (WT; gray squares) mouse RPE cells in the presence of 140 mM external thiocyanate (SCN−; KO: n = 12 cells from 8 mice of both sexes, 4.6–19 wk old; WT: n = 8 cells from 3 mice of both sexes, 7–10 wk old), (KO: n = 9 cells from 6 mice of both sexes, 4.6–19 wk old; WT: n = 20 cells from 12 mice of both sexes, 8.7–32.7 wk old), I− (KO: n = 9 cells from 6 mice of both sexes, 4.6–18.9 wk old; WT: n = 8 cells from 4 mice of both sexes, 8.7–32.7 wk old), or Cl− (KO: n = 24 cells from 11 mice of both sexes, 4.6–19.3 wk old; WT: n = 34 cells from 17 mice of both sexes, 7–32.7 wk old). Horizontal lines and error bars represent means ± SD. The difference in conductance between Slc26a7 KO and WT mouse RPE cells was significant in external SCN− (P < 0.0001, 2-way unpaired t test) but not in any of the other anions tested (P > 0.1; ns, not significant). Data for WT mouse RPE cells are from our previously published study on Slc26a7+/+ mice (10). F: comparison of relative permeabilities for SCN−, , and I− in isolated Slc26a7 KO (open circles) and WT (gray squares) mouse RPE cells. Permeability ratios (PA/PCl) were calculated from the change in reversal potential (ΔErev) produced by replacing 140 mM external Cl− with substitute anions (A) using the Goldman–Hodgkin–Katz equation (Eq. 3). Differences in relative permeability between Slc26a7 KO and WT mouse RPE cells were significant for [KO: 3.8 ± 0.5 (SE), n = 10 cells from 6 mice of both sexes, 4.6–18.9 wk old; WT: 6.3 ± 0.3 (SE), n = 20 cells from 12 mice of both sexes, 8.7–32.7 wk old; P < 0.0002, 2-way unpaired t test] but not for the other anions tested (SCN−: KO, n = 12 cells from 8 mice of both sexes, 4.6–19.2 wk old; WT, n = 8 cells from 3 mice of both sexes, 7–10 wk old; I−: KO, n = 9 cells from 6 mice of both sexes, 4.6–18.9 wk old; WT, n = 8 cells from 4 mice of both sexes, 8.7–32.7 wk old; P > 0.2, 2-way unpaired t tests). Horizontal lines and error bars represent means ± SD. Data for WT mouse RPE cells are from our previously published study on Slc26a7+/+ mice (10).
Fig. 7.
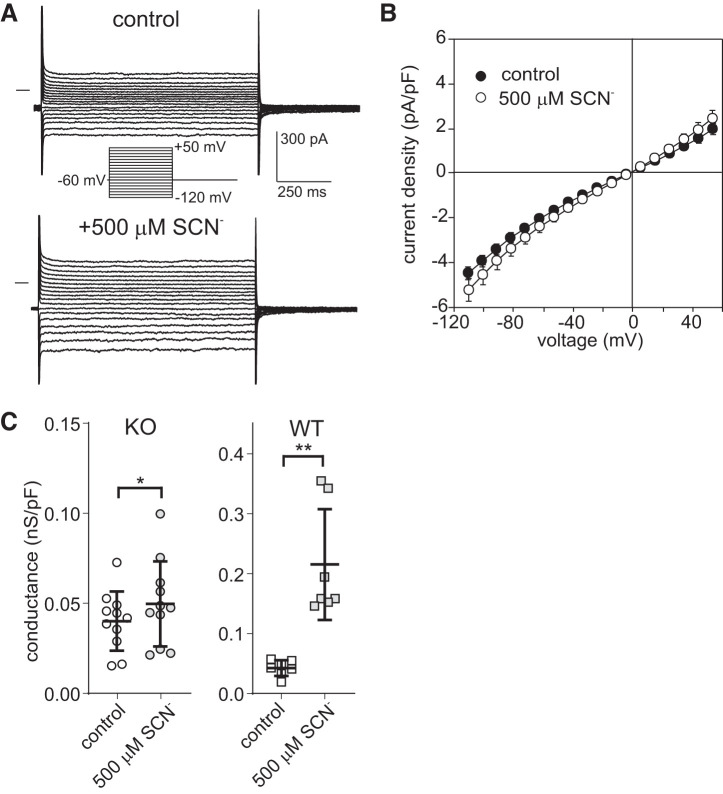
Exposure to 500 μM external thiocyanate (SCN−) produces little change in the electrophysiological properties of Slc26a7 knockout (KO) mouse retinal pigment epithelial (RPE) cells. A: families of whole cell currents evoked by voltage steps from a holding potential of −60 mV in an Slc26a7 KO RPE cell in the absence and presence of 500 μM external SCN−. Exposure to 500 μM SCN− produced small increases in inward and outward currents without changing their time course in contrast to the large transient currents that are observed in wild-type (WT) mouse RPE cells exposed to submillimolar SCN− (9). B: summary of experiments similar to those in A showing current-voltage (I-V) plots of instantaneous current obtained in the absence (closed circles) and presence (open circles) of 500 μM external SCN−. Symbols represent means, and bidirectional error bars represent SE (n = 11 cells from 5 mice of both sexes, 6–21 wk old). Five hundred micromolar SCN− produced small but significant increases in current at voltages in the range −114.5 to −84.5 mV and at +55.5 mV (P < 0.02; Šidák’s multiple comparisons test) but not at the other voltages tested (P > 0.05). C: comparison of normalized outward conductance of isolated Slc26a7 KO mouse (left) and WT mouse (right) RPE cells in the absence (open symbols) and presence (gray symbols) of 500 μM external SCN−. Horizontal lines and error bars represent means ± SD. The change in outward conductance produced by 500 μM SCN− was significantly smaller in Slc26a7 KO mouse RPE cells [0.010 ± 0.004 (SE) nS/pF, n = 11 cells from 5 mice of both sexes, 6–21 wk old] than in WT mouse RPE cells [0.173 ± 0.031 (SE) nS/pF, n = 7 cells from 2 female mice, 8 wk old; P < 0.005, unpaired t test]. Data for WT mouse RPE cells are from our previously published study on Slc26a7+/+ mice (9). *P < 0.05, **P < 0.005.
Fig. 9.
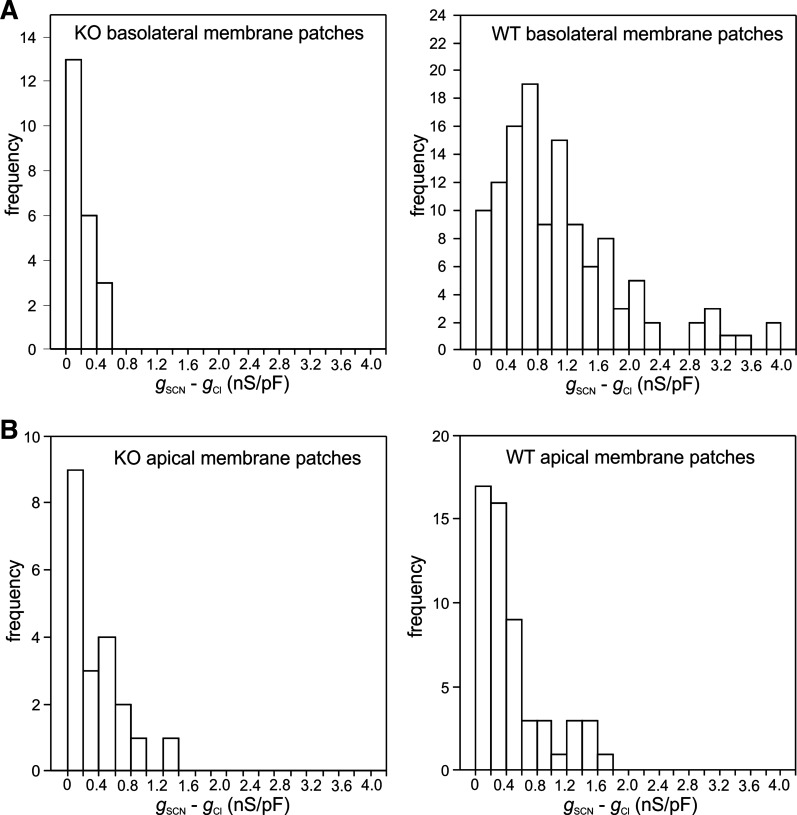
Deletion of Slc26a7 decreases the thiocyanate (SCN−)-induced increase in the conductance of the mouse retinal pigment epithelium (RPE) basolateral membrane. A: histograms showing the distribution of the change in outward conductance produced by replacing 140 mM external Cl− with SCN− (gSCN − gCl) in outside-out basolateral membrane patches from Slc26a7 knockout (KO) mouse (left) or wild-type (WT) mouse RPE cells (right). The mean values of gSCN − gCl in Slc26a7 KO RPE basolateral membrane patches [0.17 ± 0.03 (SE) nS/pF, n = 22 cells from 14 mice of both sexes, 3.9–40.0 wk old] and WT RPE basolateral membrane patches [1.14 ± 0.08 (SE) nS/pF, n = 124 cells from 48 mice of both sexes, 4.9–19.9 wk old] were significantly different (P < 0.001, 2-tailed Mann–Whitney test). B: histograms showing the distribution of gSCN − gCl in outside-out apical membrane patches from Slc26a7 KO mouse (left) or WT mouse RPE cells (right). The mean values of gSCN − gCl for Slc26a7 KO RPE apical membrane patches [0.37 ± 0.08 (SE) nS/pF, n = 20 cells from 11 mice of both sexes, 3.6–11.6 wk old] and WT mouse RPE apical membrane patches [0.46 ± 0.06 (SE) nS/pF, n = 56 cells from 25 mice of both sexes, 3.9–14.4 wk old] were not significantly different (P = 0.26, 2-tailed Mann–Whitney test). The conductance of individual patches was normalized by membrane capacitance. Data for WT mouse RPE cells are from our previously published study on Slc26a7+/+ mice (10).
Fig. 10.
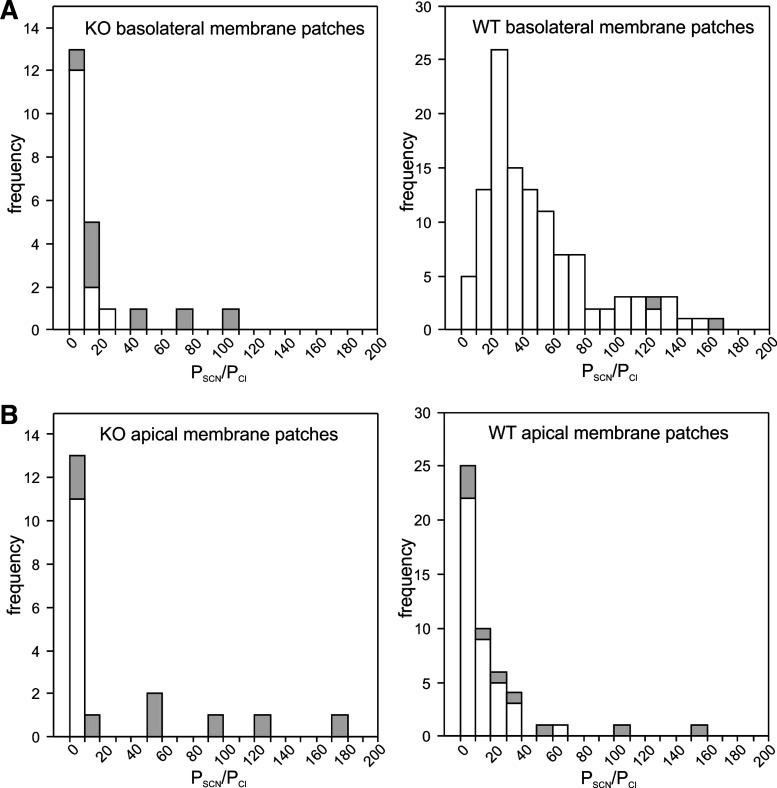
Deletion of Slc26a7 decreases the relative thiocyanate (SCN−) permeability (PSCN/PCl) of the mouse retinal pigment epithelium (RPE) basolateral membrane. A: histograms showing the distribution PSCN/PCl values for outside-out basolateral membrane patches from Slc26a7 knockout (KO) mouse (left) and wild-type (WT) mouse (right) RPE cells. Not shown in the histogram for WT mouse RPE basolateral membrane patches are PSCN/PCl values of 308.1, 309.2, 357.1, 366.3, and 519. The mean values of PSCN/PCl in Slc26a7 KO mouse RPE basolateral membrane patches [17.4 ± 5.4 (SE), n = 22 cells from 14 mice of both sexes, 3.9–40.0 wk old] and WT mouse RPE basolateral membrane patches [63.8 ± 6.8 (SE), n = 121 cells from 47 mice of both sexes, 4.9–19.9 wk old] were significantly different (P < 0.001, 2-tailed Mann–Whitney test). Open regions of bars indicate data obtained at a holding potential of −60 mV, and gray regions indicate data obtained at a holding potential of −120 mV. B: histograms showing the distribution of PSCN/PCl values for outside-out apical membrane patches from Slc26a7 KO mouse (left) and WT mouse (right) RPE cells. Not shown in the histogram for WT mouse RPE apical membrane patches are PSCN/PCl values of 263.7, 280.7, and 703.0. The mean values of PSCN/PCl Slc26a7 KO mouse RPE apical membrane patches [30.8 ± 11.7 (SE), n = 19 cells from 11 mice of both sexes, 3.6–10.6 wk old] and WT mouse RPE apical membrane patches [42.6 ± 15.1 (SE), n = 52 cells from 25 mice of both sexes, 3.6–14.4 wk old] were not significantly different (P = 0.10, 2-tailed Mann–Whitney test). Data for WT RPE cells are from our previously published study on Slc26a7+/+ mice (10).
Statistics
Statistical analysis was performed using Excel (Microsoft) or Prism 8 (GraphPad). Experiments were performed on approximately equal numbers of 4–40-wk-old male and female mice; we observed no differences between the sexes or ages and therefore combined the data for analysis. All mice of the same genotype were assumed to be identical such that cells could be treated as coming from a single population. The statistical significance of differences between groups of three or more was calculated by ANOVA followed by Šidák’s multiple comparisons test. Student’s t test or the Mann–Whitney test was used for comparisons between two groups. Probability values of P < 0.05 were considered statistically significant. The number of experiments reported (n) refers to the number of cells or membrane patches recorded. Data are presented as means ± SE unless noted otherwise.
RESULTS
qPCR Analysis
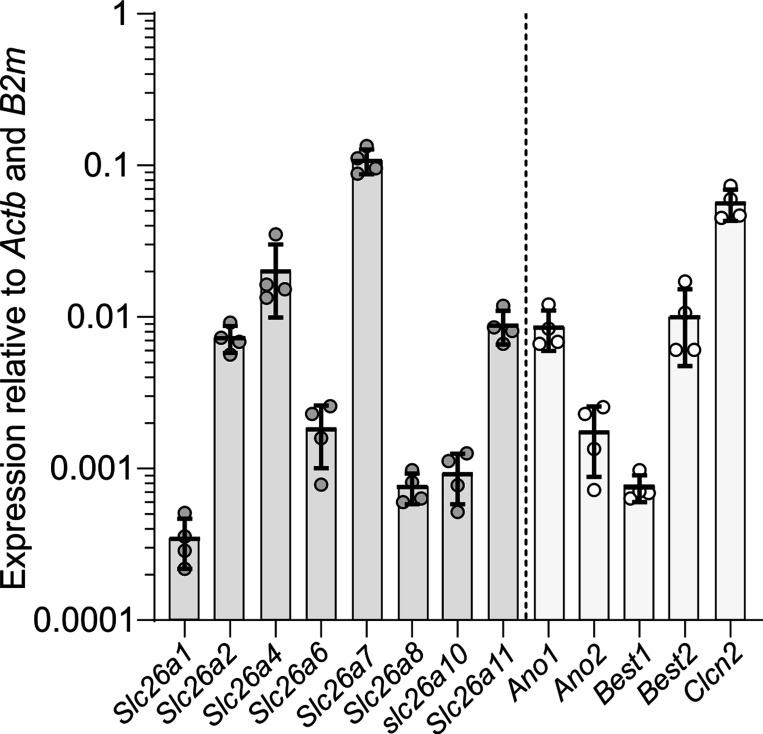
A previous gene expression profiling study identified SLC26A7 as a gene that is highly expressed in human RPE relative to photoreceptors (6). To determine the expression level of Slc26a7 in mouse RPE, we performed qPCR analysis on RNA extracted from male and female WT (Slc26a7+/+) mice using primer pairs designed to amplify cDNA reverse transcribed from transcripts of Slc26a7 and other members of the Slc26 gene family (Table 1). Figure 1 shows that among members of the Slc26 gene family, Slc26a7 was the highest expressed, with the second most abundant transcript, Slc26a4, being expressed at a fivefold lower level. For comparison, we also determined the relative expression levels of several Cl− channel genes previously reported to be expressed in the RPE. The relative expression of Slc26a7 was found to be nearly twice as high as that of Clcn2, the gene encoding ClC-2, a Cl− channel that was reported to be a component of the RPE basolateral membrane Cl− conductance (7). Transcripts for other Cl− channel genes reported to be expressed in native or cultured mammalian RPE such as Ano1 (11), Ano2 (18), Best1 (25), Best2 (36), and Cftr (5) were substantially less abundant, with their relative expression levels being >10-fold lower than Slc26a7.
Fig. 1.
Relative expression of members of the Slc26 gene family and selected Cl− channel genes in mouse retinal pigment epithelium (RPE). Expression of genes is presented relative to the average of Actb and B2m expression levels. Quantitative RT-PCR was performed separately using 4 biological replicates of cDNA reversed transcribed from mRNA pooled from RNA isolated from the RPE of wild-type mice of the following ages and sexes: six 2-mo-old males, eight 2-mo-old females, eleven 3-mo-old males, and eleven 3-mo-old females. Symbols represent relative expression values for each of these pooled samples, and the vertical bars and error bars represent means ± SD (n = 4 experiments). The relative expression levels of Slc26a3, Slc26a5, and Slc26a9 were <2 × 10−5 and are not shown.
Gene expression profiling determined that SLC26A4 and SLC26A7 are expressed in human RPE at levels 37-fold higher than in photoreceptors (6). To determine whether a similar expression pattern exists in mice, we performed qPCR analysis on two pooled samples of WT mouse retina (1 from the eyes of six 3-mo-old males and the other from the eyes of six 3-mo-old females) and compared the fold difference in Slc26a4, Slc26a7, and Kcnj13 expression in the RPE relative to retina (reference tissue). Both Slc26a7 and Kcnj13 exhibited high differential expression levels of 51-fold (range, 28–95-fold) and 52-fold (range, 29–96-fold), respectively, in the RPE compared with retina. In contrast, Slc26a4 showed only a twofold higher expression level in RPE compared with retina (results not shown).
Immunolocalization of SLC26A7
To determine the subcellular distribution of SLC26A7 in mouse RPE, we performed indirect immunofluorescence microscopy on frozen sections of retina from WT and Slc26a7 KO mice using a mouse monoclonal antibody against mouse SLC26A7 that was characterized previously (12). Figure 2, a–c, shows the immunostaining pattern of antibodies against SLC26A7 (Fig. 2a) and the monocarboxylic acid transporter MCT3 (Fig. 2b), an RPE basolateral membrane marker (30), in the distal region of a WT mouse retinal cryosection. SLC26A7 colocalized with MCT3 (Fig. 2c), establishing its polarized expression at the basolateral membrane. Apparent SLC26A7 immunoreactivity was also observed in retinal blood vessels in the inner retina, but control experiments with secondary antibodies alone showed this to be a nonspecific reaction, presumably with endogenous IgGs (results not shown). The results of an identical experiment performed on a frozen retinal section from an Slc26a7 KO mouse are shown in Fig. 2, d–f. As can be seen in Fig. 2d, SLC26A7 immunoreactivity was absent in the RPE basolateral membrane of the Slc26a7 KO mouse.
In addition to retinal cryosections, we assessed the subcellular localization of SLC26A7 in indirect immunofluorescence experiments on fixed, isolated WT mouse RPE cells. Figure 3 shows a series of DIC and confocal images obtained from isolated mouse RPE cells double labeled with antibodies against SLC26A7 and MCT3 (Fig. 3A) or SLC26A7 and Kir7.1 (Fig. 3B). Isolated mouse RPE cells retained a polarized morphology, with an apical pole displaying truncated microvilli and a smooth-appearing basolateral pole, separated by a narrowing of the cell body (Fig. 3, Aa and Ba). Consistent with the results obtained in retinal sections, SLC26A7 immunostaining colocalized with MCT3 in the basolateral membrane (Fig. 3A, b–d). Figure 3B shows results obtained in another cell double labeled with antibodies against SLC26A7 (Fig. 3Bb) and Kir7.1 (Fig. 3Bc), a K+ channel that is expressed predominantly in the RPE apical membrane (45). As can be appreciated from viewing the merged SLC26A7 and Kir7.1 fluorescence images (Fig. 3Bd), SLC26A7 immunolabeling was absent in the apical membrane but present in the basolateral membrane.
We quantified the subcellular expression patterns of SLC26A7, MCT3, and Kir7.1 by performing line scans along the major axis of fluorescence images of immunostained isolated WT mouse RPE cells and calculating the polarization ratio from the peak intensity values at the apical and basolateral membranes using a ratiometric formula (Eq. 1). Figure 3C shows epifluorescence images of a WT mouse RPE cell double labeled with antibodies against Kir7.1 (Fig. 3Ca) and SLC26A7 (Fig. 3Cc) alongside plots of fluorescence intensity versus distance obtained from line scan analysis (Fig. 3C, b and d). Kir7.1 immunofluorescence intensity had a major peak at the distal end of the apical pole (Fig. 3Cb), whereas SLC26A7 immunofluorescence intensity had a major peak at the distal end of the basolateral pole as well as a secondary peak near the junction with the apical pole. Figure 3D summarizes the results obtained in multiple isolated WT mouse RPE cells and shows that both SLC26A7 and MCT3 had negative polarization ratios averaging −0.75 ± 0.03 (SE; n = 21) and −0.69 ± 0.02 (SE; n = 21; P > 0.1, unpaired t test), respectively, indicating that the two membrane proteins are predominately expressed in the basolateral membrane. In contrast, Kir7.1 had a positive polarization ratio of 0.56 ± 0.04 (SE; n = 21), signifying that the K+ channel protein is expressed predominantly in the apical membrane. These results establish that the polarized expression of SLC26A7 in the basolateral membrane is retained in mouse RPE cells following their isolation.
Electrophysiological Effects of SCN− on RPE Cells Isolated from Slc26a7 KO Mice
Whole cell experiments.
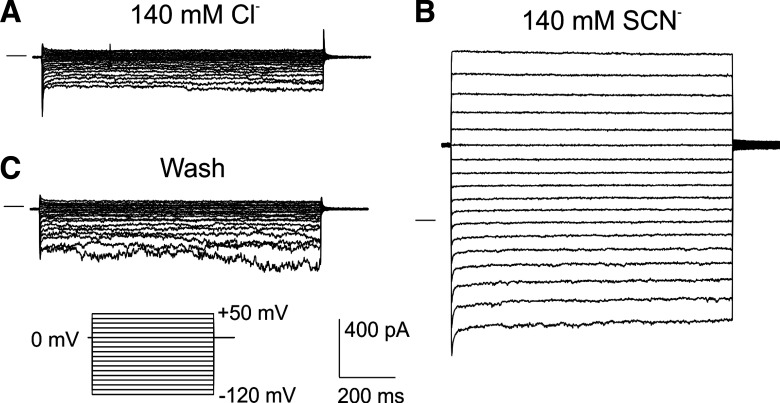
Previously, we demonstrated that the anion conductance of the WT mouse RPE cell basolateral membrane is highly selective for SCN− (10). To assess the extent to which SLC26A7 contributes to this SCN−-selective conductance, we recorded currents in RPE cells isolated from Slc26a7 KO mice in the presence of SCN− and other foreign anions. Figure 4 compares families of whole cell currents in an isolated Slc26a7 KO mouse RPE cell evoked by voltage steps from a holding potential of 0 mV in the presence of 140 mM external Cl− (Fig. 4A) and 140 mM external SCN− (Fig. 4B). The replacement of 140 mM external Cl− with SCN− produced large and reversible increases in inward and outward currents.
Fig. 4.
Effect of 140 mM thiocyanate (SCN−) on whole cell currents in an isolated Slc26a7 knockout mouse retinal pigment epithelial cell. The cell was dialyzed with an N-methyl-d-glucamine (NMDG)-Cl-based K+-free internal solution and superfused with a solution containing 140 mM NaCl (A) or 140 mM sodium thiocyanate (NaSCN; B). The effect of SCN− was reversible (C). The family of currents in C was obtained after 10 min of superfusion with 140 mM NaCl solution to ensure the complete removal of SCN−. Currents were elicited using a voltage step protocol consisting of 1-s steps from −120 to +60 mV in intervals of 10 mV from a holding potential of 0 mV. Horizontal lines to the left of the current traces indicate the zero-current level.
In subsequent experiments, we evoked currents in isolated Slc26a7 KO mouse RPE cells using voltage ramps from a holding potential of −60 mV; this protocol minimized the intracellular accumulation of test anions at positive voltages and greatly reduced the time required to test the effects of multiple anions in the same cell. The results of paired experiments testing the effect of replacing 140 mM Cl− with SCN− or other foreign anions are summarized as current-voltage (I-V) plots in Fig. 5. The replacement of Cl− with SCN− had the largest effect on whole cell current (Fig. 5A), increasing outward current at +60 mV >10-fold and shifting the reversal potential (Erev) by −54.2 ± 3.6 (SE) mV from +4.7 ± 3.3 (SE) mV to −49.5 ± 2.5 (SE) mV (n = 12, P < 0.0001, paired t test). Currents also increased in amplitude and exhibited a negative shift in Erev when Cl− was replaced with (Fig. 5B) or I− (Fig. 5C), but the effects of these anions were smaller than those of SCN−: increased outward current nearly 5-fold and shifted Erev by −30.6 ± 4.1 mV from −4.2 ± 3.0 mV to −37.2 ± 4.7 mV (n = 10, P < 0.0001), whereas I− increased outward current ~3-fold and shifted Erev by −32.1 ± 3.0 mV from −3.0 ± 3.3 mV to −35.1 ± 4.7 mV (n = 9, P < 0.0001). In contrast, replacement of Cl− with the impermeant anion isethionate (Fig. 5D) dramatically decreased outward current and produced a 50-mV positive shift in Erev from 0.9 ± 1.9 mV to 50.9 ± 2.2 mV (n = 8, P < 0.0001).
To evaluate the contribution of SLC26A7 to the anion conductance of WT mouse RPE, we calculated the outward conductance of Slc26a7 KO mouse RPE cells in the presence of the various anions using the data in Fig. 5, A–D, and compared these values with those for WT (Slc26a7+/+) mouse RPE cells recalculated from data obtained in our previous study (10). Figure 5E shows that the normalized whole cell outward conductance in the presence of 140 mM external SCN− was 84.7% smaller in Slc26a7 KO mouse RPE cells (open circles; n = 12) compared with WT mouse RPE cells (gray squares, n = 8; P < 0.0001, unpaired 2-tailed t test). In contrast, there were no statistically significant differences in outward conductance measured in the presence of 140 mM , I−, or Cl− between Slc26a7 KO mouse and WT mouse RPE cells (P > 0.13, unpaired 2-tailed t tests).
We calculated relative permeabilities (PA/PCl) for the anions (A) tested using the Goldman–Hodgkin–Katz equation (Eq. 3) from the changes in Erev produced by the anion substitutions shown in Fig. 5, A–C. The results, depicted in Fig. 5F (open circles), indicate that Slc26a7 KO mouse RPE cells have a relative permeability sequence of SCN− > ≈ I− > Cl−. To determine the effect of Slc26a7 deletion on the anion permeability of the RPE, we compared these PA/PCl values with those for WT (Slc26a7+/+) mouse RPE cells (gray squares) recalculated from our previous study (10). The mean relative permeability for [nitrate-to-chloride permeability ratio (PNO3/PCl)] was nearly 40% smaller in Slc26a7 KO mouse RPE cells [3.9 ± 0.5, n = 9] compared with WT mouse RPE cells [6.3 ± 0.3, n = 20; P = 0.002, unpaired 2-tailed t test], whereas there were no significant differences in the relative permeabilities for SCN− or I− between Slc26a7 KO mouse and WT mouse RPE cells (P > 0.2, unpaired 2-tailed t tests). Because the relative permeability for SCN− (PSCN/PCl) calculated for WT mouse RPE cells grossly underestimates the true PSCN/PCl value as a result of the depolarizing effect of the intracellular accumulation of SCN− on Erev (10), this comparison does not allow us to assess the impact of Slc26a7 deletion on the PSCN/PCl of mouse RPE cells.
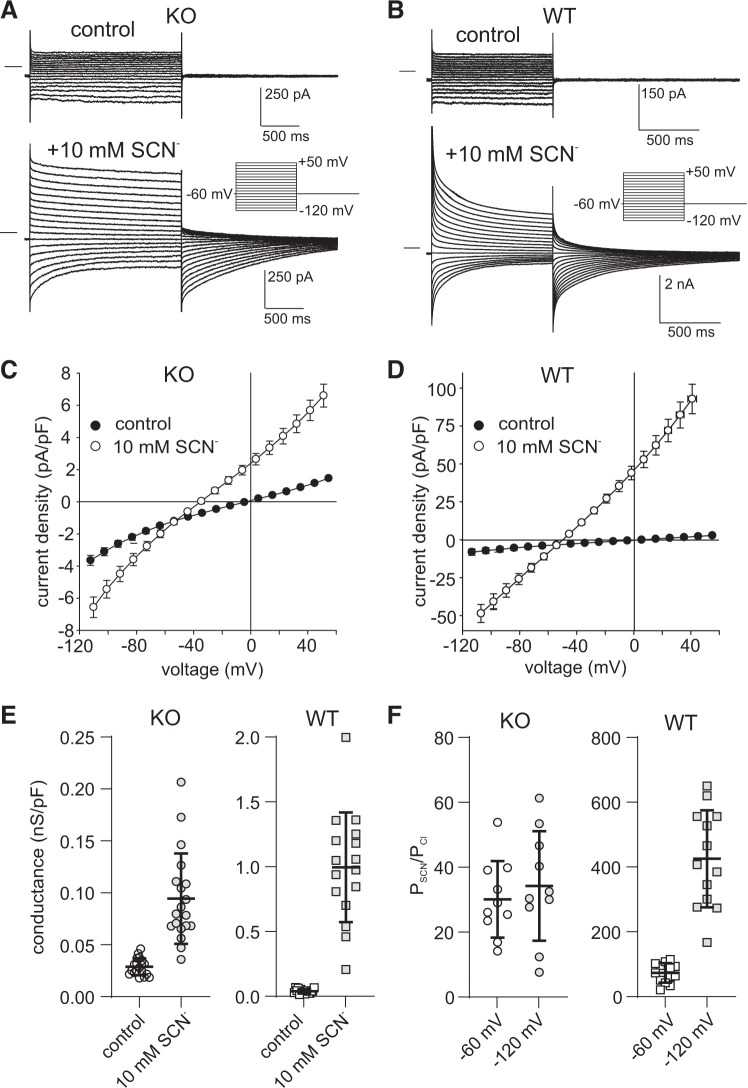
Previously, we showed that voltage steps evoked robust transient currents in WT mouse RPE cells exposed to 10 mM (10) or 500 μM (9) external SCN−. Figure 6, A and B, depicts families of whole cell currents recorded in Slc26a7 KO mouse (Fig. 6A) and WT mouse (Fig. 6B) RPE cells before and after the addition of 10 mM SCN−. In both cells, currents were time independent under control conditions, but following the replacement of 10 mM Cl− with SCN−, inward and outward currents increased in amplitude, exhibited time-dependent decay, and were followed by tail currents of opposite polarity. However, the SCN− currents in Slc26a7 KO mouse RPE cells differed from those in WT mouse RPE cells in several respects. First, the amplitudes of instantaneous inward and outward currents were substantially smaller in Slc26a7 KO mouse RPE cells than in WT mouse RPE cells. This difference can be readily seen by comparing the I-V relationships shown in Fig. 6, C and D, which summarize the current density of instantaneous currents obtained in the absence (closed circles) and presence (open circles) of 10 mM SCN− in Slc26a7 KO mouse (Fig. 6C) and WT mouse RPE cells (Fig. 6D). For example, the normalized instantaneous current evoked by voltage steps to +50 mV in the presence of 10 mM SCN− averaged +6.6 ± 0.7 pA/pF in Slc26a7 KO mouse RPE cells (n = 19) compared with an average value of +92.9 ± 9.8 pA/pF in WT mouse RPE cells (n = 9; P < 0.00005, 2-tailed unpaired t test).
Fig. 6.
The electrophysiological response of Slc26a7 knockout (KO) mouse retinal pigment epithelium (RPE) cells to 10 mM external thiocyanate (SCN−) is dramatically smaller compared with wild-type (WT) mouse RPE cells. A: families of whole cell currents evoked by voltage steps from a holding potential of −60 mV in an Slc26a7 KO mouse RPE cell in the absence and presence of 10 mM external SCN−. B: families of whole cell currents evoked by the same voltage-clamp protocol as in A in a WT mouse RPE cell in the absence and presence of 10 mM external SCN−. Note that the currents in WT mouse RPE exposed to SCN− are presented at a lower gain to account for their larger amplitude. C: summary of experiments in Slc26a7 KO mouse RPE cells similar to those in A showing current-voltage (I-V) plots of instantaneous current obtained in the absence (closed circles) and presence (open circles) of 10 mM external SCN−. Symbols represent means, and bidirectional error bars represent SE (n = 19 cells from 5 mice of both sexes, 7–12 wk old). Ten millimolar SCN− produced significant increases in current at voltages in the ranges of −114.5 to −84.5 mV and −4.5 to +55.5 mV (P < 0.02; Šidák’s multiple comparisons test). D: summary of experiments in WT mouse RPE cells similar to those in B showing I-V plots of instantaneous current obtained in the absence (closed circles) and presence (open circles) of 10 mM external SCN−. Symbols represent means, and bidirectional error bars represent SE (n = 9 cells from 1 male and 1 female mouse, 18.6 wk old). Ten millimolar SCN− produced significant increases (P < 0.002; Šidák’s multiple comparisons test) in current at voltages in the ranges of −114.5 to −84.5 mV and −24.5 to +55.5 mV. E: comparison of normalized outward conductance of RPE cells isolated from Slc26a7 KO mice (left, n = 19 cells from 5 mice of both sexes, 7–12 wk old) and WT mice (right, n = 13 cells from 6 mice of both sexes, 6.4–24 wk old) and in the absence (open symbols) and presence (gray symbols) of 10 mM external SCN−. Horizontal lines and error bars represent means ± SD. The difference in conductance between Slc26a7 KO mouse RPE cells and WT mouse RPE cells was significant in the absence (P < 0.05) and presence of external SCN− (P < 0.00001, 2-tailed unpaired t tests). Data for WT mouse RPE cells include the results from 8 cells from our previous study on Slc26a7+/+ mice (10). F: comparison of the relative permeability for SCN− of RPE cells isolated from Slc26a7 KO mice (left) and WT mice (right) determined at holding potentials (HPs) of −60 mV (open symbols) and −120 mV (gray symbols). Permeability ratios (PSCN/PCl) were calculated from the change in reversal potential (ΔErev) of instantaneous currents produced by replacing 10 mM external Cl− with SCN− using the Goldman–Hodgkin–Katz equation (Eq. 3). When HP = −60 mV, PSCN/PCl values in Slc26a7 KO mouse RPE cells [30.1 ± 3.7 (SE), n = 10 cells from 3 mice of both sexes, 7–12.5 wk old] were significantly lower than in WT mouse RPE cells [75.4 ± 8.3 (SE), n = 13 cells mice of both sexes, 6.4–18.6 wk old; P < 0.0002, 2-tailed unpaired t test]. Hyperpolarization of HP to −120 mV did not change the PSCN/PCl value in Slc26a7 KO mouse RPE cells significantly [34.2 ± 5.3 (SE), n = 10 cells from 3 mice of both sexes, 7–12.5 wk old, P > 0.05 compared with values obtained at HP = −60 mV, 2-tailed paired t test] but, as previously shown (10), resulted in a dramatically higher PSCN/PCl value in WT mouse RPE cells [437.4 ± 41.6 (SE), n = 13 cells mice of both sexes, 6.4–18.6 wk old; P < 0.0002, 2-tailed paired t test]. Data for WT mouse RPE cells include the results from 4 cells from our previous study on Slc26a7+/+ mice (10). The apparent PSCN/PCl value is underestimated in WT mouse RPE cells held at −60 mV because of the depolarizing effect of intracellular SCN− accumulation on Erev, an effect that is minimized at HP = −120 mV (10). The insensitivity of the PSCN/PCl value on HP in Slc26a7 KO RPE cells suggests that in the absence of SLC26A7 the intracellular accumulation of SCN− is relatively minor at negative potentials.
Consistent with the smaller amplitude of outward current, Fig. 6E shows that the mean normalized outward conductance in the presence of 10 mM SCN− was 92.4% smaller in Slc26a7 KO mouse RPE cells [0.095 ± 0.010 nS/pF, n = 19] than in WT mouse RPE cells [0.996 ± 0.106 nS/pF, n = 16; P < 0.0001, 2-tailed unpaired t test]. Thus, SLC26A7 contributes to most but not all of the whole cell SCN− conductance in RPE cells exposed to 140 mM or 10 mM external SCN−.
A second aspect in which Slc26a7 KO mouse and WT mouse RPE cells exposed to 10 mM SCN− differed was the extent to which currents decayed during hyperpolarizing and depolarizing voltage steps. We have shown previously that the time-dependent decay in current in WT mouse RPE cells exposed to 10 mM SCN− is not due to ion channel gating but rather results from a collapse of the electrochemical potential gradient for SCN− across the cell membrane due to the rapid intracellular accumulation or depletion of this anion (10). In Slc26a7 KO mouse RPE cells, currents evoked by hyperpolarizing voltage steps from −60 mV to −120 mV decreased 25.1 ± 3.9% in 1 s (n = 19) compared with a decline of 78.2 ± 3.9% in WT mouse RPE cells (n = 9; P < 5 × 10−9, 2-tailed unpaired t test); similarly, currents evoked by depolarizing voltage steps from −60 mV to +50 mV decreased 17.6 ± 1.0% in Slc26a7 KO mouse RPE cells in 1 s (n = 19) compared with 64.2 ± 1.9% in WT mouse RPE cells (n = 9; P < 2 × 10−11, unpaired t test). The diminished degree of current decay in Slc26a7 KO mouse RPE cells exposed to 10 mM SCN− can be attributed to the lower SCN− conductance of its cell membrane and, thus, a lesser amount of intracellular SCN− accumulation and depletion compared with WT mouse RPE cells.
Finally, the electrophysiological responses of Slc26a7 KO mouse RPE cells to 10 mM SCN− revealed a smaller relative permeability for SCN− compared with WT mouse RPE cells. We calculated PSCN/PCl from the 10 mM SCN−-induced change in Erev of instantaneous currents evoked by voltage steps from holding potentials (HPs) of −60 mV and −120 mV using the Goldman–Hodgkin–Katz equation (Eq. 3). Figure 6F summarizes the results of paired experiments on multiple Slc26a7 KO mouse RPE cells (Fig. 6F, left) and shows that mean PSCN/PCl values at HP = −60 mV [30.1 ± 3.7; open circles] and HP = −120 mV [34.2 ± 5.3; gray circles; n = 10] were not significantly different (P > 0.05, 2-tailed paired t test). The mean PSCN/PCl value in WT mouse RPE cells (Fig. 6F, right) was >3-fold higher [75.4 ± 8.3, n = 13] than in Slc26a7 KO mouse RPE cells at HP = −60 mV (open squares; P < 0.0001, 2-tailed unpaired t test) and >12-fold higher at HP = −120 mV [gray squares; 427.4 ± 41.6 (SD), n = 13; P < 0.0001, 2-tailed unpaired t test]. The difference in PSCN/PCl values for WT mouse RPE cells calculated from the data obtained at the two HPs can be accounted for by a higher intracellular SCN− concentration at −60 mV, which leads to a large underestimate of the true PSCN/PCl value (10). The insensitivity of the PSCN/PCl value in Slc26a7 KO mouse RPE cells to changes in HP suggests that intracellular SCN− accumulation does not occur at −60 mV to an appreciable extent in the absence of SLC26A7-mediated SCN− fluxes across the cell membrane.
We next tested the effect of 500 μM external SCN−, which in WT mouse RPE cells gives rise to prominent transient currents (9) and more than a fivefold increase in outer conductance (9). Figure 7A shows the results of a representative experiment on an Slc26a7 KO mouse RPE cell in which exposure to 500 μM external SCN− produced small increases in inward and outward currents without affecting their time course. Figure 7B summarizes the instantaneous I-V relationships obtained in this and 10 other Slc26a7 KO mouse RPE cells in the absence and presence of 500 μM SCN−. The addition of 500 μM SCN− to the bath produced small but significant increases in inward and outward current at voltages in the range of −114.5 to −94.5 mV and at +55.5 mV (P < 0.02, Šidák’s multiple comparisons test) but had no significant effect on Erev [control, −2.9 ± 3.8 mV; 500 µM SCN−, −2.6 ± 2.7 mV; P > 0.5, 2-way paired t test]. Figure 7C, left, shows that the addition of 500 µM SCN− to the bath produced a small increase in outward conductance [from 0.040 ± 0.005 nS/pF to 0.050 ± 0.007 nS/pF, P < 0.05, 2-way paired t test] in Slc26a7 KO mouse RPE cells. For comparison, the effect of 500 μM external SCN− on the outward conductance of WT (Slc26a7+/+) mouse RPE cells recalculated from data in our previous study (9) is also presented (Fig. 7C, right). The SCN−-induced change in outward conductance was 17-fold smaller in Slc26a7 KO mouse RPE cells [0.010 ± 0.004 nS/pF, n = 11] than in WT mouse RPE cells [0.173 ± 0.031 nS/pF, n = 7; P < 0.005, 2-tailed unpaired t test]. Taken together, the results of these whole cell patch-clamp experiments indicate that the electrophysiological response of WT mouse RPE cells to submillimolar concentrations of SCN− is mediated by SLC26A7.
Excised membrane patch experiments.
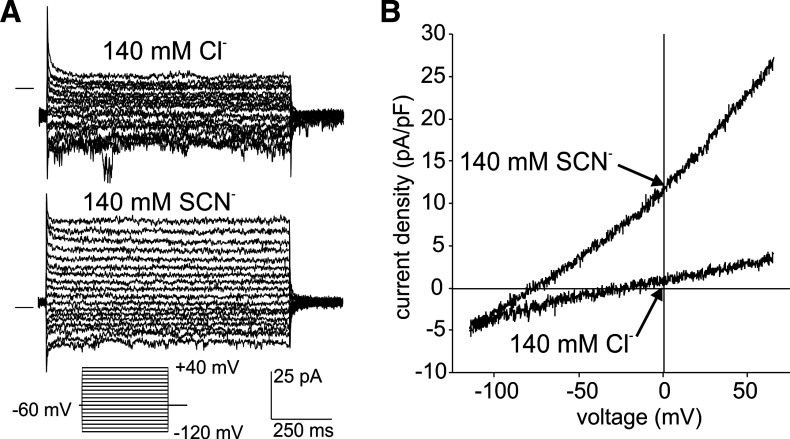
We next determined the effect of SCN− on currents in basolateral and apical membrane patches excised from Slc26a7 KO mouse RPE cells. Figure 8 depicts macroscopic currents in an outside-out basolateral membrane patch exposed to 140 mM external Cl− or SCN−. The families of currents evoked by voltage steps (Fig. 8A) show that the replacement of Cl− with SCN− increased the amplitude of outward currents without affecting their time course. The I-V plots of currents evoked in the same membrane patch by voltage ramps show that SCN− substitution for external Cl− produced an eightfold increase in current density at +60 mV from 3.4 pF/pA to 25.5 pA/pF and an increase in outward conductance from 0.041 nS/pF to 0.270 nS/pF. Coincidentally, the replacement of external Cl− with SCN− shifted Erev from −20.0 to −75.8 mV.
Fig. 8.
Macroscopic anion currents recorded from an excised outside-out basolateral membrane patch from an Slc26a7 knockout (KO) mouse retinal pigment epithelium cell. A: representative current recordings from a basolateral membrane patch evoked by voltage steps in the range −120 to +40 mV from a holding potential of −60 mV in the presence of 140 mM external NaCl (top) or 140 mM external sodium thiocyanate (NaSCN; bottom). The horizontal lines to the left of the traces indicate the zero-current level. The pipette contained 140 mM N-methyl-d-glucamine (NMDG)-Cl solution. Replacement of Cl− with thiocyanate (SCN−) increased the amplitude of outward currents. B: current-voltage plots of currents evoked by voltage ramps in the same patch in the presence of 140 mM external NaCl or 140 mM external NaSCN. The membrane potential was held at −60 mV and ramped from −120 mV to +60 mV over a period of 1 s. Replacement of Cl− with SCN− increased the outward conductance from 40.8 pS/pF to 269.7 pS/pF and shifted the reversal potential from −20.0 to −75.8 mV.
In our previous study on WT mouse RPE cells, we showed that the large conductance and permeability for SCN− of the RPE cell membrane are largely due to properties of the basolateral membrane (10). If SLC26A7 underlies the SCN− conductance of the RPE basolateral membrane, then one would expect basolateral membrane patches from Slc26a7 KO mouse RPE cells to exhibit a lower SCN− conductance compared with basolateral membrane patches from WT mouse RPE cells. This prediction was confirmed in experiments summarized in Fig. 9A, which compares the distribution of the change in conductance (gSCN − gCl) of basolateral membrane patches excised from Slc26a7 KO mouse RPE cells (Fig. 9A, left) and WT mouse RPE cells [Fig. 9A, right; data reanalyzed from our previous study (10)] produced by the equimolar replacement of 140 mM external Cl− with SCN−. The results show that the replacement of external Cl− with SCN− produced nearly a sevenfold smaller average increase in conductance in basolateral membrane patches from Slc26a7 KO mouse RPE cells [0.17 ± 0.03 (SE) nS/pF, n = 22] compared with WT mouse RPE basolateral membrane patches [1.14 ± 0.08 (SE) nS/pF, n = 114; P < 0.0001, Mann–Whitney test]. In contrast, the average change in conductance produced by SCN− substitution in apical membrane patches from Slc26a7 KO mouse RPE cells [Fig. 9B, left, 0.37 ± 0.08 (SE) nS/pF, n = 21] did not differ significantly from that in apical membrane patches from WT mouse RPE cells [Fig. 9B, right, 0.46 ± 0.06 (SE) nS/pF, n = 44; data reanalyzed from our previous study (10) P = 0.554, Mann–Whitney test].
We also measured the change in reversal potential (ΔErev) of currents produced by replacing external Cl− with SCN− in these same patches and used these values and a modified Goldman–Hodgkin–Katz equation (Eq. 3) to calculate PSCN/PCl. Figure 10A compares the distribution of PSCN/PCl values for outside-out basolateral membrane patches excised from Slc26a7 KO mouse RPE cells (Fig. 10A, left) and WT mouse RPE cells [Fig. 10A, right; data reanalyzed from our previous study (10)]. The results show that the average PSCN/PCl value was nearly sixfold smaller in RPE basolateral membrane patches from Slc26a7 KO mice compared with those from WT mice [17.4 ± 5.4, n = 22, vs. 63.8 ± 6.8, n = 121; P < 0.0001, Mann–Whitney test], whereas there was no significant difference in PSCN/PCl of RPE apical membrane patches between Slc26a7 KO mice [30.8 ± 11.7, n = 19] and WT mice [42.6 ± 15.1, n = 52; P > 0.05, Mann–Whitney test]. Thus, deletion of Slc26a7 results in a dramatic reduction in the conductance and relative permeability of the RPE basolateral membrane for SCN−, with no apparent effect on these parameters in the apical membrane.
DISCUSSION
SLC26A7, a member of the SLC26 gene family of anion channels and exchangers, has been shown to be expressed in a limited number of tissues including stomach (28), kidney (12, 27, 37), inner ear (21), and thyroid (8), where it has been proposed to function as a Cl−/ exchanger (27) or an anion channel that transports Cl− (20) or I− (17). In this study, we demonstrate that Slc26a7 is highly expressed in mouse RPE and that SLC26A7 is localized to its basolateral membrane. In addition, we show that the deletion of Slc26a7 results in a dramatic reduction in the conductance and relative permeability for SCN− in intact RPE cells and excised basolateral membrane patches, thereby identifying SLC26A7 as the likely origin of the SCN−-selective anion conductance of the RPE basolateral membrane that we characterized previously (9, 10). Overall, our findings provide insight into the localization and function of a protein encoded by a gene previously reported in a transcriptome analysis study to be highly expressed in human RPE (6).
Our quantitative PCR analysis of WT mouse RPE shows that the relative expression level of Slc26a7 is substantially higher than that of other members of the Slc26 gene family, with Slc26a4 having the second-highest level of expression. We also found that Slc26a7 is expressed at a 51-fold-higher level in the RPE than in the neural retina. A similarly high degree of differential expression was also observed for Kcnj13, whose ortholog, like SLC66A7, was found to be highly expressed in human RPE compared with photoreceptors (6). Our results lend support to the idea that within the mammalian retina, Slc26a7 is an RPE-specific gene.
Our indirect immunofluorescence microscopy results demonstrate that SLC26A7 protein is restricted to the RPE basolateral membrane, consistent with the basolateral localization of this protein in rodent α-intercalated cells in the outer medullary collecting duct (27) and gastric parietal cells (28). SLC26A7 has also been localized to recycling (44) and late endosomes (46). We did not detect SLC26A7 immunoreactivity in intracellular compartments of the RPE either in intact retina or in isolated RPE cells, but positive staining could have been obscured by melanosomes in the cytoplasm. Additional studies are needed to determine the intracellular distribution of SLC27A7 in the RPE and the mechanisms governing its trafficking to the basolateral membrane.
Acutely isolated RPE cells exhibiting a bipolar shape have been used in multiple electrophysiological studies to investigate ion channel function and regulation (13, 16, 38, 47). Although the polarized morphology of freshly isolated RPE cells is well documented, there is a paucity of information regarding their functional polarity. In the present study, we showed using indirect immunofluorescence microscopy that SLC26A7 and MCT3, a recognized RPE basolateral membrane marker (29, 30), are localized to the basolateral membrane of isolated mouse RPE cells, whereas Kir7.1 is restricted to the apical membrane, as found in situ (35, 45).
Our electrophysiological results show that RPE cells from Slc26a7 KO mice had a dramatically smaller whole cell SCN− conductance compared with WT RPE cells, consistent with a previous report that exogenously expressed human SLC26A7 has a very high conductance to SCN− (33). The residual increase in current produced by the exposure of Slc26a7 KO mouse RPE cells to 10 or 140 mM external SCN− may have been mediated by other Cl− channels reported to be expressed in the RPE such as ANO1 (11), ANO2 (18), BEST1 (25), and CFTR (5). Alternatively, the residual current may reflect the uncoupling of excitatory amino acid transporters (EAATs; 26, 34) or anion exchangers in the ClC gene family (2), several of which have been found to be expressed in native or cultured RPE (7, 22, 39).
Although heterologously expressed SLC26A7 has a high relative permeability for SCN− (33), we found that the apparent whole cell PSCN/PCl value in Slc26a7 KO mouse RPE cells exposed to 140 mM external SCN− did not differ significantly from that obtained previously in WT mouse RPE cells (10). It is important to note, however, that PSCN/PCl in intact WT mouse RPE cells was underestimated because of the depolarizing effect of intracellular SCN− accumulation on Erev and that this error was minimized when the cells were held at a large negative potential (10). In the present study, we showed that the PSCN/PCl value calculated from Erev obtained at a holding potential of −120 mV was nearly 13-fold smaller in Slc26a7 KO mouse RPE cells exposed to 10 mM external SCN− than it was in WT mouse RPE cells.
At the subcellular level, we found that the SCN− current density and relative permeability for SCN−, which are large in excised outside-out patches of basolateral membrane from WT mouse RPE cells (10), were considerably smaller in basolateral membrane patches from Slc26a7 KO mouse RPE cells. These electrophysiological findings complement our immunofluorescence results and provide strong evidence for the idea that SLC26A7 channels form the SCN−-selective conductance of the WT mouse RPE basolateral membrane.
Heterologously expressed mouse SLC26A7 has been shown to have a high conductance and large relative permeability for (20), a property that was used in another study to help establish a link between SLC26A7 and Cl− channels in mouse Reissner’s membrane epithelium (21). Consistent with this, we found that whole cell PNO3/PCl was substantially smaller in RPE cells from Slc26a7 KO mice compared with RPE cells from WT mice. On the other hand, we found no difference in the whole cell conductance of RPE cells from Slc26a7 KO mice compared with WT mice, suggesting that currents contributed by other anion channels or transporters predominate.
What is the physiological relevance of SLC26A7 in the RPE? In acid-secreting cells of the renal outer medullary collecting duct, parietal cells in stomach, and tooth ameloblasts, SLC26A7 is thought to play a role in pH regulation by acting as a Cl−/ exchanger or Cl− channel (8, 27, 28, 46). transport is a major driving force for fluid absorption by the RPE (15) and also helps remove CO2 produced by photoreceptor metabolic activity (1). Multiple transport mechanisms have been functionally identified in the RPE basolateral membrane (1, 19), but the extent to which SLC26A7 contributes to pH regulation in this epithelium remains to be determined.
Cl− channels in the RPE basolateral membrane play a central role in active Cl− absorption, which, together with and lactate transport, drives fluid absorption from the subretinal space to the choroidal blood supply (1, 4). The lack of a reduced Cl− conductance in RPE cells isolated from Slc26a7 KO mice compared with RPE cells from WT mice appears to argue against a major role for SLC26A7 in Cl− transport. However, the Cl− channel function of SLC26A7 in the RPE may depend on its interactions with auxiliary proteins or regulatory factors that become disrupted as a consequence of cell isolation and/or whole cell recording. Additional experiments in the intact RPE monolayer are warranted to elucidate the extent to which SLC26A7 participates in Cl− absorption.
Previously, we proposed that SCN− may be a natural substrate for transport via the SCN−-selective anion conductance of the RPE basolateral membrane (9), which the current study establishes to be composed of SLC26A7 channels. This idea was based on our observation that RPE cells exhibit robust currents in the presence of physiologically relevant concentrations of SCN−, which in the plasma typically range from 25 to 100 μM but can exceed 150 µM in heavy tobacco smokers (31, 32). SCN− is actively transported across multiple other epithelia by a mechanism initiated by its entry into the cell across one membrane via the sodium-iodide symporter (NIS/SLC5A5) and its exit across the opposite membrane via an anion exchanger or anion channel (14, 40). Although there are no published reports of NIS expression in the eye, indirect evidence for NIS activity in the RPE comes from the finding that iodide transport out of the vitreous humor of rabbits is blocked by , a potent NIS inhibitor (3). If NIS were expressed in the RPE apical membrane, then SLC26A7 channels could participate in the absorption of SCN− by providing a mechanism for the exit of SCN− across the basolateral membrane. Additional experiments are needed to test this transport model and ascertain its physiological significance.
In keeping with its proposed role in acid-base regulation and iodide transport, Slc26a7 KO mice display distal renal tubular acidosis and impaired gastric acid secretion (43), hypothyroidism (8), and delayed tooth enamel deposition (46). In preliminary electroretinography (ERG) experiments, we found no significant differences between WT mice and Slc26a7 KO mice at the age of 2–3 mo in the amplitudes or latencies of scotopic a-waves and B-waves or photopic B-waves (results not shown), suggesting that the functions of photoreceptors and other retinal neurons are not perturbed by the lack of SLC26A7 in the mouse RPE. Further study is needed to confirm these results and extend them by measuring the slow components of the ERG originating from the RPE (41) to determine the consequences of Slc26a7 deletion on RPE function.
GRANTS
This work was supported by NIH National Eye Institute Grants R01-EY008850 (to B. A. Hughes) and P30-EY007003 (University of Michigan Vision Core Grant), an unrestricted grant from Research to Prevent Blindness, and bridging grants from the Biomedical Research Council of the University of Michigan and the University of Michigan Office of Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.C. and B.A.H. conceived and designed research; X.C. and B.A.H. performed experiments; X.C. and B.A.H. analyzed data; X.C. and B.A.H. interpreted results of experiments; B.A.H. prepared figures; B.A.H. drafted manuscript; X.C., M.S., and B.A.H. edited and revised manuscript; X.C., M.S., and B.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Melissa Bajcz for technical assistance.
REFERENCES
- 1.Adijanto J, Banzon T, Jalickee S, Wang NS, Miller SS. CO2-induced ion and fluid transport in human retinal pigment epithelium. J Gen Physiol 133: 603–622, 2009. doi: 10.1085/jgp.200810169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alekov AK, Fahlke C. Channel-like slippage modes in the human anion/proton exchanger ClC-4. J Gen Physiol 133: 485–496, 2009. doi: 10.1085/jgp.200810155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker B. The turnover of iodide in the rabbit eye. Arch Ophthalmol 65: 832–836, 1961. doi: 10.1001/archopht.1961.01840020834017. [DOI] [PubMed] [Google Scholar]
- 4.Bialek S, Miller SS. K+ and Cl− transport mechanisms in bovine pigment epithelium that could modulate subretinal space volume and composition. J Physiol 475: 401–417, 1994. doi: 10.1113/jphysiol.1994.sp020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaug S, Quinn R, Quong J, Jalickee S, Miller SS. Retinal pigment epithelial function: a role for CFTR? Doc Ophthalmol 106: 43–50, 2003. doi: 10.1023/A:1022514031645. [DOI] [PubMed] [Google Scholar]
- 6.Booij JC, ten Brink JB, Swagemakers SM, Verkerk AJ, Essing AH, van der Spek PJ, Bergen AA. A new strategy to identify and annotate human RPE-specific gene expression. PLoS One 5: e9341, 2010. doi: 10.1371/journal.pone.0009341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bösl MR, Stein V, Hübner C, Zdebik AA, Jordt SE, Mukhopadhyay AK, Davidoff MS, Holstein AF, Jentsch TJ. Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2 Cl− channel disruption. EMBO J 20: 1289–1299, 2001. doi: 10.1093/emboj/20.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cangul H, Liao XH, Schoenmakers E, Kero J, Barone S, Srichomkwun P, Iwayama H, Serra EG, Saglam H, Eren E, Tarim O, Nicholas AK, Zvetkova I, Anderson CA, Frankl FE, Boelaert K, Ojaniemi M, Jääskeläinen J, Patyra K, Löf C, Williams ED, Soleimani M, Barrett T, Maher ER, Chatterjee VK, Refetoff S, Schoenmakers N; UK10K Consortium . Homozygous loss-of-function mutations in SLC26A7 cause goitrous congenital hypothyroidism. JCI Insight 3: e99631, 2018. doi: 10.1172/jci.insight.99631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao X, Baharozian C, Hughes BA. Electrophysiological impact of thiocyanate on isolated mouse retinal pigment epithelial cells. Am J Physiol Cell Physiol 316: C792–C804, 2019. doi: 10.1152/ajpcell.00010.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao X, Pattnaik BR, Hughes BA. Mouse retinal pigment epithelial cells exhibit a thiocyanate-selective conductance. Am J Physiol Cell Physiol 315: C457–C473, 2018. doi: 10.1152/ajpcell.00231.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dauner K, Möbus C, Frings S, Möhrlen F. Targeted expression of anoctamin calcium-activated chloride channels in rod photoreceptor terminals of the rodent retina. Invest Ophthalmol Vis Sci 54: 3126–3136, 2013. doi: 10.1167/iovs.13-11711. [DOI] [PubMed] [Google Scholar]
- 12.Dudas PL, Mentone S, Greineder CF, Biemesderfer D, Aronson PS. Immunolocalization of anion transporter Slc26a7 in mouse kidney. Am J Physiol Renal Physiol 290: F937–F945, 2006. doi: 10.1152/ajprenal.00197.2004. [DOI] [PubMed] [Google Scholar]
- 13.Fischmeister R, Hartzell HC. Volume sensitivity of the bestrophin family of chloride channels. J Physiol 562: 477–491, 2005. doi: 10.1113/jphysiol.2004.075622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fragoso MA, Fernandez V, Forteza R, Randell SH, Salathe M, Conner GE. Transcellular thiocyanate transport by human airway epithelia. J Physiol 561: 183–194, 2004. doi: 10.1113/jphysiol.2004.071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes BA, Miller SS, Machen TE. Effects of cyclic AMP on fluid absorption and ion transport across frog retinal pigment epithelium. Measurements in the open-circuit state. J Gen Physiol 83: 875–899, 1984. doi: 10.1085/jgp.83.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes BA, Steinberg RH. Voltage-dependent currents in isolated cells of the frog retinal pigment epithelium. J Physiol 428: 273–297, 1990. doi: 10.1113/jphysiol.1990.sp018212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii J, Suzuki A, Kimura T, Tateyama M, Tanaka T, Yazawa T, Arimasu Y, Chen IS, Aoyama K, Kubo Y, Saitoh S, Mizuno H, Kamma H. Congenital goitrous hypothyroidism is caused by dysfunction of the iodide transporter SLC26A7. Commun Biol 2: 270, 2019. doi: 10.1038/s42003-019-0503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keckeis S, Reichhart N, Roubeix C, Strauß O. Anoctamin2 (TMEM16B) forms the Ca2+-activated Cl− channel in the retinal pigment epithelium. Exp Eye Res 154: 139–150, 2017. doi: 10.1016/j.exer.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Kenyon E, Maminishkis A, Joseph DP, Miller SS. Apical and basolateral membrane mechanisms that regulate pHi in bovine retinal pigment epithelium. Am J Physiol Cell Physiol 273: C456–C472, 1997. doi: 10.1152/ajpcell.1997.273.2.C456. [DOI] [PubMed] [Google Scholar]
- 20.Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl− channel regulated by intracellular pH. J Biol Chem 280: 6463–6470, 2005. doi: 10.1074/jbc.M409162200. [DOI] [PubMed] [Google Scholar]
- 21.Kim KX, Sanneman JD, Kim HM, Harbidge DG, Xu J, Soleimani M, Wangemann P, Marcus DC. Slc26a7 chloride channel activity and localization in mouse Reissner’s membrane epithelium. PLoS One 9: e97191, 2014. doi: 10.1371/journal.pone.0097191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornak U, Kasper D, Bösl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104: 205–215, 2001. doi: 10.1016/S0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Lohi H, Kujala M, Makela S, Lehtonen E, Kestila M, Saarialho-Kere U, Markovich D, Kere J. Functional characterization of three novel tissue-specific anion exchangers SLC26A7, -A8, and -A9. J Biol Chem 277: 14246–14254, 2002. doi: 10.1074/jbc.M111802200. [DOI] [PubMed] [Google Scholar]
- 25.Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci USA 97: 12758–12763, 2000. doi: 10.1073/pnas.220402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melzer N, Biela A, Fahlke C. Glutamate modifies ion conduction and voltage-dependent gating of excitatory amino acid transporter-associated anion channels. J Biol Chem 278: 50112–50119, 2003. doi: 10.1074/jbc.M307990200. [DOI] [PubMed] [Google Scholar]
- 27.Petrovic S, Barone S, Xu J, Conforti L, Ma L, Kujala M, Kere J, Soleimani M. SLC26A7: a basolateral Cl−/ exchanger specific to intercalated cells of the outer medullary collecting duct. Am J Physiol Renal Physiol 286: F161–F169, 2004. doi: 10.1152/ajprenal.00219.2003. [DOI] [PubMed] [Google Scholar]
- 28.Petrovic S, Ju X, Barone S, Seidler U, Alper SL, Lohi H, Kere J, Soleimani M. Identification of a basolateral Cl−/ exchanger specific to gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 284: G1093–G1103, 2003. doi: 10.1152/ajpgi.00454.2002. [DOI] [PubMed] [Google Scholar]
- 29.Philp NJ, Yoon H, Grollman EF. Monocarboxylate transporter MCT1 is located in the apical membrane and MCT3 in the basal membrane of rat RPE. Am J Physiol Regul Integr Comp Physiol 274: R1824–R1828, 1998. doi: 10.1152/ajpregu.1998.274.6.R1824. [DOI] [PubMed] [Google Scholar]
- 30.Philp NJ, Yoon H, Lombardi L. Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. Am J Physiol Cell Physiol 280: C1319–C1326, 2001. doi: 10.1152/ajpcell.2001.280.5.C1319. [DOI] [PubMed] [Google Scholar]
- 31.Robertson AS, Burge PS, Cockrill BL. A study of serum thiocyanate concentrations in office workers as a means of validating smoking histories and assessing passive exposure to cigarette smoke. Br J Ind Med 44: 351–354, 1987. doi: 10.1136/oem.44.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saloojee Y, Vesey CJ, Cole PV, Russell MA. Carboxyhaemoglobin and plasma thiocyanate: complementary indicators of smoking behaviour? Thorax 37: 521–525, 1982. doi: 10.1136/thx.37.7.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schänzler M, Fahlke C. Anion transport by the cochlear motor protein prestin. J Physiol 590: 259–272, 2012. doi: 10.1113/jphysiol.2011.209577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider N, Cordeiro S, Machtens JP, Braams S, Rauen T, Fahlke C. Functional properties of the retinal glutamate transporters GLT-1c and EAAT5. J Biol Chem 289: 1815–1824, 2014. doi: 10.1074/jbc.M113.517177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahi PK, Liu X, Aul B, Moyer A, Pattnaik A, Denton J, Pillers DM, Pattnaik BR. Abnormal electroretinogram after Kir7.1 channel suppression suggests role in retinal electrophysiology. Sci Rep 7: 10651, 2017. doi: 10.1038/s41598-017-11034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stöhr H, Marquardt A, Nanda I, Schmid M, Weber BH. Three novel human VMD2-like genes are members of the evolutionary highly conserved RFP-TM family. Eur J Hum Genet 10: 281–284, 2002. doi: 10.1038/sj.ejhg.5200796. [DOI] [PubMed] [Google Scholar]
- 37.Vincourt JB, Jullien D, Kossida S, Amalric F, Girard JP. Molecular cloning of SLC26A7, a novel member of the SLC26 sulfate/anion transporter family, from high endothelial venules and kidney. Genomics 79: 249–256, 2002. doi: 10.1006/geno.2002.6689. [DOI] [PubMed] [Google Scholar]
- 38.Wen R, Lui GM, Steinberg RH. Whole-cell K+ currents in fresh and cultured cells of the human and monkey retinal pigment epithelium. J Physiol 465: 121–147, 1993. doi: 10.1113/jphysiol.1993.sp019669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weng TX, Godley BF, Jin GF, Mangini NJ, Kennedy BG, Yu AS, Wills NK. Oxidant and antioxidant modulation of chloride channels expressed in human retinal pigment epithelium. Am J Physiol Cell Physiol 283: C839–C849, 2002. doi: 10.1152/ajpcell.00445.2001. [DOI] [PubMed] [Google Scholar]
- 40.Wright EM. Active transport of iodide and other anions across the choroid plexus. J Physiol 240: 535–566, 1974. doi: 10.1113/jphysiol.1974.sp010622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Peachey NS, Marmorstein AD. Light-evoked responses of the mouse retinal pigment epithelium. J Neurophysiol 91: 1134–1142, 2004. doi: 10.1152/jn.00958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xin-Zhao Wang C, Zhang K, Aredo B, Lu H, Ufret-Vincenty RL. Novel method for the rapid isolation of RPE cells specifically for RNA extraction and analysis. Exp Eye Res 102: 1–9, 2012. doi: 10.1016/j.exer.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Song P, Nakamura S, Miller M, Barone S, Alper SL, Riederer B, Bonhagen J, Arend LJ, Amlal H, Seidler U, Soleimani M. Deletion of the chloride transporter Slc26a7 causes distal renal tubular acidosis and impairs gastric acid secretion. J Biol Chem 284: 29470–29479, 2009. doi: 10.1074/jbc.M109.044396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, Worrell RT, Li HC, Barone SL, Petrovic S, Amlal H, Soleimani M. Chloride/bicarbonate exchanger SLC26A7 is localized in endosomes in medullary collecting duct cells and is targeted to the basolateral membrane in hypertonicity and potassium depletion. J Am Soc Nephrol 17: 956–967, 2006. doi: 10.1681/ASN.2005111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang D, Pan A, Swaminathan A, Kumar G, Hughes BA. Expression and localization of the inwardly rectifying potassium channel Kir7.1 in native bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci 44: 3178–3185, 2003. doi: 10.1167/iovs.02-1189. [DOI] [PubMed] [Google Scholar]
- 46.Yin K, Lei Y, Wen X, Lacruz RS, Soleimani M, Kurtz I, Snead ML, White SN, Paine ML. SLC26A gene family participate in pH regulation during enamel maturation. PLoS One 10: e0144703, 2015. doi: 10.1371/journal.pone.0144703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Stanton JB, Wu J, Yu K, Hartzell HC, Peachey NS, Marmorstein LY, Marmorstein AD. Suppression of Ca2+ signaling in a mouse model of Best disease. Hum Mol Genet 19: 1108–1118, 2010. doi: 10.1093/hmg/ddp583. [DOI] [PMC free article] [PubMed] [Google Scholar]