Abstract
Introduction
Active surveillance (AS) is an accepted management strategy for low-risk prostate cancer (PCa), but its role in the management of favorable intermediate-risk PCa remains controversial. Most reports studying the role of AS for these men generally lack long-term followup and include small numbers of patients. Our objective was to report the outcomes of men diagnosed with Gleason grade groups (GGG) 2 and 3 PCa who were managed expectantly.
Methods
Using administrative datasets and pathology reports, we identified all men who were diagnosed with GGG 2 and 3 PCa and managed expectantly between 2002 and 2011 in Ontario, Canada. Outcomes and associated factors were estimated using cumulative incidence function methods and multivariable Cox regression models, respectively.
Results
We identified 926 men who were managed expectantly (AS [n=374] or watchful waiting [n=552]). The eight-year cancer-specific survival was 94% and 89% for the AS and watchful waiting cohorts, respectively. Among AS men, 266 (71%) received treatment after a followup of approximately eight years. Cumulative AS discontinuation rates at one and five years were 30.5% and 65.1%, respectively.
Conclusions
Expectant management of GGG 2 and 3 PCa may be an option for certain men. Notably for AS patients, the cancer-specific mortality at eight years was 6%, and over 65% of men underwent treatment within five years. Further studies are required to evaluate which patients, based on disease-specific features and competing health risks, would benefit most from a conservative strategy.
Introduction
Traditionally, men diagnosed with localized prostate cancer (PCa) were treated by radical prostatectomy (RP) or a form of radiotherapy.1 However, natural history studies have shown that only a minority of those with low- or intermediate-risk disease will develop metastases and/or succumb to the cancer.2 Thus, active surveillance (AS) has become an accepted strategy for low-risk disease, but debated as to its application in intermediate-risk PCa.3,4 Several large cohort studies and a randomized controlled trial have demonstrated that, for low-risk PCa, AS offers similar 10-year cancer-specific survival (CSS) compared to other well-accepted PCa treatments.5–12 Consequently, an increasing number of men are now managed this way although rates vary worldwide.1,9,13–15
Reports have suggested that AS could be applied to favorable intermediate-risk PCa given that these cancers may behave in a similar fashion to low-risk PCa.5,10,16–19 However, these experiences lack long-term followup and are generally of smaller cohorts when compared to the reports supporting AS in low-risk PCa. To our knowledge, there has not been a population-based study reporting on the long-term outcomes of Gleason Grade Groups (GGG) 2 and 3 PCa managed by AS.
Our primary objective was to report the CSS of men diagnosed with GGG 2 and 3 PCa in between 2002 and 2011 and managed expectantly, with a focus on those followed by AS. Secondary objectives were to: 1) determine the overall survival (OS) of men with GGG 2 and 3 PCa managed expectantly; 2) estimate the discontinuation rate from AS; 3) investigate characteristics associated with cancer-specific, overall and treatment-free survivals; and 4) report use of primary androgen deprivation therapy (ADT).
Methods
Study design
This was an institutional review board-approved, population-based study. Men diagnosed with PCa were identified using linked administrative databases. In Ontario, nearly all medical procedures are reimbursed by a single-payer system, the Ontario Health Insurance Plan (OHIP).20 The OHIP database was used to identify all PCa-related interventions (Supplementary Table 1; available at cuaj.ca). Transrectal or transperineal ultrasound-guided biopsy pathology reports were obtained from Cancer Care Ontario and were manually abstracted by two trained abstractors. The procedure codes and the abstracted data were then linked deterministically to several other administrative databases.
Population
The cohort consisted of men diagnosed with GGG 2 or 3 PCa in Ontario between 2002 and 2011. We excluded men whose diagnostic procedure was not a transrectal or transperineal ultrasound-guided biopsy and men with <1 year of followup. Men who were treated without a prior confirmatory biopsy (defined as the second biopsy following the diagnostic one) or with a confirmatory biopsy performed within 14 days of treatment were also excluded (i.e., biopsy likely done at the time of treatment) (Supplementary Fig. 1; available at cuaj.ca). All men who had a confirmatory biopsy with or without treatment thereafter were considered to have been managed by AS, while men who had no confirmatory biopsy and did not undergo definitive treatment were considered to have been managed by watchful waiting (WW).
All localized GGG 2 and 3 PCa were included in this study, regardless of the digital rectal exam (DRE) and/or prostate-specific antigen (PSA) levels, as these variables were not completely captured in any of the administrative databases.
Outcomes
The primary outcome measured was CSS. Secondary outcomes were OS, discontinuation of AS, and use of primary ADT. Survival outcomes were obtained using data from the Ontario Cancer Registry and from the Registered Persons Database.21,22 Cause of death was available up to December 31, 2012 while data for treatment and vital status were available up to December 31, 2014. Administrative codes used to identify treatments and use of ADT are detailed in Supplementary Table 1 (available at cuaj.ca) and have previously been shown to have high accuracy.23,24
Covariates
We used administrative databases to obtain a comprehensive set of covariates for risk adjustment. These included individual-, disease-specific, physician- and institution-level characteristics (Supplementary Table 2; available at cuaj.ca). Individual-level characteristics included age at diagnosis, year of diagnosis, neighbourhood income quintile, area of residency, initial management, and comorbidities. The aggregated diagnostic groups (ADG) score, derived from the Johns Hopkins University Adjusted Clinical Group® case mix system, was used as a proxy for the patient’s comorbidities.25 Disease-specific characteristics included PSA level and GGG at diagnosis, number of cores taken, number of positive cores, percentage of maximal core involvement at the initial and confirmatory biopsies as well as the timing of the confirmatory transrectal ultrasound-guided biopsy, where applicable. Physician-level characteristics included specialty of the treating physicians and their annual new PCa case volume, whereas institution-level characteristics included the type of treating centers and their annual new PCa case volume. The treating physician was defined as the physician who claimed the most PCa-related visits for each patient during the first 12 months after diagnosis, while the treating center was defined as the center where the patient received the majority of his PCa care during the same timeframe.
Statistical analysis
Baseline characteristics were reported using descriptive statistics and compared using Wilcoxon and Student’s t-tests for medians and means, respectively, and chi-squared tests for categorical variables.
Time on AS and time to death (where applicable) were calculated from the date of diagnosis to the date when patients experienced an event (treatment or death) or were censored (i.e., end of followup period [December 31, 2014] or lost to followup [date of last contact with OHIP]). The treatment-free, ADT-free, cancer-specific, and overall survivals were estimated using cumulative incidence function methods. Their associated factors were evaluated using Cox proportional hazard (PH) models fit for a priori-defined variables (OS) or fit with variables using a stepwise regression process (treatment-free and cancer-specific survivals) and adjusted for physician- and institution-level clusters assuming cross-classified data (i.e., physicians could work in more than one institution).26 Estimates in the multivariable models are reported as hazards ratios (HRs) with corresponding 95% confidence intervals (CIs). PH assumptions were assessed by examining residuals and with log-log plots. Fine and Gray models were also performed to account for competing risks. However, given that Cox PH and competing risk models yielded similar results, we have opted to present the Cox PH models for ease of interpretation. All statistical analyses were performed using SAS 9.4 and R version 3.1.3. All statistical tests were two-sided, and p-values <0.05 were considered statistically significant.
Results
A total of 4040 patients with GGG 2 or 3 PCa at diagnosis were identified. Of these, 3179 were excluded because they did not meet our inclusion criteria. Most (n=2179) were excluded because they received treatment without a prior confirmatory biopsy within one year of diagnosis (Supplementary Fig. 1; available at cuaj.ca). Consequently, the study cohort included 926.
Table 1 shows the demographics and disease characteristics of the cohort according to initial management. Men on WW (n=553) were significantly older than men on AS (n=374). Likewise, their median PSA at diagnosis, GGG, number of cores positive for cancer, and maximal percentage of core involvement were all significantly higher.
Table 1.
Baseline characteristics for the whole cohort and stratified by watchful waiting and active surveillance
| Variables | Total (n=926) | Watchful waiting (n=552) | Active surveillance (n=374) | p |
|---|---|---|---|---|
| Patient-specific characteristics | Ipsum | |||
| Age (years), mean (SD) | 72 (9) | 75 (8) | 67 (8) | <0.001 |
| Year of diagnosis, n (%) | ||||
| 2002–04 | 282 (31) | 203 (37) | 79 (21) | <0.001 |
| 2005–07 | 490 (53) | 295 (53) | 195 (52) | |
| 2008–11 | 154 (17) | 54 (10) | 100 (27) | |
| ADG scores, mean (SD) | 16 (12) | 18 (13) | 13 (11) | <0.001 |
| Area of residency, n (%) | ||||
| Rural | 119 (13) | 82 (15) | 37 (10) | 0.03 |
| Urban | 806 (87) | 470 (85) | 336 (90) | |
| Missing | 1 (0) | 0 (0) | 1 (0) | |
| Neighborhood income quintile, n (%) | 0.01 | |||
| 1st quintile (lowest) | 166 (18) | 107 (20) | 59 (16) | |
| 2nd quintile | 173 (19) | 108 (20) | 65 (17) | |
| 3rd quintile | 176 (19) | 115 (21) | 61 (16) | |
| 4th quintile | 197 (21) | 114 (21) | 83 (22) | |
| 5th quintile (highest) | 212 (23) | 106 (19) | 106 (28) | |
| Missing | 2 (0) | 2 (0) | 0 (0) | |
| Disease-specific characteristics | ||||
| PSA (ng/mL), median (IQR)* | 8.4 (5.8–14) | 10.3 (6.5–20) | 6.9 (5.2–9.5) | 0.004 |
| Number of cores taken at diagnostic biopsy, median (IQR)† | 10 (7–12) | 9 (6–11) | 10 (8–12) | <0.001 |
| Number of positive cores at diagnostic biopsy, mean (IQR)‡ | 4 (1–12) | 4 (1–13) | 3 (1–12) | 0.001 |
| Max. % of core at diagnostic biopsy, median (IQR)Δ | 30 (5–70) | 30 (2–70) | 25 (10–60) | 0.4 |
| Gleason grade group (GGG) at diagnosis, n (%) | <0.001 | |||
| GGG 2 (Gleason score 3+4=7) | 644 (70) | 361 (65) | 283 (76) | |
| GGG 3 (Gleason score 4+3=7) | 282 (30) | 191 (35) | 91 (24) | |
| Prostate cancer treatment-specific characteristics | ||||
| Type of primary physician¥ | <0.001 | |||
| Urologist | 808 (89) | 505 (93) | 303 (82) | |
| Radiation oncologist | 103 (11) | 35 (7) | 68 (18) | |
| Type of center¥ | <0.001 | |||
| Non-specialized cancer center | 637 (69) | 458 (83) | 179 (48) | |
| Specialized cancer center | 289 (31) | 94 (17) | 195 (52) | |
| Institution volume¥ | 0.4 | |||
| 1st tertile (lowest) | 53 (6) | 29 (5) | 27 (7) | |
| 2nd tertile | 198 (22) | 113 (21) | 82 (22) | |
| 3rd tertile (highest) | 658 (72) | 397 (74) | 261 (71) | |
| Physician volume¥ | <0.001 | |||
| 1st tertile (lowest) | 71 (8) | 29 (5) | 45 (12) | |
| 2nd tertile | 217 (24) | 120 (22) | 103 (28) | |
| 3rd tertile (highest) | 623 (38) | 391 (72) | 223 (60) | |
Data missing in 396 patients (43%);
data missing in 87 (9%) patients;
data missing in 125 patients (14%);
data missing in 281 patients (30%);
data missing in 17 physicians/institutions (2%). ADG: aggregated diagnosis groups; GGG: Gleason grade group; IQR: interquartile range; PSA: prostate-specific antigen; SD: standard deviation.
For men on AS, the median number of biopsies after diagnosis was 2 (interquartile range [IQR] 2–3), with a median time from diagnosis to confirmatory biopsy of 9.3 months (IQR 3.4–21). On these confirmatory biopsies, 27% (n=102) were downgraded to GGG 1 or were negative (Table 2).
Table 2.
Timing and outcomes of the confirmatory biopsy for men managed by active surveillance (n=374)
| Variables | Values |
|---|---|
| Time (in months) from initial to confirmatory biopsy, median (IQR) | 9.3 (3.4–21) |
| Number of cores taken at confirmatory biopsy, mean (IQR)† | 11 (3.9) |
| Number of positive cores at confirmatory biopsy, mean (SD)† | 3.9 (2.4) |
| Max. % of core at confirmatory biopsy, median (IQR)† | 30 (5–70) |
| Gleason grade group (GGG) at confirmatory biopsy, n (%) | |
| Negative or GGG 1 (Gleason score ≤6) | 102 (27) |
| GGG 2 (Gleason score 3+4=7) | 170 (45) |
| GGG 3 (Gleason score 4+3=7) | 87 (23) |
| GGG 4 or 5 (Gleason score 8–10) | 15 (4) |
| Confirmatory biopsy demonstrated: | |
| Upgrading of GGG, Yes (%) | 58 (16) |
| GGG 2 to GGG 3 | 43 (74) |
| GGG 2 to GGG 4–5 | 7 (12) |
| GGG 3 to GGG 4–5 | 8 (14) |
| Increase in number of positive cores, Yes (%) | |
| ≤ 3 to >3 | 41 (11) |
| Unknown | 136 (36) |
| Increase in max. percentage of core involvement, Yes (%) | |
| ≤ 50% to >50% | 29 (14) |
| Unknown | 201 (54) |
Data missing in 155 (41%) patients.
IQR: interquartile range; SD: standard deviation.
Survival outcomes
After a median followup of 91 months (IQR 60–116), 371 (40%) deaths were identified. When followup time was limited to December 31, 2012 (when cause of death was available), 260 (28%) deaths were identified, of which 63 (24%) were due to PCa. Significantly more deaths due to PCa were identified in the WW group than in the AS group (48 [9%] vs. 15 [4%]; p=0.006) (Supplementary Table 3; available at cuaj.ca). In the AS cohort, 7 (3%) PCa-related deaths were reported in GGG 2 after eight years, while 6 (7%) were reported in the GGG 3 (Supplementary Table 4; available at cuaj.ca). Interestingly, of all men who died from PCa, only 5 (8%) received some form of ADT during their last year of life. The five- and eight-year CSS were 98% and 94% for the AS cohort and 94% and 89% for the WW cohort, respectively. Overall, these men were four times more likely to die from causes other than PCa (Supplementary Tables 3, 4; available at cuaj.ca).
On multivariable analysis (Table 3), older age, higher GGG at diagnosis, and higher maximal percentage of core involvement at diagnosis were strong predictors of higher PCa mortality. Factors associated with overall mortality are shown in Supplementary Table 5 (available at cuaj.ca).
Table 3.
Univariable and multivariable Cox proportional hazards survival model testing for factors associated with cancer-specific survival
| Variables | Univariable HR (95%CI) |
p | Multivariable† HR (95%CI) |
p |
|---|---|---|---|---|
| Patient-specific characteristics | ||||
| Age, per 10-year increase | 1.87 (1.33–2.63) | <0.001 | 1.61 (1.14–2.28) | 0.007 |
| Year of diagnosis | ||||
| 2002–04 | REF | |||
| 2005–07 | 0.78 (0.46–1.38) | 0.4 | ||
| 2008–11 | 0.21 (0.03–1.59) | 0.13 | ||
| ADG scores, per 1-unit increase | 1.02 (1.01–1.04) | 0.01 | ||
| Area of residency (rural vs. urban) | 0.79 (0.34–1.84) | 0.6 | ||
| Neighborhood income quintile | ||||
| 1st quintile (lowest) | REF | |||
| 2nd quintile | 0.45 (0.20–0.99) | 0.04 | ||
| 3rd quintile | 0.44 (0.20–0.98) | 0.04 | ||
| 4th quintile | 0.67 (0.35–1.31) | 0.3 | ||
| 5th quintile (highest) | 0.41 (0.19–0.88) | 0.02 | ||
| Initial management (watchful waiting vs. active surveillance) | 2.18 (1.22–3.91) | 0.009 | ||
| Disease characteristics | ||||
| PSA category at diagnosis (ng/mL) | ||||
| 0–4 | REF | |||
| 4.01–10 | 0.13 (0.05–1.44) | 0.13 | ||
| >10 | 1.48 (0.35–6.36) | 0.6 | ||
| Missing | 1.42 (0.34–5.93) | 0.6 | ||
| Positive cores at diagnosis | ||||
| 1 | REF | |||
| 2 | 3.18 (0.70–15) | 0.14 | ||
| 3 | 4.14 (0.92–19) | 0.07 | ||
| >3 | 4.18 (0.99–18) | 0.05 | ||
| Missing | 4.32 (0.96–20) | 0.06 | ||
| Max. % of core involvement at diagnosis (>50% vs. ≤50%) | 2.73 (1.47–5.07) | 0.002 | 2.12 (1.13–4.01) | 0.02 |
| Gleason grade group at diagnosis (3 vs. 2) | 2.11 (1.29–3.45) | 0.003 | 1.81 (1.09–3.01) | 0.02 |
| Definitive treatment (yes vs. no) | 0.41 (0.20–0.84) | 0.01 | ||
| Prostate cancer treatment-specific characteristics | ||||
| Primary physician (urologist vs. radiation oncologist) | 1.59 (0.64–3.98) | 0.3 | ||
| Physician annual prostate cancer treatment volume | ||||
| 1st tertile (lowest) | REF | |||
| 2nd tertile | 1.66 (0.49–5.65) | 0.4 | ||
| 3rd tertile (highest) | 1.48 (0.46–4.79) | 0.5 | ||
| Specialized cancer-center (yes vs. no) | 0.41 (0.21–0.81) | 0.01 | 0.52 (0.26–1.06) | 0.07 |
| Institution annual prostate cancer treatment volume | ||||
| 1st tertile (lowest) | REF | |||
| 2nd tertile | 0.61 (0.23–1.58) | 0.3 | ||
| 3rd tertile (highest) | 0.57 (0.24–1.34) | 0.19 | ||
Variables significant in univariate model were selected for multivariable model, and a stepwise selection approach was used for the final multivariate model.
ADG: aggregated diagnosis groups; CI: confidence interval; HR: hazard ratio; PSA: prostate-specific antigen.
Treatment-free survival
After a median followup of 97 months (IQR 72–121), 266 (71%) patients had discontinued AS. Among the patients who discontinued AS, an equal number of patients were treated with RP and radiotherapy (n=119 [45%] for both therapeutic approaches). The remaining 28 (11%) patients were managed with ADT alone (median time to initiation was 26 months [IQR15–38]). Most men who discontinued AS did so following confirmatory biopsy (n=179; 67.3%). The apparent reasons for discontinuation of AS are summarized in Table 4. Of the men who underwent a RP, 25 (21.0%) were found to have a lower GGG than at diagnosis, while 22 (18.5%) were upgraded (Supplementary Table 6; available at cuaj.ca).
Table 4.
Number of transrectal ultrasound biopsy before discontinuation of active surveillance and the apparent reasons for the discontinuation (n=266 men who underwent treatment)
| Number of biopsy before discontinuation | n (%) |
|---|---|
| After second (confirmatory) biopsy | 179 (67%) |
| After third biopsy | 73 (27%) |
| After fourth biopsy | 10 (4%) |
| After fifth biopsy | 3 (0.4%) |
| After sixth biopsy | 1 (0.3%) |
| Perceived reason for discontinuation | |
| Gleason grade group (GGG) upgrade on subsequent biopsy (i.e., GGG2 to 3, GGG 2 or 3 to 4–5) | 13 (5%) |
| Tumor volume increase from baseline (i.e., positive cores ≤3 to >3 or maximal percentage core involvement from <50% to ≥50% on subsequent biopsy) | 42 (16%) |
| PSA increase from baseline (i.e., ≤10 ng/ mL at diagnosis to >10 ng/mL) | 3 (1%) |
| Not perceived reasons (i.e., no volume increase from baseline, no GGG change or GGG downgrading on subsequent biopsy, or no PSA increase) | 46 (17%) |
| Unknown (i.e., 1 of 4 variables unavailable: PSA, GGG, maximal percentage core involvement, or number of positive cores) | 162 (61%) |
PSA: prostate-specific antigen.
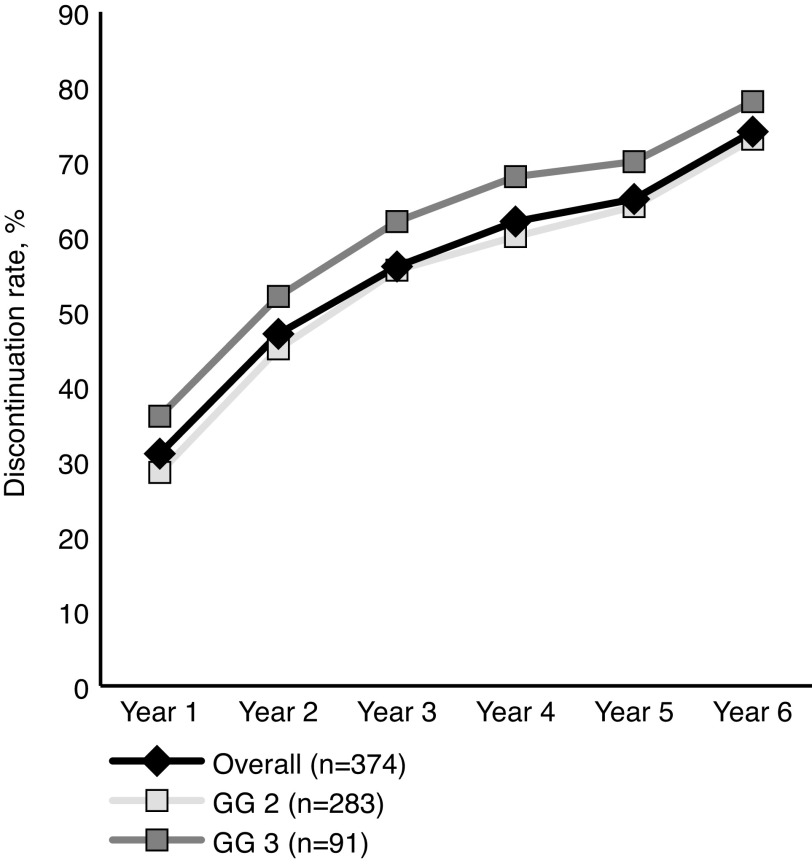
The median time to discontinuation of AS was 59 months (IQR 23–101). Cumulative discontinuation rates at one and five years were 30.5% and 65.1%, respectively. When stratified by GGG at diagnosis, the five-year discontinuation rates were 63.5% and 69.9% for GGG 2 and 3, respectively (Fig. 1). Factors associated with decreased discontinuation within the first five years were older age, being diagnosed in the earliest year of the study period, being downgraded or having a negative confirmatory biopsy, and having a lower number of positive cores at confirmatory biopsy. Patients whose primary treating physician was a urologist and patients managed in non-specialized cancer centers were also less likely to discontinue AS (Table 5).
Fig. 1.
Active surveillance discontinuation rates over 10 years. GG: Gleason grade group.
Table 5.
Factors associated with the discontinuation of active surveillance within 5 years of diagnosis
| Variables | Univariable HR (95% CI) |
p | Multivariable† HR (95% CI) |
p |
|---|---|---|---|---|
| Patient-specific characteristics | ||||
| Age, per 10 years increase | 0.63 (0.55–0.74) | <0.001 | 0.60 (0.51–0.71) | <0.001 |
| Year of diagnosis | ||||
| 2002–04 | REF | REF | ||
| 2005–07 | 0.76 (0.55–1.04) | 0.09 | 1.43 (1.00–2.05) | 0.049 |
| 2008–11 | 0.84 (0.59–1.20) | 0.4 | 1.79 (1.18–2.72) | 0.006 |
| ADG scores, per 1-unit increase | 0.99 (0.98–1.00) | 0.11 | ||
| Area of residency (rural vs. urban) | 1.31 (0.86–1.96) | 0.19 | ||
| Neighborhood Income quintile | ||||
| 1st quintile | REF | |||
| 2nd quintile | 1.33 (0.85–2.09) | 0.2 | ||
| 3rd quintile | 1.34 (0.85–2.10) | 0.2 | ||
| 4th quintile | 1.19 (0.77–1.83) | 0.4 | ||
| 5th quintile | 1.35 (0.90–2.04) | 0.15 | ||
| Disease characteristics at diagnosis | ||||
| PSA category at diagnosis (ng/mL) | ||||
| 0–4 | REF | |||
| 4.01–10 | 1.35 (0.74–2.46) | 0.3 | ||
| >10 | 1.77 (0.91–3.41) | 0.09 | ||
| Missing | 1.29 (0.71–2.35) | 0.4 | ||
| Number of positive cores at diagnosis | ||||
| 1 | REF | |||
| 2 | 1.02 (0.65–1.57) | 0.9 | ||
| 3 | 1.44 (0.93–2.23) | 0.10 | ||
| >3 | 1.44 (0.99–2.09) | 0.06 | ||
| Missing | 1.19 (0.74–1.93) | 0.5 | ||
| Max. % of core involvement at diagnostic (>50% vs. ≤50%) | 1.19 (0.82–1.73) | 0.4 | 0.79 (0.54–1.18) | 0.3 |
| Gleason grade group at diagnosis (3 vs. 2) | 1.22 (0.92–1.63) | 0.16 | 1.33 (0.98–1.82) | 0.06 |
| Disease characteristics at confirmatory biopsy | ||||
| Gleason grade group at confirmatory | ||||
| 2 or 3 | REF | REF | ||
| 4 or 5 (upgraded) | 1.08 (0.60–1.93) | 0.8 | 1.30 (0.70–2.42) | 0.4 |
| 1 (downgraded) | 0.55 (0.39–0.80) | 0.002 | 0.61 (0.44–0.99) | 0.02 |
| Negative | 0.37 (0.21–0.66) | <0.001 | 0.33 (0.18–0.61) | <0.001 |
| Number of positive cores at confirmatory | ||||
| 1 | REF | REF | ||
| 2 | 1.23 (0.67–2.38) | 0.5 | 1.03 (0.52–2.02) | 0.9 |
| 3 | 1.84 (1.02–3.31) | 0.04 | 2.06 (1.08–3.09) | 0.03 |
| 3+ | 1.76 (1.04–2.96) | 0.03 | 1.56 (0.86–2.82) | 0.14 |
| Missing | 1.99 (1.19–3.35) | 0.009 | 2.59 (1.40–4.78) | 0.002 |
| Max. % of core involvement at confirmatory biopsy (>50% vs. ≤50%) | 1.14 (0.84–1.55) | 0.4 | ||
| Prostate cancer treatment-specific characteristics | ||||
| Primary physician (urologist vs. radiation oncologist | 0.41 (0.31–0.56) | <0.001 | 0.43 (0.29–0.61) | <0.001 |
| Physician annual prostate cancer treatment volume | ||||
| 1st tertile (lowest) | REF | |||
| 2nd tertile | 1.28 (0.75–1.86) | 0.5 | ||
| 3rd tertile (highest) | 1.31 (0.87–1.99) | 0.19 | ||
| Specialized cancer-center (yes vs. no) | 1.59 (1.23–2.05) | <0.001 | 1.35 (1.00–1.82) | 0.048 |
| Institution annual prostate cancer treatment volume | ||||
| 1st tertile (lowest) | REF | |||
| 2nd tertile | 1.10 (0.63–1.93) | 0.7 | ||
| 3rd tertile (highest) | 1.20 (0.72–2.01) | 0.5 | ||
Variables significant in univariate model were selected for multivariable model, and a stepwise selection approach was used for the final multivariate model. ADG: aggregated diagnosis group; CI: confidence interval; HR: hazard ratio; PSA: prostate specific antigen.
Discussion
The role of AS for GGG 2 and 3 remains controversial. To our knowledge, this is the largest population-based study describing the outcomes of expectant management for these men. Our results demonstrated that 4% and 11% of men managed by AS and by WW, respectively, died of PCa during the first eight years of followup. Unsurprisingly, PCa-related deaths were more common among men with GGG 3 diseases than among men with GGG 2 cancers. Interestingly, of these men, only 8% received some form of ADT during their last year of life. Therefore, one could speculate that although PCa was specified as their cause of death, it is entirely plausible that many died of other causes. Nevertheless, even with this possibility in mind, men managed expectantly were four times more likely to die from non-PCa related causes.
Additionally, over 65% of men on AS were treated within five years, including 6% with primary ADT. Factors associated with AS discontinuation included age, year of diagnosis, and total number of positive cores at the confirmatory biopsy. Interestingly, men treated by radiation oncologists or in dedicated cancer centers were more likely to undergo treatment during followup. Although this could indicate a practice pattern, it is also plausible that the association is more reflective of a referral pattern than a true treatment philosophy. In addition to these aforementioned factors, our results also demonstrated that men who were downgraded to GGG 1 or who had a negative confirmatory biopsy were significantly less likely to discontinue AS within five years of diagnosis.
The survival outcomes reported here are in line with several previously published reports. These studies have reported cancer-specific mortality rates for men with intermediate-risk PCa managed by AS varying from 0–4% after a followup ranging from 28–80 months.7,11,16–19 Importantly, the outcomes reported by these studies are no different to the outcomes of men with similar disease who have undergone treatment. Based on data from the National Prostate Cancer Register of Sweden, Stattin et al have reported a 10-year CSS of 96.6% and 96.2% after RP and radiation therapy, respectively.27 Similar numbers (95% CSS) were reported by Stephenson et al at 10-year followup for patients who underwent a RP for GGG 2 and 3 PCa.28 Thus, the evidence suggests that, at the very least, a subset of GGG 2 and 3 PCa patients could be managed with AS while avoiding some of the potential complications associated with PCa treatments.
In spite of the reassuring survival outcomes, the definitive treatment rate was higher than that published in previous reports, with rates historically varying from 29–61%.7,11, 16–19 One of the possible explanations for our higher rates, in addition to longer followup when compared to previous publications, is the fact our study only included men that were considered as intermediate-risk PCa based solely on their GGG and not on their PSA level or DRE. In comparison, in the previously reported studies of men with intermediate-risk PCa managed by AS, the proportion of men included with GGG 2 and 3 PCa varied from 22–63%.7,11,16–19 Regardless, one needs to remember that nearly 30% of men in our cohort avoided the potential complications of PCa treatment by choosing AS.
Although this study reports the outcomes of the largest GGG 2 and 3 PCa cohort managed expectantly, it is not devoid of limitations. The study was based on administrative databases and lacks the granularity of prospective studies. Because of this, data for some variables were incomplete (PSA values, number of positive cores at confirmatory biopsy, etc.) or were not captured (DRE findings, metastatic state, etc.). Likewise, as the cause of death was not available after 2012, our results may have underestimated the proportion of patients who died from PCa during the study period. As demonstrated by our rates of upgrading at confirmatory biopsy, and as suggested by others, it is entirely possible that the initial biopsy underestimated the true extent of the disease.29 Additionally, 6% of patients were started on primary ADT during followup, which is thought to be a proxy for metastatic disease. Therefore, it is possible that longer followup would have found more PCa-related deaths and/or metastatic diseases. The study also lacks information on family history, race, and use of diagnostic imaging, such as multiparametric magnetic resonance imaging. Moreover, we defined AS patients as those who received a confirmatory biopsy. This definition likely introduced a certain selection bias, as it is well-known that not all AS patients will undergo a confirmatory biopsy.16,30 Lastly, this study lacks a comparative treatment arm. Consequently, this limited our conclusions with regard to which patients were ideal candidates for AS and what the triggers for intervention should be. Nevertheless, our results indicated that older men and men with more favorable findings on the diagnostic biopsy (i.e., GGG 2 and ≤50% maximal core involvement) were less likely to die from PCa and, thus, may potentially be better candidates.
Conclusions
Expectant management of GGG 2 and 3 PCa remains an option for certain men, as many will succumb to a non-PCa-related death. Nevertheless, men on AS had a 6% cancer-specific mortality at eight years after diagnosis, and more than 65% of them were treated within five years. It is clear this option should not be applied to all GGG 2 and 3 patients. Further studies are required to evaluate which subgroup of patients would benefit most from a conservative approach. Men with GGG 2 and 3 PCa opting for this strategy should fully understand the potential benefits and harms of this approach and the high likelihood of eventually undergoing treatment.
Supplementary Information
Footnotes
See cuaj.ca for supplementary data
Competing interests: Dr. Richard has been an advisory board member for BMS and Sanofi; a speakers’ bureau member for Abbvie, Amgen Astellas, Ferring, and Janssen; and has participated in clinical trials supported by Calithera and Lidds Pharma. Dr. Martin has been a consultant for Abbvie, Amgen, Astellas, Bayer, Janssen, Roche, and Sanofi. Dr. Finelli has been an advisory board member for Abbvie, Astellas, Bayer, Ipsen, Janssen, Sanofi, and TerSera; and has participated in clinical trials supported by Astellas, Bayer, and Janssen. The remaining authors report no competing personal or financial interests related to this work.
Funding: This study was supported by Prostate Cancer Canada (through the Movember Foundation funding) and the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
This paper has been peer-reviewed.
References
- 1.Cooperberg M, Carroll P. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA. 2015;314:80–2. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts and figures. 2017. Dec 31, [Accessed Jan 20, 2020]. 2017. Available at: www.cancer.org.
- 3.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: Risk stratification, shared decision-making, and care options. J Urol. 2018;199:683–90. doi: 10.1016/j.juro.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 4.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term followup of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 6.Klotz L, Zhang L, Lam A, et al. Clinical results of a long-term followup of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 7.Bul M, van den Bergh R, Zhu X, et al. Outcomes of initially expectantly managed patients with low- or intermediate-risk screen-detected localized prostate cancer. BJU Int. 2012;110:1672–7. doi: 10.1111/j.1464-410X.2012.11434.x. [DOI] [PubMed] [Google Scholar]
- 8.Eggener S, Mueller A, Berglund R, et al. A multi-institutional evaluation of active surveillance for low-risk prostate cancer. J Urol. 2013;189:S19–26. doi: 10.1016/j.juro.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Loeb S, Folkvaljon Y, Makarov D, et al. Five-year nationwide followup study of active surveillance for prostate cancer. Eur Urol. 2015;67:233–8. doi: 10.1016/j.eururo.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selvadurai E, Singhera M, Thomas K, et al. Medium-term outcomes of active surveillance for localized prostate cancer. Eur Urol. 2013;64:981–7. doi: 10.1016/j.eururo.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active surveillance program for favorable-risk prostate cancer. J Clin Oncol. 2015;33:3379–85. doi: 10.1200/JCO.2015.62.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman K, Niu J, Shen Y, et al. Physician variation in management of low-risk prostate cancer: A population-based cohort study. JAMA Intern Med. 2014;174:1450–9. doi: 10.1001/jamainternmed.2014.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richard PO, Alibhai SM, Panzarella T, et al. The uptake of active surveillance for the management of prostate cancer: A population-based analysis. Can Urol Assoc J. 2016;10:333–8. doi: 10.5489/cuaj.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weerakoon M, Papa N, Lawrentschuk N, et al. The current use of active surveillance in an Australian cohort of men: A pattern of care analysis from the Victorian Prostate Cancer Registry. BJU Int. 2015;115:50–6. doi: 10.1111/bju.13049. [DOI] [PubMed] [Google Scholar]
- 16.Cooperberg MR, Cowan JE, Hilton JF, et al. Outcomes of active surveillance for men with intermediaterisk prostate cancer. J Clin Oncol. 2011;29:228–34. doi: 10.1200/JCO.2010.31.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munsunru HB, Yamamoto T, Klotz L, et al. Active surveillance for intermediate-risk prostate cancer: Survival outcomes in the Sunnybrook experience. J Uol. 2016;196:1651–8. doi: 10.1016/j.juro.2016.06.102. [DOI] [PubMed] [Google Scholar]
- 18.Newcomb LF, Thompson IM, Jr, Boyer HD, et al. Outcomes of active surveillance for clinically localized prostate cancer in the prospective, multi-institutional Canary PASS cohort. J Uol. 2016;2:313–20. doi: 10.1016/j.juro.2015.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whalen MJ, Pak JS, Lascano D, et al. Oncologic outcomes of definitive treatments for low- and intermediaterisk prostate cancer after a period of active surveillance. Clin Genitourin Cancer. 2018;16:e425–35. doi: 10.1016/j.clgc.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Robles S, Marrett L, Clarke E, et al. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol. 1988;41:495–501. doi: 10.1016/0895-4356(88)90052-2. [DOI] [PubMed] [Google Scholar]
- 21.Juurlink D, Preyra C, Croxford R, et al. Canadian Institute for Health Information discharge abstract database: A validation study. Toronto: Institute for Clinical Evaluative Sciences; 2006. [Google Scholar]
- 22.Williams J, Young W. Inventory of studies on the accuracy of Canadian health administrative databases. ICES technical report. 1996 [Google Scholar]
- 23.Alibhai S. Do age and comorbidity influence the treatment of localized prostate cancer? Toronto, Ontario: University of Toronto; 2001. [Google Scholar]
- 24.Bhindi B. Prostate cancer as a metabolic disease — is prostate cancer diagnosis associated with worse cardiovascular outcomes? Toronto, Ontario: University of Toronto; 2014. [Google Scholar]
- 25.Austin P, van Walraven C. The mortality risk score and the ADG score. Two points-based scoring systems for the Johns Hopkins aggregated diagnosis groups to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49:940–7. doi: 10.1097/MLR.0b013e318229360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leckie G. Cross-classified multilevel models - Concepts. LEMMA VLE. 2013;12:1–60. [Google Scholar]
- 27.Stattin P, Holmberg E, Johansson JE, et al. Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden followup study. J Natl Cancer Inst. 2010;102:950–8. doi: 10.1093/jnci/djq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300–5. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gearman DJ, Morlacco A, Cheville JC, et al. Comparison of pathological and oncologic outcomes of favourable-risk Gleason score 3 + 4 and low-risk Gleason score 6 prostate cancer: Considerations for active surveillance. J Urol. 2018;199:1188–95. doi: 10.1016/j.juro.2017.11.116. [DOI] [PubMed] [Google Scholar]
- 30.Hefermehl LJ, Disteldorf D, Lehmann K. Acknowledging unreported problems with active surveillance for prostate cancer: A prospective, single-center, observational study. BMJ Open. 2016;6:e010191. doi: 10.1136/bmjopen-2015-010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.